Controlling the Ductile/Fragile Behavior of a 3D-Printed PLA-BaTiO3 Biocomposite by PBS Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
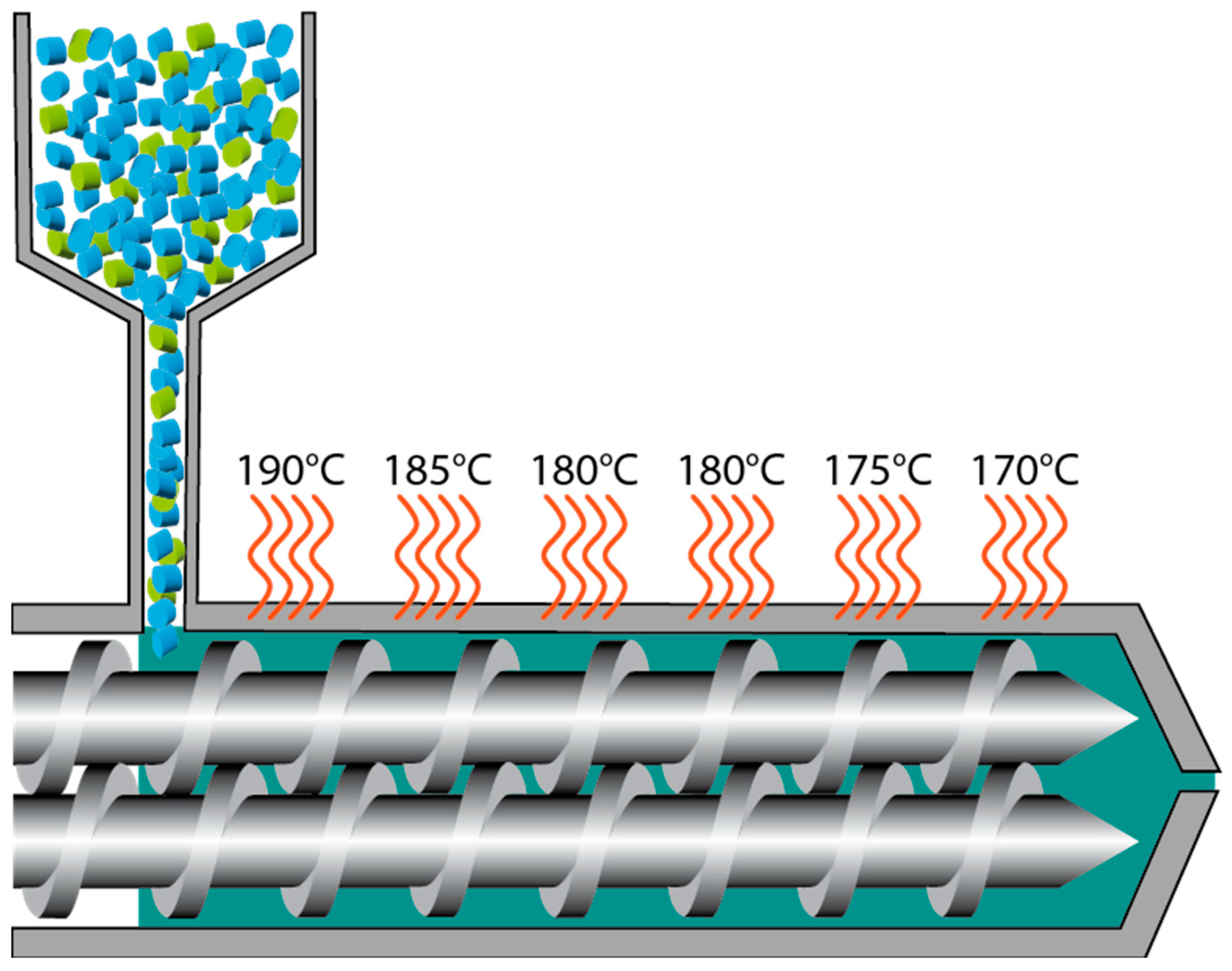
Processing: Preparation of Compounds, Filaments, and Samples
2.3. Characterization Methods
2.3.1. Mechanical Analysis
2.3.2. Thermal and Microstructural Analysis
2.3.3. Morphological Analysis
3. Results and Discussion
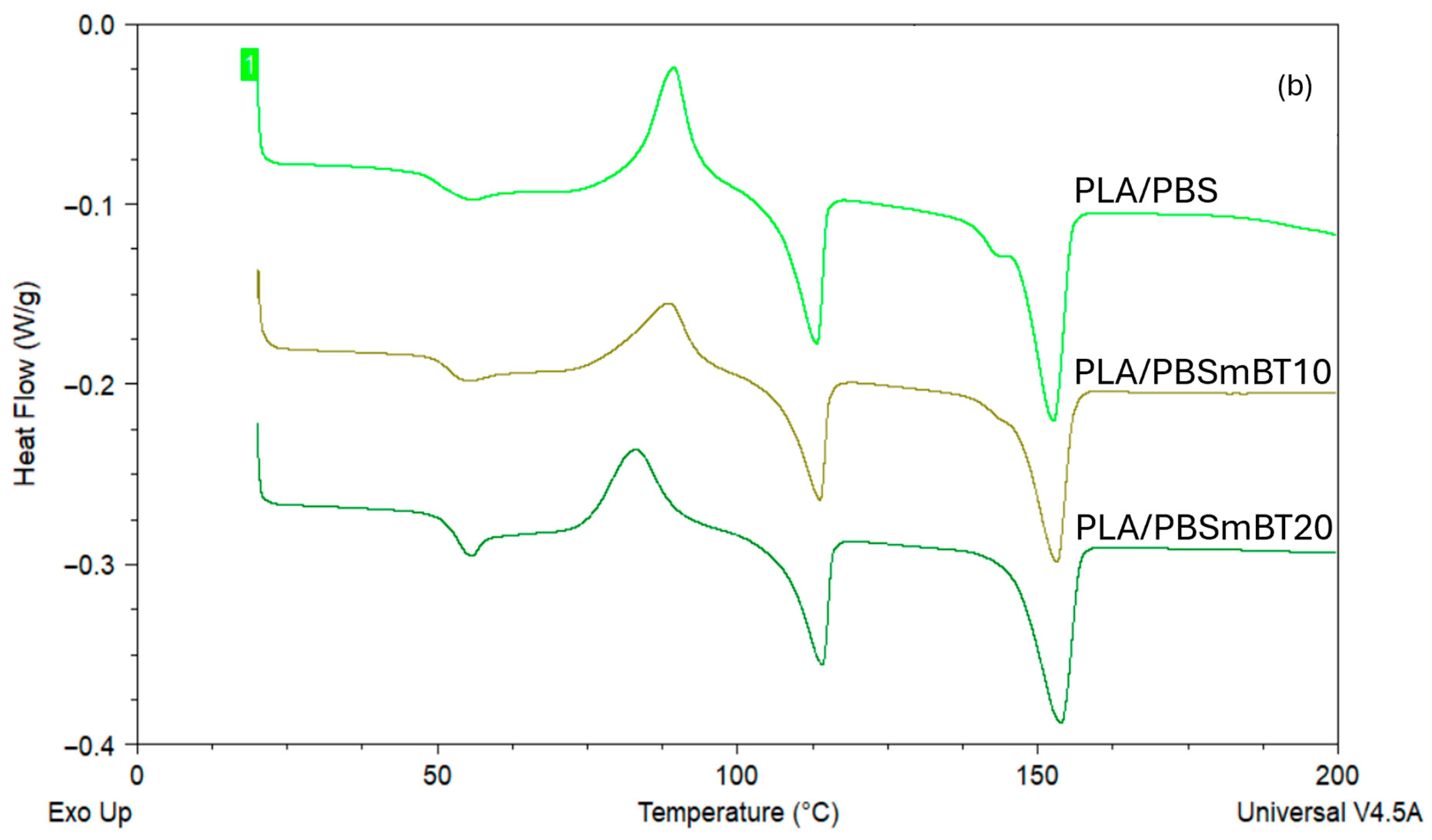
3.1. Thermal Analysis
3.2. Compression Tests
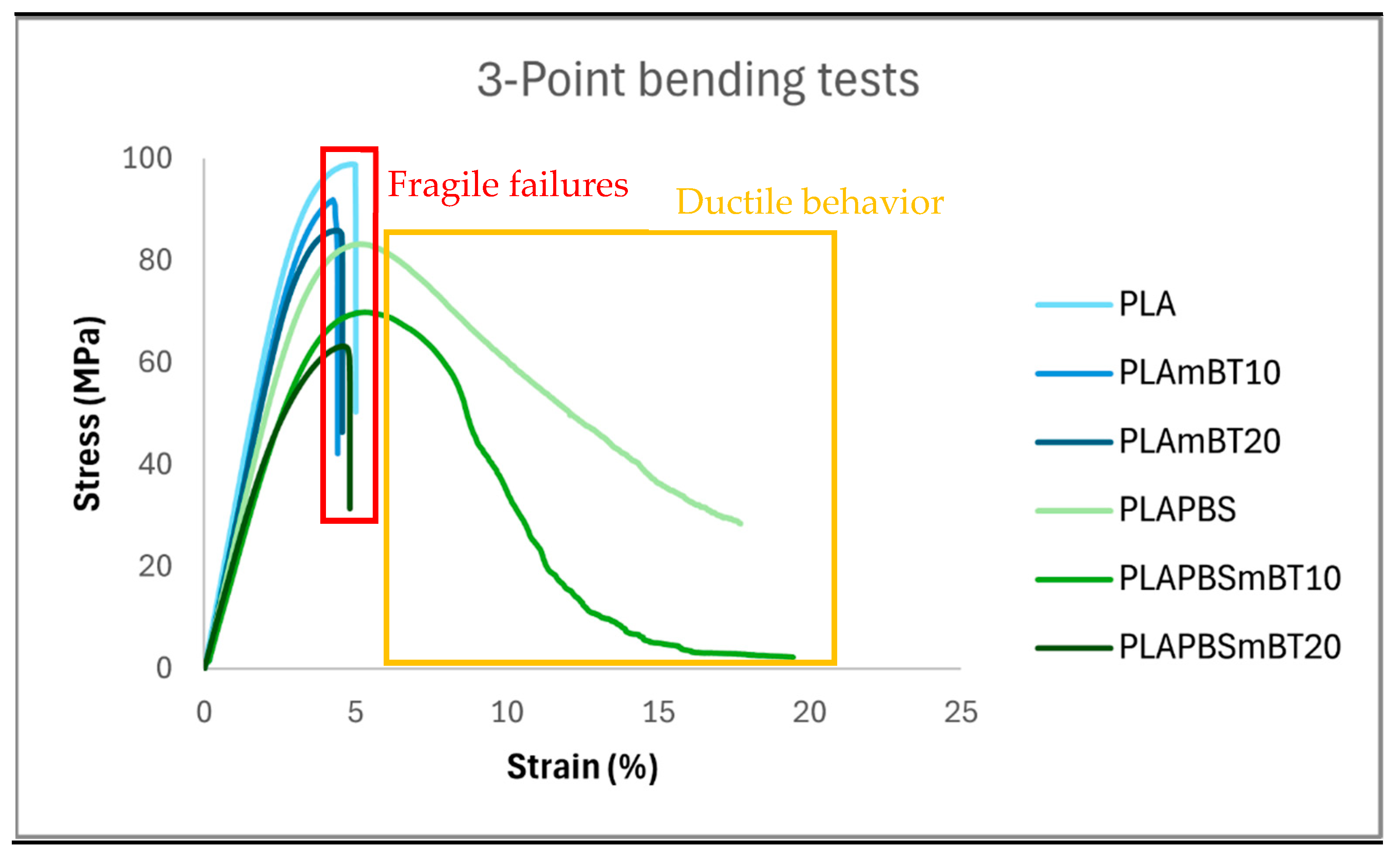
3.3. Three-Point Bending Tests
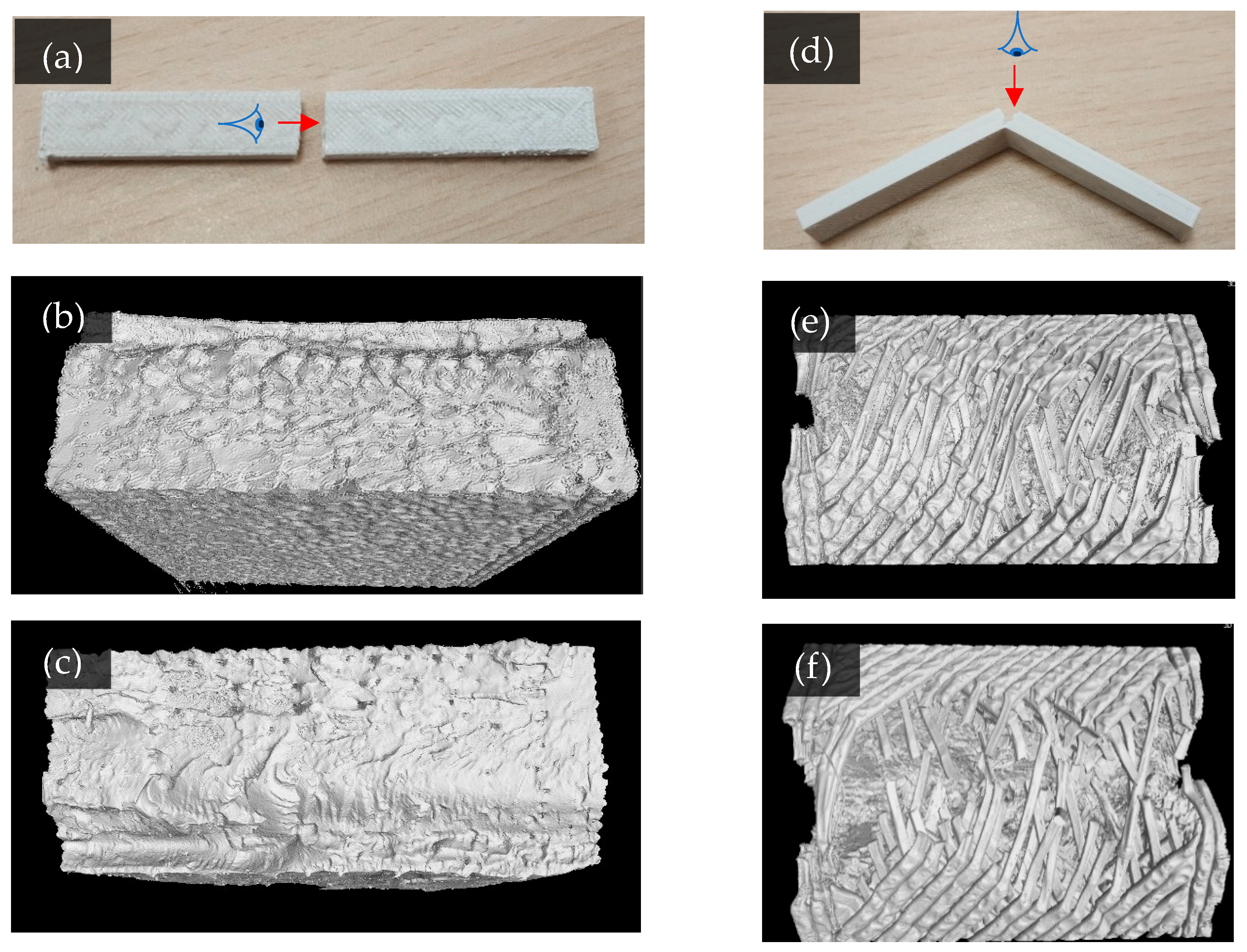
3.4. Fracture Morphology After Bending Tests
3.5. Comparison with PLA/PCL/HAp Biomaterials
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PLA | Poly(lactide) |
| PBS | Poly(butylene succinate) |
| mBT | Micro Barium Titanate |
References
- Knight, S.R.; Aujla, R.; Biswas, S.P. Total Hip Arthroplasty-over 100 Years of Operative History. Orthop. Rev. 2011, 3, e16. [Google Scholar]
- Zhu, M.H.; Cai, Z.B.; Li, W.; Yu, H.Y.; Zhou, Z.R. Fretting in Prosthetic Devices Related to the Human Body. Tribol. Int. 2009, 42, 1360–1364. [Google Scholar] [CrossRef]
- Piao, C.; Wu, D.; Luo, M.; Ma, H. Stress Shielding Effects of Two Prosthetic Groups after Total Hip Joint Simulation Replacement. J. Orthop. Surg. Res. 2014, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Guo, X.; Cai, N.; Dong, Y. Novel Fabrication Method of Porous Poly(Lactic Acid) Scaffold with Hydroxyapatite Coating. Mater. Lett. 2012, 74, 197–199. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- MBCP|Substitut Osseux Synthétique de Phosphate de Calcium. Available online: https://biomatlante.com/fr/produits/mbcp-substitut-osseux-synthetique (accessed on 8 July 2022).
- MBCP Putty-In’ossTM|Substitut Osseux Synthétique Biphasé Malléable. Available online: https://biomatlante.com/fr/produits/mbcp-putty-in-oss (accessed on 15 October 2021).
- Tappa, K.; Jammalamadaka, U.; Weisman, J.; Ballard, D.; Wolford, D.; Pascual-Garrido, C.; Wolford, L.; Woodard, P.; Mills, D. 3D Printing Custom Bioactive and Absorbable Surgical Screws, Pins, and Bone Plates for Localized Drug Delivery. J. Funct. Biomater. 2019, 10, 17. [Google Scholar] [CrossRef]
- Danoux, C.B.; Barbieri, D.; Yuan, H.; de Bruijn, J.D.; van Blitterswijk, C.A.; Habibovic, P. In Vitro and in Vivo Bioactivity Assessment of a Polylactic Acid/Hydroxyapatite Composite for Bone Regeneration. Biomatter 2014, 4, e27664. [Google Scholar] [CrossRef]
- Khare, D. Electrical Stimulation and Piezoelectric Biomaterials for Bone Tissue Engineering Applications. Biomaterials 2020, 258, 25. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, S.; Qi, F.; Zan, J.; Liu, G.; Zhao, Z.; Shuai, C. Graphene-Assisted Barium Titanate Improves Piezoelectric Performance of Biopolymer Scaffold. Mater. Sci. Eng. C 2020, 116, 111195. [Google Scholar] [CrossRef]
- Guillot-Ferriols, M.; Lanceros-Méndez, S.; Gómez Ribelles, J.L.; Gallego Ferrer, G. Electrical Stimulation: Effective Cue to Direct Osteogenic Differentiation of Mesenchymal Stem Cells? Biomater. Adv. 2022, 138, 212918. [Google Scholar] [CrossRef]
- Orkwis, J.A.; Wolf, A.K.; Mularczyk, Z.J.; Bryan, A.E.; Smith, C.S.; Brown, R.; Krutko, M.; McCann, A.; Collar, R.M.; Esfandiari, L.; et al. Mechanical Stimulation of a Bioactive, Functionalized PVDF-TrFE Scaffold Provides Electrical Signaling for Nerve Repair Applications. Biomater. Adv. 2022, 140, 213081. [Google Scholar] [CrossRef]
- Gomes, M.R.; Castelo Ferreira, F.; Sanjuan-Alberte, P. Electrospun Piezoelectric Scaffolds for Cardiac Tissue Engineering. Biomater. Adv. 2022, 137, 212808. [Google Scholar] [CrossRef]
- Tavangar, M.; Heidari, F.; Hayati, R.; Tabatabaei, F.; Vashaee, D.; Tayebi, L. Manufacturing and Characterization of Mechanical, Biological and Dielectric Properties of Hydroxyapatite-Barium Titanate Nanocomposite Scaffolds. Ceram. Int. 2020, 46, 9086–9095. [Google Scholar] [CrossRef]
- Jiao, H.; Zhao, K.; Bian, T.; Tang, Y. Hydrothermal Synthesis and Properties Characterization of Barium Titanate/Hydroxyapatite Spherical Nanocomposite Materials. J. Alloys Compd. 2017, 715, 73–82. [Google Scholar] [CrossRef]
- Wu, J.; Jiao, C.; Yu, H.; Naqvi, S.M.R.; Ge, M.; Cai, K.; Liang, H.; Liu, J.; Zhao, J.; Tian, Z.; et al. 3D Printed Barium Titanate/Calcium Silicate Composite Biological Scaffold Combined with Structural and Material Properties. Biomater. Adv. 2024, 158, 213783. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Caetano, G.; Vyas, C.; Blaker, J.; Diver, C.; Bártolo, P. Polymer-Ceramic Composite Scaffolds: The Effect of Hydroxyapatite and β-Tri-Calcium Phosphate. Materials 2018, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Bittner, S.M.; Smith, B.T.; Diaz-Gomez, L.; Hudgins, C.D.; Melchiorri, A.J.; Scott, D.W.; Fisher, J.P.; Mikos, A.G. Fabrication and Mechanical Characterization of 3D Printed Vertical Uniform and Gradient Scaffolds for Bone and Osteochondral Tissue Engineering. Acta Biomater. 2019, 90, 37–48. [Google Scholar] [CrossRef]
- Pitjamit, S.; Thunsiri, K.; Nakkiew, W.; Wongwichai, T.; Pothacharoen, P.; Wattanutchariya, W. The Possibility of Interlocking Nail Fabrication from FFF 3D Printing PLA/PCL/HA Composites Coated by Local Silk Fibroin for Canine Bone Fracture Treatment. Materials 2020, 13, 1564. [Google Scholar] [CrossRef]
- Hassanajili, S.; Karami-Pour, A.; Oryan, A.; Talaei-Khozani, T. Preparation and Characterization of PLA/PCL/HA Composite Scaffolds Using Indirect 3D Printing for Bone Tissue Engineering. Mater. Sci. Eng. C 2019, 104, 109960. [Google Scholar] [CrossRef]
- Su, S.; Kopitzky, R.; Tolga, S.; Kabasci, S. Polylactide (PLA) and Its Blends with Poly(Butylene Succinate) (PBS): A Brief Review. Polymers 2019, 11, 1193. [Google Scholar] [CrossRef]
- Delamarche, E.; Mattlet, A.; Livi, S.; Gérard, J.-F.; Bayard, R.; Massardier, V. Tailoring Biodegradability of Poly(Butylene Succinate)/Poly(Lactic Acid) Blends With a Deep Eutectic Solvent. Front. Mater. 2020, 7, 7. [Google Scholar] [CrossRef]
- Harada, M.; Ohya, T.; Iida, K.; Hayashi, H.; Hirano, K.; Fukuda, H. Increased Impact Strength of Biodegradable Poly(Lactic Acid)/Poly(Butylene Succinate) Blend Composites by Using Isocyanate as a Reactive Processing Agent. J. Appl. Polym. Sci. 2007, 106, 1813–1820. [Google Scholar] [CrossRef]
- Ojansivu, M.; Johansson, L.; Vanhatupa, S.; Tamminen, I.; Hannula, M.; Hyttinen, J.; Kellomäki, M.; Miettinen, S. Knitted 3D Scaffolds of Polybutylene Succinate Support Human Mesenchymal Stem Cell Growth and Osteogenesis. Stem Cells Int. 2018, 2018, 5928935. [Google Scholar] [CrossRef] [PubMed]
- ISO 604; Plastics—Determination of Compressive Properties. Association Française de Normalisation: La Plaine Saint-Denis, France, 2004.
- ISO 178; Plastics—Determination of Flexural Properties. Association Française de Normalisation: La Plaine Saint-Denis, France, 2019.
- Lin, C.; Liu, L.; Liu, Y.; Leng, J. 4D Printing of Shape Memory Polybutylene Succinate/Polylactic Acid (PBS/PLA) and Its Potential Applications. Compos. Struct. 2022, 279, 114729. [Google Scholar] [CrossRef]
- Mystiridou, E.; Patsidis, A.C.; Bouropoulos, N. Development and Characterization of 3D Printed Multifunctional Bioscaffolds Based on PLA/PCL/HAp/BaTiO3 Composites. Appl. Sci. 2021, 11, 4253. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Pham, V.H.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite Nano Bioceramics Optimized 3D Printed Poly Lactic Acid Scaffold for Bone Tissue Engineering Application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Shi, J.; Ye, H.; Zhou, Q. Distinctive Tensile Properties of the Blends of Poly(l-Lactic Acid) (PLLA) and Poly(Butylene Succinate) (PBS). J. Polym. Environ. 2018, 26, 1737–1744. [Google Scholar] [CrossRef]



| Compound | Desired Ceramic Percentage | Measured Ceramic Percentage |
|---|---|---|
| PLAmBT10 | 10% | 10 ± 0.3% |
| PLAmBT20 | 20% | 20 ± 0.4% |
| PLA/PBSmBT10 | 10% | 12 ± 0.3% |
| PLA/PBSmBT20 | 20% | 20 ± 0.4% |
| Material | Tg PLA (°C) | Tcc PLA (°C) | Tm PLA (°C) | χc PLA (%) | Tm PBS (°C) | χc PBS (%) |
|---|---|---|---|---|---|---|
| PLA | 52.5 ± 0.1 | 95.7 ± 0.1 | 153.1 ± 0.1 | 3.4 ± 0.8 | - | - |
| PLAmBT10 | 55.1 ± 0.1 | 92.3 ± 0.2 | 154.7 ± 0.1 | 3.6 ± 1.2 | - | - |
| PLAmBT20 | 56.8 ± 0.2 | 92.8 ± 0.3 | 153.2 ± 0.2 | 3.4 ± 0.5 | - | - |
| PLA/PBS | 49.9 ± 0.2 | 89.4 ± 0.8 | 152.7 ± 1.1 | 10.8 ± 1.4 | 113.3 ± 0.2 | 33.6 ± 1.5 |
| PLA/PBSmBT10 | 51.9 ± 0.1 | 88.8 ± 0.2 | 153.4 ± 0.7 | 11.8 ± 1.2 | 113.5 ± 0.6 | 33 ± 1.2 |
| PLA/PBSmBT20 | 53.6 ± 0.2 | 83.5 ± 0.7 | 153.2 ± 0.6 | 11.5 ± 1.2 | 114.5 ± 0.6 | 36.7 ± 1.5 |
| Material | Young Modulus (MPa) | Elastic Limit (MPa) | Elastic Deformation (%) |
|---|---|---|---|
| PLA | 1673 ± 29 | 64 ± 0.7 | 4.93 ± 0.43 |
| PLAmBT10 | 1864 ± 35 | 80 ± 0.5 | 5.99 ± 0.17 |
| PLAmBT20 | 1906 ± 109 | 72 ± 2.4 | 4.86 ± 0.24 |
| PLA/PBS | 1625 ± 38 | 71 ± 1.8 | 6.57 ± 0.24 |
| PLA/PBSmBT10 | 1744 ± 20 | 67 ± 0.8 | 5.61 ± 0.10 |
| PLA/PBSmBT20 | 1630 ± 12 | 69 ± 1.3 | 6.58 ± 0.3 |
| Material | Flexural Modulus (MPa) | Elastic Limit (MPa) | Elastic Deformation (%) |
|---|---|---|---|
| PLA | 3211 ± 66 | 100 ± 1 | 4.85 ± 0.12 |
| PLAmBT10 | 2930 ± 141 | 90 ± 3 | 4.41 ± 0.13 |
| PLAmBT20 | 3178 ± 201 | 90 ± 3 | 4.17 ± 0.14 |
| PLA/PBS | 2472 ± 60 | 83 ± 1 | 5.19 ± 0.19 |
| PLA/PBSmBT10 | 2266 ± 306 | 73 ± 9 | 5.18 ± 0.34 |
| PLA/PBSmBT20 | 2208 ± 126 | 63 ± 1 | 4.42 ± 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burel, P.; Ragoubi, M.; Millet, P.; Alix, S.; Gattin, R. Controlling the Ductile/Fragile Behavior of a 3D-Printed PLA-BaTiO3 Biocomposite by PBS Addition. J. Compos. Sci. 2025, 9, 491. https://doi.org/10.3390/jcs9090491
Burel P, Ragoubi M, Millet P, Alix S, Gattin R. Controlling the Ductile/Fragile Behavior of a 3D-Printed PLA-BaTiO3 Biocomposite by PBS Addition. Journal of Composites Science. 2025; 9(9):491. https://doi.org/10.3390/jcs9090491
Chicago/Turabian StyleBurel, Paul, Mohamed Ragoubi, Pierre Millet, Sébastien Alix, and Richard Gattin. 2025. "Controlling the Ductile/Fragile Behavior of a 3D-Printed PLA-BaTiO3 Biocomposite by PBS Addition" Journal of Composites Science 9, no. 9: 491. https://doi.org/10.3390/jcs9090491
APA StyleBurel, P., Ragoubi, M., Millet, P., Alix, S., & Gattin, R. (2025). Controlling the Ductile/Fragile Behavior of a 3D-Printed PLA-BaTiO3 Biocomposite by PBS Addition. Journal of Composites Science, 9(9), 491. https://doi.org/10.3390/jcs9090491







