Porous Resorcinol-Formaldehyde Resins
Abstract
1. Introduction
2. Materials and Methods
3. Results
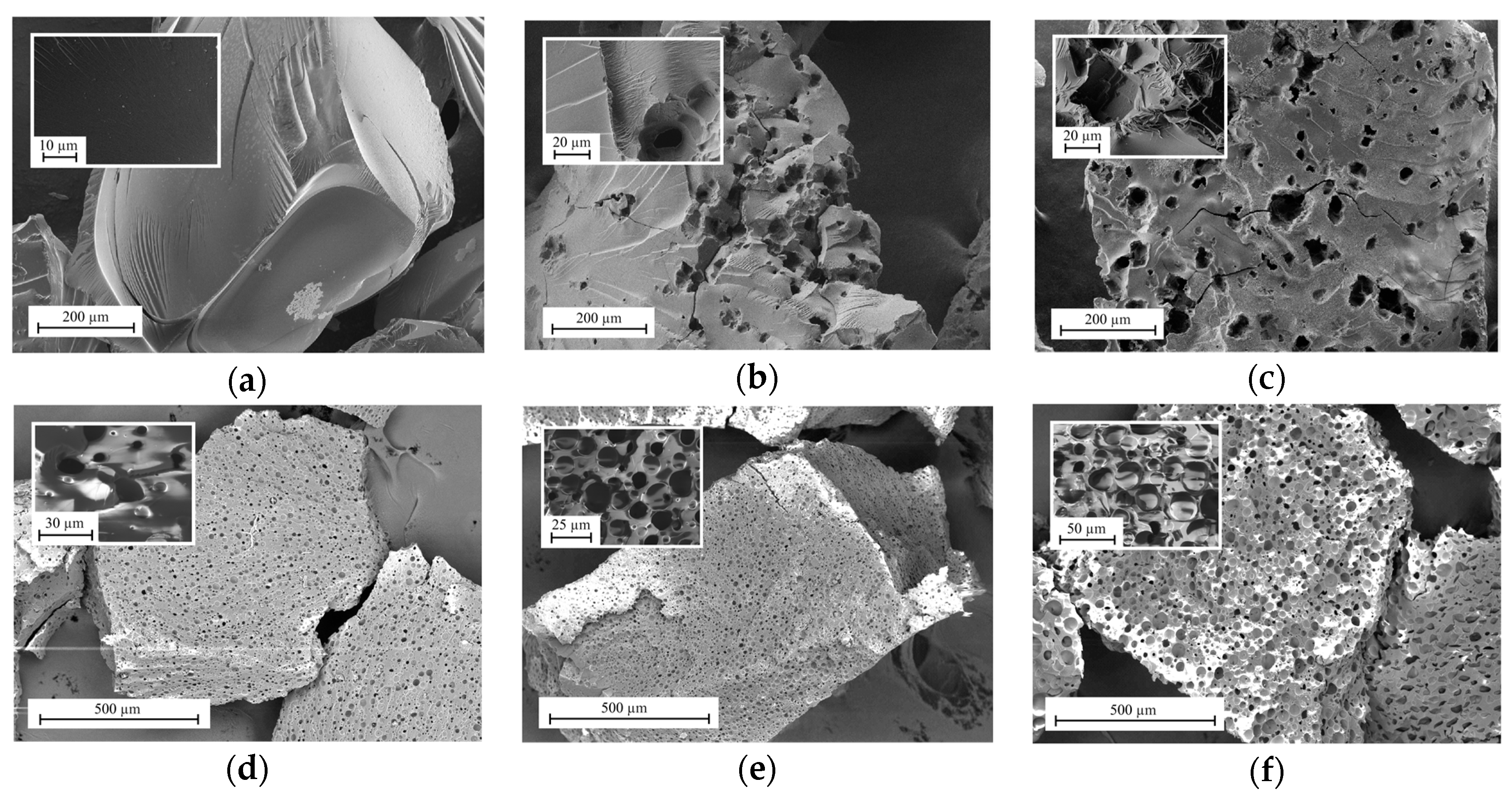
3.1. Characterization of Ion-Exchange Resins
3.2. Sorption Kinetics
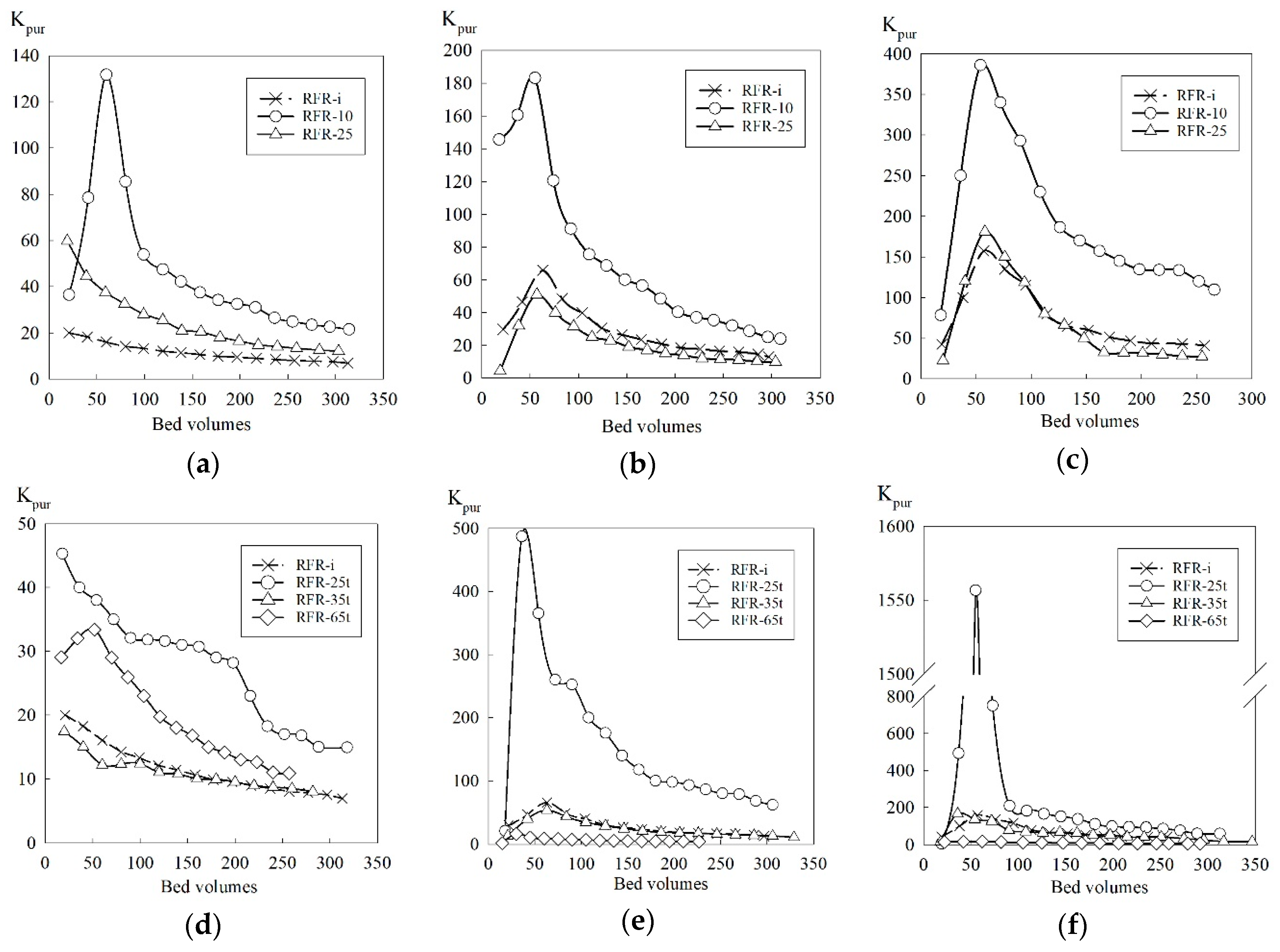
3.3. Sorption of Cs-137 under Dynamic Conditions
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Logunov, M.V.; Karpov, V.I.; Druzhinin, N.E.; Tananaev, I.G. Approaches to the processing of high-level pulps accumulated at the FSUE “PA Mayak”. Vop. Radiat. Bezopasnosti 2011, 18–27. (In Russian) [Google Scholar]
- Duignan, M.R.; Nash, C.A. Removal of Cesium from Savannah River Site Waste with Spherical Resorcinol Formaldehyde Ion Exchange Resin: Experimental Tests. Sep. Sci. Technol. 2010, 45, 1828–1840. [Google Scholar] [CrossRef]
- Hassan, N.M.; Adu-Wusu, K. Cesium Removal from Hanford Tank Waste Solution Using Resorcinol-Formaldehyde Resin. Solvent Extr. Ion Exch. 2005, 23, 375–389. [Google Scholar] [CrossRef]
- Russel, R.L.; Fiskum, S.K.; Jagoda, L.K.; Poloski, A.P. AP-101 Diluted Feed (Envelope A) Simulant Development Report; Technical Report; Battelle, Pacific Northwest Division: Richland, WA, USA, 2003. Available online: https://www.pnnl.gov/rpp-wtp/documents/WTP-RPT-057.pdf (accessed on 9 November 2018).
- Logunov, M.V.; Karpov, V.I.; Tananaev, I.G. Stabilization of the thermophysical state and examination of some storage tanks of highly active pulps at the FSUE “PA Mayak”. Vop. Radiat. Bezopasnosti 2011, 18–27. (In Russian) [Google Scholar]
- Omelchuk, V.V.; Stahiv, M.R.; Savkin, A.E.; Fedorov, D.A.; Kornev, V.I. Development of technologies and processing of bottoms at Kola NPP. At. Energy 2007, 34–37. (In Russian) [Google Scholar]
- Gibert, O.; Valderrama, C.; Peterkóva, M.; Cortina, J.L. Evaluation of Selective Sorbents for the Extraction of Valuable Metal Ions (Cs, Rb, Li, U) from Reverse Osmosis Rejected Brine. Solvent Extr. Ion Exch. 2010, 28, 543–562. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Mikheev, S.V.; Gelis, V.M.; Kozlitin, E.A. Sorption of cesium on ferrocyanide sorbents from highly saline solutions. Radiochemistry 2009, 51, 298–300. [Google Scholar] [CrossRef]
- Prout, W.E.; Russell, E.R.; Groh, H.J. Ion exchange absorption of cesium by potassium hexacyanocobalt (II) ferrate (II). J. Inorg. Nucl. Chem. 1965, 27, 473–479. [Google Scholar] [CrossRef]
- Ernest, M.V.; Bibler, J.P.; Whitley, R.D.; Wang, N.-H.L. Development of a Carousel Ion-Exchange Process for Removal of Cesium-137 from Alkaline Nuclear Waste. Ind. Eng. Chem. Res. 1997, 36, 2775–2788. [Google Scholar] [CrossRef]
- Hassan, N.M.; Adu-Wusu, K.; Marra, J.C. Resorcinol-formaldehyde adsorption of cesium from Hanford waste solutions, Part I. Batch equilibrium study. J. Radioanal. Nucl. Chem. 2004, 262, 579–586. [Google Scholar] [CrossRef]
- Milyutin, V.V.; Mikheev, S.V.; Gelis, V.M.; Kononenko, O.A. Coprecipitation of microamounts of cesium with precipitates of transition metal ferrocyanides in alkaline solutions. Radiochemistry 2009, 51, 295–297. [Google Scholar] [CrossRef]
- Sharygin, L.M.; Muromskii, A.Y.; Moiseev, V.E.; Tsekh, A.R.; Vaver, A.V. Sorption purification of liquid radioactive wastes from nuclear power plants. At. Energy 1997, 83, 493–499. [Google Scholar] [CrossRef]
- Miller, H.S.; Kline, G.E. Reactions of Cesium in Trace Amounts with Ion-exchange Resins. J. Am. Chem. Soc. 1951, 73, 2741–2743. [Google Scholar] [CrossRef]
- Fiskum, S.K.; Blanchard, D.L.; Steele, M.J.; Thomas, K.K.; Trang-Le, T.; Thorson, M.R. Spherical Resorcinol-Formaldehyde Resin Testing for Cesium Removal from Hanford Tank Waste Simulant. Sep. Sci. Technol. 2006, 41, 2461–2474. [Google Scholar] [CrossRef]
- Brown, G.N.; Russell, R.L.; Peterson, R.A. Small-Column Cesium Ion Exchange Elution Testing of Spherical Resorcinol-Formaldehyde; Technical Report; Pacific Northwest National Laboratory: Richland, WA, USA, 2011. Available online: https://www.pnnl.gov/rpp-wtp/documents/WTP-RPT-210.pdf (accessed on 9 November 2018).
- Fiskum, S.K.; Augspurger, B.S.; Brooks, K.P.; Buchmiller, W.C.; Russell, R.L.; Schweiger, M.J.; Snow, L.A.; Steele, M.J.; Thomas, K.K.; Wallace, D.E.; et al. Comparison Testing of Multiple Resorcinol-Formaldehyde Resins for the River Protection Project—Waste Treatment Plant; Technical Report; Savannah River Technology Center, Department of Energy 1000 Independence Ave.: SW Washington, DC, USA, 2004; p. 226. Available online: https://www.pnnl.gov/rpp-wtp/documents/WTP-RPT-103.pdf (accessed on 9 November 2018).
- Raj, K.; Prasad, K.K.; Bansal, N.K. Radioactive waste management practices in India. Nucl. Eng. Des. 2006, 236, 914–930. [Google Scholar] [CrossRef]
- Hubler, T.L.; Franz, J.A.; Shaw, W.J.; Bryan, S.A.; Hallen, R.T.; Brown, G.N.; Bray, L.A.; Linehan, J.C. Synthesis, Structural Characterization, and Performance Evaluation of Resorcinol-Formaldehyde (R-F) Ion-Exchange Resin; Technical Report; Pacific Northwest Lab.: Richland, WA, USA, 1995; Available online: https://digital.library.unt.edu/ark:/67531/metadc622721/ (accessed on 9 November 2018).
- Arm, S.T.; Blanchard, D.L.; Weier, D.R. Aging Effects of Stored SuperLig®644 Ion Exchange Resin; Technical Report; Battelle-Pacific Northwest Division, 902 Battelle Blvd: Richland, WA, USA, 2004. Available online: https://www.pnnl.gov/rpp-wtp/documents/WTP-RPT-093.pdf (accessed on 9 November 2018).
- Hubler, T.L.; Franz, J.A. Chemical Derivatization of Resorcinol-Formaldehyde Resin Leading to Enhanced Chemical/oxidative Stability of the Resin; Technical Report; Pacific Northwest National Laboratory: Richland, WA, USA, 1996; Available online: https://digital.library.unt.edu/ark:/67531/metadc686585/ (accessed on 9 November 2018).
- Shelkovnikova, L.A.; Gavlina, O.T.; Ivanov, V.A. Stability of phenol-formaldehyde ion-exchange sorbents in aqueous solutions. Russ. J. Phys. Chem. A 2011, 85, 1652–1659. [Google Scholar] [CrossRef]
- Arm, S.T.; Blanchard, D.L. Pre-conditioning and Regeneration Requirements of Ground Gel Resorcinol Formaldehyde Ion Exchange Resin; Technical Report; Battelle—Pacific Northwest Division: Richland, WA, USA, 2004; p. 57. Available online: https://www.pnnl.gov/rpp-wtp/documents/WTP-RPT-104.pdf (accessed on 9 November 2018).
- Tretyakov, V.A.; Kondrutsky, D.A.; Bobrov, A.F.; Milyutin, V.V.; Nesterov, A.G. Method of Obtaining Sorbent for Selective Extraction of Cesium. Russia Patent No. RU2521379C1; Application date 13 February 2013, published 27 June 2014,
- Silverstein, M.S. PolyHIPEs: Recent advances in emulsion-templated porous polymers. Prog. Polym. Sci. 2014, 39, 199–234. [Google Scholar] [CrossRef]
- Egorin, A.M.; Tutov, M.V.; Didenko, N.A.; Slobodyuk, A.B.; Marinin, D.V.; Avramenko, V.A. Effect of parameters of thermal treatment of resorcinol-formaldehyde resins on their chemical stability and Cs-137 uptake efficiency. J. Radioanal. Nucl. Chem. 2015, 304, 281–286. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites; kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Reichenberg, D. Properties of Ion-Exchange Resins in Relation to their Structure. III. Kinetics of Exchange. J. Am. Chem. Soc. 1953, 75. [Google Scholar] [CrossRef]
- Doğan, M.; Özdemir, Y.; Alkan, M. Adsorption kinetics and mechanism of cationic methyl violet and methylene blue dyes onto sepiolite. Dyes Pigments 2007, 75, 701–713. [Google Scholar] [CrossRef]
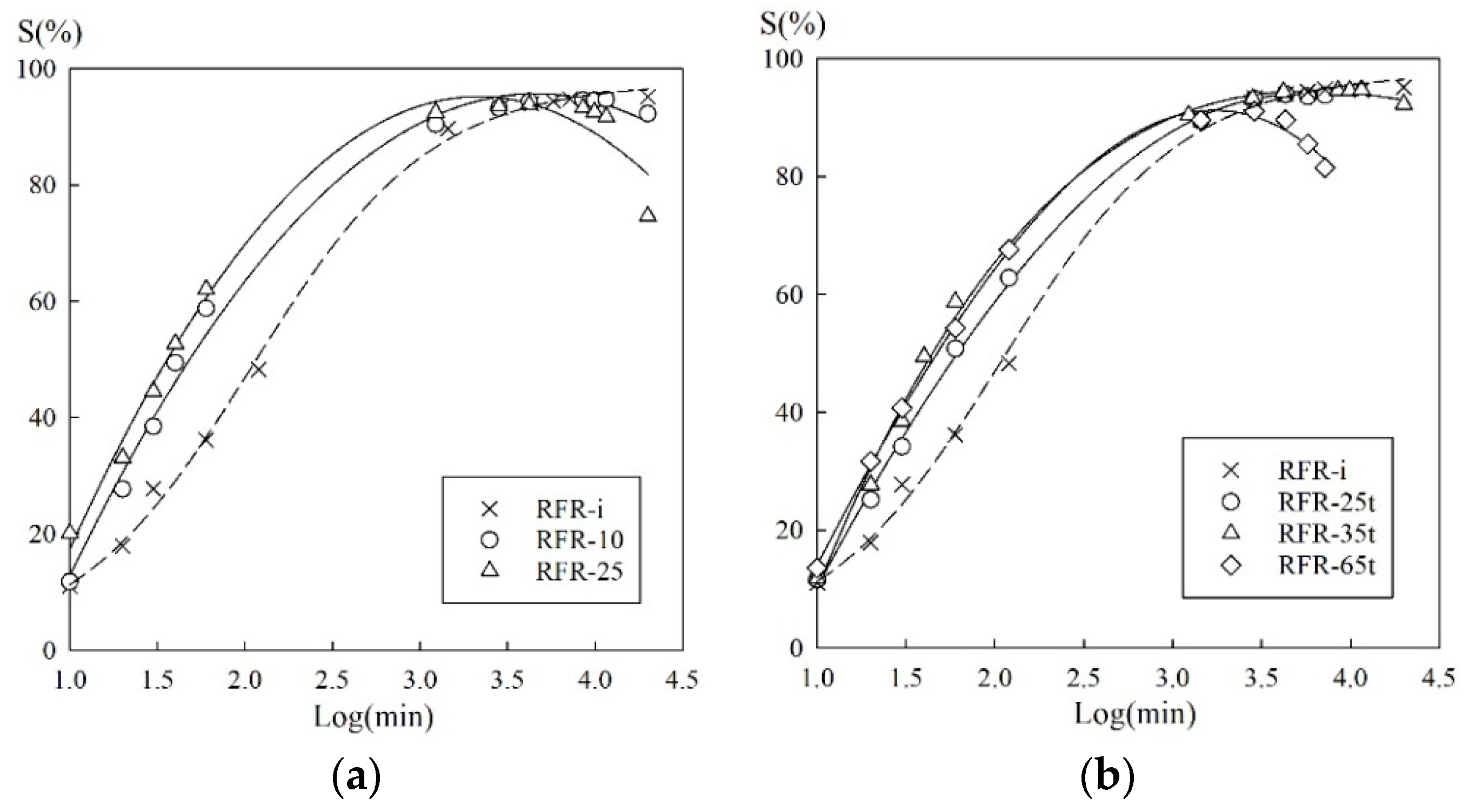
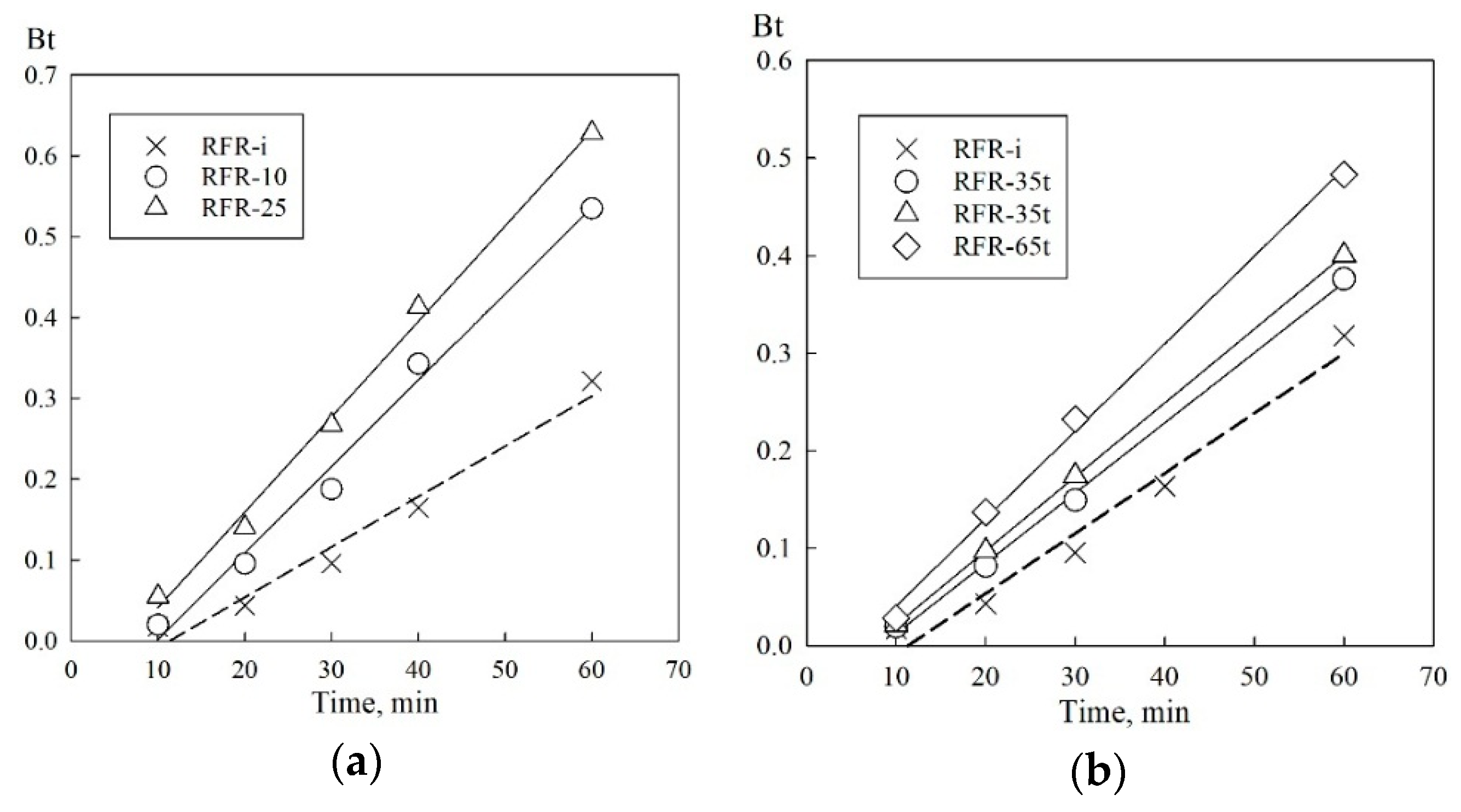
| Sample | R2 | Di × 107 (cm2 min−1) | t1/2 (min) |
|---|---|---|---|
| RFR-i | 0.98 | 8.8 | 47 |
| RFR-10 | 0.99 | 15.2 | 27 |
| RFR-25 | 0.99 | 16.8 | 28 |
| RFR-25t | 0.99 | 10.2 | 41 |
| RFR-35t | 0.99 | 10.8 | 39 |
| RFR-65t | 0.99 | 12.8 | 32 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Egorin, A.; Tokar, E.; Tutov, M.; Portnyagin, A. Porous Resorcinol-Formaldehyde Resins. Colloids Interfaces 2019, 3, 7. https://doi.org/10.3390/colloids3010007
Egorin A, Tokar E, Tutov M, Portnyagin A. Porous Resorcinol-Formaldehyde Resins. Colloids and Interfaces. 2019; 3(1):7. https://doi.org/10.3390/colloids3010007
Chicago/Turabian StyleEgorin, Andrei, Eduard Tokar, Mikhail Tutov, and Arseniy Portnyagin. 2019. "Porous Resorcinol-Formaldehyde Resins" Colloids and Interfaces 3, no. 1: 7. https://doi.org/10.3390/colloids3010007
APA StyleEgorin, A., Tokar, E., Tutov, M., & Portnyagin, A. (2019). Porous Resorcinol-Formaldehyde Resins. Colloids and Interfaces, 3(1), 7. https://doi.org/10.3390/colloids3010007





