Bioactive Properties of Chitosan/Nanocellulose Films Loaded with Sage Essential Oil: From In Vitro Study to In Situ Application in Shelf-Life Extension of Fresh Poultry Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Bionanocomposite Production
2.3. In Vitro Bionanocomposite Study
2.3.1. Specific Migration of Antioxidant Compounds
2.3.2. Antioxidant Activity
2.3.3. Antimicrobial Activity—Colony-Forming Unit Counting Method
2.4. In Situ Application of Bionanocomposites as Primary Packaging of Poultry Meat
2.4.1. Fresh Poultry Meat Preparation
2.4.2. Microbiological Growth
2.4.3. Physicochemical Characterization
2.4.4. Lipid Oxidation (TBARS Index)
2.5. Statistical Analysis
3. Results
3.1. In Vitro Bionanocomposite Study
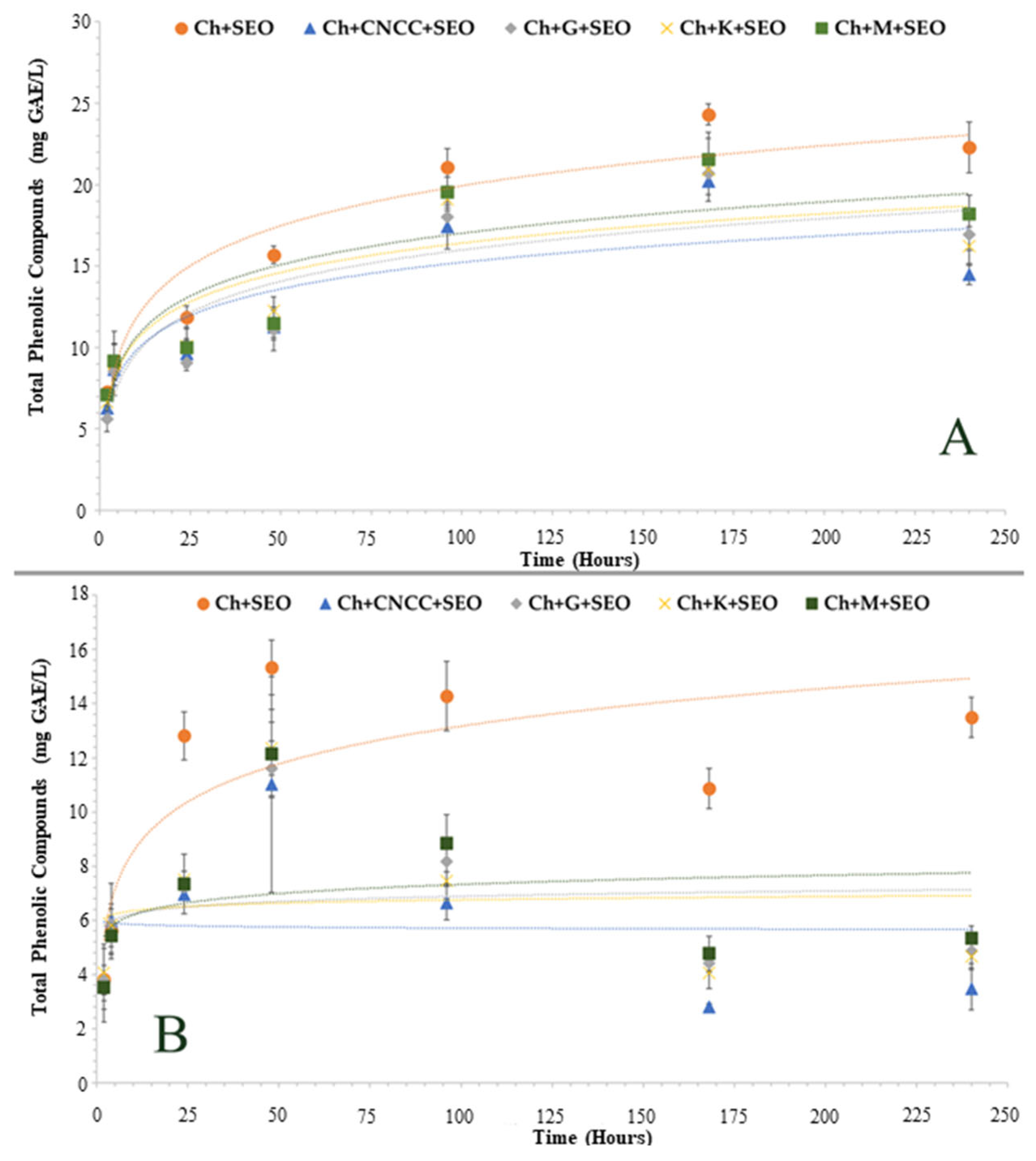
3.1.1. Specific Migration of Antioxidant Compounds
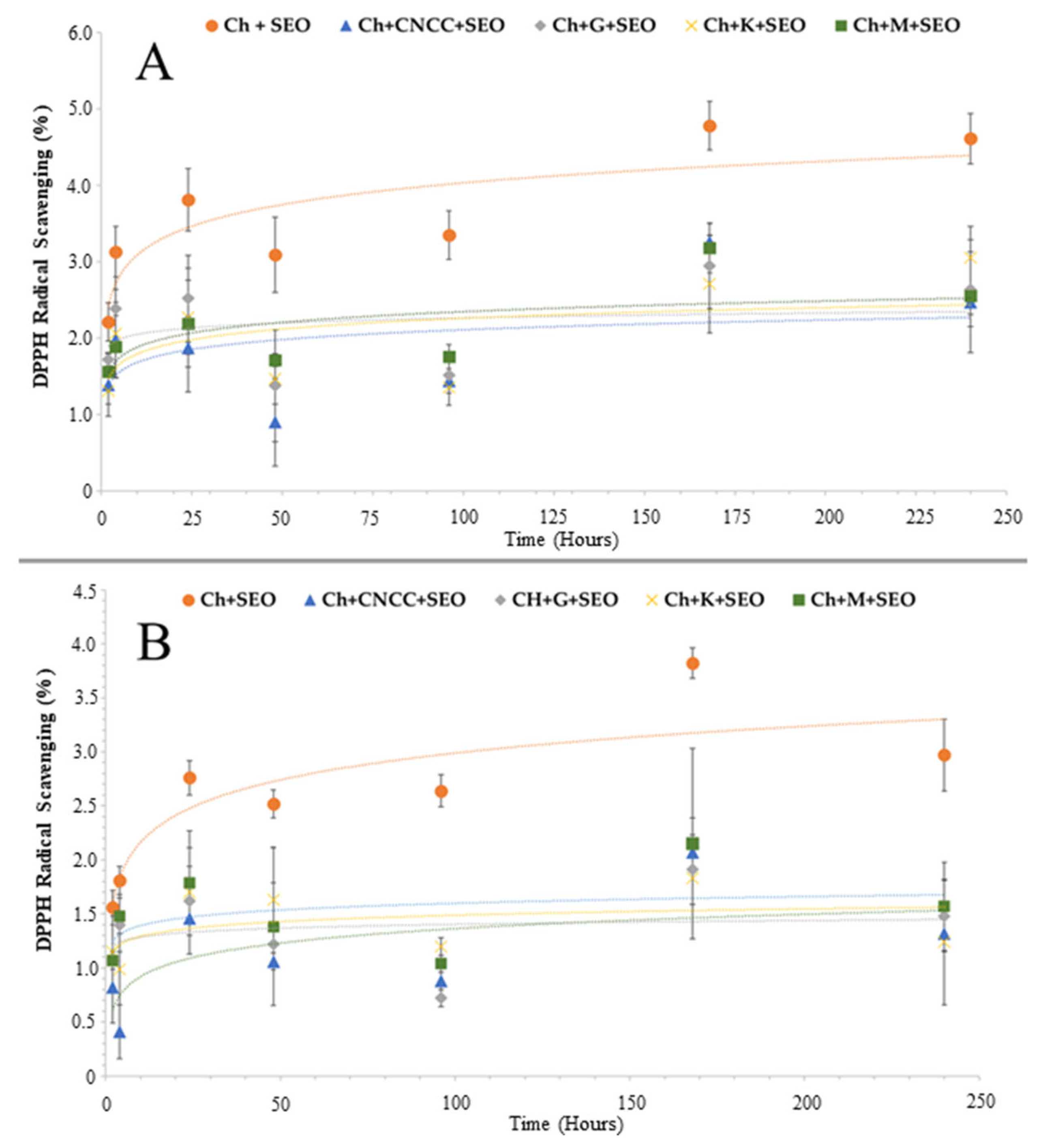
3.1.2. Antioxidant Activity
3.1.3. Antimicrobial Activity—Colony-Forming Unit Counting Method
3.2. “In Situ” Meat Characterization—Shelf-Life Study
3.2.1. Microbiological Growth
3.2.2. Physicochemical Characterization
Meat Color
Moisture Content
pH and Total Titratable Acidity
Total Volatile Basic Nitrogen
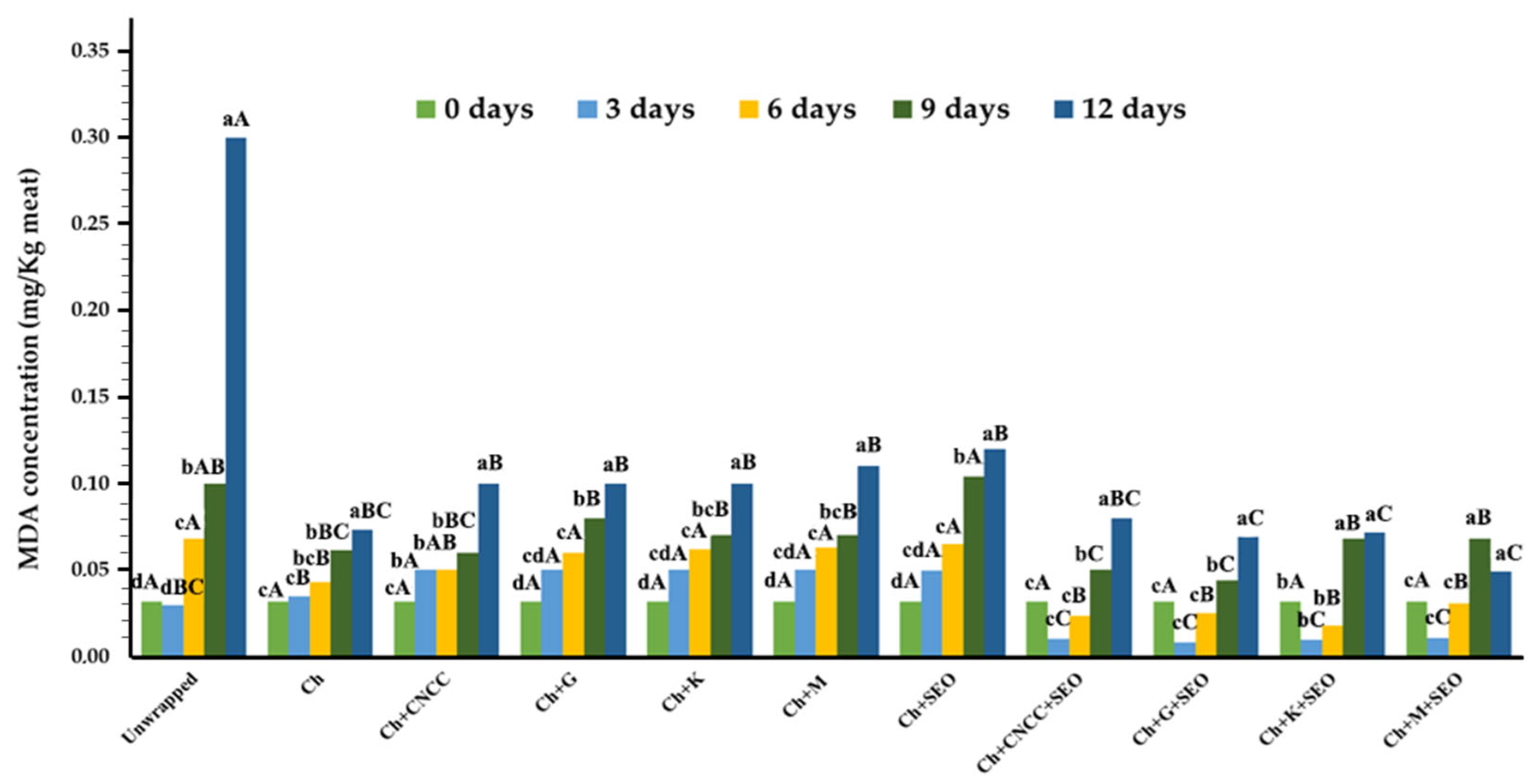
Lipid Oxidation (TBARS Index)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Otoni, C.G.; Azeredo, H.M.C.; Mattos, B.D.; Beaumont, M.; Correa, D.S.; Rojas, O.J. The Food–Materials Nexus: Next Generation Bioplastics and Advanced Materials from Agri-Food Residues. Adv. Mater. 2021, 33, 2102520. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.R.A.; Souza, V.G.L.; Fuciños, P.; Pastrana, L.; Fernando, A.L. Methodologies to Assess the Biodegradability of Bio-Based Polymers—Current Knowledge and Existing Gaps. Polymers 2022, 14, 1359. [Google Scholar] [CrossRef]
- Lau, W.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating Scenarios toward Zero Plastic Pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable Polymers from Renewable Resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vásquez, M. Chitosan for Food Packaging: Recent Advances in Active and Intelligent Films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan Based Nanocomposite Films and Coatings: Emerging Antimicrobial Food Packaging Alternatives. Trends Food Sci. Technol. 2020, 97, 196–209. [Google Scholar] [CrossRef]
- Rodrigues, C.; Polesca, C.; Bicalho, I.; Souza, V.G.L.; Coelhoso, I.; Fernando, A.L. Quality Preservation and Shelf-Life Extension of Prickly Pear (Opuntia ficus-indica L. Mill) Using Edible Coatings. Foods 2025, 14, 161. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.; de Paula, C.D.; Souza, V.G.L.; Fernando, A.L.; Coelhoso, I. Understanding the Barrier and Mechanical Behavior of Different Nanofillers in Chitosan Films for Food Packaging. Polymers 2021, 13, 721. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Bourbon, A.I.; Costa, M.J.; Maciel, L.C.; Pastrana, L.; Vicente, A.A.; Cerqueira, M.A. Active Carboxymethylcellulose-Based Edible Films: Influence of Free and Encapsulated Curcumin on Films’ Properties. Foods 2021, 10, 1512. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Behera, K.; Chang, Y.H.; Chiu, F.C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers 2020, 12, 202. [Google Scholar] [CrossRef]
- Ahankari, S.S.; Subhedar, A.R.; Bhadauria, S.S.; Dufresne, A. Nanocellulose in Food Packaging: A Review. Carbohydr. Polym. 2021, 255, 117479. [Google Scholar] [CrossRef] [PubMed]
- Pires, J.R.A.; Souza, V.G.L.; Fernando, A.L. Production of Nanocellulose from Lignocellulosic Biomass Wastes: Prospects and Limitations. In Innovation, Engineering and Entrepreneurship; Machado, J., Soares, F., Veiga, G., Eds.; Lecture Notes in Electrical Engineering; Springer International Publishing: Cham, Switzerland, 2019; Volume 505, pp. 719–725. ISBN 978-3-319-91333-9. [Google Scholar]
- Vosoughi, N.; Gomarian, M.; Ghasemi Pirbalouti, A.; Khaghani, S.; Malekpoor, F. Essential Oil Composition and Total Phenolic, Flavonoid Contents, and Antioxidant Activity of Sage (Salvia officinalis L.) Extract under Chitosan Application and Irrigation Frequencies. Ind. Crops Prod. 2018, 117, 366–374. [Google Scholar] [CrossRef]
- Branislav, Š.; Pavlić, B.; Zeković, Z.; Tomović, V.; Ikonić, P.; Džinić, N. The Effect of Essential Oil and Extract from Sage (Salvia officinalis L.) Herbal Dust (Food Industry by-Product) on the Oxidative and Microbiological Stability of Fresh Pork Sausages. LWT-Food Sci. Technol. 2018, 89, 749–755. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Afshari, A.; Aminzare, M.; Raeisi, M.; Zeinali, T. Effect of Different Types of Active Biodegradable Films Containing Lactoperoxidase System or Sage Essential Oil on the Shelf Life of Fish Burger during Refrigerated Storage. LWT-Food Sci. Technol. 2020, 117, 108633. [Google Scholar] [CrossRef]
- EMA. Assessment Report on Salvia officinalis L., folium and Salvia officinalis L., aetheroleum; EMA: London, UK, 2016.
- Costa, S.M.; Ferreira, D.P.; Teixeira, P.; Ballesteros, L.F.; Teixeira, J.A.; Fangueiro, R. Active Natural-Based Films for Food Packaging Applications: The Combined Effect of Chitosan and Nanocellulose. Int. J. Biol. Macromol. 2021, 177, 241–251. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Souza, V.G.L.; Gomes, L.A.; Coelhoso, I.M.; Godinho, M.H.; Fernando, A.L. Micro and Nanocellulose Extracted from Energy Crops as Reinforcement Agents in Chitosan Films. Ind. Crops Prod. 2022, 186, 115247. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Rodrigues, C.; Ferreira, L.; Pires, J.R.A.; Duarte, M.P.; Coelhoso, I.; Fernando, A.L. In Vitro Bioactivity of Novel Chitosan Bionanocomposites Incorporated with Different Essential Oils. Ind. Crops Prod. 2019, 140, 111563. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Chung, D.; Papadakis, S.E.; Yam, K.L. Simple Models for Assessing Migration from Food-Packaging Films. Food Addit. Contam. 2002, 19, 611–617. [Google Scholar] [CrossRef]
- Nouri, A.; Yaraki, M.T.; Ghorbanpour, M.; Agarwal, S.; Gupta, V.K. Enhanced Antibacterial Effect of Chitosan Film Using Montmorillonite/CuO Nanocomposite. Int. J. Biol. Macromol. 2017, 109, 1219–1231. [Google Scholar] [CrossRef] [PubMed]
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 17410:2019; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. ISO: Geneva, Switzerland, 2019.
- ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. ISO: Geneva, Switzerland, 2017.
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 20th ed.; AOAC: San Diego, CA, USA, 2016. [Google Scholar]
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López-Santoveña, F.; Aranda-Catalá, V. TBA Test by an Extractive Method Applied to “Paté”. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Ali, S.; Liu, D.; Guo, M. Emerging Chitosan-Essential Oil Films and Coatings for Food Preservation—A Review of Advances and Applications. Carbohydr. Polym. 2021, 273, 118616. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Activity of Chitosan-Montmorillonite Bionanocomposites Incorporated with Rosemary Essential Oil: From in Vitro Assays to Application in Fresh Poultry Meat. Food Hydrocoll. 2019, 89, 241–252. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; de Melo, N.R.; Andrade, M.; Sanches-Silva, A. Potential of Migration of Active Compounds from Protein-Based Films with Essential Oils to a Food and a Food Simulant. Packag. Technol. Sci. 2017, 30, 791–798. [Google Scholar] [CrossRef]
- Montero, Y.; Souza, A.G.; Oliveira, É.R.; Rosa, S. Nanocellulose Functionalized with Cinnamon Essential Oil: A Potential Application in Active Biodegradable Packaging for Strawberry. Sustain. Mater. Technol. 2021, 29, e00289. [Google Scholar] [CrossRef]
- Esparza, I.; Cimminelli, M.J.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Stability of Phenolic Compounds in Grape Stem Extracts. Antioxidants 2020, 9, 720. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Rodrigues, P.F.; Duarte, M.P.; Fernando, A.L. Antioxidant Migration Studies in Chitosan Films Incorporated with Plant Extracts. J. Renew. Mater. 2018, 6, 548–558. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Jiang, W. Development of Antioxidant Chitosan Film with Banana Peels Extract and Its Application as Coating in Maintaining the Storage Quality of Apple. Int. J. Biol. Macromol. 2020, 154, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, G.G.; Del Nobile, M.A.; Panizza, A.; Corbo, M.R.; Nicolais, L. A General Approach to Describe the Antimicrobial Agent Release from Highly Swellable Films Intended for Food Packaging Applications. J. Control Release 2003, 90, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, L.; Cháfer, M.; González-Martínez, C.; Chiralt, A.; Desobry, S. Study of the Release of Limonene Present in Chitosan Films Enriched with Bergamot Oil in Food Simulants. J. Food Eng. 2011, 105, 138–143. [Google Scholar] [CrossRef]
- Abbasi, A.; Mohammad, K.; Ahmad, M.; Mohammad, A.; Sourestani, M. Nutritive Composition, Growth, Biochemical Traits, Essential Oil Content and Compositions of Salvia officinalis L. Grown in Different Nitrogen Levels in Soilless Culture. J. Soil Sci. Plant Nutr. 2021, 21, 3320–3332. [Google Scholar] [CrossRef]
- Cuceu, A.P.; Tofană, M.; Socaci, S.A.; Vârban, D.; Nagy, M.; Borş, M.-D.; Fărcaş, A. Essential Oil Composition, Phenolic Content and Antioxidant Activity in Romanian Salvia officinalis L. J. Agroaliment. Process. Technol. 2015, 21, 241–246. [Google Scholar]
- O’Neil, M.J. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals, 15th ed.; Chemistry, R.S., Ed.; Royal Society of Chemistry: Cambridge, UK, 2013; ISBN 9781849736701. [Google Scholar]
- Yalkowsky, S.H.; He, Y.; Jain, P. Handbook of Aqueous Solubility Data, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780367384173. [Google Scholar]
- Lan, W.; Wang, S.; Zhang, Z.; Liang, X.; Liu, X.; Zhang, J. Development of Red Apple Pomace Extract/Chitosan-Based Films Reinforced by TiO2 Nanoparticles as a Multifunctional Packaging Material. Int. J. Biol. Macromol. 2021, 168, 105–115. [Google Scholar] [CrossRef]
- Surendhiran, D.; Chandra, V.; Park, J.; Chun, B. Fabrication of Chitosan-Based Food Packaging Film Impregnated with Turmeric Essential Oil (TEO)-Loaded Magnetic-Silica Nanocomposites for Surimi Preservation. Int. J. Biol. Macromol. 2022, 203, 650–660. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and Antioxidant Properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L.; Lamiaceae) Essential Oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Navajas, Y.R.; Zapata, E.S.; Fernández-López, J.; Pérez-Álvarez, J.A. Antioxidant Activity of Essential Oils of Five Spice Plants Widely Used in a Mediterranean Diet. Flavour Fragr. J. 2009, 25, 13–19. [Google Scholar] [CrossRef]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A Review of Molecular Structure, Bioactivities and Interactions with the Human Body and Micro-Organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Pires, J.R.A.; Almeida, K.M.; Augusto, A.S.; Vieira, É.T.; Fernando, A.L.; Souza, V.G.L. Application of Biocomposite Films of Chitosan/Natural Active Compounds for Shelf Life Extension of Fresh Poultry Meat. J. Compos. Sci. 2022, 6, 342. [Google Scholar] [CrossRef]
- Maria Pelissari, F.; Andrade-Mahecha, M.M.; José, P.; Cecilia, F. Nanocomposites Based on Banana Starch Reinforced with Cellulose Nanofibers Isolated from Banana Peels. J. Colloid Interface Sci. 2017, 505, 154–167. [Google Scholar] [CrossRef]
- Samapundo, S.; De Baenst, I.; Aerts, M.; Cnockaert, M.; Devlieghere, F.; Van Damme, P. Tracking the Sources of Psychrotrophic Bacteria Contaminating Chicken Cuts during Processing. Food Microbiol. 2019, 81, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Norrrahim, M.N.F.; Nurazzi, N.M.; Jenol, M.A.; Farid, M.A.A.; Janudin, N.; Ujang, F.A.; Yasim-Anuar, T.A.T.; Najmuddin, S.U.F.S.; Ilyas, R.A. Emerging Development of Nanocellulose as an Antimicrobial Material: An Overview. Mater. Adv. 2021, 2, 3538–3551. [Google Scholar] [CrossRef]
- Jannatyha, N.; Shojaee-aliabadi, S.; Moslehishad, M.; Moradi, E. Comparing Mechanical, Barrier and Antimicrobial Properties of Nanocellulose/CMC and Nanochitosan/CMC Composite Films. Int. J. Biol. Macromol. 2020, 164, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Cegielka, A.; Hac-Szymanczuk, E.; Piwowarek, K.; Dasiewicz, K.; Slowinski, M.; Wronska, K. The Use of Bioactive Properties of Sage Preparations to Improve the Storage Stability of Low-Pressure Mechanically Separated Meat from Chickens. Poult. Sci. 2019, 98, 5045–5053. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of Essential Oils as Antimicrobial Agents against Spoilage and Pathogenic Microorganisms in Meat Products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, P.F.; Lopes, A.A.S.; Fernandes, F.M.B.; Duarte, M.P.; Coelhoso, I.M.; Fernando, A.L. Bionanocomposites of Chitosan/Montmorillonite Incorporated with Rosmarinus officinalis Essential Oil: Development and Physical Characterization. Food Packag. Shelf Life 2018, 16, 148–156. [Google Scholar] [CrossRef]
- Pires, J.R.A.; de Souza, V.G.L.; Fernando, A.L. Chitosan/Montmorillonite Bionanocomposites Incorporated with Rosemary and Ginger Essential Oil as Packaging for Fresh Poultry Meat. Food Packag. Shelf Life 2018, 17, 142–149. [Google Scholar] [CrossRef]
- Bonilla, J.; Vargas, M.; Atarés, L.; Chiralt, A. Effect of Chitosan Essential Oil Films on the Storage-Keeping Quality of Pork Meat Products. Food Bioprocess Technol. 2014, 7, 2443–2450. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; Hernández, M.; Chiralt, A. Antimicrobial Activity of Polysaccharide Films Containing Essential Oils. Food Control 2011, 22, 1302–1310. [Google Scholar] [CrossRef]
- Matei, P.M.; Buzón-Durán, L.; Pérez-Lebeña, E.; Martín-Gil, J.; Iacomi, B.M.; Ramos-Sánchez, M.C.; Martín-Ramos, P. In Vitro Antifungal Activity of Chitosan-Polyphenol Conjugates against Phytophthora cinnamomi. AgriEngineering 2020, 2, 72–77. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial Chitosan Conjugates: Current Synthetic Strategies and Potential Applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef]
- Sedlaříková, J.; Doležalová, M.; Egner, P.; Pavlačková, J.; Krejčí, J.; Rudolf, O.; Peer, P. Effect of Oregano and Marjoram Essential Oils on the Physical and Antimicrobial Properties of Chitosan Based Systems. Int. J. Polym. Sci. 2017, 2017, 2593863. [Google Scholar] [CrossRef]
- The Commision of the European Communities. COMMISSION REGULATION (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; Elsevier: Amsterdam, The Netherlands, 2005; Volume L338. [Google Scholar]
- Marmion, M.; Ferone, M.T.; Whyte, P.; Scannell, A.G.M. The Changing Microbiome of Poultry Meat; from Farm to Fridge. Food Microbiol. 2021, 99, 103823. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L. Packaging of Flesh Foods. In Food Packaging: Principles and Practice; CRC Press: Boca Raton, FL, USA, 2013; pp. 445–476. [Google Scholar]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.d.L.S.; Gomide, L.A.d.M.; Ramos, E.M. Selection of a Chitosan Gelatin-Based Edible Coating for Color Preservation of Beef in Retail Display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef]
- Singh, P.; Wani, A.A.; Saengerlaub, S.; Langowski, H.-C. Understanding Critical Factors for the Quality and Shelf-Life of MAP Fresh Meat: A Review. Crit. Rev. Food Sci. Nutr. 2011, 51, 146–177. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Oiseth, S.K.; Purslow, P.P.; Warner, R.D. A Structural Approach to Understanding the Interactions between Colour, Water-Holding Capacity and Tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of Broiler Chicken Meat Quality and Factors Affecting Them: A Review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Knorr, D. Recovery and Utilization of Chitin and Chitosan in Food Processing Waste Management. Food Technol. 1991, 45, 114–122. [Google Scholar]
- Ünal, K.; Babaoglu, A.S.; Karakaya, M. Effect of Oregano, Sage and Rosemary Essential Oils on Lipid Oxidation and Color Properties of Minced Beef during Refrigerated Storage. J. Essent. Oil Bear. Plants 2014, 17, 797–805. [Google Scholar] [CrossRef]
- Dave, D.; Ghaly, A.E. Meat Spoilage Mechanisms and Preservation Techniques: A Critical Review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar] [CrossRef]
- Warner, R.D. The Eating Quality of Meat—IV Water-Holding Capacity and Juiciness. In Lawrie’s Meat Science; Toldra, F., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 419–459. ISBN 978-0-08-100694-8. [Google Scholar]
- Souza, V.G.L.; Mello, I.P.; Khalid, O.; Pires, J.R.A.; Rodrigues, C.; Alves, M.M.; Santos, C.; Fernando, A.L.; Coelhoso, I. Strategies to Improve the Barrier and Mechanical Properties of Pectin Films for Food Packaging: Comparing Nanocomposites with Bilayers. Coatings 2022, 12, 108. [Google Scholar] [CrossRef]
- Souza, V.; Rodrigues, C.; Valente, S.; Pimenta, C.; Pires, J.; Alves, M.; Santos, C.; Coelhoso, I.; Fernando, A. Eco-Friendly ZnO/Chitosan Bionanocomposites Films for Packaging of Fresh Poultry Meat. Coatings 2020, 10, 110. [Google Scholar] [CrossRef]
- Chauhan, S.S.; Lemaster, M.N.; Clark, D.L.; Foster, M.K.; Miller, C.E.; England, E.M. Glycolysis and PH Decline Terminate Prematurely in Oxidative Muscles despite the Presence of Excess Glycogen. Meat Muscle Biol. 2019, 3, 254–264. [Google Scholar] [CrossRef]
- Matarneh, S.K.; England, E.M.; Scheffler, T.L.; Gerrard, D.E. The Conversion of Muscle to Meat. In Lawrie’s Meat Science; Toldrá, F., Ed.; Woodhead Publishing: Sawston, UK, 2017; pp. 159–185. [Google Scholar]
- Bekhit, A.E.A.; Holman, B.W.B.; Giteru, S.G.; Hopkins, D.L. Total Volatile Basic Nitrogen (TVB-N) and Its Role in Meat Spoilage: A Review. Trends Food Sci. Technol. 2021, 109, 280–302. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef]
- Tyl, C.; Sadler, G.D. PH and Titratable Acidity. In Food Analysis; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 389–406. [Google Scholar]
- Grashorn, M.A. Research into Poultry Meat Quality. Br. Poult. Sci. 2010, 51, 60–67. [Google Scholar] [CrossRef]
- Cheng, Y.; Hu, J.; Wu, S. Chitosan Based Coatings Extend the Shelf-Life of Beef Slices during Refrigerated Storage. LWT 2021, 138, 110694. [Google Scholar] [CrossRef]
- Mehrabi, F.A.; Sharifi, A.; Ahvazi, M. Effect of Chitosan Coating Containing Nepeta Pogonosperma Extract on Shelf Life of Chicken Fillets during Chilled Storage. Food Sci. Nutr. 2021, 9, 4517–4528. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of Multifunctional Food Packaging Films Based on Chitosan, TiO2 Nanoparticles and Anthocyanin-Rich Black Plum Peel Extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Çoban, O.E.; Patir, B.; Özpolat, E.; Kuzgun, N.K. Improving the Quality of Fresh Rainbow Trout by Sage Essential Oil and Packaging Treatments. J. Food Saf. 2016, 36, 299–307. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Tajik, H.; Jafarie, S.; Kaboudari, A. Effect of Salvia officinalis L. Extract on Chemical, Microbial, Sensory and Shelf Life of Rainbow Trout Fillet. Food Sci. Biotechnol. 2019, 28, 1499–1506. [Google Scholar] [CrossRef]
- Wan, A.; Xu, Q.; Sun, Y.; Li, H. Antioxidant Activity of High Molecular Weight Chitosan and N,O-Quaternized Chitosans. J. Agric. Food Chem. 2013, 61, 6921–6928. [Google Scholar] [CrossRef]
- Chen, X.; Chen, W.; Lu, X.; Mao, Y.; Luo, X.; Liu, G.; Zhu, L.; Zhang, Y. Effect of Chitosan Coating Incorporated with Oregano or Cinnamon Essential Oil on the Bacterial Diversity and Shelf Life of Roast Duck in Modified Atmosphere Packaging. Food Res. Int. 2021, 147, 110491. [Google Scholar] [CrossRef]
- Przybyszewska, A.; Barbosa, C.H.; Pires, F.; Pires, J.R.A.; Rodrigues, C.; Galus, S.; Souza, V.G.L.; Alves, M.M.; Santos, C.F.; Coelhoso, I.; et al. Packaging of Fresh Poultry Meat with Innovative and Sustainable ZnO/Pectin Bionanocomposite Films—A Contribution to the Bio and Circular Economy. Coatings 2023, 13, 1208. [Google Scholar] [CrossRef]
- Contini, L.; De Souza, T.; Isadora, Z.; Brazolin, F.; Wesley, J.; Mariangela, S.; Silva, F.; Lopes, P.S.; Araújo, K.; Rosemary, S.; et al. Antioxidant Chitosan Film Containing Lemongrass Essential Oil as Active Packaging for Chicken Patties. J. Food Process. Preserv. 2021, 46, e16136. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Langroodi, A.M. Chitosan Coatings Incorporated with Propolis Extract and Zataria Multi Flora Boiss Oil for Active Packaging of Chicken Breast Meat. Int. J. Biol. Macromol. 2019, 141, 401–409. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Aminzare, M.; Raeisi, M.; Afshari, A.; Mirza, A.; Mohammadreza, A. Comparative Evaluation of Edible Films Impregnated with Sage Essential Oil or Lactoperoxidase System: Impact on Chemical and Sensory Quality of Carp Burgers. J. Food Process. Preserv. 2019, 43, e14070. [Google Scholar] [CrossRef]
- Ferreira, C.S.R.; Henrique, B.; Saqueti, F.; Daniele, P.; Martins, J.; Ant, M.; Carla, A.; Martha, J.; Mikcha, G.; Oliveira, O. Effect of Salvia (Salvia officinalis) on the Oxidative Stability of Salmon Hamburgers. LWT 2022, 154, 112867. [Google Scholar] [CrossRef]
- Kenar, M.; Ozogul, F.; Kuley, E. Effects of Rosemary and Sage Tea Extracts on the Sensory, Chemical and Microbiological Changes of Vacuum-Packed and Refrigerated Sardine (Sardina pilchardus) Fillets. Int. J. Food Sci. Technol. 2010, 45, 2366–2372. [Google Scholar] [CrossRef]
- Akyüz, E.; Başkan, K.S.; Tütem, E.; Apak, R. Novel Protein-Based Solid-Biosensor for Determining pro-Oxidant Activity of Phenolic Compounds. J. Agric. Food Chem. 2017, 65, 5821–5830. [Google Scholar] [CrossRef] [PubMed]


| Specimen | Bionanocomposite Formulation Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| Chitosan (% w/v FFD) | Glycerol (% w/w Ch) | Tween® 80 (% w/v SEO) | CNCCs (% w/w Ch) | CNC G (% w/w Ch) | CNC K (% w/w Ch) | CNC M (% w/w Ch) | SEO (% v/v FFD) | |
| Ch | 1.5 | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ch + CNCC | 1.5 | 30 | 0 | 2.5 | 0 | 0 | 0 | 0 |
| Ch + G | 1.5 | 30 | 0 | 0 | 2.5 | 0 | 0 | 0 |
| Ch + K | 1.5 | 30 | 0 | 0 | 0 | 2.5 | 0 | 0 |
| Ch + M | 1.5 | 30 | 0 | 0 | 0 | 0 | 2.5 | 0 |
| Ch + SEO | 1.5 | 30 | 0.2 | 0 | 0 | 0 | 0 | 1 |
| Ch + CNCC + SEO | 1.5 | 30 | 0.2 | 2.5 | 0 | 0 | 0 | 1 |
| Ch + G + SEO | 1.5 | 30 | 0.2 | 0 | 2.5 | 0 | 0 | 1 |
| Ch + K + SEO | 1.5 | 30 | 0.2 | 0 | 0 | 2.5 | 0 | 1 |
| Ch + M + SEO | 1.5 | 30 | 0.2 | 0 | 0 | 0 | 2.5 | 1 |
| Diffusion Coefficient (cm2/s) | Maximum Diffusion/Total Incorporated | |||
|---|---|---|---|---|
| Sample | Ethanol 50% | Ethanol 95% | Ethanol 50% | Ethanol 95% |
| Ch + SEO * | 4.4 × 10−11 | 8.9 × 10−11 | 0.85 | 0.75 |
| Ch + CNCC + SEO | 4.0 × 10−11 | 3.7 × 10−11 | 0.71 | 0.54 |
| Ch + G + SEO | 4.2 × 10−11 | 3.8 × 10−11 | 0.73 | 0.57 |
| Ch + K + SEO | 4.3 × 10−11 | 4.8 × 10−11 | 0.73 | 0.60 |
| Ch + M + SEO | 4.6 × 10−11 | 5.6 × 10−11 | 0.76 | 0.60 |
| Bacillus cereus | Salmonella Choleraesuis | |||
|---|---|---|---|---|
| Sample | LogCFU/mL | Logarithmic Reduction | LogCFU/mL | Logarithmic Reduction |
| Control | 9.2 ± 0.4 | 9.7 ± 0.2 a* | ||
| Ch | <1 | >8.2 ± 0.4 | 3.7 ± 0.3 c | 6.0 ± 0.3 a |
| Ch + SEO | <1 | >8.2 ± 0.4 | 4.5 ± 0.1 b | 5.1 ± 0.1 b |
| Ch + CNCC + SEO | <1 | >8.2 ± 0.4 | 4.0 ± 0.3 c | 5.7 ± 0.3 a |
| Parameters | Storage Days | Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unwrapped | Ch * | Ch + CNCC | Ch + G | Ch + K | Ch + M | Ch + SEO | Ch + CNCC + SEO | Ch + G+SEO | Ch + K+SEO | Ch + M+SEO | ||
| TMAM (Log CFU/g meat) | 0 | 2.11 ± 0.21 cA | 2.11 ± 0.21 cA | 2.11 ± 0.21 dA | 2.11 ± 0.21 dA | 2.11 ± 0.21 dA | 2.11 ± 0.21 dA | 2.11 ± 0.21 cA | 2.11 ± 0.21 cA | 2.11 ± 0.21 dA | 2.11 ± 0.21 cA | 2.11 ± 0.21 cA |
| 3 | 7.26 ± 0.08 bA | 5.35 ± 0.33 bB | 4.28 ± 0.21 cBC | 3.53 ± 0.33 cC | 3.70 ± 0.20 cC | 3.88 ± 0.13 cC | 5.93 ± 0.62 bAB | 4.88 ± 0.28 bBC | 3.85 ± 0.20 cC | 4.00 ± 0.49 bC | 4.34 ± 0.33 bBC | |
| 6 | 8.91 ± 0.11 aA | 6.26 ± 0.32 bB | 5.47 ± 0.25 bB | 5.11 ± 0.11 bB | 5.30 ± 0.17 bB | 5.51 ± 0.36 bB | 5.97 ± 0.10 bB | 6.50 ± 0.17 aB | 5.51 ± 0.15 bB | 5.90 ± 0.76 aB | 5.16 ± 0.39 bB | |
| 9 | 9.74 ± 0.36 aA | 7.02 ± 0.06 aB | 7.06 ± 0.26 aB | 6.34 ± 0.34 aB | 6.43 ± 0.36 aB | 6.88 ± 0.28 aB | 7.30 ± 0.18 aB | 6.98 ± 0.73 aB | 6.61 ± 0.25 abB | 6.57 ± 0.29 aB | 6.70 ± 0.13 aB | |
| 12 | 9.81 ± 0.37 aA | 7.11 ± 0.11 aB | 7.70 ± 0.34 aB | 7.04 ± 0.26 aB | 7.10 ± 0.21 aB | 7.02 ± 0.15 aB | 7.52 ± 0.29 aB | 7.66 ± 0.42 aB | 7.23 ± 0.18 aB | 6.92 ± 0.31 aB | 6.88 ± 0.17 aB | |
| TPAM (Log CFU/g meat) | 0 | 2.31 ± 0.49 cA | 2.31 ± 0.49 cA | 2.31 ± 0.49 dA | 2.31 ± 0.49 dA | 2.31 ± 0.49 dA | 2.31 ± 0.49 dA | 2.31 ± 0.49 cA | 2.31 ± 0.49 cA | 2.31 ± 0.49 dA | 2.31 ± 0.49 dA | 2.31 ± 0.49 dA |
| 3 | 7.29 ± 0.06 bA | 5.53 ± 0.26 bB | 3.74 ± 0.34 cC | 3.61 ± 0.20 cC | 3.35 ± 0.17 cC | 3.70 ± 0.49 cC | 5.97 ± 0.55 bAB | 4.35 ± 0.41 bBC | 3.01 ± 0.04 cC | 3.04 ± 0.56 cC | 3.64 ± 0.46 cC | |
| 6 | 9.16 ± 0.19 aA | 6.51 ± 0.39 abB | 5.44 ± 0.05 bB | 5.43 ± 0.04 bB | 5.66 ± 0.11 bB | 5.34 ± 0.20 bB | 6.09 ± 0.50 bB | 6.91 ± 0.53 aB | 5.91 ± 0.21 bB | 5.97 ± 0.60 bB | 5.56 ± 0.21 bB | |
| 9 | 9.24 ± 0.47 aA | 6.81 ± 0.40 aB | 6.75 ± 0.70 aB | 6.26 ± 0.25 abB | 6.17 ± 0.10 baB | 6.30 ± 0.11 abB | 7.39 ± 0.03 aB | 7.62 ± 0.43 aB | 6.63 ± 0.05 aB | 6.43 ± 0.08 aB | 6.48 ± 0.11 aB | |
| 12 | 9.58 ± 0.01 aA | 7.23 ± 0.18 aB | 6.97 ± 0.29 aB | 7.47 ± 0.37 aB | 7.53 ± 0.51 aB | 7.28 ± 0.46 aB | 7.81 ± 0.41 aB | 7.70 ± 0.59 aB | 6.73 ± 0.10 aB | 6.80 ± 0.21 aB | 6.73 ± 0.10 aB | |
| Enterobacteriaceae (Log CFU/g meat) | 0 | 2.26 ± 0.12 dA | 2.26 ± 0.12 bA | 2.26 ± 0.12 cA | 2.26 ± 0.12 cA | 2.26 ± 0.12 cA | 2.26 ± 0.12 cA | 2.26 ± 0.12 cA | 2.26 ± 0.12 cA | 2.26 ± 0.12 cA | 2.26 ± 0.12 cA | 2.26 ± 0.12 cA |
| 3 | 6.06 ± 0.17 cA | 3.45 ± 0.71 bB | 2.52 ± 0.21 cB | 2.64 ± 0.08 cB | 2.74 ± 0.10 cB | 2.58 ± 0.17 cB | 3.11 ± 0.21 cB | 3.14 ± 0.12 bcB | 2.36 ± 0.12 cB | 2.58 ± 0.55 cB | 2.88 ± 0.10 bcB | |
| 6 | 7.00 ± 0.06 bA | 5.49 ± 0.09 aB | 4.14 ± 0.43 bBC | 3.61 ± 0.33 bC | 3.35 ± 0.25 bC | 3.53 ± 0.18 bC | 5.11 ± 0.21 bB | 4.36 ± 0.08 bBC | 3.65 ± 0.03 bC | 3.65 ± 0.44 bC | 3.27 ± 0.10 bC | |
| 9 | 8.89 ± 0.13 aA | 5.71 ± 0.21 aC | 6.17 ± 0.37 aBC | 5.56 ± 0.49 aC | 5.97 ± 0.20 aC | 5.51 ± 0.06 aC | 5.56 ± 0.10 abC | 6.48 ± 0.14 aB | 5.86 ± 0.17 aC | 5.87 ± 0.03 aC | 5.67 ± 0.06 aC | |
| 12 | 9.41 ± 0.32 aA | 6.11 ± 0.21 aB | 6.92 ± 0.24 aB | 6.46 ± 0.32 aB | 6.53 ± 0.04 aB | 6.16 ± 0.26 aB | 6.30 ± 0.49 aB | 7.18 ± 0.47 aB | 6.13 ± 0.04 aB | 6.29 ± 0.15 aB | 6.11 ± 0.06 aB | |
| Parameters | Storage Days | Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unwrapped | Ch | Ch + CNCC | Ch + G | Ch + K | Ch + M | Ch + SEO | Ch + CNCC + SEO | Ch + G+SEO | Ch + K+SEO | Ch + M+SEO | ||
| Hue angle (degrees) | 0 | 45 ± 1 cA | 45 ± 1 bA | 45 ± 1 bA | 45 ± 1 cA | 45 ± 1 cA | 45 ± 1 bA | 45 ± 1 cA | 45 ± 1 cA | 45 ± 1 cA | 45 ± 1 cA | 45 ± 1 cA |
| 3 | 43 ± 2 cB | 48 ± 2 bAB | 50 ± 3 aAB | 49 ± 1 bAB | 48 ± 1 bAB | 46 ± 1 bAB | 52 ± 0 bA | 44 ± 1 cB | 50 ± 1 bAB | 49 ± 2 bAB | 48 ± 2 bAB | |
| 6 | 45 ± 0 cB | 46 ± 2 bAB | 46 ± 0 bAB | 48 ± 1 bAB | 48 ± 2 bAB | 47 ± 2 bAB | 52 ± 1 bA | 49 ± 3 bAB | 51 ± 2 bA | 49 ± 2 bAB | 49 ± 1 bAB | |
| 9 | 50 ± 1 bAB | 48 ± 1 bAB | 45 ± 0 bC | 47 ± 0 bB | 46 ± 0 bB | 45 ± 1 bBC | 50 ± 0 bAB | 52 ± 1 bAB | 53 ± 2 bA | 51 ± 1 bAB | 49 ± 1 bAB | |
| 12 | 56 ± 2 aB | 55 ± 1 aB | 50 ± 1 aC | 53 ± 1 aBC | 51 ± 2 aBC | 52 ± 2 aBC | 56 ± 2 aB | 68 ± 3 aA | 69 ± 4 aA | 66 ± 7 aAB | 71 ± 1 aA | |
| Lightness (L*) | 0 | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA | 54 ± 1 aA |
| 3 | 47 ± 1 bB | 45 ± 1 bBC | 47 ± 0 bB | 46 ± 1 bB | 47 ± 0 bB | 47 ± 1 bB | 45 ± 0 cC | 47 ± 0 bB | 50 ± 0 bA | 50 ± 1 bA | 49 ± 1 bAB | |
| 6 | 46 ± 0 bB | 44 ± 0 bC | 47 ± 0 bB | 46 ± 1 bB | 46 ± 1 bB | 47 ± 1 bB | 46 ± 0 bB | 49 ± 1 bAB | 50 ± 1 bA | 49 ± 1 bAB | 48 ± 1 bAB | |
| 9 | 48 ± 1 bB | 49 ± 1 bBC | 47 ± 1 bBC | 46 ± 1 bBC | 47 ± 0 bB | 48 ± 1 bB | 44 ± 1 bcC | 49 ± 0 bA | 49 ± 1 bAB | 50 ± 0 bA | 49 ± 0 bAB | |
| 12 | 52 ± 2 aAB | 48 ± 2 bB | 49 ± 1 bB | 49 ± 2 bB | 48 ± 2 bB | 49 ± 2 bB | 47 ± 1 bB | 56 ± 2 aA | 56 ± 2 aA | 55 ± 2 aA | 57 ± 1 aA | |
| Moisture content (%) | 0 | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA | 74.7 ± 0.3 aA |
| 3 | 76.2 ± 2.6 aA | 69.4 ± 0.1 bAB | 69.6 ± 0.6 bAB | 70.2 ± 1.1 bAB | 71.1 ± 2.6 bAB | 70.8 ± 2.1 bAB | 65.8 ± 2.0 bcB | 68.4 ± 4.1 bAB | 65.8 ± 1.0 cB | 66.8 ± 2.1 bcB | 67.7 ± 1.3 bAB | |
| 6 | 74.7 ± 0.1 aA | 65.5 ± 2.9 cB | 66.6 ± 0.2 cB | 68.4 ± 1.3 bAB | 69.4 ± 0.4 bAB | 70.3 ± 2.0 bAB | 65.2 ± 0.3 cB | 69.9 ± 2.8 bAB | 70.6 ± 0.9 bAB | 65.8 ± 1.0 cB | 68.8 ± 3.3 bAB | |
| 9 | 76.6 ± 0.4 aA | 69.7 ± 1.0 bB | 68.7 ± 0.6 bB | 70.5 ± 0.2 bB | 69.8 ± 0.6 bB | 69.1 ± 1.2 bB | 69.4 ± 0.8 bB | 69.4 ± 0.6 bB | 69.2 ± 1.3 bcB | 69.7 ± 1.3 bB | 72.4 ± 5.9 bB | |
| 12 | 77.3 ± 0.1 aA | 67.9 ± 0.1 bcB | 67.8 ± 1.0 bB | 69.4 ± 0.8 bB | 70.3 ± 1.5 bB | 71.5 ± 2.1 bB | 68.9 ± 1.9 bB | 69.0 ± 0.4 bB | 66.9 ± 0.6 cB | 68.7 ± 0.4 bB | 69.5 ± 0.8 bB | |
| Parameters | Storage Days | Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unwrapped | Ch | Ch + CNCC | Ch + G | Ch + K | Ch + M | Ch + SEO | Ch + CNCC + SEO | Ch + G+SEO | Ch + K+SEO | Ch + M+SEO | ||
| pH | 0 | 6.0 ± 0.0 cA* | 6.0 ± 0.0 bA | 6.0 ± 0.0 bA | 6.0 ± 0.0 bA | 6.0 ± 0.0 bA | 6.0 ± 0.0 bA | 6.0 ± 0.0 aA | 6.0 ± 0.0 aA | 6.0 ± 0.0 aA | 6.0 ± 0.0 aA | 6.0 ± 0.0 aA |
| 3 | 5.9 ± 0.1 cA | 5.9 ± 0.0 bA | 6.1 ± 0.1 bA | 6.0 ± 0.0 bA | 5.9 ± 0.1 bA | 6.0 ± 0.0 bA | 5.8 ± 0.0 aA | 5.7 ± 0.1 aA | 5.6 ± 0.1 aA | 5.5 ± 0.0 aA | 5.8 ± 0.0 aA | |
| 6 | 6.7 ± 0.0 bA | 5.9 ± 0.1 bB | 6.3 ± 0.0 bAB | 6.1 ± 0.0 bB | 6.0 ± 0.0 bB | 6.1 ± 0.0 bB | 5.8 ± 0.0 aBC | 5.6 ± 0.0 aC | 5.5 ± 0.0 aC | 5.4 ± 0.2 aC | 5.5 ± 0.1 aC | |
| 9 | 7.0 ± 0.1 abA | 5.8 ± 0.1 bBC | 6.3 ± 0.0 bB | 6.3 ± 0.0 bB | 6.1 ± 0.0 bB | 6.0 ± 0.1 bB | 5.6 ± 0.1 aC | 5.6 ± 0.1 aC | 5.6 ± 0.0 aC | 5.5 ± 0.2 aC | 5.5 ± 0.0 aC | |
| 12 | 7.3 ± 0.2 aA | 6.3 ± 0.0 aB | 6.6 ± 0.1 aB | 6.7 ± 0.1 aB | 6.5 ± 0.0 aB | 6.4 ± 0.1 aB | 5.8 ± 0.1 aC | 5.7 ± 0.0 aC | 5.6 ± 0.1 aC | 5.5 ± 0.1 aC | 5.7 ± 0.2 aC | |
| Titratable acidity (% oleic acid equivalent) | 0 | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA | 1.5 ± 0.2 aA |
| 3 | 1.0 ± 0.2 bA | 1.0 ± 0.2 bA | 1.2 ± 0.2 bA | 1.1 ± 0.0 bA | 1.0 ± 0.1 bA | 1.1 ± 0.0 bA | 1.1 ± 0.1 bA | 1.0 ± 0.0 bA | 1.0 ± 0.2 bA | 1.0 ± 0.1 bA | 1.0 ± 0.0 bA | |
| 6 | 0.9 ± 0.0 bA | 1.1 ± 0.4 bA | 1.1 ± 0.2 bA | 0.9 ± 0.1 bA | 1.0 ± 0.0 bA | 1.0 ± 0.1 bA | 0.9 ± 0.2 bA | 1.1 ± 0.1 bA | 1.0 ± 0.0 bA | 1.0 ± 0.0 bA | 1.2 ± 0.1 bA | |
| 9 | 0.4 ± 0.0 cB | 0.8 ± 0.2 bA | 0.9 ± 0.0 bA | 0.8 ± 0.1 bA | 0.9 ± 0.2 bA | 1.0 ± 0.1 bA | 1.0 ± 0.1 bA | 1.2 ± 0.1 bA | 1.0 ± 0.2 bA | 1.1 ± 0.2 bA | 1.1 ± 0.2 bA | |
| 12 | 0.6 ± 0.3 cB | 0.9 ± 0.0 bA | 0.8 ± 0.1 bAB | 0.9 ± 0.1 bAB | 0.7 ± 0.2 bAB | 0.8 ± 0.1 bAB | 1.0 ± 0.1 bA | 1.0 ± 0.1 bA | 1.0 ± 0.0 bA | 1.0 ± 0.2 bA | 1.0 ± 0.0 bA | |
| TVB-N (mg/kg meat) | 0 | 14.1 ± 1.0 cA | 14.1 ± 1.0 cA | 14.1 ± 1.0 cA | 14.1 ± 1.0 cA | 14.1 ± 1.0 cA | 14.1 ± 1.0 bA | 14.1 ± 1.0 bA | 14.1 ± 1.0 bA | 14.1 ± 1.0 bA | 14.1 ± 1.0 bA | 14.1 ± 1.0 bA |
| 6 | 60.6 ± 0.3 bA | 38.0 ± 1.9 bB | 41.3 ± 1.1 bB | 42.4 ± 1.5 bB | 40.9 ± 2.3 bB | 43.6 ± 3.8 aB | 38.7 ± 6.7 aB | 44.4 ± 3.1 aB | 37.6 ± 3.5 aB | 40.2 ± 1.8 aB | 38.4 ± 3.2 aB | |
| 12 | 87.8 ± 15.4 aA | 59.7 ± 2.8 aB | 52.1 ± 3.1 aC | 50.7 ± 3.6 aC | 49.2 ± 6.7 aC | 48.5 ± 5.6 aC | 42.1 ± 5.4 aC | 43.8 ± 3.6 aC | 44.9 ± 5.2 aC | 47.6 ± 2.5 aC | 47.2 ± 3.4 aC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pires, J.R.A.; Pereira, R.; Paz, S.; Gomes, L.A.; Souza, V.G.L.; Godinho, M.H.; Duarte, M.P.; Fernando, A.L. Bioactive Properties of Chitosan/Nanocellulose Films Loaded with Sage Essential Oil: From In Vitro Study to In Situ Application in Shelf-Life Extension of Fresh Poultry Meat. J. Compos. Sci. 2025, 9, 428. https://doi.org/10.3390/jcs9080428
Pires JRA, Pereira R, Paz S, Gomes LA, Souza VGL, Godinho MH, Duarte MP, Fernando AL. Bioactive Properties of Chitosan/Nanocellulose Films Loaded with Sage Essential Oil: From In Vitro Study to In Situ Application in Shelf-Life Extension of Fresh Poultry Meat. Journal of Composites Science. 2025; 9(8):428. https://doi.org/10.3390/jcs9080428
Chicago/Turabian StylePires, João R. A., Raquel Pereira, Sara Paz, Leandro A. Gomes, Victor G. L. Souza, Maria H. Godinho, Maria P. Duarte, and Ana L. Fernando. 2025. "Bioactive Properties of Chitosan/Nanocellulose Films Loaded with Sage Essential Oil: From In Vitro Study to In Situ Application in Shelf-Life Extension of Fresh Poultry Meat" Journal of Composites Science 9, no. 8: 428. https://doi.org/10.3390/jcs9080428
APA StylePires, J. R. A., Pereira, R., Paz, S., Gomes, L. A., Souza, V. G. L., Godinho, M. H., Duarte, M. P., & Fernando, A. L. (2025). Bioactive Properties of Chitosan/Nanocellulose Films Loaded with Sage Essential Oil: From In Vitro Study to In Situ Application in Shelf-Life Extension of Fresh Poultry Meat. Journal of Composites Science, 9(8), 428. https://doi.org/10.3390/jcs9080428









