What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach?
Abstract
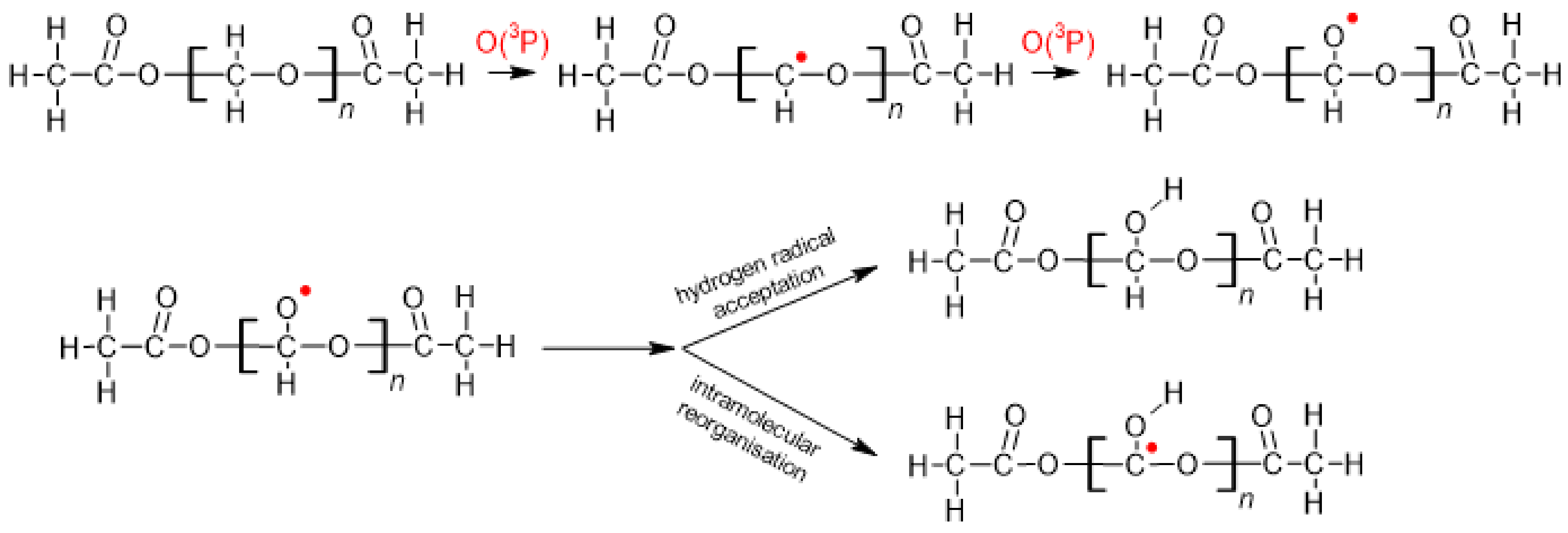
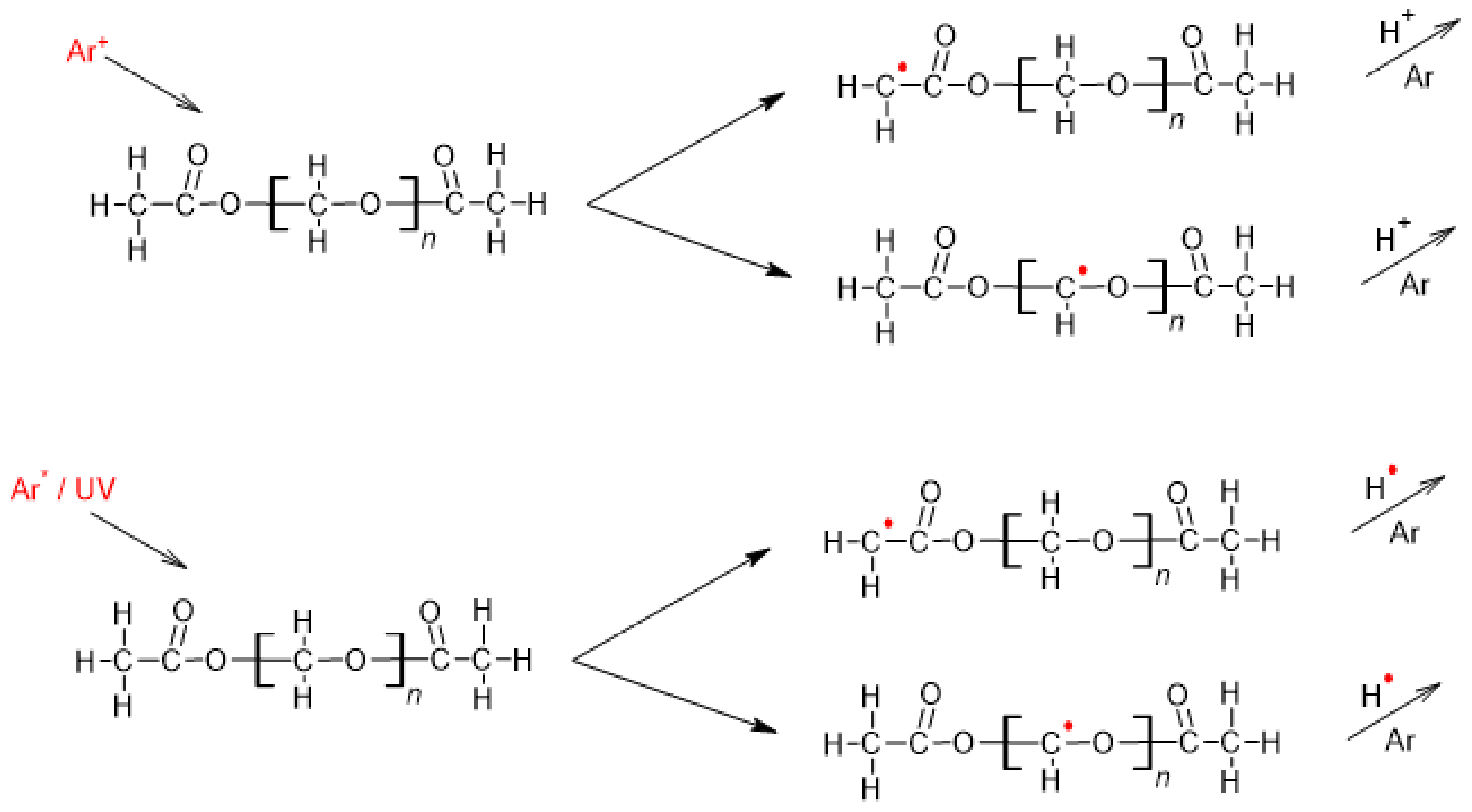
1. Introduction
2. Materials and Methods
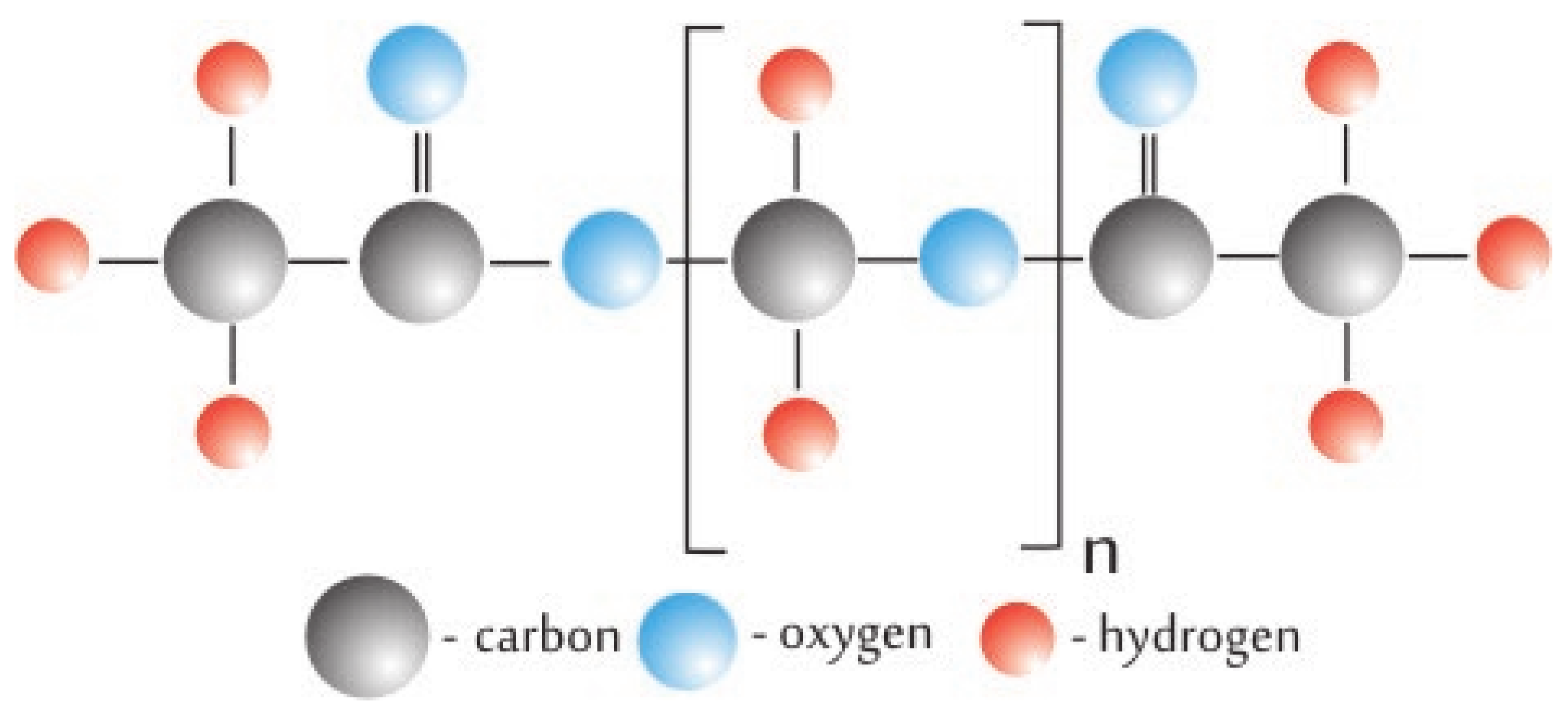
2.1. Sample Preparation
2.2. Plasma Activation
2.3. Contact Angle Measurements
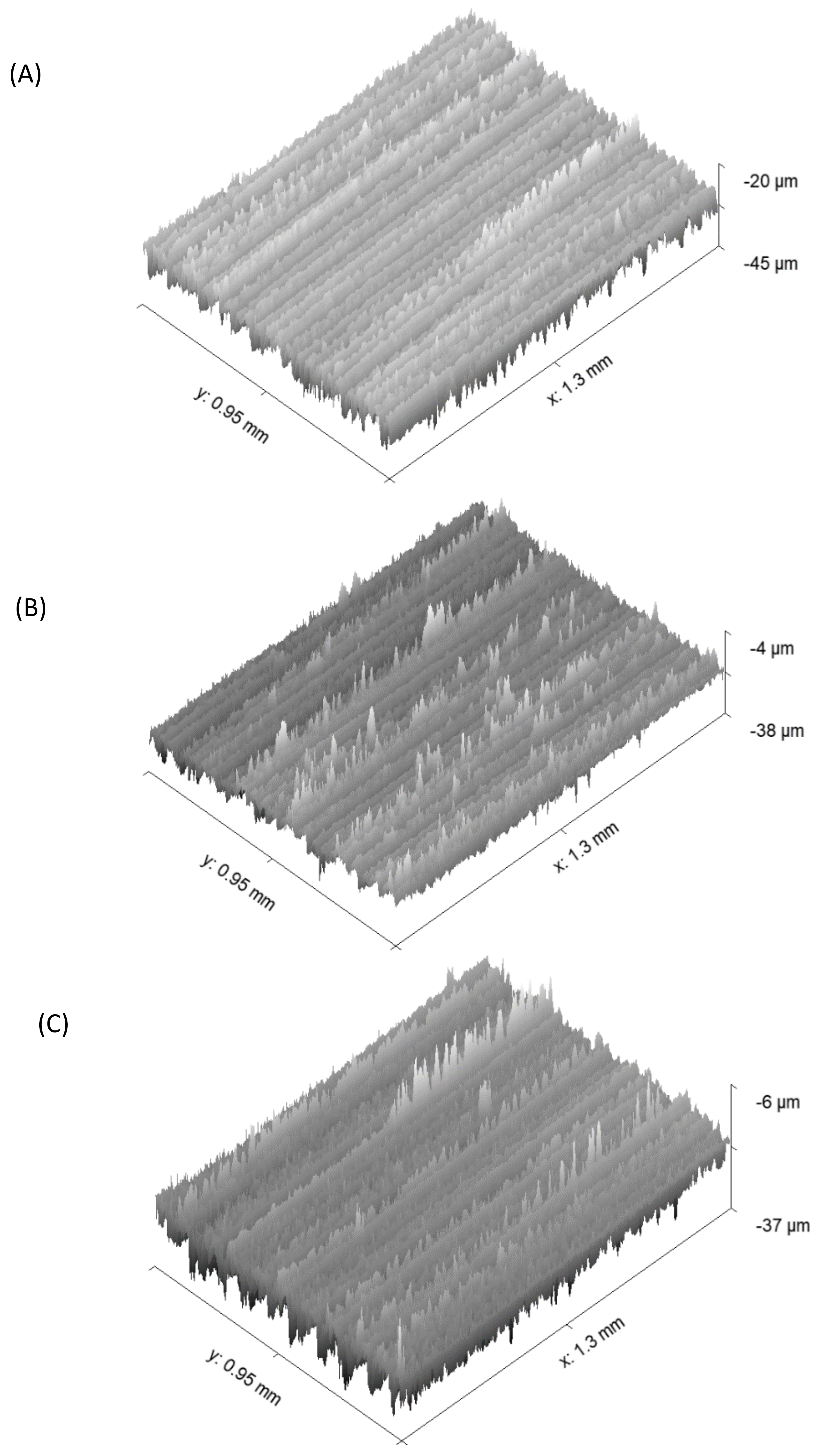
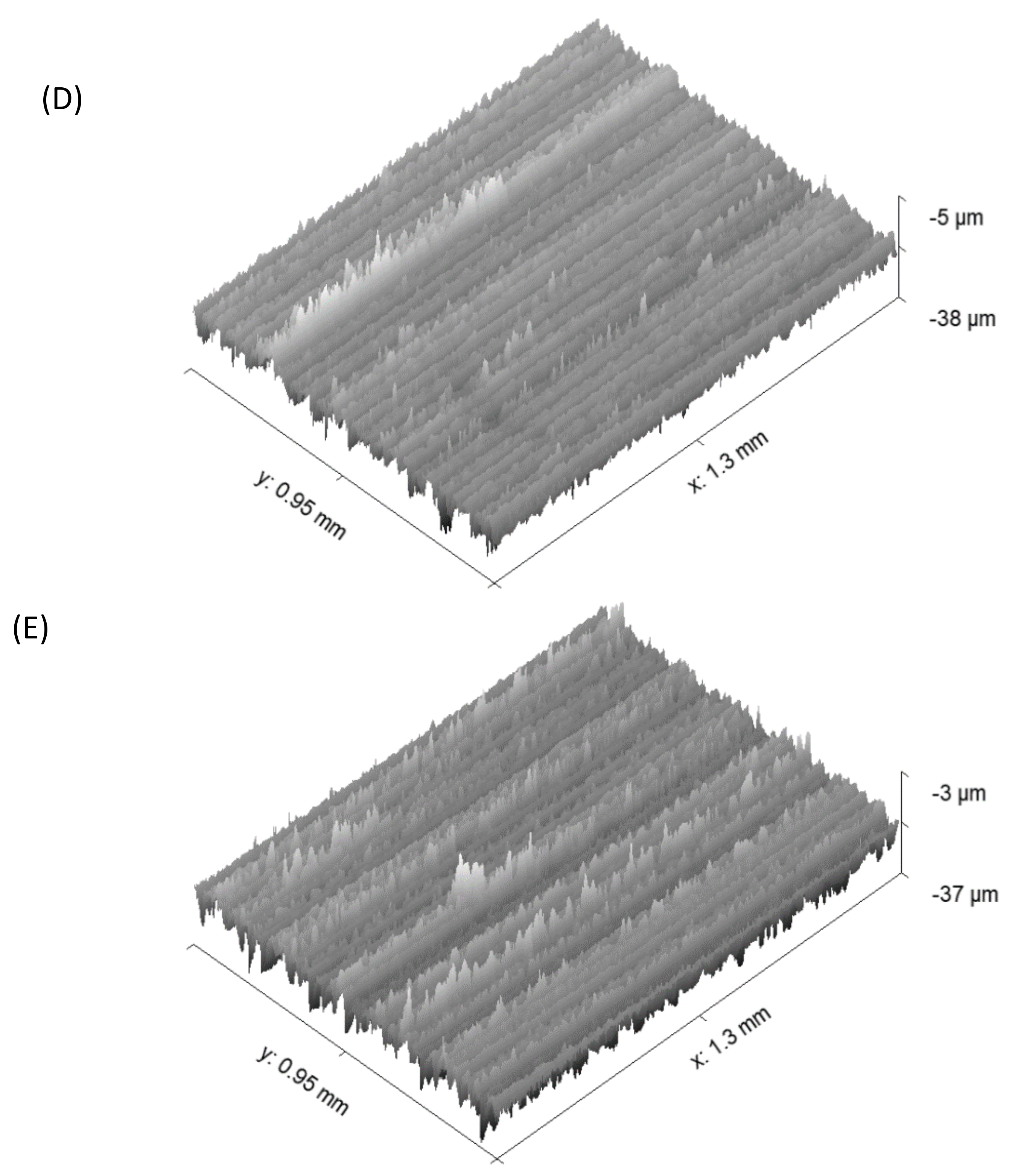
2.4. Optical Profilometry
- -
- the arithmetic mean deviation of the profile from the mean line, measured along the measuring or elementary section: Ra;
- -
- the Root Mean Square deviation standard from the vertical mean value of measurement coordinates: Rq;
- -
- the distance between the deepest valley and the highest elevation: Rt
- -
- Roughness parameters were calculated using WYKO—Vison software, and the 3d images were obtained using GWYDDION software.
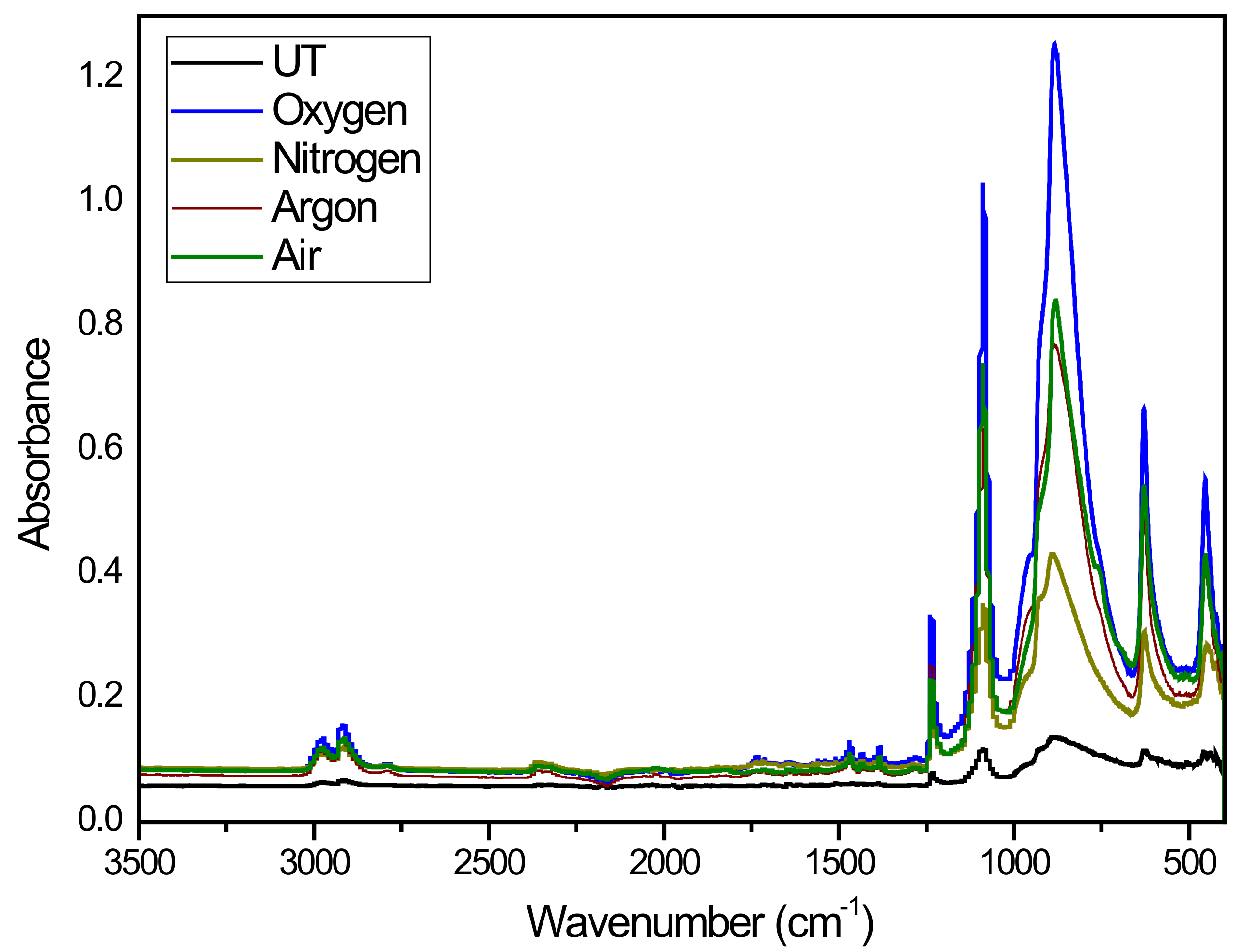
2.5. Infrared Spectroscopy (IR-ATR)
2.6. X-ray Photoelectron Spectroscopy (XPS)
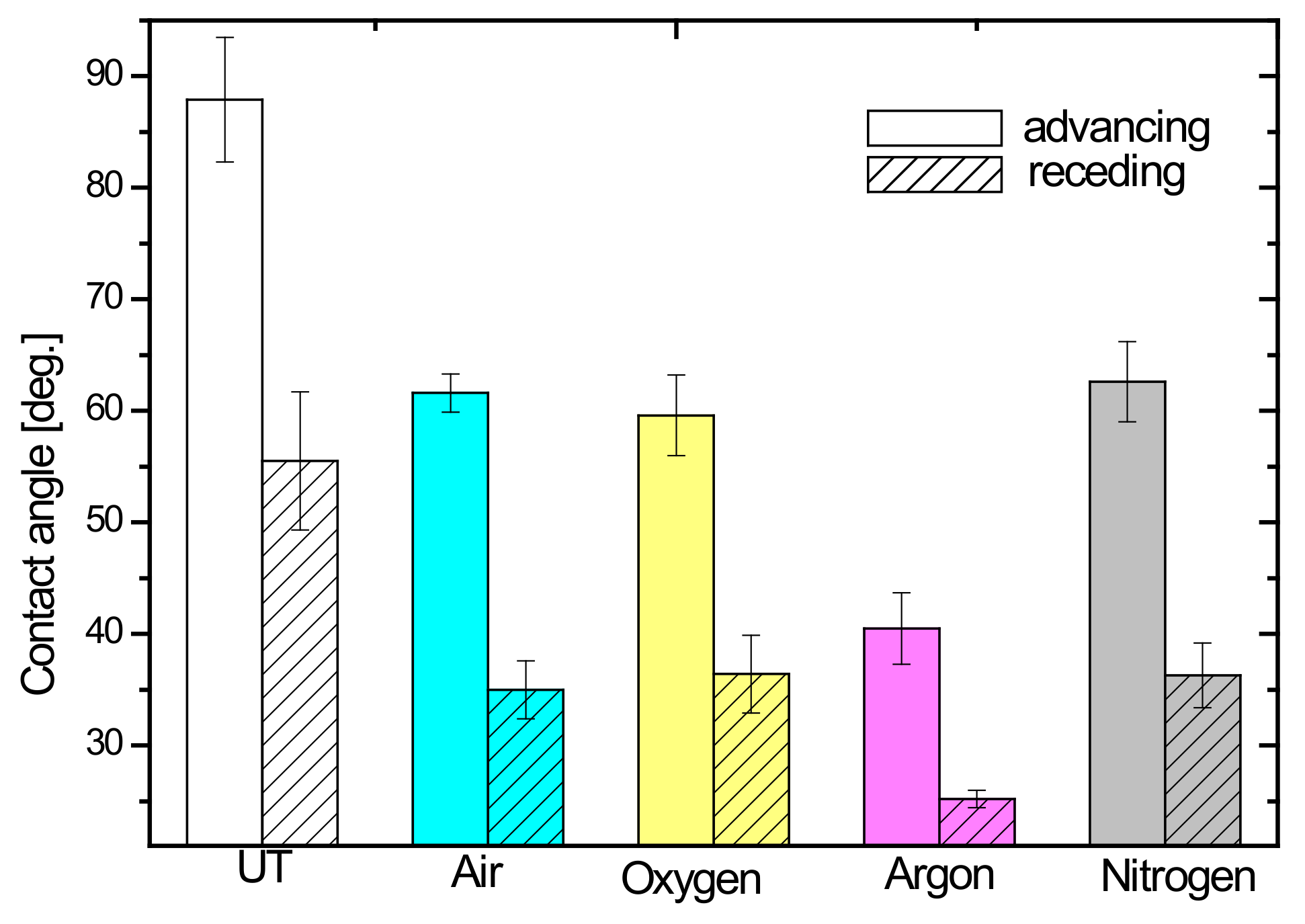
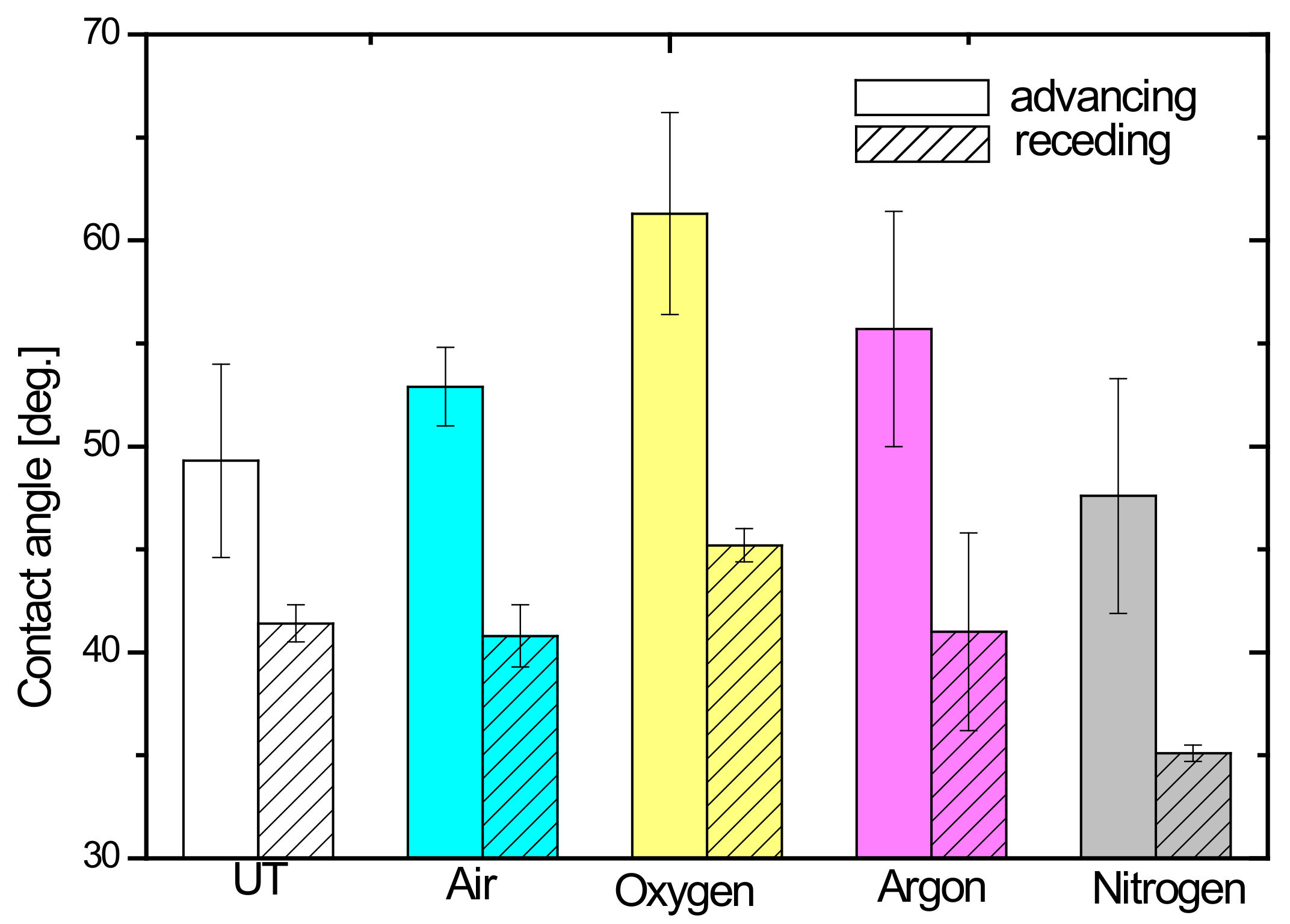
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Żenkiewicz, M. Methods for the calculation of surface free energy of solids. J. Achiev. 2007, 24, 137–145. [Google Scholar]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Della Volpe, C.; Hołysz, L.; Marmur, A.; Siboni, S. Contact angles: History of over 200 years of open questions. Surf. Innov. 2020, 8, 3–27. [Google Scholar] [CrossRef]
- Chibowski, E. On some relations between advancing, receding and Young’s contact angles. Adv. Colloid Interface Sci. 2007, 133, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E. Apparent contact angles for reactive wetting of smooth, rough, and heterogeneous surfaces calculated from the variational principles. J. Colloid Interface Sci. 2019, 537, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Chibowski, E. Contact angle hysteresis due to a film present behind the drop. In Contact Angle, Wettability and Adhesion, 2nd ed.; Mittal, K.L., Ed.; VSP: Utrecht, The Netherlands, 2002; Volume 2, pp. 265–288. [Google Scholar]
- Chibowski, E. On the interpretation of contact angle hysteresis. J. Adhes. Sci. Technol. 2002, 16, 1367–1404. [Google Scholar] [CrossRef]
- van Oss, C.J.; Good, R.J.; Chaudhury, M.K. The role of van der Waals forces and hydrogen bonds in “hydrophobic interactions” between biopolymers and low energy surfaces. J. Colloid Interface Sci. 1986, 111, 378–390. [Google Scholar] [CrossRef]
- van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Additive and nonadditive surface tension components and the interpretation of contact angles. Langmuir 1988, 4, 884–891. [Google Scholar] [CrossRef]
- van Oss, C.J.; Good, R.J.; Chaudhury, M.K. Interfacial Lifshitz-van der Waals and polar interactions in macroscopic systems. Chem. Rev. 1988, 88, 927–941. [Google Scholar] [CrossRef]
- Schmitt, M.; Heib, F. High-precision drop shape analysis on inclining flat surfaces: Introduction and comparison of this special method with commercial contact angle analysis. J. Chem. Phys. 2013, 139, 1–10. [Google Scholar] [CrossRef]
- Schmitt, M.; Heib, F. More Appropriate Procedure to Measure and Analyse Contact Angles/Drop Shape Behaviours. In Advances in Contact Angle, Wettability and Adhesion, 3rd ed.; Mittal, K.L., Ed.; Wiley-Scrivener: Beverly, MA, USA, 2018; Volume 3, pp. 3–57. [Google Scholar]
- Perèz-Huertas, S.; Terpiłowski, K.; Tomczyńska-Mleko, M.; Mleko, S.; Szajnecki, Ł. Time-based changes in surface properties of poly(ethylene terephthalate) activated with air and argon-plasma treatments. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 322–329. [Google Scholar] [CrossRef]
- Rymuszka, K.; Terpiłowski, K.; Borowski, P.; Hołysz, L. Time-dependent changes of surface properties of polyether ether ketone caused by air plasma treatment. Polym. Int. 2016, 65, 827–834. [Google Scholar] [CrossRef]
- Pèrez-Huertas, S.; Terpilowski, K.; Tomczyńska-Mleko, M.; Mleko, S. Surface modification of albumin/gelatin films gelled on low-temperature plasma-treated polyethylene terephthalate plates. Plasma Process Polym. 2020, 3, 1900171. [Google Scholar] [CrossRef]
- Available online: https://omnexus.specialchem.com/selection-guide/polyacetal-polyoxymethylene-pom-plastic (accessed on 12 January 2021).
- Available online: https://www.relyon-plasma.com/glossary/polyoxymethylene (accessed on 5 October 2020).
- Gesche, R.; Kovacs, R.; Scherer, J. Mobile plasma activation of polymer using the plasma gun. Surf. Coat. Technol. 2005, 200, 544–547. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Chibowski, E.; Terpiłowski, K. Comparison of Apparent Surface Free Energy of Some Solids Determined by Different Approaches. In Contact Angle, Wettability and Adhesion; Mittal, K.L., Ed.; CRC Press: London, UK, 2009; Volume 6, pp. 283–299. [Google Scholar]
- Terpiłowski, K.; Wiącek, A.E.; Jurak, M. Influence of nitrogen plasma treatment on the wettability of polyetheretherketone and deposited chitosan layers. Adv. Polym. Technol. 2018, 37, 1557–1569. [Google Scholar] [CrossRef]
- Fowkes, F.M. Attractive forces at interfaces. Ind. Eng. Chem. 1964, 56, 40–52. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Hołysz, L.; Chibowski, E. Surface free energy of sulfur—Revisited II. Samples solidified against different solid surfaces. J. Colloid Interface Sci. 2008, 319, 505–513. [Google Scholar] [CrossRef]
- Jurak, M.; Chibowski, B. Surface free energy and topography of mixed lipid layers on mica. Colloids Surf. B 2010, 75, 165–174. [Google Scholar] [CrossRef]
- Chibowski, E. Some problems of characterization of a solid surface via the surface free energy changes. Adsorp. Sci. Technol. 2017, 35, 647–659. [Google Scholar] [CrossRef]
- Stammitti-Scarpone, A.; Acosta, E.J. Solid-liquid-liquid wettability and its prediction with surface free energy models. Adv. Colloid Interface Sci. 2019, 264, 28–46. [Google Scholar] [CrossRef]
- Chibowski, E.; Perea-Carpio, R. Problems of contact angle and solid surface free energy determination. Adv. Colloid Interface Sci. 2002, 98, 245–264. [Google Scholar] [CrossRef]
- Filipić, A.; Gutierrezi-Aguiree, I.; Primc, G.; Mozetič, M.; Dobnik, D. Cold Plasma, a New Hope in the Field of Virus Inactivation. Trends Biotechnol. 2020, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.; Fuzhi, L.; Li, B.; Kwok, D.Y. Wettability Interpretation of Oxygen Plasma Modified Poly(methyl methacrylate). Langmuir 2004, 20, 10919–10927. [Google Scholar] [CrossRef] [PubMed]
- Karimian, D.; Yadollahi, B.; Zendehdel, M.; Mirkhani, V. Efficient dye-sensitized solar cell with a pure thin film of a hybrid polyoxometalate covalently attached organic dye as a working electrode in a cobalt redox mediator system. RSC Adv. 2015, 5, 76875–76882. [Google Scholar] [CrossRef]
- Gernot, M.; Wallner, G.M.; Geretschläger, K.J.; Hintersteiner, I.; Buchberger, W. Characterization of polyoxymethylene for backsheets of photovoltaic modules. J. Plast. 2016, 33, 345–360. [Google Scholar]
- Li, Y.; Zhou, T.; Chen, Z.; Hui, J.; Li, L.; Zhang, A. Non-isothermal crystallization process of polyoxymethylene studied by two-dimensional correlation infrared spectroscopy. Polymer 2011, 52, 2059–2069. [Google Scholar] [CrossRef]
| Sample | γtotCAH(W,DM,G) | γtotCAH(W) | γtotCAH(DM) | γtotCAH(W) − γtotCAH(DM) | γtotLWAB(W,DM,G) | γLW | γ− | γ+ | γAB |
|---|---|---|---|---|---|---|---|---|---|
| UT | 38.1 ± 4.0 | 37.1 ± 5.8 | 42.5 ± 2.2 | - | 36.0 ± 2.8 | 34.6 ± 3.7 | 6.7 ± 7.0 | 0.3 | 2.1 ± 1.4 |
| Air | 42.9 ± 4.5 | 48.8 ± 1.7 | 40.5 ± 3.2 | 7.6 ± 1.5 | 38.7 ± 2.5 | 36.6 ± 3.4 | 22.2 ± 0.6 | 0.4 | 6.0 ± 1.4 |
| Oxygen | 42.6 ± 6.5 | 49.9 ± 2.9 | 40.7 ± 4.7 | 9.1 ± 1.8 | 33.2 ± 3.0 | 27.8 ± 2.8 | 30.2 ± 1.5 | 0.2 | 5.3 ± 0.2 |
| Argon | 44.6 ± 4.0 | 61.4 ± 4.5 | 38.9 ± 4.7 | 22.5 ± 0.2 | 31.0 ± 3.3 | 31.0 ± 3.3 | 68.4 ± 3.6 | - | - |
| Nitrogen | 42.1 ± 4.0 | 47.5 ± 2.7 | 41.1 ± 3.3 | 6.4 ± 0.6 | 37.0 ± 3.8 | 35.6 ± 3.1 | 23.6 ± 0.9 | 0.03 | 1.5 ± 0.6 |
| Species | UT | Argon Plasma | Oxygen Plasma |
|---|---|---|---|
| C-O-C (main POM molecular unit) | 40.6 | 36.3 | 36.7 |
| C-O-C (epoxy groups) | 7.2 | 14.9 | 14.3 |
| C-H sp3 (aliphatic carbon) | 6.6 | 3.8 | 4.0 |
| C-C sp3 (aliphatic carbon) | 12.3 | 4.0 | 5.0 |
| C=O (carbonyl group) | 11.7 | 14.8 | 14.7 |
| Species | UT | Argon Plasma | Oxygen Plasma |
|---|---|---|---|
| C-O-C (main POM unit) | 61.3 | 61.8 | 65.4 |
| OH-C (hydroxyl groups bound to aliphatic carbon) | 9.8 | 8.8 | 10.4 |
| O-C=O (carboxyl groups) | 26.6 | 24.5 | 23.1 |
| Ra | Rq | Rt | |
|---|---|---|---|
| UT | 2.6 ± 0.4 | 3.3 ± 0.4 | 32.8 ± 7.3 |
| Air | 2.1 ± 0.4 | 2.7 ± 0.4 | 30.8 ± 5.8 |
| Oxygen | 2.2 ± 0.2 | 2.9 ± 0.1 | 34.6 ± 3.7 |
| Argon | 2.2 ± 0.01 | 2.9 ± 0.02 | 34.0 ± 1.4 |
| Nitrogen | 2.5 ± 0.1 | 3.2 ± 0.1 | 32.9 ± 1.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpiłowski, K.; Hołysz, L.; Chodkowski, M.; Clemente Guinarte, D. What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach? Colloids Interfaces 2021, 5, 4. https://doi.org/10.3390/colloids5010004
Terpiłowski K, Hołysz L, Chodkowski M, Clemente Guinarte D. What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach? Colloids and Interfaces. 2021; 5(1):4. https://doi.org/10.3390/colloids5010004
Chicago/Turabian StyleTerpiłowski, Konrad, Lucyna Hołysz, Michał Chodkowski, and David Clemente Guinarte. 2021. "What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach?" Colloids and Interfaces 5, no. 1: 4. https://doi.org/10.3390/colloids5010004
APA StyleTerpiłowski, K., Hołysz, L., Chodkowski, M., & Clemente Guinarte, D. (2021). What Can You Learn about Apparent Surface Free Energy from the Hysteresis Approach? Colloids and Interfaces, 5(1), 4. https://doi.org/10.3390/colloids5010004





