Equilibrium, Kinetic, and Thermodynamic Studies of Cationic Dyes Adsorption on Corn Stalks Modified by Citric Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Adsorbent
2.2.2. Infrared Analysis
2.2.3. Adsorption Studies
2.2.4. Desorption Studies
2.2.5. Kinetic Studies
2.2.6. Adsorption Isotherms
2.2.7. Adsorption Thermodynamics
- Van der Waals areas of solvent and dye molecules were calculated using software package ChemAxon Marvin 5.2 [26]: so (H2O) = 0.0959 nm2, s (MB) = 1.01 nm2, s (MG) = 1.19 nm2.
- The values of ΔSo and ΔHo were evaluated from the intercept and slope of the van ’t Hoff plot of ln Ko vs. 1/T, respectively [17], assuming that ΔHo and ΔSo are temperature independent from 293–333 K.
2.2.8. Error Analysis
3. Results and Discussion
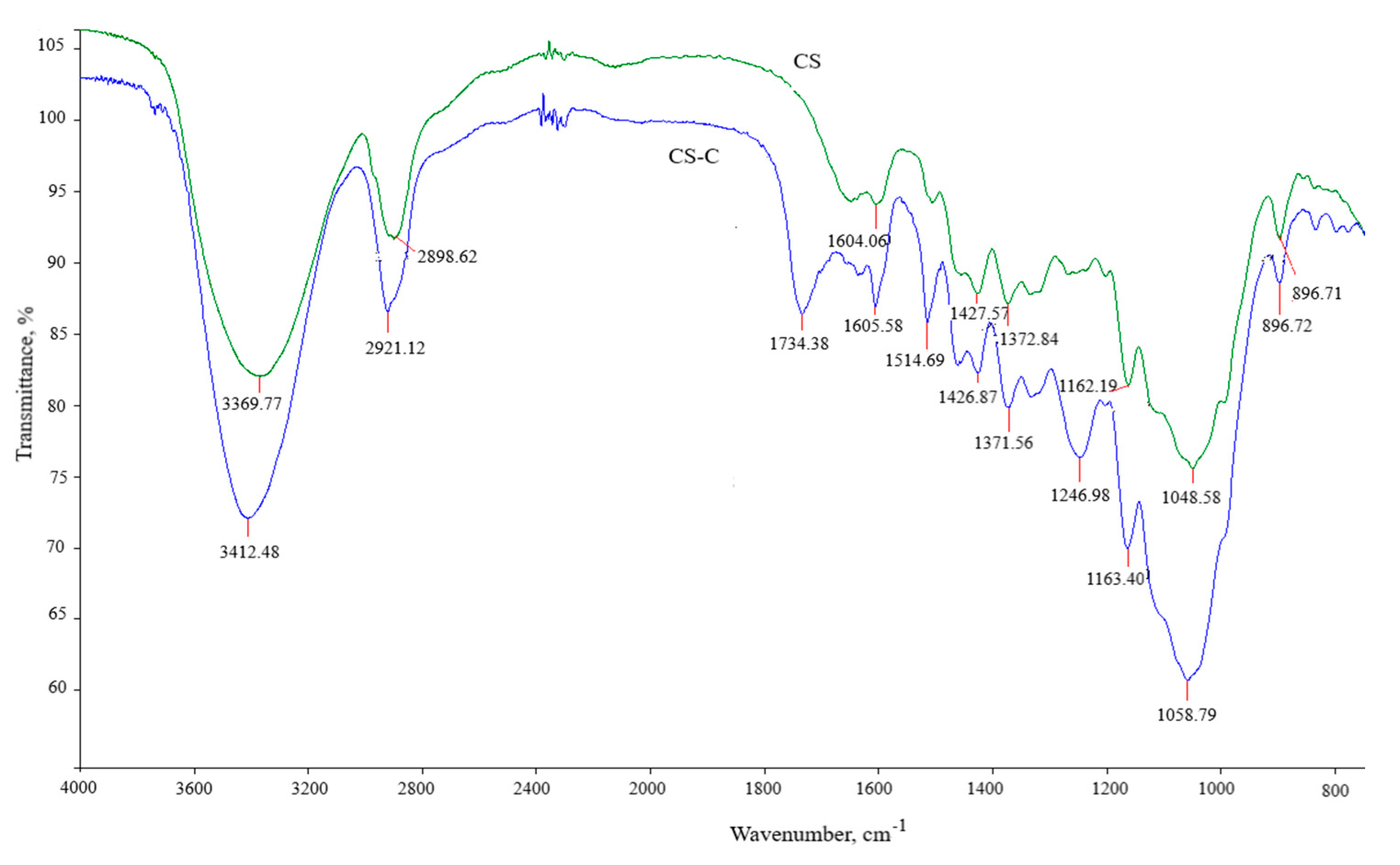
3.1. Characterization of Adsorbent CS-C
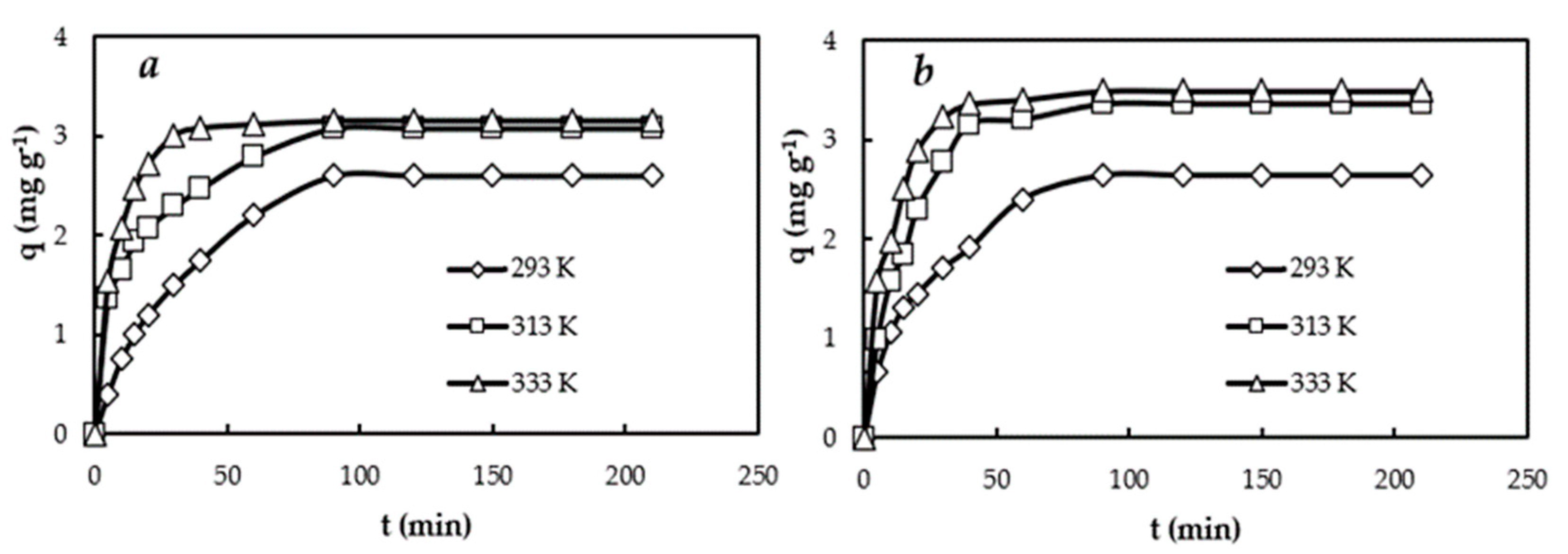
3.2. Kinetic Studies
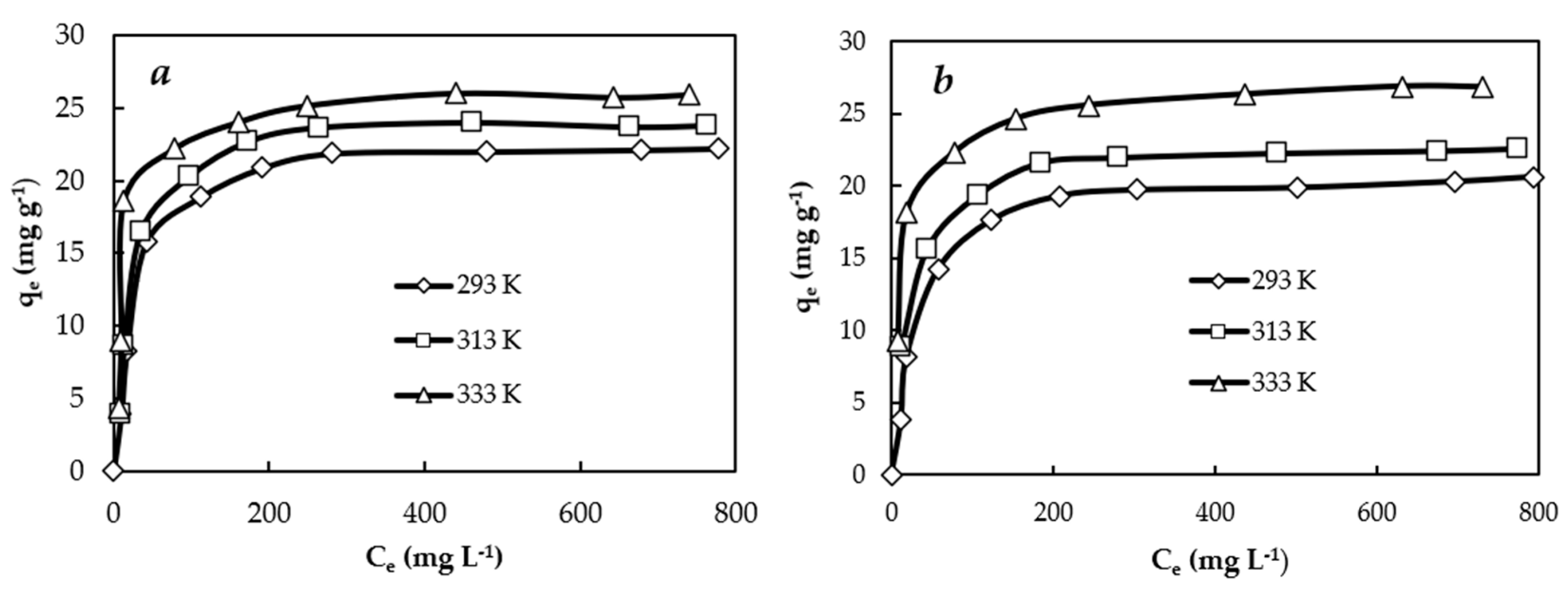
3.3. Equilibrium Studies
3.4. Thermodynamic Studies
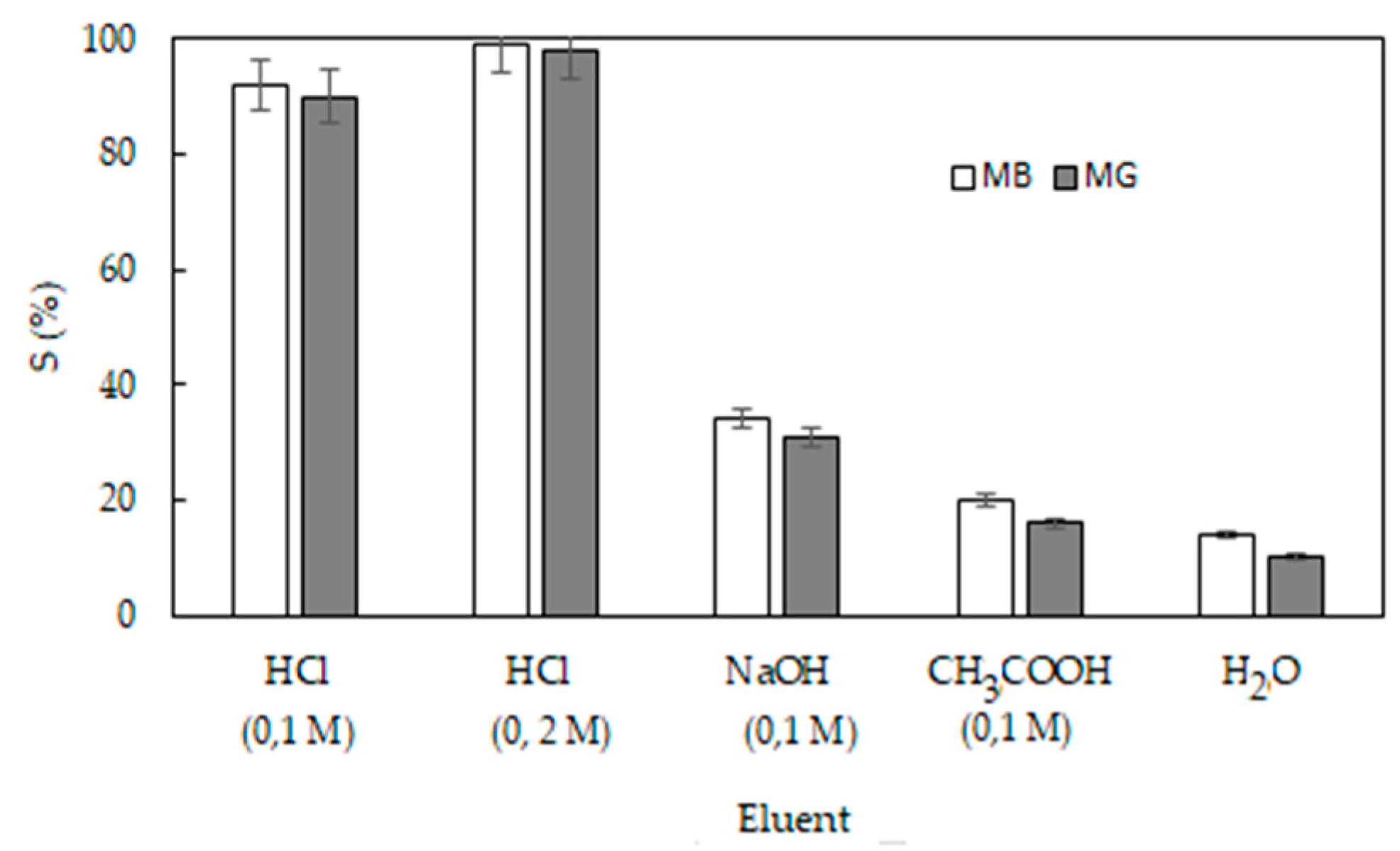
3.5. Desorption Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bushra, R.; Mohamad, S.; Alias, Y.; Jin, Y.; Ahmad, M. Current approaches and methodologies to explore the perceptive adsorption mechanism of dyes on low-cost agricultural waste—A review. Microporous Mesoporous Mater. 2021, 319, 111040. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Adsorptive potential of modified plant-based adsorbents for sequestration of dyes and heavy metals from wastewater—A review. J. Water Process Eng. 2021, 42, 102148. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, A. Enhanced adsorption of Gentian Violet dye from water using lignocellulosic agricultural waste modified with di- and tri-carboxylic acids: Artificial intelligence modeling, practical comprehension, mechanistic and regeneration analyses. J. Environ. Chem. Eng. 2021, 9, 105578. [Google Scholar] [CrossRef]
- Lima, D.R.; Klein, L.; Dotto, G.L. Application of ultrasound modified corn straw as adsorbent for Malachite Green removal from synthetic and real effluents. Environ. Sci. Pollut. Res. 2017, 24, 21484–21495. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhao, Y.; Lin, T.; Li, Y.; Zhou, R.; Peng, Y. Adsorption performance and mechanism of Methylene Blue by H3PO4-modified corn stalks. J. Environ. Chem. Eng. 2019, 7, 103398. [Google Scholar] [CrossRef]
- Chowdhury, S.; Saha, P.D. Citric acid modified wheat bran as a potential adsorbent for removal of Cu(II) and Malachite Green from aqueous solutions. Desalination Water Treat. 2013, 51, 6038–6048. [Google Scholar] [CrossRef]
- Chikri, R.; Elhadiri, N.; Benchanaa, M.; El Maguana, Y. Efficiency of sawdust as low-cost adsorbent for dyes removal. J. Chem. 2020, 2020, 8813420. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, Y.; Xue, L.; Sun, H.; Guo, Z.; Zhang, Y.; Yang, L. Carboxylic acid functionalized sesame straw: A sustainable cost-effective bioadsorbent with superior dye adsorption capacity. Bioresour. Technol. 2017, 238, 675–683. [Google Scholar] [CrossRef]
- Gong, R.; Jin, Y.; Chen, F.; Chen, J.; Liu, Z. Enhanced Malachite Green removal from aqueous solution by citric acid modified rice straw. J. Hazard. Mater. 2006, 137, 865–870. [Google Scholar] [CrossRef]
- Gong, R.; Zhong, K.; Hu, Y.; Chen, J.; Zhu, G. Thermochemical esterifying citric acid onto lignocellulose for enhancing Methylene Blue sorption capacity of rice straw. J. Environ. Manag. 2008, 88, 875–880. [Google Scholar] [CrossRef]
- Fathy, N.A.; El-Shafey, O.I.; Khalil, L.B. Effectiveness of alkali-acid treatment in enhancement the adsorption capacity for rice straw: The removal of Methylene Blue dye. Phys. Chem. 2013, 2013, 208087. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, H.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavy-metal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef]
- Gong, R.; Zhu, S.; Zhang, D.; Chen, J.; Ni, S.; Guan, R. Adsorption behavior of cationic dyes on citric acid esterifying wheat straw: Kinetic and thermodynamic profile. Desalination 2008, 230, 220–228. [Google Scholar] [CrossRef]
- Han, R.; Zhang, L.; Song, C.; Zhang, M.; Zhu, H.; Zhang, L. Characterization of modified wheat straw, kinetic and equilibrium study about Copper ion and Methylene Blue adsorption in batch mode. Carbohydr. Polym. 2010, 79, 1140–1149. [Google Scholar] [CrossRef]
- Sajab, M.S.; Chia, C.H.; Zakaria, S.; Jani, S.M.; Ayob, M.K.; Chee, K.L.; Khiew, P.S.; Chiu, W.S. Citric acid modified kenaf core fibres for removal of Methylene Blue from aqueous solution. Bioresour. Technol. 2011, 102, 7237–7243. [Google Scholar] [CrossRef]
- Makeswari, M.; Santhi, T.; Ezhilarasi, M.R. Adsorption of Methylene Blue dye by citric acid modified leaves of Ricinus communis from aqueous solutions. J. Chem. Pharm. Res. 2016, 8, 452–462. Available online: https://www.jocpr.com/archive/jocpr-volume-8-issue-7-year-2016.html (accessed on 21 October 2021).
- Wang, P.; Ma, Q.; Hu, D.; Wang, L. Adsorption of Methylene Blue by a low-cost biosorbent: Citric acid modified peanut shell. Desalin. Water Treat. 2016, 57, 10261–10269. [Google Scholar] [CrossRef]
- Zou, W.; Bai, H.; Gao, S.; Li, K.; Zhao, X.; Han, R. Investigations on the batch performance of cationic dyes adsorption by citric acid modified peanut husk. Desalination Water Treat. 2012, 49, 41–56. [Google Scholar] [CrossRef]
- Chen, L.; Ramadan, A.; Lu, L.; Shao, W.; Luo, F.; Chen, J. Biosorption of Methylene Blue from aqueous solution using lawny grass modified with citric acid. J. Chem. Eng. Data 2011, 56, 3392–3399. [Google Scholar] [CrossRef]
- Yan, J.; Lan, G.; Qiu, H.; Chen, C.; Liu, Y.; Du, G.; Zhang, J. Adsorption of heavy metals and Methylene Blue from aqueous solution with citric acid modified peach stone. Sep. Sci. Technol. 2018, 53, 1678–1688. [Google Scholar] [CrossRef] [Green Version]
- Soldatkina, L.M.; Zavrichko, M.A. Application of agriculture waste as biosorbents for dye removal from aqueous solutions. Chem. Phys. Surf. Technol. 2013, 4, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Yulianti, E.; Mahmudah, R.; Khalifah, S.N.; Prasetyo, A.; Irviyanti, A.S.; Romadhoni, A.F.; Yudisputra, G.P. Modification of corn stalk using citric acid as biosorbent for methylene blue and malachite green. IOP Conf. Ser. Earth Environ. Sci. 2020, 456, 012015. [Google Scholar] [CrossRef] [Green Version]
- Kieush, L.; Koveria, A.; Hrubyak, A.; Rachiy, B.I. Corn stalks as ecofriendly sources for carbon nanomaterials. In Proceedings of the 8th International Conference on “Nanomaterials: Applications & Properties”, Zatoka, Ukraine, 9–14 September 2018; p. 19210806. [Google Scholar] [CrossRef]
- Cheșcă, A.M.; Tofănică, B.M.; Puiţel, A.C.; Nicu, R.; Gavrilescu, D. Environmentally friendly cellulosic fibers from corn stalks. Environ. Eng. Manag. J. 2018, 17, 1765–1771. [Google Scholar] [CrossRef]
- Soldatkina, L.M.; Zavrichko, M.A. Obtaining of adsorbents using citric acid modification of plant waste. Odesa Natl. Univ. Herald. Chem. 2019, 18, 47–59. [Google Scholar] [CrossRef]
- Soldatkina, L.M.; Zavrichko, M.A. Adsorption of anionic dyes on corn stalks modified by polyaniline: Kinetics and thermodynamic studies. Chem. Phys. Technol. Surf. 2017, 8, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Vučurović, V.M.; Razmovski, R.N.; Miljić, U.D.; Puškaš, V.S.J. Removal of cationic and anionic azo dyes from aqueous solutions by adsorption on maize stem tissue. J. Taiwan Inst. Chem. Eng. 2014, 45, 1700–1708. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane bagasse pith. Desalination 2008, 223, 152–161. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, L.; Gao, C.; Ma, J.; Ma, X.; Han, R. Adsorption of Copper Ions and Methylene Blue in a single and binary system on wheat straw. J. Chem. Eng. Data 2009, 54, 3229–3234. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yua, Z. Performance, kinetics, and equilibrium of Methylene Blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef]
- Suvendu, M.; Debasis, R.; Prosenjit, S.; Deepu, G.; Sabu, T. Rapid Methylene Blue adsorption using modified lignocellulosic materials. Process Saf. Environ. Prot. 2017, 107, 346–356. [Google Scholar] [CrossRef]
| Adsorbent | Dye | Maximum Adsorption Capacity 1 (mg g−1) | Best Kinetic Model | Best Isotherm Model | Nature | Reference |
|---|---|---|---|---|---|---|
| Rice straw | Malachite Green | 256.41 (293 K) | Pseudo- First order | Langmuir and Freundlich | - | [9] |
| Rice straw | Methylene Blue | 270.3 (293 K) | Pseudo- first order | Langmuir | - | [10] |
| Rice straw | Methylene Blue | 135.1 (308 K) | Pseudo- second order | Tempkin | Spontaneous | [11] |
| Rice straw | Methylene Blue | 164.16 (298 K) | Pseudo- second order | - | - | [12] |
| Wheat straw | Methylene Blue | 312.5 2 | Pseudo- second order | Langmuir | Endothermic and Spontaneous | [13] |
| Wheat straw | Methylene Blue | 396.9 (293 K) 432.8 (303 K) 450.0 (323 K) | Pseudo- second order | Langmuir and Freundlich | Endothermic and Spontaneous | [14] |
| Wheat straw | Crystal Violet | 227.27 2 | Pseudo- second order | Langmuir | Endothermic and Spontaneous | [13] |
| Wheat bran | Malachite Green | 67.547 (293 K) 64.781 (303 K) 61.165 (323 K) | Pseudo- second order | Langmuir | Exothermic and Spontaneous | [6] |
| Sesame straw | Methylene Blue | 650 (298 K) | Pseudo- second order | Langmuir | Endothermic and Spontaneous | [8] |
| Kenaf core fibers | Methylene Blue | 103.1 (293 K) 128.2 (313 R) 131.6 (333 K) | Pseudo- second order | Langmuir | Endothermic and Spontaneous | [15] |
| Leaves of Ricinus communis | Methylene Blue | 333.33 2 | Pseudo- second order | Temkin | - | [16] |
| Peanut shell | Methylene Blue | 120.48 (303 K) 119.05 (313 K) 108.69 (323 K) | Pseudo- second order | Freundlich | Exothermic and Spontaneous | [17] |
| Peanut shell | Methylene Blue | 99.41 (283 K) 111.38 (298 K) 129.83 (313 K) | Pseudo- second order | Langmuir | Endothermic and Spontaneous | [18] |
| Peanut shell | Neutral Red | 112.72 (283 K) 131.8 6(298 K) 151.24 (313 K) | Pseudo- first order | Freundlich | Endothermic and Spontaneous | [18] |
| Seeds of Abelmoschus esculentus | Gentian Violet | 211.46 (288 K) 245.82 (303 K) 253.29 (318 K) | Pseudo- first order | Redlich- Peterson | Endothermic and Spontaneous | [3] |
| Grass | Methylene Blue | 301.1 2 | Pseudo- first order | Langmuir | - | [19] |
| Peach stone | Methylene Blue | 178.25 (303 K) | Pseudo- second order | Freundlich | - | [20] |
| Barley straw | Methylene Blue | - | Pseudo-second order | - | - | [21] |
| Jerusalem artichoke stalks | Methylene Blue | - | Pseudo-second order | - | - | [21] |
| Corn stalks | Malachite Green | 16.47 2 | Pseudo-second order | Langmuir | - | [22] |
| Adsorbent | Specific Surface Area (m2 g−1) | pHpzc | COOH (mmol g−1) |
|---|---|---|---|
| CS | 22.1 | 5.4 | 0.7 |
| CS-C | 45.3 | 3.3 | 3.5 |
| Kinetic Model | Parameter | MB | MG | ||||
|---|---|---|---|---|---|---|---|
| 293 (K) | 313 (K) | 333 (K) | 293 (K) | 313 (K) | 333 (K) | ||
| Experimental | te (min) | 90 | 90 | 90 | 90 | 90 | 90 |
| data | qeexp (mg g−1) | 2.60 | 3.07 | 3.15 | 2.63 | 3.36 | 3.49 |
| Pseudo-first order | qecalc (mg g−1) | 2.57 | 1.94 | 1.91 | 2.43 | 3.00 | 2.25 |
| k1∙102 (min−1) | 2.98 | 3.12 | 7.30 | 3.62 | 5.45 | 6.09 | |
| R2 | 0.9918 | 0.9924 | 0.9578 | 0.9628 | 0.9416 | 0.9420 | |
| SE | 0.09 | 1.23 | 1.32 | 0.24 | 0.38 | 1.32 | |
| χ2 | 0.05 | 15.7 | 11.61 | 0.54 | 0.60 | 10.43 | |
| Pseudo-second order | qecalc (mg g−1) | 3.06 | 3.25 | 3.22 | 2.91 | 3.57 | 3.60 |
| k2∙102 (g mg−1 min−1) | 1.14 | 3.13 | 8.70 | 1.98 | 2.81 | 5.53 | |
| R2 | 0.9950 | 0.9989 | 0.9996 | 0.9970 | 0.9981 | 0.9994 | |
| SE | 0.12 | 0.12 | 0.16 | 0.11 | 0.18 | 0.18 | |
| χ2 | 0.07 | 0.10 | 0.12 | 0.05 | 0.15 | 0.13 | |
| Elovich | α (mg g−1 min−1) | 0.21 | 1.02 | 2.35 | 0.31 | 0.56 | 1.48 |
| β (g mg −1) | 1.31 | 1.67 | 1.73 | 1.45 | 1.12 | 1.39 | |
| R2 | 0.9773 | 0.9905 | 0.9036 | 0.9936 | 0.9633 | 0.9232 | |
| SE | 0.10 | 0.06 | 0.20 | 0.08 | 0.21 | 0.23 | |
| χ2 | 0.15 | 0.01 | 0.14 | 0.04 | 0.13 | 0.16 | |
| Weber and Morris | kid (mg g−1 min−1/2) | 0.30 | 0.23 | 0.21 | 0.27 | 0.33 | 0.26 |
| I (mg g−1) | 0.20 | 0.99 | 1.53 | 0.19 | 0.61 | 1.40 | |
| R2 | 0.9950 | 0.9822 | 0.7477 | 0.9814 | 0.8740 | 0.7935 | |
| SE | 0.40 | 0.07 | 0.24 | 0.31 | 0.26 | 0.27 | |
| χ2 | 0.83 | 0.02 | 0.24 | 0.58 | 0.29 | 0.28 | |
| Isotherm Model | Parameter | MB | MG | ||||
|---|---|---|---|---|---|---|---|
| 293 (K) | 313 (K) | 333 (K) | 293 (K) | 313 (K) | 333 (K) | ||
| Langmuir | qm (mg g−1) | 23.15 | 24.81 | 26.60 | 21.41 | 23.53 | 27.55 |
| KL·102 (L mg−1) | 3.6 | 4.0 | 5.8 | 3.0 | 3.6 | 5.4 | |
| R2 | 0.9999 | 0.9982 | 0.9993 | 0.9989 | 0.9978 | 0.9996 | |
| SE | 1.16 | 1.52 | 2.86 | 0.88 | 1.58 | 1.93 | |
| χ2 | 1.08 | 1.76 | 5.33 | 0.73 | 2.15 | 2.37 | |
| Freundlich | 1/n | 0.33 | 0.33 | 0.28 | 0.34 | 0.32 | 0.29 |
| KF (mg1 − 1/n L1/n g−1) | 3.05 | 3.36 | 4.95 | 2.69 | 3.25 | 4.80 | |
| R2 | 0.7878 | 0.7560 | 0.6946 | 0.8020 | 0.7386 | 0.7545 | |
| SE | 4.11 | 4.75 | 5.13 | 3.74 | 4.17 | 4.84 | |
| χ2 | 7.76 | 9.64 | 12.99 | 6.02 | 8.40 | 10.29 | |
| Temkin | KT (L g–1) | 0.63 | 0.65 | 1.77 | 0.48 | 0.66 | 1.44 |
| b (kJ mol–1) | 0.503 | 0.610 | 0.704 | 0.646 | 0.654 | 0.661 | |
| R2 | 0.8946 | 0.8770 | 0.8226 | 0.9102 | 0.8759 | 0.8890 | |
| SE | 5.35 | 2.79 | 3.62 | 1.95 | 2.59 | 2.96 | |
| χ2 | 9.47 | 4.09 | 7.26 | 2.26 | 3.87 | 4.66 | |
| Dye | T (K) | Ko 10−4 | −ΔGo (kJ mol−1) | ΔHo (kJ mol−1) | ΔSo (J K−1 mol−1) |
|---|---|---|---|---|---|
| MB | 293 | 6.1 | 26.8 | 9.5 | 124 |
| 313 | 6.7 | 28.9 | |||
| 333 | 9.8 | 31.8 | |||
| MG | 293 | 4.9 | 26.3 | 11.8 | 130 |
| 313 | 5.9 | 28.6 | |||
| 333 | 8.8 | 31.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldatkina, L.; Yanar, M. Equilibrium, Kinetic, and Thermodynamic Studies of Cationic Dyes Adsorption on Corn Stalks Modified by Citric Acid. Colloids Interfaces 2021, 5, 52. https://doi.org/10.3390/colloids5040052
Soldatkina L, Yanar M. Equilibrium, Kinetic, and Thermodynamic Studies of Cationic Dyes Adsorption on Corn Stalks Modified by Citric Acid. Colloids and Interfaces. 2021; 5(4):52. https://doi.org/10.3390/colloids5040052
Chicago/Turabian StyleSoldatkina, Liudmyla, and Marianna Yanar. 2021. "Equilibrium, Kinetic, and Thermodynamic Studies of Cationic Dyes Adsorption on Corn Stalks Modified by Citric Acid" Colloids and Interfaces 5, no. 4: 52. https://doi.org/10.3390/colloids5040052
APA StyleSoldatkina, L., & Yanar, M. (2021). Equilibrium, Kinetic, and Thermodynamic Studies of Cationic Dyes Adsorption on Corn Stalks Modified by Citric Acid. Colloids and Interfaces, 5(4), 52. https://doi.org/10.3390/colloids5040052







