Characterization of Chitosan/Hyaluronan Complex Coacervates Assembled by Varying Polymers Weight Ratio and Chitosan Physical-Chemical Composition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
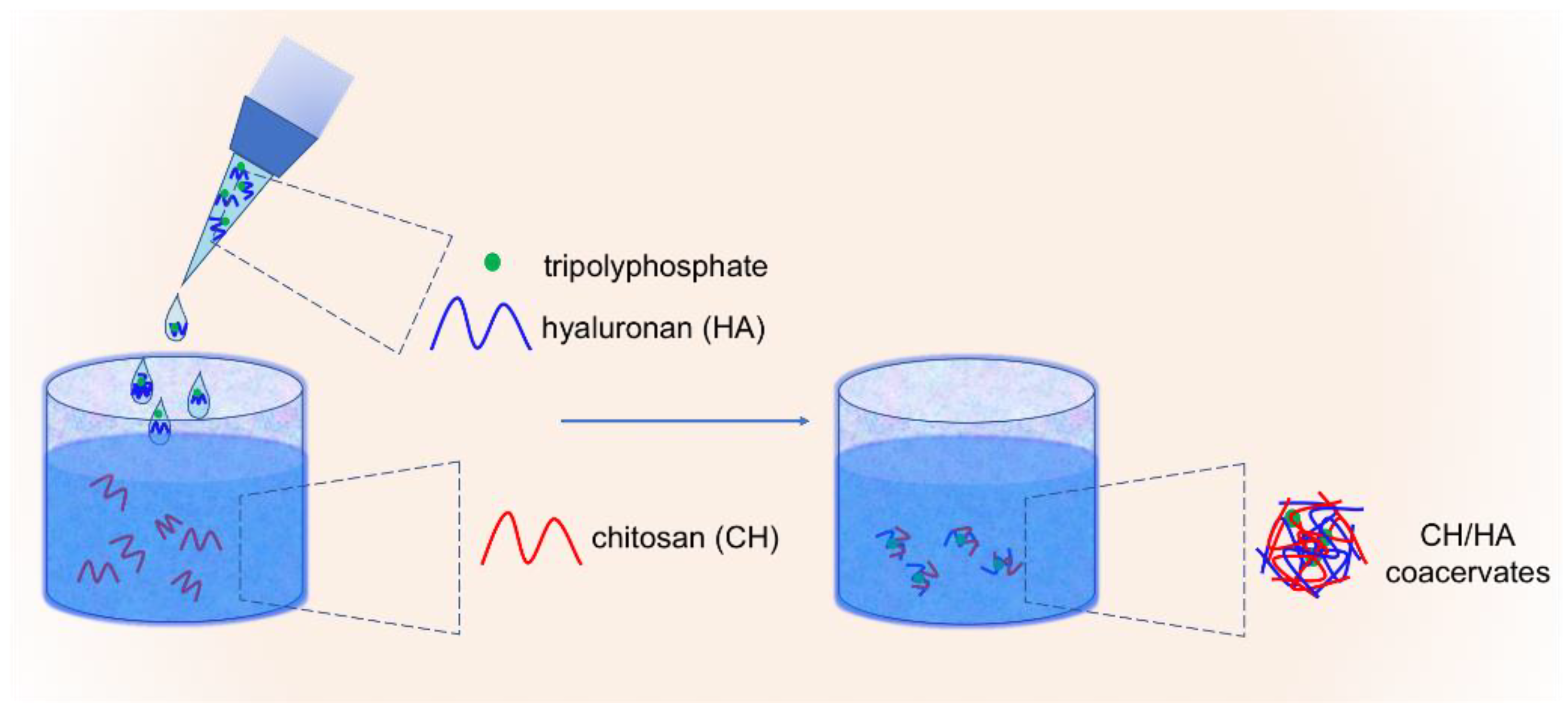
2.2. Preparation of Complex Coacervates
2.3. Physical-Chemical Characterization of Complex Coacervates
Dynamic Light Scattering (DLS) Analyses
3. Results and Discussion
4. Conclusions
- (i)
- Low acetylation as well as low polycation molecular weight determine a true liquid-liquid phase separation without formation of aggregates; in these conditions, the dilute phase (the supernatant) is in equilibrium with the dense phase (the coacervate);
- (ii)
- Chitosan-to-hyaluronan mass ratio directs colloids surface charge;
- (iii)
- Chitosan physical-chemical features influence colloids dimension and homogeneity.
Author Contributions
Conflicts of Interest
References
- Perugini, L.; Cinelli, G.; Cofelice, M.; Ceglie, A.; Lopez, F.; Cuomo, F. Effect of the coexistence of sodium caseinate and Tween 20 as stabilizers of food emulsions at acidic pH. Colloids Surf. B Biointerfaces 2018, 168, 163–168. [Google Scholar] [CrossRef]
- Kroll, J. Food Colloids Proteins, Lipids and Polysaccharides; Dickinson, E., Bergenståhl, B., Eds.; WILEY-VCH Verlag: Weinheim, Germany, 1997. [Google Scholar]
- Dickinson, E. On the road to understanding and control of creaminess perception in food colloids. Food Hydrocoll. 2018, 77, 372–385. [Google Scholar] [CrossRef]
- McClements, D.J. The future of food colloids: Next-generation nanoparticle delivery systems. Curr. Opin. Colloid Interface Sci. 2017, 28, 7–14. [Google Scholar] [CrossRef]
- Dickinson, E. Colloids in Food: Ingredients, Structure, and Stability. Annu. Rev. Food Sci. Technol. 2015, 6, 211–233. [Google Scholar] [CrossRef]
- Gutiérrez, T.J. Chitosan: Derivatives, Composites and Applications. In Chitosan: Derivatives, Composites and Applications; Ahmed, S., Ikram, S., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 183–232. [Google Scholar]
- Dutta, J.; Tripathi, S.; Dutta, P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systematic study needs for food applications. Food Sci. Technol. Int. 2012, 18, 3–34. [Google Scholar] [CrossRef]
- Guo, M.; Yadav, M.P.; Jin, T.Z. Antimicrobial edible coatings and films from micro-emulsions and their food applications. Int. J. Food Microbiol. 2017, 263, 9–16. [Google Scholar] [CrossRef]
- Lekshmi, R.G.K.; Rahima, M.; Chatterjee, N.S.; Tejpal, C.S.; Anas, K.K.; Vishnu, K.V.; Sarika, K.; Asha, K.K.; Anandan, R.; Suseela, M. Chitosan—Whey protein as efficient delivery system for squalene: Characterization and functional food application. Int. J. Biol. Macromol. 2019, 135, 855–863. [Google Scholar] [CrossRef]
- de Farias, B.S.; Sant’Anna Cadaval Junior, T.R.; de Almeida Pinto, L.A. Chitosan-functionalized nanofibers: A comprehensive review on challenges and prospects for food applications. Int. J. Biol. Macromol. 2019, 123, 210–220. [Google Scholar] [CrossRef]
- Vårum, K.M.; Smidsrød, O. Structure-Property Relationship in Chitosans. In Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Nilsen-Nygaard, J.; Strand, S.P.; Vårum, K.M.; Draget, K.I.; Nordgård, C.T. Chitosan: Gels and interfacial properties. Polymers (Basel) 2015, 7, 552–579. [Google Scholar] [CrossRef]
- Cuomo, F.; Cofelice, M.; Venditti, F.; Ceglie, A.; Miguel, M.; Lindman, B.; Lopez, F. In-vitro digestion of curcumin loaded chitosan-coated liposomes. Colloids Surfaces B Biointerfaces 2018, 168, 29–34. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Racine, L.; Texier, I.; Auzély-Velty, R. Chitosan-based hydrogels: Recent design concepts to tailor properties and functions. Polym. Int. 2017, 66, 981–998. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Aramwit, P.; Ekasit, S.; Yamdech, R. The development of non-toxic ionic-crosslinked chitosan-based microspheres as carriers for the controlled release of silk sericin. Biomed. Microdevices 2015, 17, 1–9. [Google Scholar] [CrossRef]
- Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; Paoletti, S.; Donati, I.; Sacco, P.; Furlani, F.; de Marzo, G.; Marsich, E.; et al. Concepts for Developing Physical Gels of Chitosan and of Chitosan Derivatives. Gels 2018, 4, 67. [Google Scholar] [CrossRef]
- Liu, Q.; Sacco, P.; Marsich, E.; Furlani, F.; Arib, C.; Djaker, N.; Lamy De La Chapelle, M.; Donati, I.; Spadavecchia, J. Lactose-Modified Chitosan Gold(III)-PEGylated Complex-Bioconjugates: From Synthesis to Interaction with Targeted Galectin-1 Protein. Bioconjug. Chem. 2018, 29, 3352–3361. [Google Scholar] [CrossRef]
- Cok, M.; Viola, M.; Vecchies, F.; Sacco, P.; Furlani, F.; Marsich, E.; Donati, I. N-isopropyl chitosan. A pH- and thermo-responsive polysaccharide for gel formation. Carbohydr. Polym. 2020, 230, 115641. [Google Scholar] [CrossRef]
- Cok, M.; Sacco, P.; Porrelli, D.; Travan, A.; Borgogna, M.; Marsich, E.; Paoletti, S.; Donati, I. Mimicking mechanical response of natural tissues. Strain hardening induced by transient reticulation in lactose-modified chitosan (chitlac). Int. J. Biol. Macromol. 2018, 106, 656–660. [Google Scholar] [CrossRef]
- Vecchies, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Porrelli, D.; Donati, I.; Turco, G.; Paoletti, S.; Marsich, E. Complex Coacervates between a Lactose-Modified Chitosan and Hyaluronic Acid as Radical-Scavenging Drug Carriers. Biomacromolecules 2018, 19, 3936–3944. [Google Scholar] [CrossRef]
- Alves, N.M.; Mano, J.F. Chitosan derivatives obtained by chemical modifications for biomedical and environmental applications. Int. J. Biol. Macromol. 2008, 43, 401–414. [Google Scholar] [CrossRef]
- Lim, S.H.; Hudson, S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydr. Res. 2004, 339, 313–319. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Marsich, E.; Donati, I.; Paoletti, S. Highly monodisperse colloidal coacervates based on a bioactive lactose-modified chitosan: From synthesis to characterization. Carbohydr. Polym. 2017, 174, 360–368. [Google Scholar] [CrossRef]
- Kizilay, E.; Kayitmazer, A.B.; Dubin, P.L. Complexation and coacervation of polyelectrolytes with oppositely charged colloids. Adv. Colloid Interface Sci. 2011, 167, 24–37. [Google Scholar] [CrossRef]
- Antonov, M.; Mazzawi, M.; Dubin, P.L. Entering and exiting the protein-polyelectrolyte coacervate phase via nonmonotonic salt dependence of critical conditions. Biomacromolecules 2010, 11, 51–59. [Google Scholar] [CrossRef]
- Umerska, A.; Paluch, K.J.; Inkielewicz-Stepniak, I.; Santos-Martinez, M.J.; Corrigan, O.I.; Medina, C.; Tajber, L. Exploring the assembly process and properties of novel crosslinker-free hyaluronate-based polyelectrolyte complex nanocarriers. Int. J. Pharm. 2012, 436, 75–87. [Google Scholar] [CrossRef]
- Zambito, Y.; Felice, F.; Fabiano, A.; Di Stefano, R.; Di Colo, G. Mucoadhesive nanoparticles made of thiolated quaternary chitosan crosslinked with hyaluronan. Carbohydr. Polym. 2013, 92, 33–39. [Google Scholar] [CrossRef]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-based nanoparticles by chitosan and gum arabic polyelectrolyte complexation as carriers for curcumin. Food Hydrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Almalik, A.; Donno, R.; Cadman, C.J.; Cellesi, F.; Day, P.J.; Tirelli, N. Hyaluronic acid-coated chitosan nanoparticles: Molecular weight-dependent effects on morphology and hyaluronic acid presentation. J. Control. Release 2013, 172, 1142–1150. [Google Scholar] [CrossRef]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronan-based nanocarriers for transmucosal delivery of macromolecules. Macromol. Biosci. 2008, 8, 441–450. [Google Scholar] [CrossRef]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2016–2024. [Google Scholar] [CrossRef]
- de la Fuente, M.; Seijo, B.; Alonso, M.J. Design of novel polysaccharidic nanostructures for gene delivery. Nanotechnology 2008, 19, 075105. [Google Scholar] [CrossRef]
- Lallana, E.; Rios De La Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef]
- Sacco, P.; Decleva, E.; Tentor, F.; Menegazzi, R.; Borgogna, M.; Paoletti, S.; Kristiansen, K.A.; Vårum, K.M.; Marsich, E. Butyrate-Loaded Chitosan/Hyaluronan Nanoparticles: A Suitable Tool for Sustained Inhibition of ROS Release by Activated Neutrophils. Macromol. Biosci. 2017, 17. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent development of chitosan-based polyelectrolyte complexes with natural polysaccharides for drug delivery. Int. J. Biol. Macromol. 2014, 64, 353–367. [Google Scholar] [CrossRef]
- Oyarzun-Ampuero, F.A.; Brea, J.; Loza, M.I.; Torres, D.; Alonso, M.J. Chitosan-hyaluronic acid nanoparticles loaded with heparin for the treatment of asthma. Int. J. Pharm. 2009, 381, 122–129. [Google Scholar] [CrossRef]
- Furlani, F.; Sacco, P.; Decleva, E.; Menegazzi, R.; Donati, I.; Paoletti, S.; Marsich, E. Chitosan Acetylation Degree Influences the Physical Properties of Polysaccharide Nanoparticles: Implication for the Innate Immune Cells Response. ACS Appl. Mater. Interfaces 2019, 11, 9794−9803. [Google Scholar] [CrossRef]
- Sacco, P.; Cok, M.; Asaro, F.; Paoletti, S.; Donati, I. The role played by the molecular weight and acetylation degree in modulating the stiffness and elasticity of chitosan gels. Carbohydr. Polym. 2018, 196, 405–413. [Google Scholar] [CrossRef]



| FA | (mL/g) | |||
|---|---|---|---|---|
| 0.16 | 110 | 30,000 | 198 | 152 |
| 681 | 220,000 | 1111 | ||
| 1026 | 340,000 | 1717 | ||
| 0.46 | 340 | 100,000 | 200 | 500 |
| 650 | 210,000 | 1050 | ||
| 920 | 300,000 | 1500 | ||
| 0.63 | 300 | 90,000 | >201 | 448 |
| 550 | 170,000 | 846 | ||
| 950 | 310,000 | 1542 |
| FA | CH:HA Weight Ratio | Notes | |
|---|---|---|---|
| 30,000 | 1:2 | no aggregation | |
| 2:1 | no aggregation | ||
| 0.16 | 220,000 | 1:2 | no aggregation |
| 2:1 | no aggregation | ||
| 340,000 | 1:2 | no aggregation | |
| 2:1 | limited aggregation | ||
| 100,000 | 1:2 | no aggregation | |
| 2:1 | no aggregation | ||
| 0.46 | 210,000 | 1:2 | no aggregation |
| 2:1 | no aggregation | ||
| 300,000 | 1:2 | no aggregation | |
| 2:1 | limited aggregation | ||
| 90,000 | 1:2 | no aggregation | |
| 2:1 | no aggregation | ||
| 0.63 | 170,000 | 1:2 | limited aggregation |
| 2:1 | no aggregation | ||
| 310,000 | 1:2 | no aggregation | |
| 2:1 | limited aggregation |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlani, F.; Donati, I.; Marsich, E.; Sacco, P. Characterization of Chitosan/Hyaluronan Complex Coacervates Assembled by Varying Polymers Weight Ratio and Chitosan Physical-Chemical Composition. Colloids Interfaces 2020, 4, 12. https://doi.org/10.3390/colloids4010012
Furlani F, Donati I, Marsich E, Sacco P. Characterization of Chitosan/Hyaluronan Complex Coacervates Assembled by Varying Polymers Weight Ratio and Chitosan Physical-Chemical Composition. Colloids and Interfaces. 2020; 4(1):12. https://doi.org/10.3390/colloids4010012
Chicago/Turabian StyleFurlani, Franco, Ivan Donati, Eleonora Marsich, and Pasquale Sacco. 2020. "Characterization of Chitosan/Hyaluronan Complex Coacervates Assembled by Varying Polymers Weight Ratio and Chitosan Physical-Chemical Composition" Colloids and Interfaces 4, no. 1: 12. https://doi.org/10.3390/colloids4010012
APA StyleFurlani, F., Donati, I., Marsich, E., & Sacco, P. (2020). Characterization of Chitosan/Hyaluronan Complex Coacervates Assembled by Varying Polymers Weight Ratio and Chitosan Physical-Chemical Composition. Colloids and Interfaces, 4(1), 12. https://doi.org/10.3390/colloids4010012








