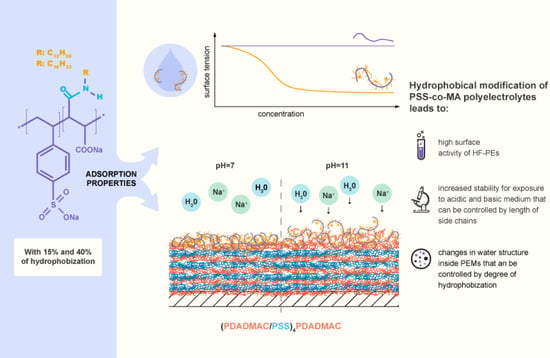
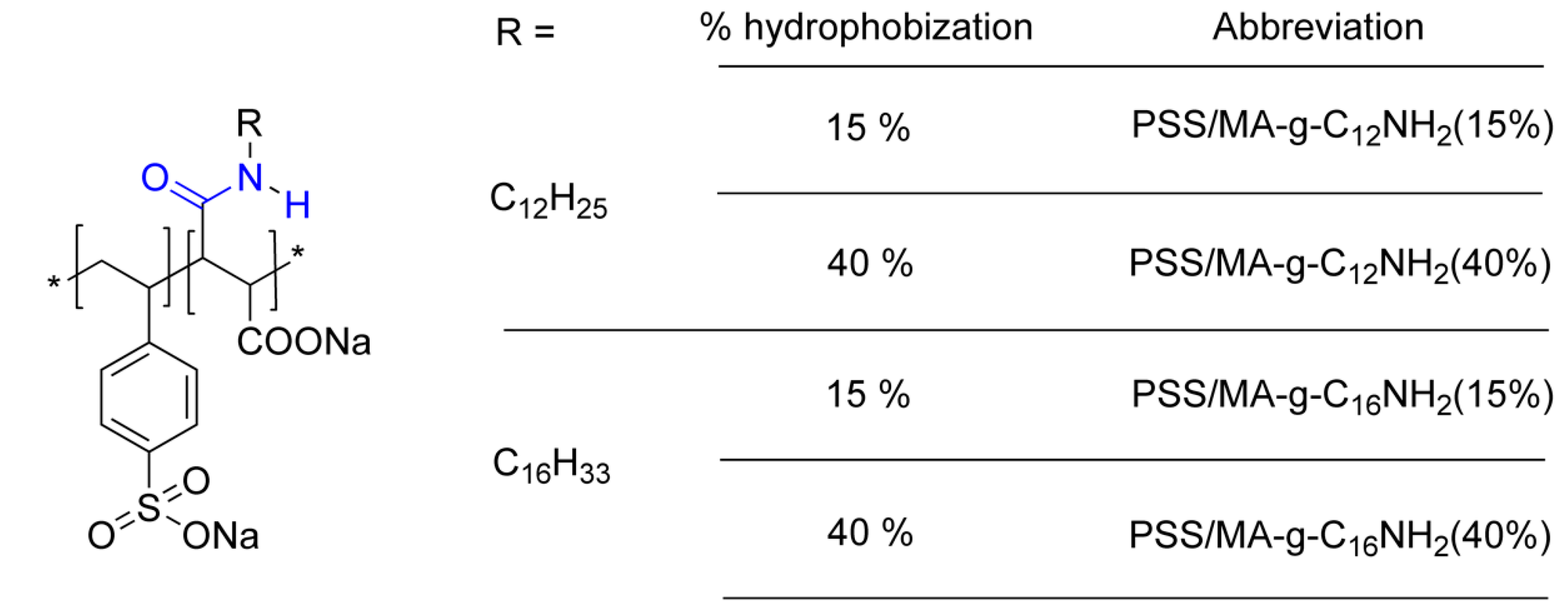
Adsorption Properties of Soft Hydrophobically Functionalized PSS/MA Polyelectrolytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface/Interfacial Tension
2.2. Quartz Crystal Microbalance with Dissipation (QCM-D) Measurements of the Mass of the Adsorbed Polymer
2.3. Contact Angle Analysis
3. Results and Discussion
3.1. Adsorption at Fluid Interfaces
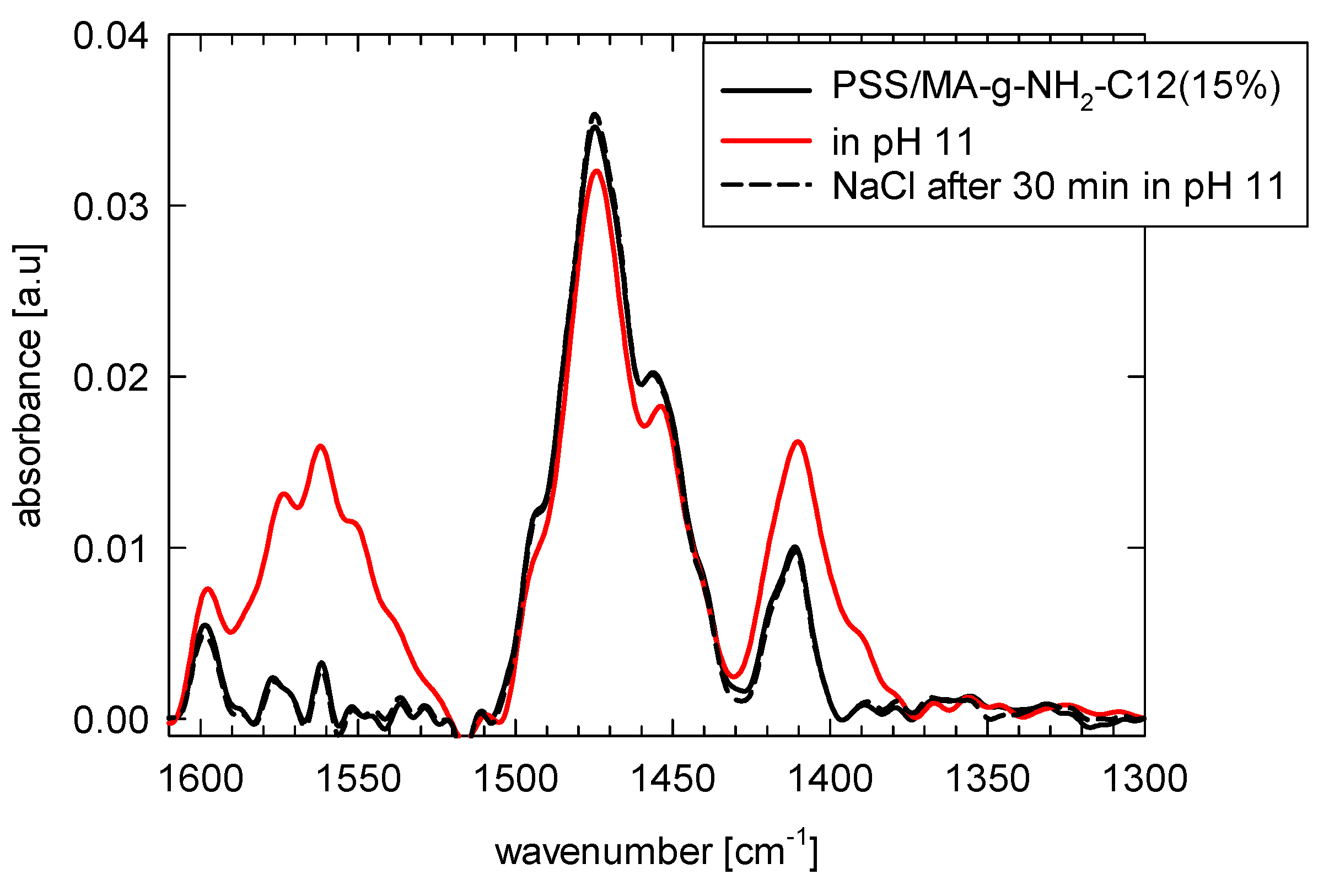
3.2. Adsorption at Solid Surface Covered with Polyelectrolyte Multilayer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kötz, J.; Kosmella, S.; Beitz, T. Self-assembled polyelectrolyte systems. Prog. Polym. Sci. 2001, 26, 1199–1232. [Google Scholar] [CrossRef]
- Olea, A.F. Hydrophobic Polyelectrolytes. In Ionic Interactions in Natural and Synthetic Macromolecules; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 211–233. ISBN 9780470529270. [Google Scholar]
- Seantier, B.; Deratani, A. Polyelectrolytes at Interfaces: Applications and Transport Properties of Polyelectrolyte Multilayers in Membranes. In Ionic Interactions in Natural and Synthetic Macromolecules; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 683–726. ISBN 9780470529270. [Google Scholar]
- Lamch, Ł.; Pucek, A.; Kulbacka, J.; Chudy, M.; Jastrzębska, E.; Tokarska, K.; Bułka, M.; Brzózka, Z.; Wilk, K.A. Recent progress in the engineering of multifunctional colloidal nanoparticles for enhanced photodynamic therapy and bioimaging. Adv. Colloid Interface Sci. 2018, 261, 62–81. [Google Scholar] [CrossRef] [PubMed]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Lamch, Ł.; Ronka, S.; Warszyński, P.; Wilk, K.A. NMR studies of self-organization behavior of hydrophobically functionalized poly(4-styrenosulfonic-co-maleic acid) in aqueous solution. J. Mol. Liq. 2020, 308, 112990. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. (Eds.) Multilayer Thin Films; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012; Volumes 1–2, ISBN 9783527646746. [Google Scholar]
- Sukhorukov, G.B.; Donath, E.; Lichtenfeld, H.; Knippel, E.; Knippel, M.; Budde, A.; Möhwald, H. Layer-by-layer self assembly of polyelectrolytes on colloidal particles. Colloids Surfaces Physicochem. Eng. Asp. 1998, 137, 253–266. [Google Scholar] [CrossRef]
- Szczepanowicz, K.; Hoel, H.J.; Szyk-Warszynska, L.; Bielańska, E.; Bouzga, A.M.; Gaudernack, G.; Simon, C.; Warszynski, P. Formation of Biocompatible Nanocapsules with Emulsion Core and Pegylated Shell by Polyelectrolyte Multilayer Adsorption. Langmuir 2010, 26, 12592–12597. [Google Scholar] [CrossRef]
- Picart, C.; Caruso, F.; Voegel, J.-C. (Eds.) Layer-by-Layer Films for Biomedical Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; ISBN 9783527675869. [Google Scholar]
- Richardson, J.J.; Bjornmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348, aaa2491. [Google Scholar] [CrossRef]
- Antipov, A.A.; Sukhorukov, G.B. Polyelectrolyte multilayer capsules as vehicles with tunable permeability. Adv. Colloid Interface Sci. 2004, 111, 49–61. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Szyk-Warszyńska, L.; Lamch, Ł.; Weżgowiec, J.; Warszyński, P.; Wilk, K.A. Benefits of pH-responsive polyelectrolyte coatings for carboxymethyl cellulose-based microparticles in the controlled release of esculin. Mater. Sci. Eng. C 2021, 118, 111397. [Google Scholar] [CrossRef]
- Sadman, K.; Wang, Q.; Chen, Y.; Keshavarz, B.; Jiang, Z.; Shull, K.R. Influence of Hydrophobicity on Polyelectrolyte Complexation. Macromolecules 2017, 50, 9417–9426. [Google Scholar] [CrossRef]
- Leermakers, F.A.M.; Léonforte, F.; Luengo, G.S. Structure and Colloidal Stability of Adsorption Layers of Macrocycle, Linear, Comb, Star, and Dendritic Macromolecules. Macromolecules 2020, 53, 7322–7334. [Google Scholar] [CrossRef]
- Borisov, O.V.; Halperin, A. Self-assembly of polysoaps. Curr. Opin. Colloid Interface Sci. 1998, 3, 415–421. [Google Scholar] [CrossRef]
- Borisov, O.V.; Halperin, A. Micelles of Polysoaps. Langmuir 1995, 11, 2911–2919. [Google Scholar] [CrossRef]
- Morishima, Y.; Kobayashi, T.; Nozakura, S. Amphiphilic Polyelectrolytes with Various Hydrophobic Groups: Intramolecular Hydrophobic Aggregation in Aqueous Solution. Polym. J. 1989, 21, 267–274. [Google Scholar] [CrossRef]
- Dobrynin, A.V.; Rubinstein, M. Hydrophobically Modified Polyelectrolytes in Dilute Salt-Free Solutions. Macromolecules 2000, 33, 8097–8105. [Google Scholar] [CrossRef]
- Lamch, Ł.; Ronka, S.; Moszyńska, I.; Warszyński, P.; Wilk, K.A. Hydrophobically Functionalized Poly(Acrylic Acid) Comprising the Ester-Type Labile Spacer: Synthesis and Self-Organization in Water. Polymers 2020, 12, 1185. [Google Scholar] [CrossRef]
- Huang, J.; Morin, F.J.; Laaser, J.E. Charge-Density-Dominated Phase Behavior and Viscoelasticity of Polyelectrolyte Complex Coacervates. Macromolecules 2019, 52, 4957–4967. [Google Scholar] [CrossRef]
- Biswas, A.; Cheng, H.N.; Kim, S.; Alves, C.R.; Furtado, R.F. Hydrophobic Modification of Cashew Gum with Alkenyl Succinic Anhydride. Polymers 2020, 12, 514. [Google Scholar] [CrossRef]
- Poujol, S.; Pinguet, F.; Bressole, F.; Boustta, M.; Vert, M. Molecular Microencapsulation: Paclitaxel Formations in Aqueous Medium Using Hydrophobized Poly(L-Lysine Citramide Imide). J. Bioact. Compat. Polym. 2000, 15, 99–114. [Google Scholar] [CrossRef]
- Domurado, D.; Vert, M. Bioresorbable polyelectrolyte amphiphiles as nanosized carriers for lipophilic drug solubilization and delivery. J. Biomater. Sci. Polym. Ed. 2007, 18, 287–301. [Google Scholar] [CrossRef]
- Locatelli-Champagne, C.; Suau, J.-M.; Guerret, O.; Pellet, C.; Cloitre, M. Versatile Encapsulation Technology Based on Tailored pH-Responsive Amphiphilic Polymers: Emulsion Gels and Capsules. Langmuir 2017, 33, 14020–14028. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Bazylińska, U.; Pietkiewicz, J.; Szyk-Warszyńska, L.; Wilk, K.A.; Warszyński, P. Biocompatible long-sustained release oil-core polyelectrolyte nanocarriers: From controlling physical state and stability to biological impact. Adv. Colloid Interface Sci. 2015, 222, 678–691. [Google Scholar] [CrossRef] [PubMed]
- Tjipto, E.; Quinn, J.F.; Caruso, F. Assembly of Multilayer Films from Polyelectrolytes Containing Weak and Strong Acid Moieties. Langmuir 2005, 21, 8785–8792. [Google Scholar] [CrossRef]
- Gong, X. Controlling surface properties of polyelectrolyte multilayers by assembly pH. Phys. Chem. Chem. Phys. 2013, 15, 10459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Q.; Ma, T.; Liu, Q.; Wu, S.; Hua, K.; Zhang, C.; Chen, M.; Cui, Y. Enhanced Stability of Gold Magnetic Nanoparticles with Poly(4-styrenesulfonic acid-co-maleic acid): Tailored Optical Properties for Protein Detection. Nanoscale Res. Lett. 2017, 12, 547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Su, P.-G.; Cheng, K.-H. Self-assembly of polyelectrolytic multilayer thin films of polyelectrolytes on quartz crystal microbalance for detecting low humidity. Sens. Actuators B Chem. 2009, 142, 123–129. [Google Scholar] [CrossRef]
- Maza, E.; Tuninetti, J.S.; Politakos, N.; Knoll, W.; Moya, S.; Azzaroni, O. pH-responsive ion transport in polyelectrolyte multilayers of poly(diallyldimethylammonium chloride) (PDADMAC) and poly(4-styrenesulfonic acid-co-maleic acid) (PSS-MA) bearing strong- and weak anionic groups. Phys. Chem. Chem. Phys. 2015, 17, 29935–29948. [Google Scholar] [CrossRef]
- Magny, B.; Iliopoulos, I.; Audebert, R.; Piculell, L.; Lindman, B. Interactions between hydrophobically modified polymers and surfactants. In Trends in Colloid and Interface Science VI; Steinkopff: Darmstadt, Germany, 2007; pp. 118–121. [Google Scholar]
- Para, G.; Jarek, E.; Warszynski, P. The Hofmeister series effect in adsorption of cationic surfactants—Theoretical description and experimental results. Adv. Colloid Interface Sci. 2006, 122, 39–55. [Google Scholar] [CrossRef]
- Bruckenstein, S.; Shay, M. Experimental aspects of use of the quartz crystal microbalance in solution. Electrochim. Acta 1985, 30, 1295–1300. [Google Scholar] [CrossRef]
- Elżbieciak-Wodka, M.; Kolasińska-Sojka, M.; Nowak, P.; Warszyński, P. Comparison of permeability of poly(allylamine hydrochloride)/and poly(diallyldimethylammonium chloride)/poly(4-styrenesulfonate) multilayer films: Linear vs. exponential growth. J. Electroanal. Chem. 2015, 738, 195–202. [Google Scholar] [CrossRef]
- Szyk-Warszyńska, L.; Raszka, K.; Warszyński, P. Interactions of Casein and Polypeptides in Multilayer Films Studied by FTIR and Molecular Dynamics. Polymers 2019, 11, 920. [Google Scholar] [CrossRef]
- Théodoly, O.; Ober, R.; Williams, C.E. Adsorption of hydrophobic polyelectrolytes at the air/water interface: Conformational effect and history dependence. Eur. Phys. J. E 2001, 5, 51–58. [Google Scholar] [CrossRef]
- Barraza, R.G.; Olea, A.F.; Martinez, F.; Ruiz-Tagle, I. Adsorption of hydrophobically modified polyelectrolytes at the -octane/water interface. J. Colloid Interface Sci. 2003, 261, 559–564. [Google Scholar] [CrossRef]
- Kolasińska, M.; Warszyński, P. The effect of nature of polyions and treatment after deposition on wetting characteristics of polyelectrolyte multilayers. Appl. Surf. Sci. 2005, 252, 759–765. [Google Scholar] [CrossRef]
- Elzbieciak, M.; Kolasinska, M.; Warszynski, P. Characteristics of polyelectrolyte multilayers: The effect of polyion charge on thickness and wetting properties. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 321, 258–261. [Google Scholar] [CrossRef]
- Gregoriou, V.G.; Hapanowicz, R.; Clark, S.L.; Hammond, P.T. Infrared Studies of Novel Optically Responsive Materials: Orientation Characteristics of Sulfonated Polystyrene/Poly(Diallyldimethylammonium Chloride) Ionic Polymer Multilayers on Patterned Self-Assembled Monolayers. Appl. Spectrosc. 1997, 51, 470–476. [Google Scholar] [CrossRef]
- Thouvenin, M.; Linossier, I.; Sire, O.; Péron, J.-J.; Vallée-Réhel, K. Structural and Dynamic Approach of Early Hydration Steps in Erodable Polymers by ATR−FTIR and Fluorescence Spectroscopies. Macromolecules 2002, 35, 489–498. [Google Scholar] [CrossRef]
- Ho, T.T.M.; Bremmell, K.E.; Krasowska, M.; MacWilliams, S.V.; Richard, C.J.E.; Stringer, D.N.; Beattie, D.A. In Situ ATR FTIR Spectroscopic Study of the Formation and Hydration of a Fucoidan/Chitosan Polyelectrolyte Multilayer. Langmuir 2015, 31, 11249–11259. [Google Scholar] [CrossRef]
- Lasagabaster, A.; Abad, M.J.; Barral, L.; Ares, A. FTIR study on the nature of water sorbed in polypropylene (PP)/ethylene alcohol vinyl (EVOH) films. Eur. Polym. J. 2006, 42, 3121–3132. [Google Scholar] [CrossRef]
- Rivas, B.L.; Seguel, G.V.; Geckeler, K.E. Synthesis, characterization, and properties of polychelates of poly(styrene sulfonic acid-co-maleic acid) with Co(II), Cu(II), Ni(II), and Zn(II). J. Appl. Polym. Sci. 2002, 85, 2546–2551. [Google Scholar] [CrossRef]





| Copolymer | Mass of Copolymer (ng/cm2) pH = 7 | Film Mass Increment with Respect to Deposited at pH = 7 (ng/cm2) | |||
|---|---|---|---|---|---|
| pH = 3 | pH = 7 Rinsing after pH = 3 | pH = 11 | pH = 7 Rinsing after pH = 11 | ||
| PSS/MA | 247 | −210 | −19 | +75 | −60 |
| PSS/MA-g-C12NH2 (15%) | 217 | −125 | +46 | +75 | +34 |
| PSS/MA-g-C12NH2 (40%) | 219 | −150 | +27 | +47 | −30 |
| PSS/MA-g-C16NH2 (15%) | 227 | −135 | +52 | +22 | −7 |
| PSS/MA-g-C16NH2 (40%) | 193 | −160 | +30 | +13 | +9 |
| Polymer at the Top Layer | PSS | PDADMAC | PSS/MA | PSS/MA-g-C12NH2 (15%) | PSS/MA-g-C12NH2 (40%) | PSS/MA-g-C16NH2 (15%) | PSS/MA-g-C16NH2 (40%) |
|---|---|---|---|---|---|---|---|
| Contact angle (deg) | 14 ± 4 | 35 ± 8 | 15 ± 4 | 18 ± 3 | 16 ± 3 | 24 ± 2 | 21 ± 5 |
| Contact angle after pH = 11 (deg) | - | 23 ± 4 | 23 ± 5 | 29 ± 4 | 23 ± 6 | 25 ± 5 | 23 ± 6 |
| Intensity Difference of PSS Characteristic Bands (a.u) | |||||
|---|---|---|---|---|---|
| Adsorbed Copolymer | 1008 cm−1 | 1035 cm−1 | 1125 cm−1 | 1190 cm−1 | Sum |
| PSS/MA | 0.08 | 0.12 | 0.24 | 0.71 | 1.15 |
| PSS/MA-g-C12NH2 (15%) | 0.13 | 0.17 | 0.27 | 0.87 | 1.44 |
| PSS/MA-g-C12NH2 (40%) | 0.06 | 0.03 | 0.33 | 1.08 | 1.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarek, E.; Krasińska-Krawet, Z.; Kruk, T.; Lamch, Ł.; Ronka, S.; Wilk, K.A.; Warszyński, P. Adsorption Properties of Soft Hydrophobically Functionalized PSS/MA Polyelectrolytes. Colloids Interfaces 2021, 5, 3. https://doi.org/10.3390/colloids5010003
Jarek E, Krasińska-Krawet Z, Kruk T, Lamch Ł, Ronka S, Wilk KA, Warszyński P. Adsorption Properties of Soft Hydrophobically Functionalized PSS/MA Polyelectrolytes. Colloids and Interfaces. 2021; 5(1):3. https://doi.org/10.3390/colloids5010003
Chicago/Turabian StyleJarek, Ewelina, Zofia Krasińska-Krawet, Tomasz Kruk, Łukasz Lamch, Sylwia Ronka, Kazimiera A. Wilk, and Piotr Warszyński. 2021. "Adsorption Properties of Soft Hydrophobically Functionalized PSS/MA Polyelectrolytes" Colloids and Interfaces 5, no. 1: 3. https://doi.org/10.3390/colloids5010003
APA StyleJarek, E., Krasińska-Krawet, Z., Kruk, T., Lamch, Ł., Ronka, S., Wilk, K. A., & Warszyński, P. (2021). Adsorption Properties of Soft Hydrophobically Functionalized PSS/MA Polyelectrolytes. Colloids and Interfaces, 5(1), 3. https://doi.org/10.3390/colloids5010003