Dense Phases of γ-Gliadins in Confined Geometries
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions of γ-Gliadins
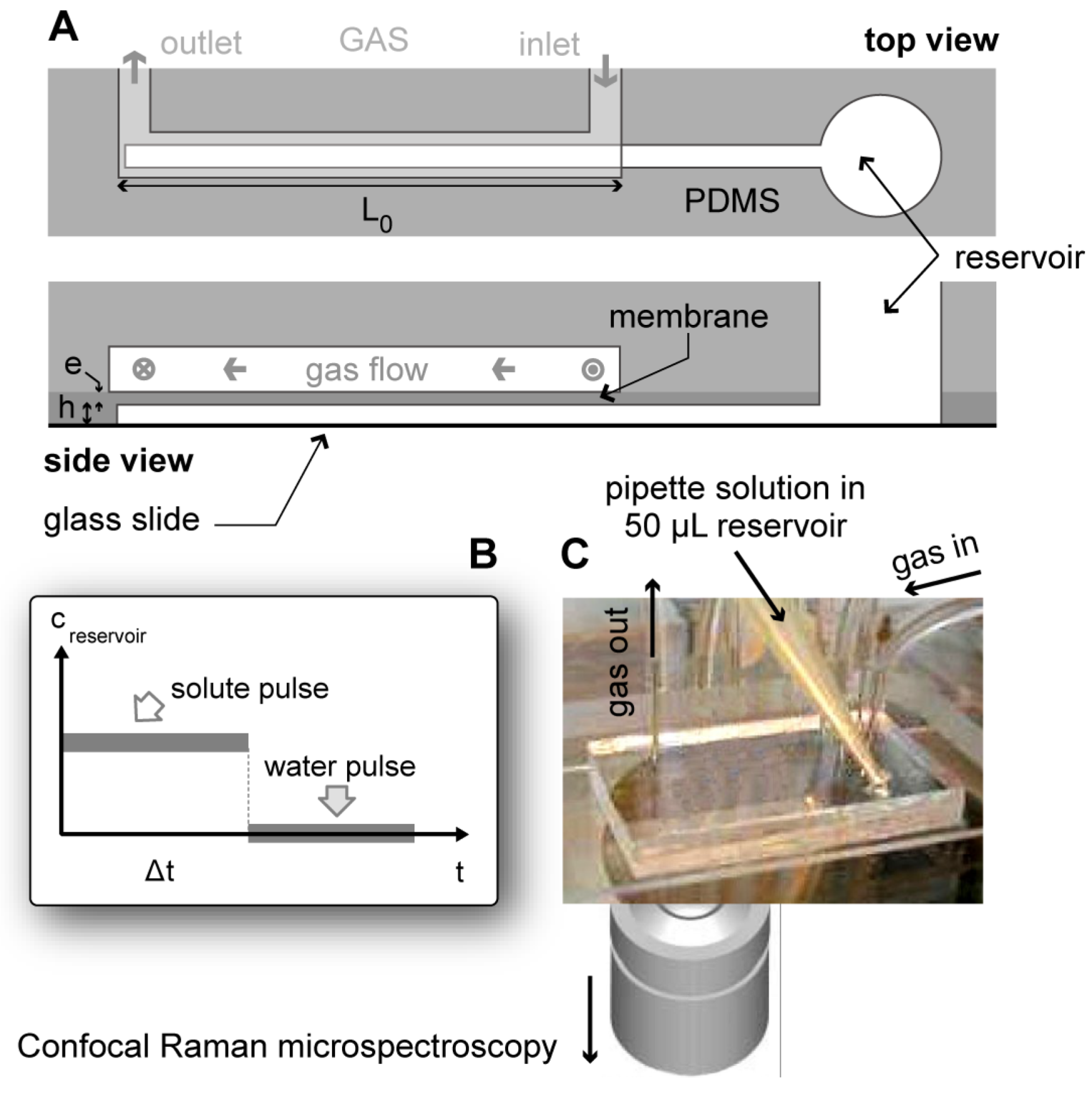
2.2. Microfluidic Device and Kinetic Inspection of Phase Diagram
2.3. Microscopy and Spectroscopy
3. Results
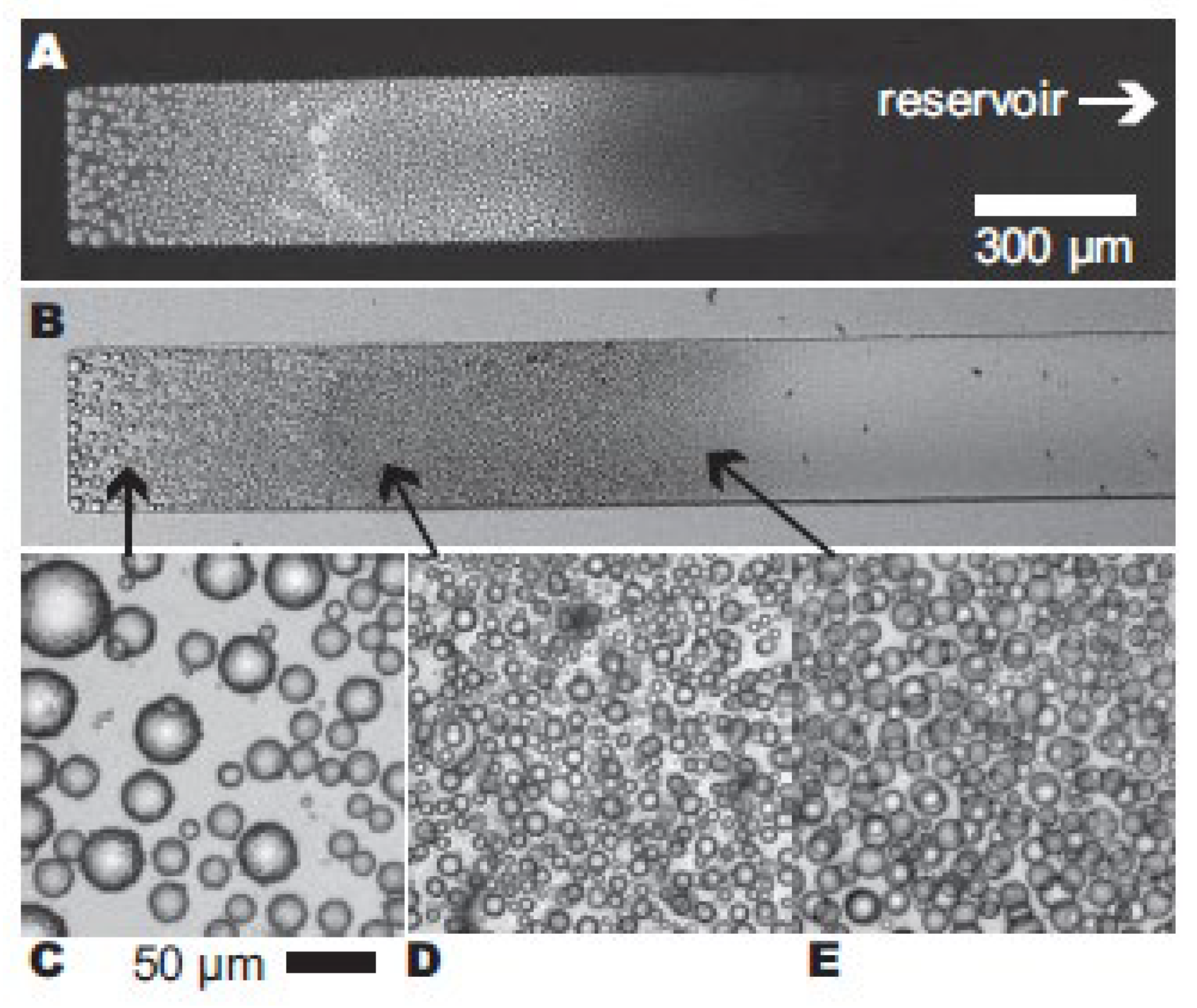
3.1. Microscopic Observations of γ-Gliadin Accumulation in Pure Water
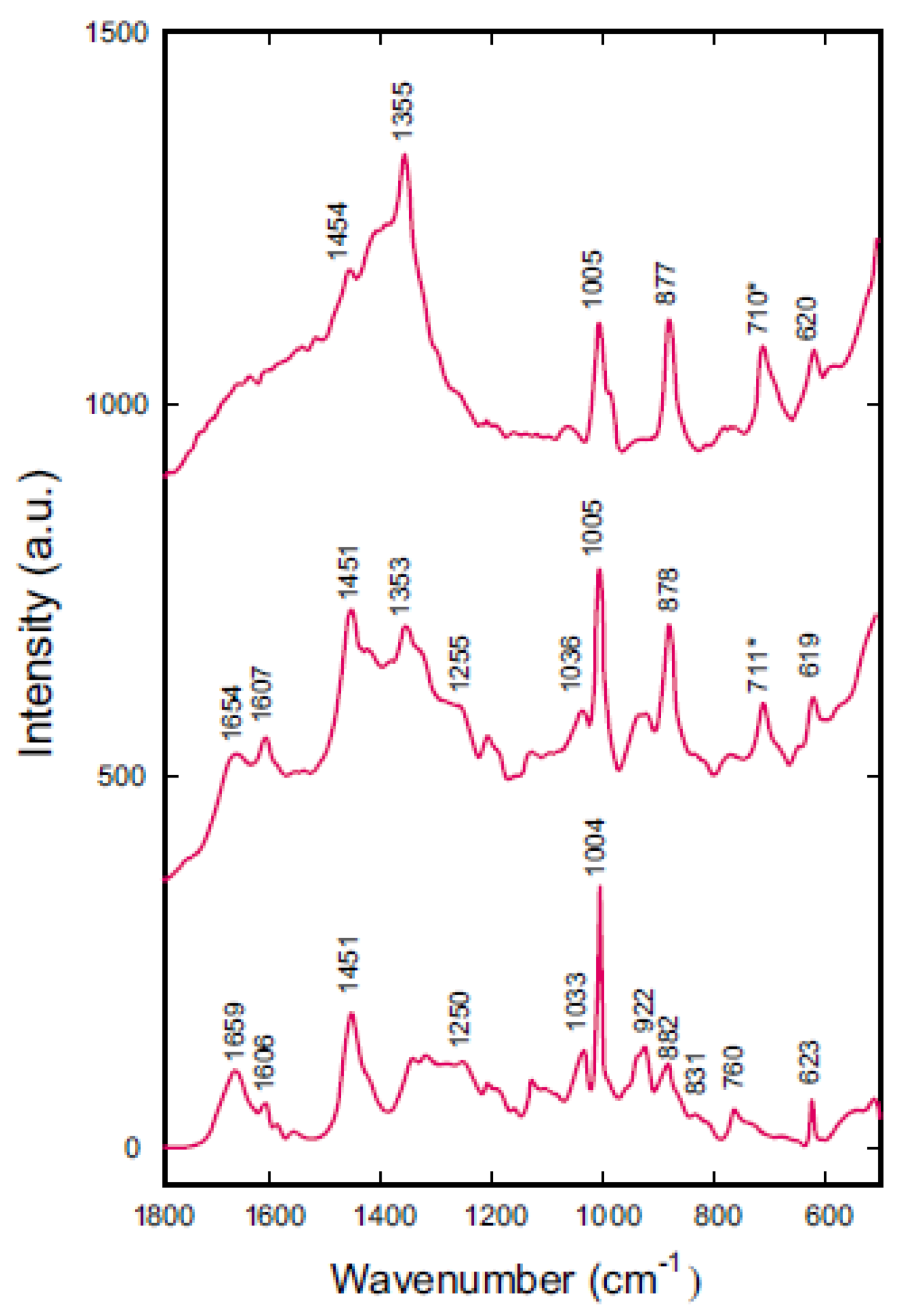
3.2. Raman Scattering in the Dense Phase of γ-Gliadins
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, M.; Khatkar, B.S. Structural and functional properties of wheat storage proteins: A review. J. Food Sci. Technol.-Mysore 2005, 42, 455–471. [Google Scholar]
- Redl, A.; Guilbert, S.; Morel, M.H. Heat and shear mediated polymerisation of plasticized wheat gluten protein upon mixing. J. Cereal Sci. 2003, 38, 105–114. [Google Scholar] [CrossRef]
- Andreani, L.; Cercena, R.; Ramos, B.G.Z.; Soldi, V. Development and characterization of wheat gluten microspheres for use in a controlled release system. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2009, 29, 524–531. [Google Scholar] [CrossRef]
- Orecchioni, A.M.; Duclairoir, C.; Renard, D.; Nakache, E. Gliadin characterization by sans and gliadin nanoparticle growth modelization. J. Nanosci. Nanotechnol. 2006, 6, 3171–3178. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S. The prolamin storage proteins of cereal seeds—Structure and evolution. Biochem. J. 1990, 267, 1–12. [Google Scholar] [CrossRef]
- Loussert, C.; Popineau, Y.; Mangavel, C. Protein bodies ontogeny and localization of prolamin components in the developing endosperm of wheat caryopses. J. Cereal Sci. 2008, 47, 445–456. [Google Scholar] [CrossRef]
- Thomson, N.H.; Miles, M.J.; Popineau, Y.; Harries, J.; Shewry, P.; Tatham, A.S. Small angle X-ray scattering of wheat seed-storage proteins: Alpha-, gamma- and omega-gliadins and the high molecular weight (HMW) subunits of glutenin. Biochim. Biophys. Acta-Protein Struct. Mol. Enzymol. 1999, 1430, 359–366. [Google Scholar] [CrossRef]
- Tatham, A.S.; Shewry, P.R. The conformation of wheat gluten proteins—The secondary structures and thermal stabilities of alpha-gliadins, beta-gliadins, gamma-gliadins and omega-gliadins. J. Cereal Sci. 1985, 3, 103–113. [Google Scholar] [CrossRef]
- Tatham, A.S.; Masson, P.; Popineau, Y. Conformational stuides of peptides derived by the enzymic-hydrolysis of a gamma-type gliadin. J. Cereal Sci. 1990, 11, 1–13. [Google Scholar] [CrossRef]
- Sahli, L.; Renard, D.; Sole-Jamault, V.; Giuliani, A.; Boire, A. Role of protein conformation and weak interactions on gamma-gliadin liquid-liquid phase separation. Sci. Rep. 2019, 9, 13391. [Google Scholar] [CrossRef] [PubMed]
- Mameri, H.; Brossard, C.; Gaudin, J.C.; Gohon, Y.; Paty, E.; Beaudouin, E.; Moneret-Vautrin, D.A.; Drouet, M.; Sole, V.; Wien, F.; et al. Structural Basis of IgE Binding to alpha- and gamma-Gliadins: Contribution of Disulfide Bonds and Repetitive and Nonrepetitive Domains. J. Agric. Food Chem. 2015, 63, 6546–6554. [Google Scholar] [CrossRef]
- Pezolet, M.; Bonenfant, S.; Dousseau, F.; Popineau, Y. Conformation of wheat gluten proteins—Comparison between functional and solution states as determined by infrared-spectroscopy. Febs Lett. 1992, 299, 247–250. [Google Scholar] [CrossRef]
- Matsuoka, K.; Bednarek, S.Y. Protein transport within the plant cell endomembrane system: An update. Curr. Opin. Plant Biol. 1998, 1, 463–469. [Google Scholar] [CrossRef]
- Tosi, P.; Parker, M.; Gritsch, C.S.; Carzaniga, R.; Martin, B.; Shewry, P.R. Trafficking of storage proteins in developing grain of wheat. J. Exp. Bot. 2009, 60, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Hinz, G. Sorting of proteins to storage vacuoles: How many mechanisms? Trends Plant Sci. 2005, 10, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Vitale, A.; Galili, G. The endomembrane system and the problem of protein sorting. Plant Physiol. 2001, 125, 115–118. [Google Scholar] [CrossRef]
- Hara-Nishimura, I.; Matsushima, R.; Shimada, T.; Nishimura, M. Diversity and formation of endoplasmic reticulum-derived compartments in plants. Are these compartments specific to plant cells? Plant Physiol. 2004, 136, 3435–3439. [Google Scholar] [CrossRef]
- Rosenberg, N.; Shimoni, Y.; Altschuler, Y.; Levanony, H.; Volokita, M.; Galili, G. Wheat (Triticum aestivum L.) gamma-gliadin accumulates in dense protein bodies within the endoplasmic reticulum of yeast. Plant Physiol. 1993, 102, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Altschuler, Y.; Rosenberg, N.; Harel, R.; Galili, G. The N-terminal and C-terminal regions regulate the transport of wheat gamma-gliadin through the endoplasmic-reticulum in xenopus oocytes. Plant Cell 1993, 5, 443–450. [Google Scholar] [CrossRef]
- Galili, G.; Altschuler, Y.; Levanony, H.; Giorinisilfen, S.; Shimoni, Y.; Shani, N.; Karchi, H. Assembly and transport of wheat storage proteins. J. Plant Physiol. 1995, 145, 626–631. [Google Scholar] [CrossRef]
- Francin-Allami, M.; Saumonneau, A.; Lavenant, L.; Bouder, A.; Sparkes, I.; Hawes, C.; Popineau, Y. Dynamic trafficking of wheat gamma-gliadin and of its structural domains in tobacco cells, studied with fluorescent protein fusions. J. Exp. Bot. 2011, 62, 4507–4520. [Google Scholar] [CrossRef] [PubMed]
- Boire, A.; Sanchez, C.; Morel, M.H.; Lettinga, M.P.; Menut, P. Dynamics of liquid-liquid phase separation of wheat gliadins. Sci. Rep. 2018, 8, 14441. [Google Scholar] [CrossRef]
- Sahli, L.; Boire, A.; Sole-Jamault, V.; Rogniaux, H.; Giuliani, A.; Roblin, P.; Renard, D. New exploration of the gamma-gliadin structure through its partial hydrolysis. Int. J. Biol. Macromol. 2020, 165, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.X.; Sapirstein, H.D.; Bushuk, W. Salt-induced disaggregation/solubilization of gliadin and glutenin proteins in water. J. Cereal Sci. 1996, 24, 241–246. [Google Scholar] [CrossRef]
- Simpson, L.W.; Good, T.A.; Leach, J.B. Protein folding and assembly in confined environments: Implications for protein aggregation in hydrogels and tissues. Biotechnol. Adv. 2020, 42, 107573. [Google Scholar] [CrossRef]
- Thewissen, B.G.; Celus, I.; Brijs, K.; Delcour, J.A. Foaming Properties of Wheat Gliadin. J. Agric. Food Chem. 2011, 59, 1370–1375. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.C.A.; Orsel, R.; Hamer, R.J. Competitive adsorption behaviour of wheat flour components and emulsifiers at an air-water interface. J. Cereal Sci. 1997, 25, 175–183. [Google Scholar] [CrossRef]
- Poirier, A.; Banc, A.; Stocco, A.; In, M.; Ramos, L. Multistep building of a soft plant protein film at the air-water interface. J. Colloid Interface Sci. 2018, 526, 337–346. [Google Scholar] [CrossRef]
- Haward, S.J.; Shewry, P.R.; Miles, M.J.; McMaster, T.J. Direct Real-Time Imaging of Protein Adsorption onto Hydrophilic and Hydrophobic Surfaces. Biopolymers 2010, 93, 74–84. [Google Scholar] [CrossRef]
- Banc, A.; Desbat, B.; Renard, D.; Popineau, Y.; Mangavel, C.; Navailles, L. Exploring the Interactions of Gliadins with Model Membranes: Effect of Confined Geometry and Interfaces. Biopolymers 2009, 91, 610–622. [Google Scholar] [CrossRef]
- Banc, A.; Desbat, B.; Renard, D.; Popineau, Y.; Mangavel, U.; Navailles, L. Structure and orientation changes of omega- and gamma-gliadins at the air-water interface: A PM-IRRAS Spectroscopy and Brewster angle microscopy study. Langmuir 2007, 23, 13066–13075. [Google Scholar] [CrossRef][Green Version]
- Cochereau, R.; Renard, D.; Nous, C.; Boire, A. Semi-permeable vesicles produced by microfluidics to tune the phase behaviour of encapsulated macromolecules. J. Colloid Interface Sci. 2020, 580, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Salmon, J.B.; Leng, J. Application of microevaporators to dynamic exploration of the phase diagram. J. Appl. Phys. 2010, 107, 84905. [Google Scholar] [CrossRef]
- Leng, J.; Lonetti, B.; Tabeling, P.; Joanicot, M.; Ajdari, A. Microevaporators for kinetic exploration of phase diagrams. Phys. Rev. Lett. 2006, 96, 084503. [Google Scholar] [CrossRef]
- Fischer, H.; Polikarpov, I.; Craievich, A.F. Average protein density is a molecular-weight-dependent function. Protein Sci. 2004, 13, 2825–2828. [Google Scholar] [CrossRef] [PubMed]
- Loussert, C. Accumulation et Assemblage des Prolamines au Cours du Développement du Gran de blé: Approches Microscopiques et Protéomiques; Université de Nantes: Nantes, France, 2008. [Google Scholar]
- Tu, A. Raman Spectroscopy in Biology: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 1982; p. 464. [Google Scholar]
- Banc, A.; Desbat, B.; Cavagnat, D. Ab Initio Calculations of Proline Vibrations with and without Water: Consequences on the Infrared Spectra of Proline-Rich Proteins. Appl. Spectrosc. 2011, 65, 817–819. [Google Scholar] [CrossRef] [PubMed]
- Harada, I.; Miura, T.; Takeuchi, H. Origin of the doublet at 1360 and 1340 cm-1 in the Raman-spectra of tryptophan and related-compounds. Spectrochim. Acta Part A-Mol. Biomol. Spectrosc. 1986, 42, 307–312. [Google Scholar] [CrossRef]
- Clark, R.; Hester, R. Spectroscopy of Biological Systems; John Wiley & Sons: Hoboken, NJ, USA, 1986; p. 570. [Google Scholar]
- Tanaka, M.; Young, R.J. Polarised Raman spectroscopy for the study of molecular orientation distributions in polymers. J. Mater. Sci. 2006, 41, 963–991. [Google Scholar] [CrossRef]
- Prinsen, P.; Odijk, T. Optimized Baxter model of protein solutions: Electrostatics versus adhesion. J. Chem. Phys. 2004, 121, 6525–6537. [Google Scholar] [CrossRef]
- Piazza, R.; Peyre, V.; Degiorgio, V. “Sticky hard spheres” model of proteins near crystallization: A test based on the osmotic compressibility of lysozyme solutions. Phys. Rev. E 1998, 58, R2733–R2736. [Google Scholar] [CrossRef]
- Fine, B.M.; Lomakin, A.; Ogun, O.O.; Benedek, G.B. Static structure factor and collective diffusion of globular proteins in concentrated aqueous solution. J. Chem. Phys. 1996, 104, 326–335. [Google Scholar] [CrossRef]
- Gogelein, C.; Nagele, G.; Tuinier, R.; Gibaud, T.; Stradner, A.; Schurtenberger, P. A simple patchy colloid model for the phase behavior of lysozyme dispersions. J. Chem. Phys. 2008, 129, 85102. [Google Scholar] [CrossRef]
- Giacometti, A.; Gazzillo, D.; Pastore, G.; Das, T.K. Numerical study of a binary Yukawa model in regimes characteristic of globular proteins in solutions. Phys. Rev. E 2005, 71, 031108. [Google Scholar] [CrossRef] [PubMed]
- Piston, F.; Dorado, G.; Martin, A.; Barro, F. Cloning of nine gamma-gliadin mRNAs (cDNAs) from wheat and the molecular characterization of comparative transcript levels of gamma-gliadin subclasses. J. Cereal Sci. 2006, 43, 120–128. [Google Scholar] [CrossRef]
- Altschuler, Y.; Galili, G. Role of conserved cysteines of a wheat gliadin in its transport and assembly into protein bodies in xenopus-oocytes. J. Biol. Chem. 1994, 269, 6677–6682. [Google Scholar] [CrossRef]
- Monteiro, A.M.F.; Areas, E.P.G.; Schroder, A.; Fa, N. Gliadin effect on fluctuation properties of phospholipid giant vesicles. Colloids Surf. B-Biointerfaces 2004, 34, 53–57. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banc, A.; Navailles, L.; Leng, J.; Renard, D. Dense Phases of γ-Gliadins in Confined Geometries. Colloids Interfaces 2021, 5, 51. https://doi.org/10.3390/colloids5040051
Banc A, Navailles L, Leng J, Renard D. Dense Phases of γ-Gliadins in Confined Geometries. Colloids and Interfaces. 2021; 5(4):51. https://doi.org/10.3390/colloids5040051
Chicago/Turabian StyleBanc, Amélie, Laurence Navailles, Jacques Leng, and Denis Renard. 2021. "Dense Phases of γ-Gliadins in Confined Geometries" Colloids and Interfaces 5, no. 4: 51. https://doi.org/10.3390/colloids5040051
APA StyleBanc, A., Navailles, L., Leng, J., & Renard, D. (2021). Dense Phases of γ-Gliadins in Confined Geometries. Colloids and Interfaces, 5(4), 51. https://doi.org/10.3390/colloids5040051






