1. Introduction
The concept of a monomolecular layer is widely used in thermodynamics [
1,
2]. Typically, one has in mind an adsorption layer of some substance at the interface of any material. The molecules forming the monolayer create some surface (two-dimensional) pressure [
3]. The connection between the two-dimensional pressure and other parameters of the monolayer (density of molecules, temperature) is determined by the equation of state. The first two-dimensional equation of the state for the monolayer at low concentrations has been proposed by Traube [
4]. This equation describes some ideal two-dimensional monolayer:
πA = RT, where
π is the two-dimensional pressure and A is the area per one molecule [
5]. De Boer [
6] proposed an empirical equation of state
π(A-b
2) = iRT, where parameter b
2 denotes the area per single molecule in a tightly packed layer, i is a measure of the attractive forces.
To date, different equations have been proposed for two-dimensional systems: power-type equations based on the virial expansions, the Frumkin logarithmic equation, and the Van der Waals (VdW) equation [
7].
Recently, a new two-dimensional equation of the state of the monolayer was proposed [
3]:
The first term under the integral takes into account the orientation effect (h is the dimensionless thickness of the monolayer) of asymmetric molecules. The value depends on the type of molecular interaction, corresponds to the dimensionless two-dimensional pressure, and θ to the degree of filling the surface. Molecular interactions are always calculated through the centers of mass of particles, and therefore two-dimensional pressure is determined not by the real area of the monolayer, but by the size of the area in which the centers of mass of the particles move. This means that the excluded area aex must be understood as the area that is inaccessible to the centers of mass. Similarly, a particle with a center of mass at rest (but rotating along the surface and therefore round) creates an excluded area in the form of a circle with a radius equal to the sum of the radii of the particle at rest and the moving particle approaching it. If all particles are of the same size and move independently (the case of a one-component two-dimensional gas), then a stationary particle of radius r creates an excluded area, which is four times the landing area of the particle. Taking this into account, the exclusion factor fex as the ratio of excluded and landing areas: is introduced.
For a monolayer of neutral, spherically symmetric molecules, Equation (1) has the form:
The integration of Equation (2) depends on the choice of the exclusion factor fex and is the dimensionless constant of intermolecular interaction.
If
fex = constant, the integration of Equation (2) leads to the Frumkin and Planck equation [
8,
9]:
When
fex = 4 this is a two-dimensional analogue of the Planck equation, and for
fex = 1 this is the Frumkin equation [
3]. The definition of
will be given below.
As a first approximation, the exclusion factor
fex was chosen in the form [
3]:
Then the integration of Equation (2) with (4) yields the two-dimensional equation of VdW
As a next approximation, the
fex dependence [
3] was proposed:
where
κ is a constant (
κ ~ 1.144).
Then, the integration of Equation (2) with (6) yields the two-dimensional equation [
3]:
The parameter
β is a quantity that depends on the excluded area:
β = −1428. The correlations between
κ and
β are described in Rusanov [
3].
Equation (7) allows us to describe the first three virial coefficients quite accurately.
Although such equations are usually considered two-dimensional, the three-dimensional aspect of real monolayers is inevitably present. For example, this is due to the influence of the surrounding bulk phase, the change of the orientation of non-spherical molecules in the monolayer with the change of the two-dimensional pressure. Therefore, generally speaking, the equation of state for the monolayer should be considered quasi-two-dimensional.
The description of the surface in terms of two dimensions is certainly appropriate if bumps and dips in the surface have extensions much larger than the size of any molecule. Such is no longer true when the irregularities of the surface are comparable to the size of adsorbate molecules. Consequently, to describe the irregular surface one should consider its dimension as an appropriate value between two and three.
Let us now explain the determination of the surface (two-dimensional) pressure. The monolayer is a real, in general, three-dimensional medium under the influence of molecular interactions. The influence of external fields will not be considered. A monolayer of spherically symmetric, neutral molecules on a geometrically and energetically homogeneous surface corresponds to a Euclidean space.
The mechanical state, the surface pressure of the monolayer, is determined by specifying a stress tensor or a pressure tensor. These tensors differ only in sign. For molecular systems, the pressure tensor is more generally accepted. In the general case (Adsorption on a fractal surface) the conformal conditions for a monolayer on a surface are not satisfied. Therefore, it is not possible to determine two-dimensional pressure in the framework of the classical Gibbs definition. Therefore, the authors use the definition of surface pressure as: with free energy F.
Two tangential components of the tensor of excess surface pressure, in general, determine the surface two-dimensional pressure. The task of strictly calculating the tensor components of the surface pressure is a very difficult one and practically impossible for the analysis of real (non-model) adsorption systems. Therefore, the only possible version for the calculation of surface pressure is the calculation of the value from the pressure of the experimental adsorption isotherm [
6].
To correctly describe the state of a monolayer in a two-dimensional Euclidean space in a wide range of parameter changes, various approximations must be made. These approximations are not always strictly justified. When describing unordered media by the methods of fractal geometry, one fundamental assumption is made, namely the hypothesis of scale-invariant structures. This hypothesis allows us to construct a theory of disordered media using renormalization group methods. This approach is universal, but is associated with large mathematical (computational) difficulties. These difficulties were overcome by Mandelbrot’s introduction of the concepts of fractal geometry. Thus, the description of the thermodynamic system, a quasi-two-dimensional layer of molecules, contains almost no assumptions or approximations.
The aim of this work is the modification of the two-dimensional VdW equation to a quasi-two-dimensional one. This procedure is based on the concept of the fractal nature of the monolayer–solid interface.
2. Theory
The surface pressure is generally defined as work required to change the surface area of the adsorbed phase on unit value at constant V, N, and T.
The definition of the surface pressure through the grand partition function is as follows [
10]:
where Ξ
=Ξ/Ξ
, Ξ
is the grand partition function for not adsorbed gas in the bulk phase.
The virial expansion method is theoretically exact specifically in the considered case of ethane adsorption on activated carbon. The logarithm of the grand partition function can be expanded in a series [
10]:
Then, the equation for the surface pressure can be obtained by the substitution of the expansion in Equation (9) into Equation (8) [
10]:
where
Na is the number of the adsorbed gas molecules and
B2D,
C2D,… are the two-dimensional virial coefficients. These coefficients are also determined for the three-dimensional case. For a large
N, it is impossible to calculate
exactly. Model approaches are necessary. However, the difficulties of calculation of the higher virial coefficients limit the applicability of this method by small values of the surface density of matter [
10].
The equation for the surface pressure isotherm at high densities can be obtained as the two-dimensional VdW equation. In the thermodynamics of adsorption, it is more convenient to use the canonical partition function [
10]. The equation that relates the canonical partition function with two-dimensional (surface) pressure has the form [
10]:
where the configuration interval is given by:
.
Let us give some clarifications concerning the value Va. The whole volume V of the gas phase in a thermostat can be divided into two parts: (1) a small region Va near the surface of the adsorbent, where the gas density differs substantially from the density in the bulk phase; (2) the part of the volume Vd, in which gas properties do not differ from the properties of the gas in the main volume.
The configuration integral can be represented in two parts:
- (1)
three-dimensional integrals for an individual molecule on the surface;
- (2)
a configuration integral in which the integration is carried out in the plane of the parallel surface.
The configuration integral
is calculated by Equation (12):
In Equation (12)
, the amplitude of the free oscillations of the molecule is perpendicular to the surface;
is the magnitude of the energy of the molecule at the minimum of the potential well. The integral
is defined as follows [
10]:
In Equation (13),
is the energy of interaction of the
i-th molecule with the
j-th molecule. In case of moving (or restrictedly moving) films (here: adsorption layers) the dependence
on
can be neglected. This approximation is valid for the isotherm under consideration. With the Lennard-Jones potential as the interaction potential of the molecules, the integral in Equation (13) is transformed to the form:
In Equation (14),
0 is the average energy of lateral interactions per molecule. Then combining Equations (12) and (14), the configuration integral
will be written in the form:
where
is an excluded area of the molecule in the adsorption layer,
is the average energy of intermolecular interactions in the potential field of the adsorbent. In Equation (15), it is assumed that
where
σ (molecular diameter) and
ε (depth of potential well) are the parameters of the Lennard-Jones potential for molecules in the monolayer [
6].
Let us give some comments concerning the parameter
. According to Equation (18), the value of the parameter
depends only on the molecular diameter
σ. However, in real adsorption monolayers, the parameter
is defined as the area accessible for the molecule at the maximum density of the monolayer. The maximum density of the monolayer is determined by the type of molecules, by the properties of adsorbent and by the temperature. Let us denote the number of molecules in the monolayer at maximum filling for a given temperature as
Nm; the number of molecules adsorbed at given temperature and pressure
p is denoted as
N. In this case, the number of sites available for adsorption will be equal to
Nm-
N. The number of molecules
N and
Nm are associated with the areas occupied by a molecule by means of relations [
6]:
Usually, the values
Nm and
N are determined experimentally as the number of moles per unit weight of the adsorbate and are denoted as
Vm and
V respectively. Then, from Equations (20) and (21), we obtain the area occupied by one molecule at an arbitrary
p in a dense monolayer:
where
V and
are the adsorption values at pressure
p and in maximum dense monolayer, respectively.
In the Equations (22) and (23), is the specific surface area of adsorbent. Thus, in future, we determine the value for each individual isotherm by Formula (23). The same conclusions must be made concerning parameter , as well. The parameters and are determined from the experimental isotherm in each case separately.
Substituting Equations (15)–(17) into Equation (11), we obtain the well-known VdW equation:
Then, substituting Equations (22) and (23) into Equation (24), we rewrite Equation (24) in the form:
It is also convenient to consider the equation of state of a monolayer in the dimensionless form:
In this case, the strictly two-dimensional equation of state has the form:
It should be noted that the Equations (16) and (17) are obtained for a homogeneous surface. The topological dimension of the interface between the monolayer and the surface of the adsorbent is equal to two. For inhomogeneous adsorbent surfaces, the dimension of the interface between the monolayer and the surface is defined as fractal, with a fractal dimension
DF with
[
11].
Now, consider the VdW equation for a fractal surface. In order to correctly apply Equations (16) and (17) to the fractal surface, it is necessary to take into consideration the effect of the fractal dimension of the surface on the value of area occupied by a molecule. Consider two types of adsorbent surface: homogenous, with the topological dimension
D = 2, and rough, with the fractal dimension
DF > 2. On each surface, let us define an area bounded by the perimeter L. Suppose that the perimeters for homogeneous and rough surfaces are the same. Then the following equality is fulfilled [
12]:
In the future, we will use the notation
. Accordingly, the value of the excluded area of the molecule in the monolayer on the fractal surface will be equal to:
Now it is necessary to rewrite Equations (16) and (17), taking into account Equations (28). Then the integral for the monolayer on the fractal surface should be written as:
Substituting Equation (29) into (11) we obtain:
It is also convenient to consider the equation of state of a monolayer in the dimensionless form:
The strictly two-dimensional equation of state, Equation (27), contains one parameter , whereas the quasi-two-dimensional equation of state, Equation (31), contains two parameters: α and .
In deriving Equation (30), the influence of the degree of disorder of the adsorbent (fractal dimension) on the properties of Equation (30) is strictly shown. In this case, the dependence of the excluded volume (the first term of Equation (30)) and of the molecular interaction (the second term of Equation (30)) on the fractal dimension DF is strictly obtained. If all the states of the adsorption medium are equally probable, DF = 2 and Equation (30) reduces to the classical equation of van der Waals.
3. Results and Discussion
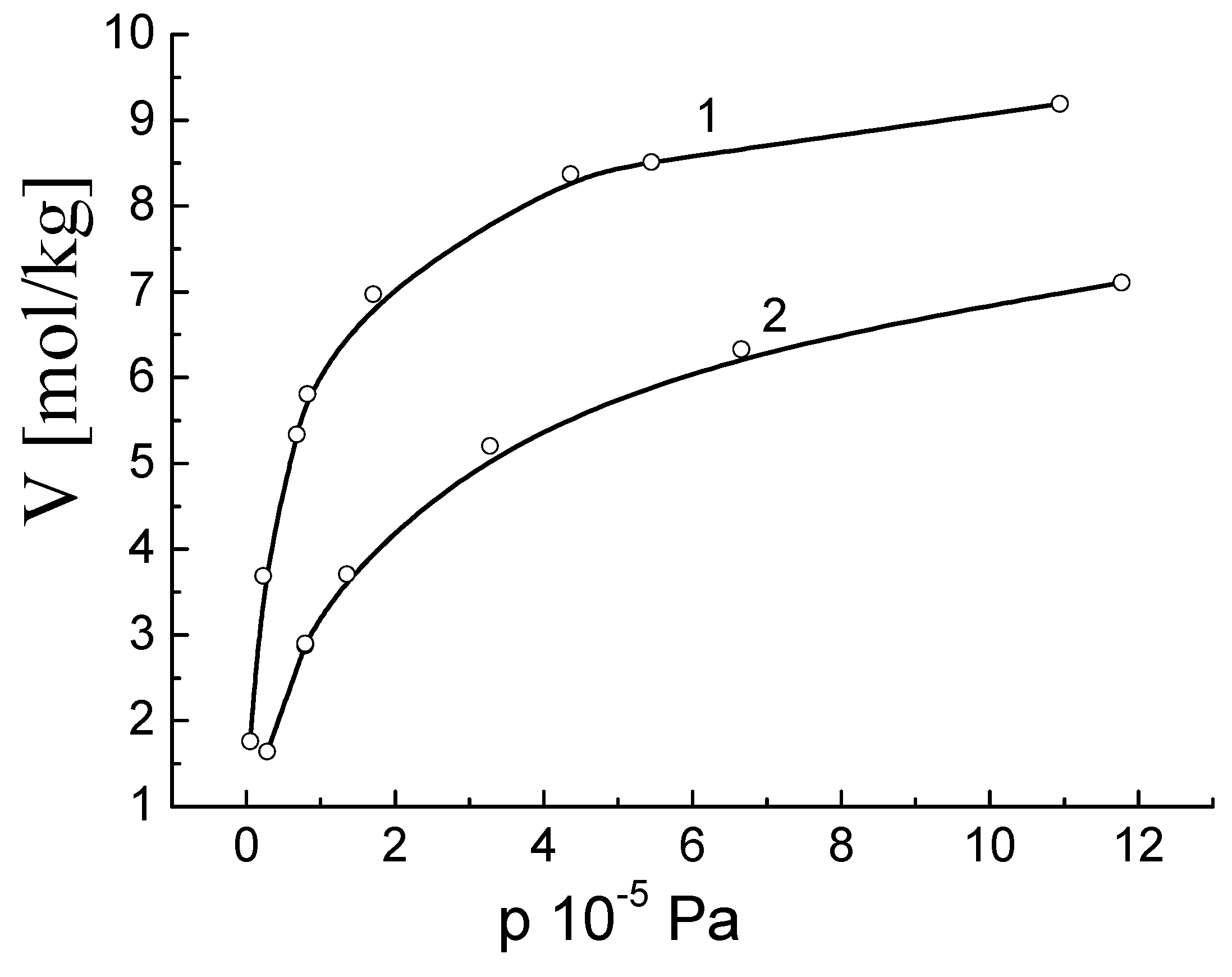
As an example, consider the adsorption isotherms
a(
T,
p) of ethane at
T = 273.15 K (sample №1) and
T = 323.15 K (sample №2) on activated carbon sample with specific surface S
BET = 1440 m
2/g (
Figure 1). In
Figure 1, the points denote the experimental values [
13,
14] and the lines are computer fittings to the Toth equation.
The isotherms of the surface pressure
are calculated using the adsorption isotherms as follows [
6]:
Further, we will determine the value of the surface pressure π, calculated by Formula (25), as the experimental value and denote as
. We assume that we do not know
a priori the equation of the adsorption isotherm
a(
T,
p), but we use Equations (25) and (30) with free parameters for alpha,
. The surface pressure
will be calculated by Equation (32) using Newton-Kotes numerical integration formulas [
15]. For further analysis, it is necessary to represent the surface pressure isotherm
in the form
, where
, where the parameter
is determined by the adsorption isotherm
a(
T,
p) [
6].
For the adsorption isotherm of ethane on activated carbon (S = 1440 m
2/g) at
T = 273.15 K (sample №1) the following values were obtained:
For sample 2:
The results of comparative graphical analysis of the calculated and experimental data are presented in the form of graphs:
Figure 2 represents the results of analysis for sample №1 and
Figure 3 for sample №2.
Figure 2 and
Figure 3 are constructed as follows. Ordinates of quantities
Y are calculated from Equation (33) of the knots of the function
V(
p). As the knots of the function, the values of the argument (the value of the pressure) at which the adsorption was measured were chosen.
For sample 1, Equation (27) describes the experimental surface pressure isotherm for with a maximum relative deviation of 9.5% and for sample for with a maximum relative deviation of 9.7%.
The quasi-two-dimensional Equation (31) describes the experimental surface pressure isotherm for sample №1 for with a maximum relative deviation of 8.5% and for sample 2 for with a maximum relative deviation of 8.7%.
Earlier, the designation
was introduced. Consequently, the fractal dimension of the interface between the monolayer and the surface of the adsorbent is
for sample 1 and is
for sample 2. It is interesting to check the results of the calculation of these fractal dimensions obtained with the quasi-two-dimensional Equation (31). In earlier published work, the Langmuir adsorption isotherm equation has been investigated in the form [
16]:
In Equation (34),
V and
Vm are the adsorption values at pressure
p and in the maximum dense monolayer, respectively;
b is an equilibrium constant for
. If
β = 1, Equation (34) turns into the Langmuir equation corresponding to the adsorption on a homogeneous surface without taking into account the interaction between the adsorbate molecules. It has also been proven rigorously that the parameter
β and fractal dimension
DF of the interface between the monolayer and the adsorbent surface are related by the equality:
[
11].
According to Equation (34), sample 1 is characterized by the parameter value β = 0.68. For sample 2, we found β = 0.76. Consequently, the fractal dimension of the interface between the monolayer and the adsorbent surface for sample 1 is DF = 2.32 and for sample 2 is DF = 2.24. These results agree well with the values of the fractal dimension determined from quasi-two-dimensional Equation (31).
Now let us give some comments regarding the interval of θ, for which Equation (31) describes the surface pressure isotherm. To do this, it is convenient to analyze the isotherms for the Graham functions [
17]:
Figure 4 shows the function
of the adsorption isotherm for sample №1. The dots denote the values calculated by Formula (35) based on the experimental adsorption isotherm.
Such behavior of
is typical for the monolayer on non-uniform surface taking into account the interaction of adsorbed molecules.
Figure 4 clearly shows that the function
is not monotonic: it reaches a minimum at
and then increases sharply. The domain of applicability of the quasi-two- dimensional VdW equation is limited by the monotonic domain of the function
.