Influence of Preparation Methods on the Physicochemical and Functional Properties of NiO-CeO2/Al2O3 Catalysts
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Catalysts
2.3. Catalytic Tests
2.4. Catalyst Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ciuparu, D.; Lyubovsky, M.R.; Altman, E.; Pfefferle, L.D.; Datye, A. Catalytic combustion of methane over palladium-based catalysts. Catal. Rev. 2002, 44, 593. [Google Scholar] [CrossRef]
- Chin, Y.-H.; Buda, C.; Neurock, M.; Iglesia, E. Reactivity of chemisorbed oxygen atoms and their catalytic consequences during CH4-O2 catalysis on supported Pt clusters. J. Am. Chem. Soc. 2011, 133, 15958–15978. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, R.; Wu, L.; Wanke, S.E.; Hayes, R.E. Kinetics of methane combustion over Pt and Pt−Pd catalysts. Chem. Eng. Res. Des. 2012, 90, 1930–1942. [Google Scholar] [CrossRef]
- Monai, M.; Montini, T.; Gorte, R.J.; Fornasiero, P. Catalytic oxidation of methane: Pd and Beyond. Eur. J. Inorg. Chem. 2018, 25, 2884–2893. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, H.; Law, C.K. Kinetics of catalytic oxidation of methane, ethane and propane over palladium oxide. Combust. Flame 2014, 161, 1048–1054. [Google Scholar] [CrossRef]
- Borisov, V.A.; Nedosekov, A.S.; Sigayeva, S.S.; Suprunov, G.I.; Vershinin, V.I.; Tsyrulnikov, P.G. Deep oxidation of methane on palladic catalysts on suppliers ZrO2, CeO2, ZrO2-CeO2,CeO2-CuO on stainless steel prepared with the method of plasma drawing. Procedia Eng. 2015, 113, 124–130. [Google Scholar] [CrossRef]
- Jiang, D.; Khivantsev, K.; Wang, Y. Low-temperature methane oxidation for efficient emission control in natural gas vehicles: Pd and Beyond. ACS Catal. 2020, 10, 14304–14314. [Google Scholar] [CrossRef]
- Banerjee, A.C. Catalytic Oxidation of Methane. Catalysts 2021, 11, 944. [Google Scholar] [CrossRef]
- Roche, V.; Revel, R.; Vernoux, P. Electrochemical promotion of YSZ monolith honeycomb for deep oxidation of methane. Catal. Commun. 2010, 11, 1076–1080. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, S.; Lu, J.; Luo, M. Effect of structural properties of mesoporous Co3O4 catalysts on methane combustion. Chem. Res. Chin. Univ. 2016, 32, 808–811. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Xue, B. Catalytic combustion of methane over M (Ni, Co, Cu) supported on ceria–magnesia. Fuel Process. Technol. 2009, 90, 652. [Google Scholar] [CrossRef]
- Rogozhnikov, V.N.; Kulikov, A.V.; Potemkin, D.I.; Glotov, A.P.; Zasypalov, G.O.; Snytnikov, P.V. Structured catalytic burner for deep oxidation of hydrocarbons. Catal. Commun. 2021, 149, 106198. [Google Scholar] [CrossRef]
- Hao, Y.-J.; Tian, L.-G.; Duan, E.; Liu, J.; Qi, T.-Y.; Kong, W.-Q.; Qi, X.-H.; Liu, X.; Liu, Y.; Zhao, J.; et al. Low-temperature methane oxidation triggered by peroxide radicals over noble-metal-free MgO catalyst. ACS Appl. Mater. Int. 2020, 12, 21761–21771. [Google Scholar] [CrossRef]
- Isupova, L.A.; Kulikovskaya, N.A.; Saputina, N.F.; Gerasimov, E.Y.; Tsybulya, S.V. La1–xCaxFeO3–δ (x=0–1) Perovskites prepared by the pechini method: Catalytic activity in deep methane and CO oxidation. Kinet. Catal. 2015, 56, 781–787. [Google Scholar] [CrossRef]
- Shu, Y.; Wang, M.; Duan, X.; Liu, D.; Yang, S.; Zhang, P. Low-temperature total oxidation of methane by pore- and vacancy-engineered NiO catalysts. AIChE J. 2022, 68, e17664. [Google Scholar] [CrossRef]
- Torralba, R.; Corro, G.; Rosales, F.; Bañuelos, F.; Pal, U.; Olivares-Xometl, O.; Guilleminot, E.; Fierro, J.L.G. Total oxidation of methane over sulfur poisoning resistant Pt/ZrO2 catalyst: Effect of Pt2+–Pt4+ and Pt2+–Zr4+ dipoles at metal-support Interface. Catal. Lett. 2021, 151, 1592–1603. [Google Scholar] [CrossRef]
- Halipova, O.S.; Kuznetsova, S.A.; Galanov, S.I. Synthesis of deep methane oxidation catalysts based on SnO2–CeO2. Inorg. Mater. 2016, 52, 372–377. [Google Scholar] [CrossRef]
- Bawa, S.G.; Pankajakshan, A.; Waldron, C.; Cao, E.; Galvanin, F.; Gavriilidis, A. Rapid screening of kinetic models for methane total oxidation using an automated gas phase catalytic micro reactor platform. Chemistry-Methods 2023, 3, e202200049. [Google Scholar] [CrossRef]
- Pu, Z.; Zhou, H.; Zheng, Y.; Huang, W.; Li, X. Enhanced methane combustion over Co3O4 catalysts prepared by a facile precipitation method: Effect of aging time. Appl. Surf Sci. 2017, 410, 14–21. [Google Scholar] [CrossRef]
- Borisov, V.A.; Anoshkina, E.A.; Sigayeva, S.S.; Suprunov, G.I.; Nedosekov, A.S.; Ivanov, A.L.; Vershinin, V.I.; Shlyapin, D.A.; Tsyrulnikov, P.G. Deep oxidation of methane on Pd/Al2O3, Pd/Al2O3-CeO2 and Pd/Al2O3-MnO2 catalysts with metal alloy supports obtained by plasma deposition. AIP Conf. Proc. Oil Gas Eng. 2018, 2007, 020004. [Google Scholar] [CrossRef]
- Mihai, M.-A.; Culita, D.C.; Atkinson, I.; Papa, F.; Popescu, I.; Marcu, I.-C. Unraveling mechanistic aspects of the total oxidation of methane over Mn, Ni and Cu spinel cobaltites via in situ electrical conductivity measurements. Appl. Catal. A Gen. 2021, 611, 117901. [Google Scholar] [CrossRef]
- Schwartz, W.R.; Ciuparu, D.; Pfefferle, L.D. Combustion of Methane over Palladium-Based Catalysts: Catalytic Deactivation and Role of the Support. J. Phys. Chem. C 2012, 116, 8587–8593. [Google Scholar] [CrossRef]
- Sun, C.; Zhao, K.; Yi, Z. Research progress in catalytic total oxidation of Methane. J. Inorg. Mater. 2023, 38, 1245–1256. [Google Scholar] [CrossRef]
- Shao, C.; Li, W.; Lin, Q.; Huang, Q.; Pi, D. Low temperature complete combustion of lean methane over cobalt-nickel mixed-oxide catalysts. Energy Technol. 2016, 5, 604–610. [Google Scholar] [CrossRef]
- Popescu, I.; Tanchoux, N.; Tichit, D.; Marcu, I.-C. Total oxidation of methane over supported CuO: Influence of the MgxAlyO support. Appl. Catal. A Gen. 2017, 538, 81–90. [Google Scholar] [CrossRef]
- Gu, H.; Lan, J.; Hu, H.; Jia, F.; Ai, Z.; Zhang, L.; Liu, X. Surface oxygen vacancy-dependent molecular oxygen activation for propane combustion over α-MnO2. J. Hazard. Mater. 2023, 460, 132499. [Google Scholar] [CrossRef]
- Lim, T.H.; Cho, S.J.; Yang, H.S.; Engelhard, M.H.; Kim, D.H. Effect of Co/Ni ratios in cobalt nickel mixed oxide catalysts on methane combustion. Appl. Catal. A Gen. 2015, 505, 62–69. [Google Scholar] [CrossRef]
- Ding, M.-Y.; Tu, J.-Y.; Wang, T.-J.; Ma, L.-L.; Wang, C.-G.; Chen, L.-G. Bio-syngas methanation towards synthetic natural gas (SNG) over highly active Al2O3–CeO2 supported Ni catalyst. Fuel Process. Technol. 2015, 134, 480–486. [Google Scholar] [CrossRef]
- Zheng, W.; Zhang, J.; Ge, Q.; Xu, H.; Li, W. Effects of CeO2 addition on Ni/Al2O3 catalysts for the reaction of ammonia decomposition to hydrogen. Appl. Catal. B Environ. 2008, 80, 98–105. [Google Scholar] [CrossRef]
- Li, Z.; Li, B.; Li, Z.; Rong, X. The Promoter Action of CeO2 for the Ni/Al2O3-Catalyzed Methanation of CO2. Kinet. Catal. 2015, 56, 329–334. [Google Scholar] [CrossRef]
- Hu, Z.; Qiu, S.; You, Y.; Guo, Y.; Guo, Y.; Wang, L.; Zhan, W.; Lu, G. Hydrothermal synthesis of NiCeOx nanosheets and its application to the total oxidation of propane. Appl. Catal. B Environ. 2018, 225, 110–120. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, J.; Ma, G.; Xu, Y.; Zhang, J.; Samart, C.; Ding, M. Ni nanocatalysts supported on mesoporous Al2O3–CeO2 for CO2 methanation at low temperature. RSC Adv. 2020, 10, 2067–2072. [Google Scholar] [CrossRef]
- Solsona, B.; Garcia, T.; Aylón, E.; Dejoz, A.M.; Vázquez, I.; Agouram, S.; Davies, T.E.; Taylor, S.H. Promoting the activity and selectivity of high surface area Ni–Ce–O mixed oxides by gold deposition for VOC catalytic combustion. Chem. Eng. J. 2011, 15, 271–278. [Google Scholar] [CrossRef]
- Yu, J.; Cao, Q.; Li, Y.; Long, X.; Yang, S.; Clark, J.K.; Nakabayashi, M.; Shibata, N.; Delaunay, J.-J. Defect-Rich NiCeOx Electrocatalyst with Ultrahigh Stability and Low Overpotential for Water Oxidation. ACS Catal. 2019, 9, 1605–1611. [Google Scholar] [CrossRef]
- Yergaziyeva, G.; Kutelia, E.; Dossumov, K.; Gventsadze, D.; Jalabadze, N.; Dzigrashvili, T.; Nadaraia, L.; Tsurtsumia, O.; Anissova, M.; Mambetova, M.; et al. Effect of lanthanum oxide on the activity Ni-Co/diatomite catalysts in dry reforming of methane. Eurasian Chem.-Technol. J. 2023, 25, 21–32. [Google Scholar] [CrossRef]
- Dossumov, K.; Ergazieva, G.E.; Ermagambet, B.T.; Myltykbaeva, L.K.; Telbaeva, M.M.; Mironenko, A.V.; Mambetova, M.M.; Kassenova, Z. Morphology and catalytic properties of cobalt-containing catalysts synthesized by different means. Russ. J. Phys. Chem. A 2020, 94, 880–882. [Google Scholar] [CrossRef]
- Dossumov, K.; Yergazyieva, G.Y.; Myltykbayeva, L.K.; Suyunbayev, U.; Asanov, N.A.; Gyulmaliev, A.M. Oxidation of methane over polyoxide catalysts. Coke Chem. 2015, 58, 178–183. [Google Scholar] [CrossRef]
- Dossumov, K.; Yergazieva, G.Y.; Myltykbaieva, L.K.; Asanov, N.A. Effect of Co, Ce, and La oxides as modifying additives on the activity of an NiO/-Al2O3 catalyst in the oxidation of methane to give synthesis gas. Theor. Exp. Chem. 2016, 52, 119–122. [Google Scholar] [CrossRef]
- Dou, J.; Tang, Y.; Nie, L.; Andolina, C.M.; Zhang, X.; House, S.; Li, Y.; Yang, J.; Tao, F. Complete Oxidation of Methane on Co3O4/CeO2 Nanocomposite: A Synergic Effect. Catal. Today 2018, 311, 48–55. [Google Scholar] [CrossRef]
- Tang, C.; Sun, B.; Sun, J.; Hong, X.; Deng, Y.; Gao, F.; Dong, L. Solid state preparation of NiO-CeO2 catalyst for NO reduction. Catal. Today 2017, 281, 575–582. [Google Scholar] [CrossRef]
- Jalowiecki-Duhamel, L.; Zarrou, H.; D’Huysser, A. Hydrogen production at low temperature from methane on cerium and nickel based mixed oxides. Inter. J. Hydrogen Energy 2008, 33, 5527–5534. [Google Scholar] [CrossRef]
- Solsona, B.; Concepción, P.; Hernández, S.; Demicol, B.; Nieto, J.M.L. Oxidative dehydrogenation of ethane over NiO–CeO2 mixed oxides catalysts. Catal. Today 2012, 180, 51–58. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.-C. Deep Oxidation of Methane Over Manganese Oxide Modified by Mg, Ca, Sr and Ba Additives. React. Kinet. Catal. Lett. 2000, 71, 263–271. [Google Scholar] [CrossRef]
- Choi, S.O.; Ahn, I.Y.; Moon, S.H. In Young Ahn, Sang Heup Moon., Effect of CeO2-addition sequence on the performance of CeO2-modified Ni/Al2O3 catalyst in autothermal reforming of iso-octane. Korean J. Chem. Eng. 2009, 5, 1252–1258. [Google Scholar] [CrossRef]
- Shah, M. Development of versatile Ni–Ce-based catalyst for syngas production through dry reforming and catalytic partial oxidation of methane. Int. J. Hydrogen Energy 2025, 105, 505–520. [Google Scholar] [CrossRef]
- Stoian, M.; Rogé, V.; Lazar, L.; Maurer, T.; Védrine, J.C.; Marcu, I.-C.; Fechete, I. Total Oxidation of Methane on Oxide and Mixed Oxide Ceria-Containing Catalysts. Catalysts 2021, 11, 427. [Google Scholar] [CrossRef]
- Dossumov, K.; Ergazieva, G.E.; Ermagambet, B.T.; Telbayeva, M.M.; Mambetova, M.M.; Myltykbayeva, L.K.; Kassenova, Z.M. Role of ceria in several energy-related catalytic transformations. Chem. Pap. 2020, 74, 373–388. [Google Scholar] [CrossRef]
- Song, T.; Zhang, P.; Wang, T.; Ali, A.; Zeng, H. Alkali-assisted fabrication of holey carbon nitride nanosheet with tunable conjugated system for efficient visible-light-driven water splitting. Appl. Catal. B Environ. 2018, 224, 877–885. [Google Scholar] [CrossRef]
- Wu, J.; Xiong, Q.; Liang, J.; He, Q.; Yang, D.; Deng, R.; Chen, Y. Degradation of benzotriazole by DBD plasma and peroxymonosulfate: Mechanism, degradation pathway and potential toxicity. Chem. Eng. J. 2020, 384, 123300. [Google Scholar] [CrossRef]
- Dossumov, K.; Ergazieva, G.E.; Myltykbaeva, L.K.; Telbaeva, M.M.; Batyrbaev, A.T. Effect of MoO3 on the catalytic properties of NiO/Al2O3 in the carbon dioxide conversion of methane. Theor. Exp. Chem. 2019, 55, 137–142. [Google Scholar] [CrossRef]
- Worth, D.J.; Stettler, M.E.J.; Dickinson, P.; Hegarty, K.; Boies, A.M. Characterization and Evaluation of Methane Oxidation Catalysts for Dual-Fuel Diesel and Natural Gas Engines. Emiss. Control. Sci. Technol. 2016, 2, 204–214. [Google Scholar] [CrossRef]
- Monjezi, R.; Bouriakova, A.; Bjelić, A.; Heynderickx, P.M.; Heynderickx, G.J.; Poelman, D.; Giraudon, J.-M.; Lamonier, J.-F.; Morent, R.; Thybaut, J.W. Mechanistic insights into methane total oxidation over Cu/Hydroxyapatite catalyst synthesized with β-Cyclodextrin assistance. Chem. Eng. J. 2024, 489, 151324. [Google Scholar] [CrossRef]
- Caravaggio, G.; Nossova, L.; Turnbull, M.J. Nickel-magnesium mixed oxide catalyst for low temperature methane oxidation. J. Chem. Eng. 2021, 405, 126862. [Google Scholar] [CrossRef]
- Du, Z.; Ganesapillai, M.; Abdullah, A. Research progress on low-concentration methane oxidation using palladium-based catalysts, Web of Conferences. EDP Sci. 2023, 385, 04033. [Google Scholar] [CrossRef]
- Derekaya, F.; Bulagay, E. Total oxidation of methane over the LaNi1-xMxO3 (M:Mn,Ag,Cu,Co) perovskites. Res. Artic. Chem. Eng. 2021, 47, 6325–6339. [Google Scholar] [CrossRef]
- Choya, A.; de Rivas, B.; González-Velasco, J.R.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Oxidation of lean methane over cobalt catalysts supported on ceria/alumina. Appl. Catal. A Gen. 2020, 591, 117381. [Google Scholar] [CrossRef]
- Florén, C.-R.; Demirci, C.; Carlsson, P.-A.; Creaser, D.; Skoglundh, M. Total oxidation of methane over Pd/Al2O3 at pressures from 1 to 10 atm. Catal. Sci. Technol. 2020, 10, 5480–5486. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wang, Z.; Du, W.; Zhu, G. Morphological effect of CeO2 catalysts on their catalytic performance in lean methane combustion. Chem. Lett. 2020, 49, 461–464. [Google Scholar] [CrossRef]
- Huang, X.; Li, J.; Wang, J.; Li, Z.; Xu, J. Catalytic combustion of methane over a highly active and stable NiO/CeO2 catalyst. Front. Chem. Sci. Eng. 2020, 14, 534–545. [Google Scholar] [CrossRef]
- Corro, G.; Torralba, R.; Pal, U.; Olivares-Xometl, O.; Luis, J.; Fierro, G. Total Oxidation of Methane over Pt/Cr2O3 Catalyst at Low Temperature: Effect of Pt0−Ptx+ Dipoles at the Metal−Support Interface. J. Phys. Chem. C 2019, 123, 2882–2893. [Google Scholar] [CrossRef]
- Darda, S.; Pachatouridou, E.; Lappas, A.; Iliopoulou, E. Effect of preparation method of Co-Ce catalysts on CH4 combustion. Catalysts 2019, 9, 219. [Google Scholar] [CrossRef]
- Al-Aani, H.M.S.; Iro, E.; Chirra, P.; Fechete, I.; Badea, M.; Negrilă, C.; Marcu, I.C. CuxCeMgAlO mixed oxide catalysts derived from multicationic LDH precursors for methane total oxidation. Appl. Catal. A Gen. 2019, 586, 117215. [Google Scholar] [CrossRef]
- Li, D.; Li, K.; Xu, R.; Wang, H.; Tian, D.; Wei, Y.; Zhu, X.; Zeng, C.; Zeng, L. Ce1−xFexO2−δ catalysts for catalytic methane combustion: Role of oxygen vacancy and structural dependence. Catal. Today 2018, 318, 73–85. [Google Scholar] [CrossRef]
- Thaicharoensutcharittham, S.; Meeyoo, V.; Kitiyanan, B.; Rangsunvigit, P.; Rirksomboon, T. Catalytic combustion of methane over NiO/Ce0.75Zr0.25O2 catalyst. Catal. Commun. 2009, 10, 673–677. [Google Scholar] [CrossRef]


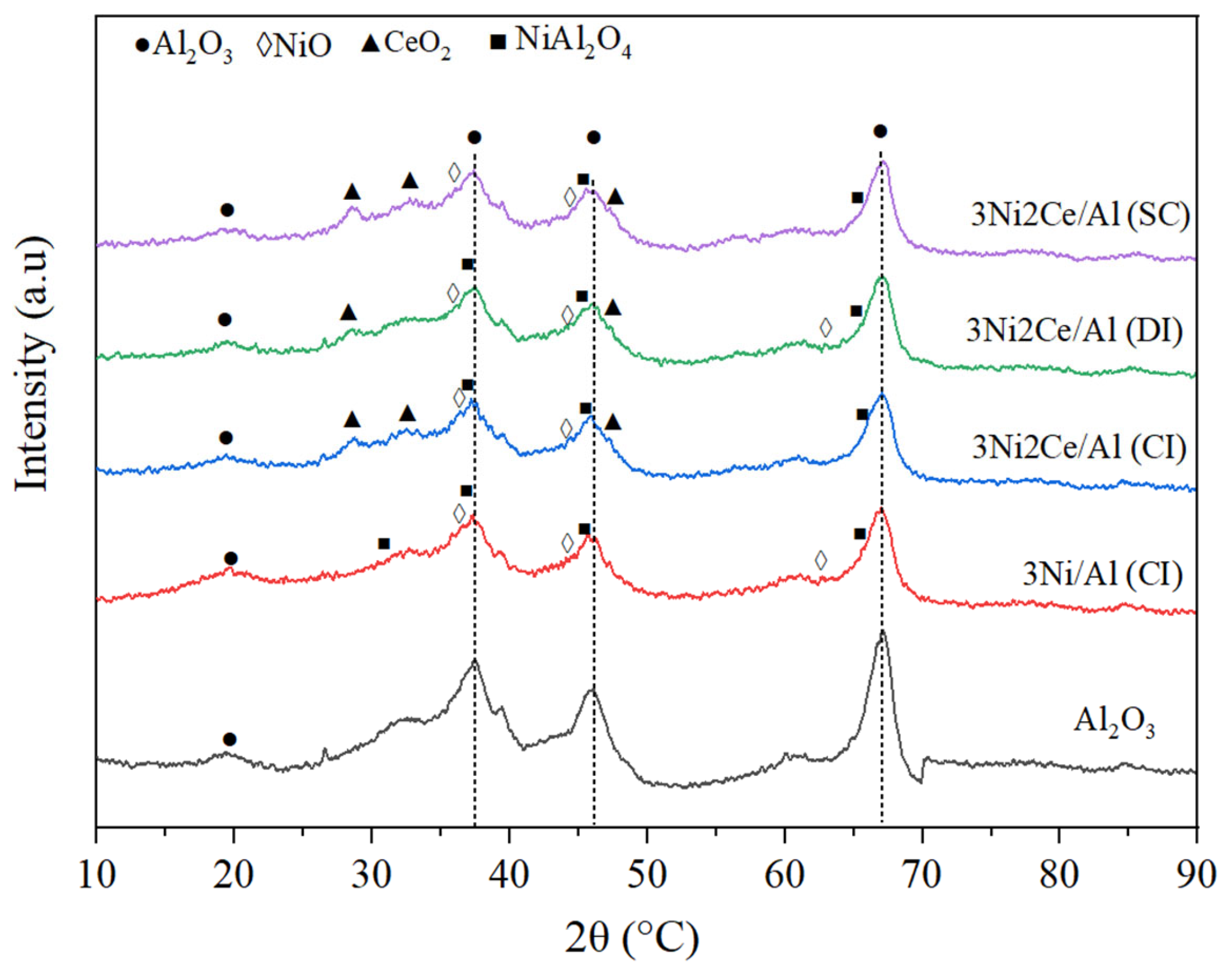

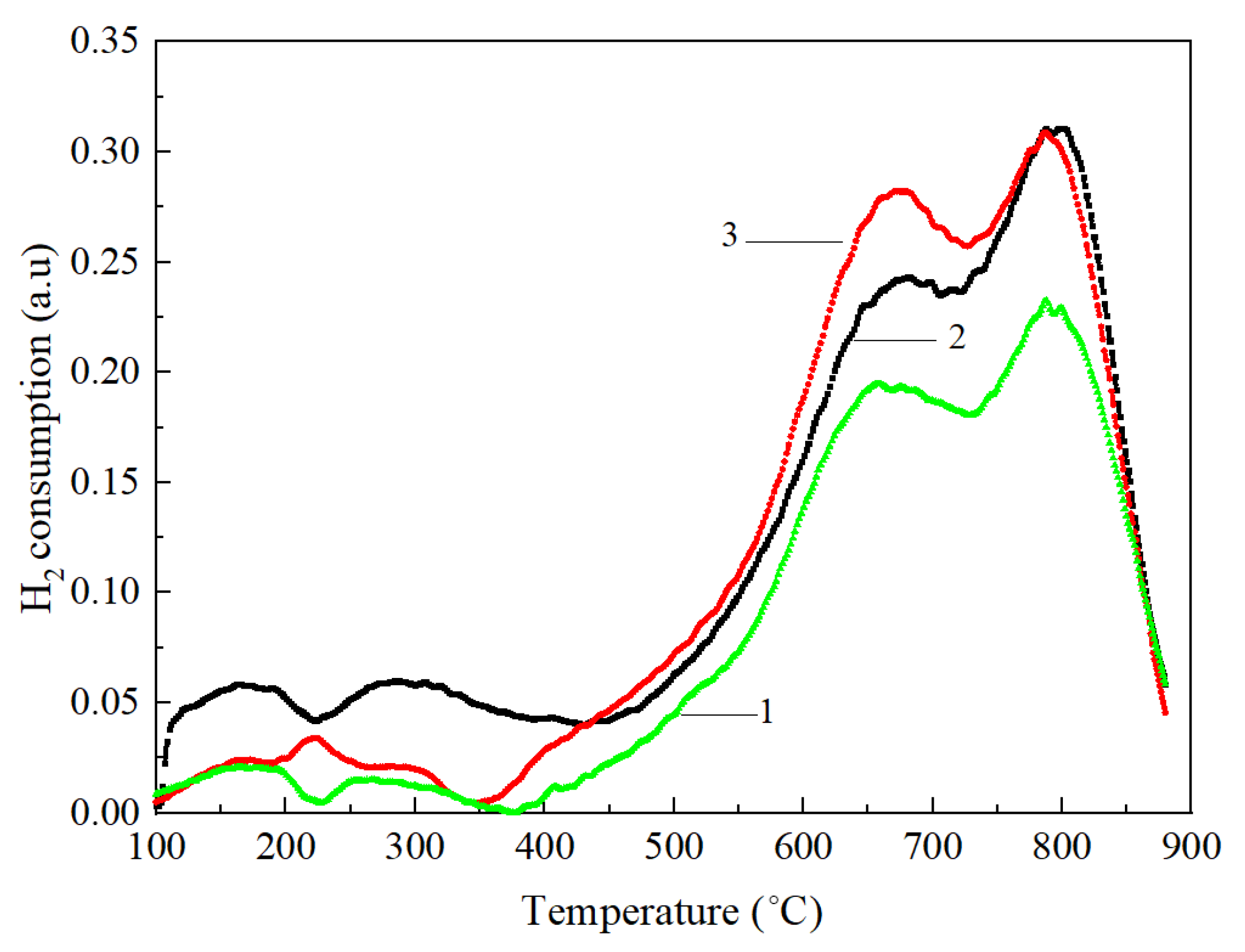

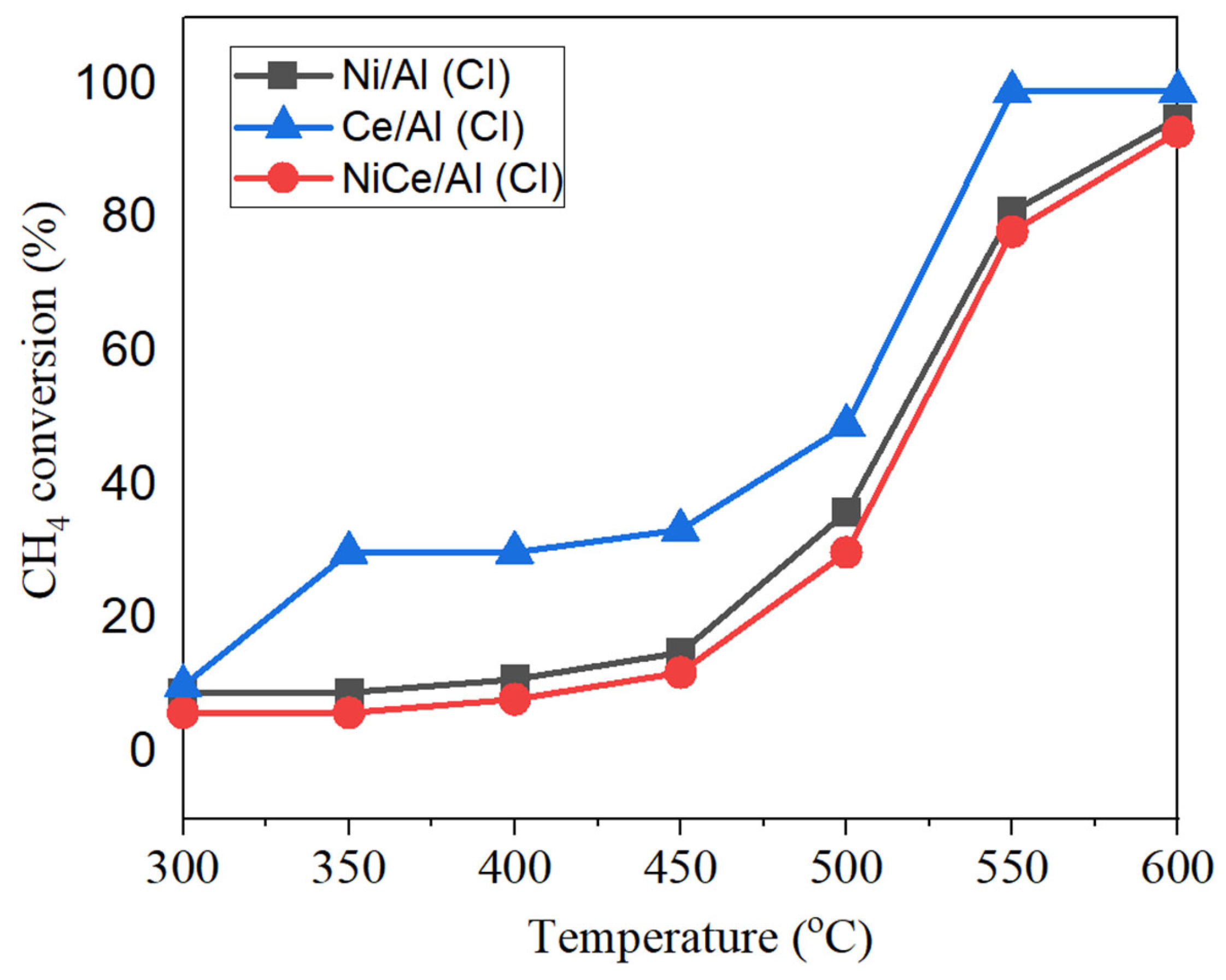

| Samples | <400 °C, % | 400–850 °C, % | >850 °C, % |
|---|---|---|---|
| 3Ni2Ce/Al (SC) | 15.0 | 80.0 | 5.0 |
| 3Ni2Ce/Al (CI) | 33.0 | 60.0 | 7.0 |
| 3Ni2Ce/Al (DI) | 40.0 | 54.0 | 6.0 |
| Year | Sample | Calcination Temperature and Time | Initial Gases | Space Velocity, h−1 | Process Temperature, °C | Conversion of CH4, % | Preparation Catalyst | Ref. |
|---|---|---|---|---|---|---|---|---|
| 2025 | 3Ni2Ce/Al (SC) | 550 °C for 6 h | N2:CH4:O2 = 78:2.5:19.5 | 2000 | 500 600 | 82 100 | capillary impregnation | This work |
| 2009 | NiO/Ce0.75Zr0.25O2 | He:CH4:O2 = 87.5:3.0:10 | 39,000 | 600 | 60 | wetness impregnation | [52] | |
| 2018 | Ce0.6Fe0.4O2-δ | 500 °C for 2 h | N2:CH4:O2 = 89:1.0:20 | 30,000 | 500 | 75 | wetness impregnation | [53] |
| 2019 | Co/CeO2-H | 500 °C for 5 h | He:CH4:O2 = 89.5:0.5:10 | 25,000 | 600 | 90 | wetness impregnation | [54] |
| 2019 | Cu(15) CeMgAlO | 750 °C for 8 h | CH4:Air = 1.0:99.0 | 16,000 | 550 | 90 | coprecipitation | [55] |
| 2019 | Pt/γ-Al2O3 | 600 °C for 4 h | N2:CH4:O2 = 89.8:0.2:10 | 30,000 | 550 | 100 | impregnating | [56] |
| 2020 | Pd/Al2O3 | 600 °C for 2 h | Ar:CH4:O2 = 97:1.0:2.0 | 450 | 20 | incipient wetness impregnation | [57] | |
| 2020 | CeO2-S | 500 °C for 4 h | Ar:CH4:O2 = 80:1.0:19 | 20,000 | 550 | 97 | wet impregnation | [58] |
| 2020 | NiO/CeO2 | 450 °C for 4 h | Ar:CH4:O2 = 95:1.0:4.0 | 15,000 | 600 | 100 | wetness impregnation | [59] |
| 2020 | Co/20Ce-Al | 600 °C for 4 h | N2:CH4:O2 = 89:1.0:10 | 15,000 | 600 | 95 | basic precipitation | [60] |
| 2020 | Ni9Mg | 550 °C for 6 h | He:CH4:O2 = 89:1.0:10 | 10,000 | 600 | 94 | co-precipitation | [61] |
| 2021 | LaNi0.5Mn0.5O3 | 800 °C for 4 h | He:CH4:O2 = 88:1.0:21 | 45,000 | 560 | 50 | coprecipitation and Pechini sol–gel | [62] |
| 2023 | Pd-CeO2-cube catalyst | - | 0.05% to 0.5% of CH4 leakage in marine natural gas | 36,000–120,000 | 500 | 100 | wet impregnation | [63] |
| 2024 | Cu/HAP | - | Ar:CH4:O2 = 95:1.0:4.0 | 39,000 | 600 | 100 | co-precipitation | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myltykbayeva, L.; Mambetova, M.; Anissova, M.; Makayeva, N.; Dossumov, K.; Yergaziyeva, G. Influence of Preparation Methods on the Physicochemical and Functional Properties of NiO-CeO2/Al2O3 Catalysts. J. Compos. Sci. 2025, 9, 446. https://doi.org/10.3390/jcs9080446
Myltykbayeva L, Mambetova M, Anissova M, Makayeva N, Dossumov K, Yergaziyeva G. Influence of Preparation Methods on the Physicochemical and Functional Properties of NiO-CeO2/Al2O3 Catalysts. Journal of Composites Science. 2025; 9(8):446. https://doi.org/10.3390/jcs9080446
Chicago/Turabian StyleMyltykbayeva, Laura, Manshuk Mambetova, Moldir Anissova, Nursaya Makayeva, Kusman Dossumov, and Gaukhar Yergaziyeva. 2025. "Influence of Preparation Methods on the Physicochemical and Functional Properties of NiO-CeO2/Al2O3 Catalysts" Journal of Composites Science 9, no. 8: 446. https://doi.org/10.3390/jcs9080446
APA StyleMyltykbayeva, L., Mambetova, M., Anissova, M., Makayeva, N., Dossumov, K., & Yergaziyeva, G. (2025). Influence of Preparation Methods on the Physicochemical and Functional Properties of NiO-CeO2/Al2O3 Catalysts. Journal of Composites Science, 9(8), 446. https://doi.org/10.3390/jcs9080446






