Enhancing Polycaprolactone with Levulinic Acid-Extracted Lignin: Toward Sustainable Bio-Based Polymer Blends
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Lignin Fractionation and Extraction from Pine Wood Sawdust Residues
2.2.2. PCL–Lignin Blends
2.2.3. Water Absorption
2.2.4. Water Solubility
2.2.5. Contact Angle
2.2.6. Antioxidant Properties
2.2.7. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2.8. Mechanical Properties
2.2.9. Scanning Electron Microscopy (SEM)
2.2.10. Thermogravimetric Analysis (TGA)
2.2.11. Statistical Analysis
3. Results and Discussion
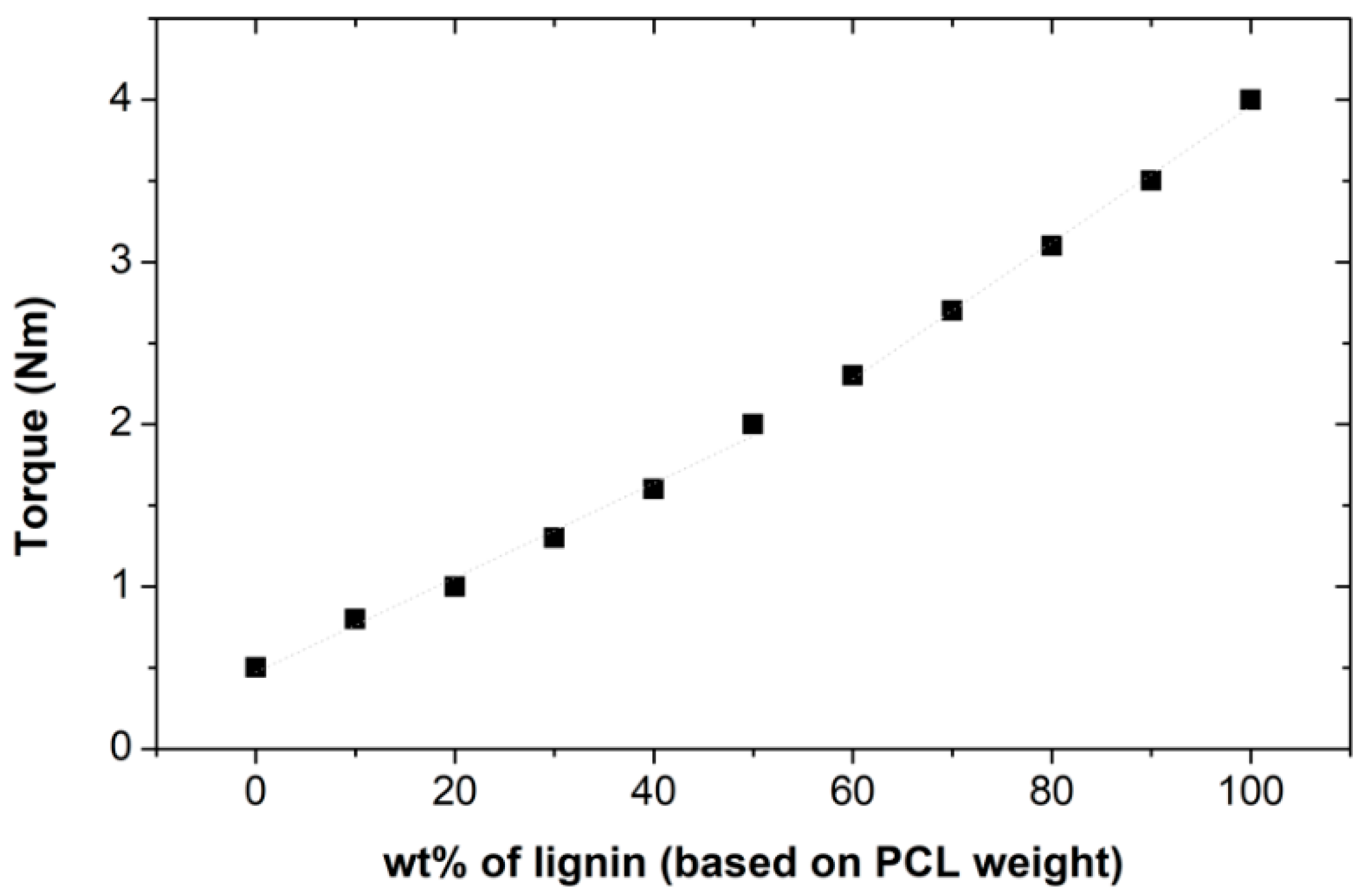
3.1. PCL–Lignin Blends: Torque Rheometry and FTIR
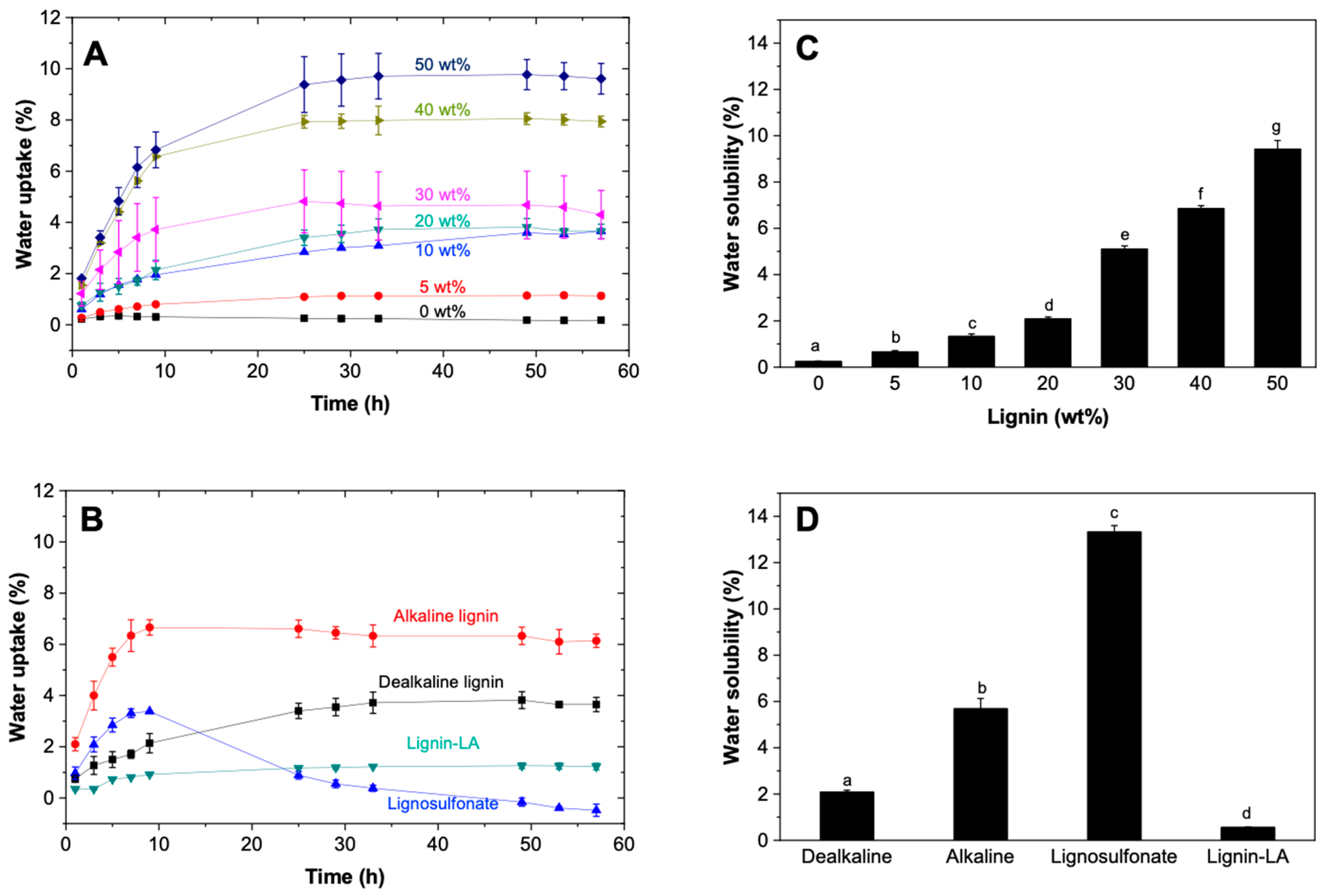
3.2. Water Uptake and Water Solubility PLC–Lignin Blends
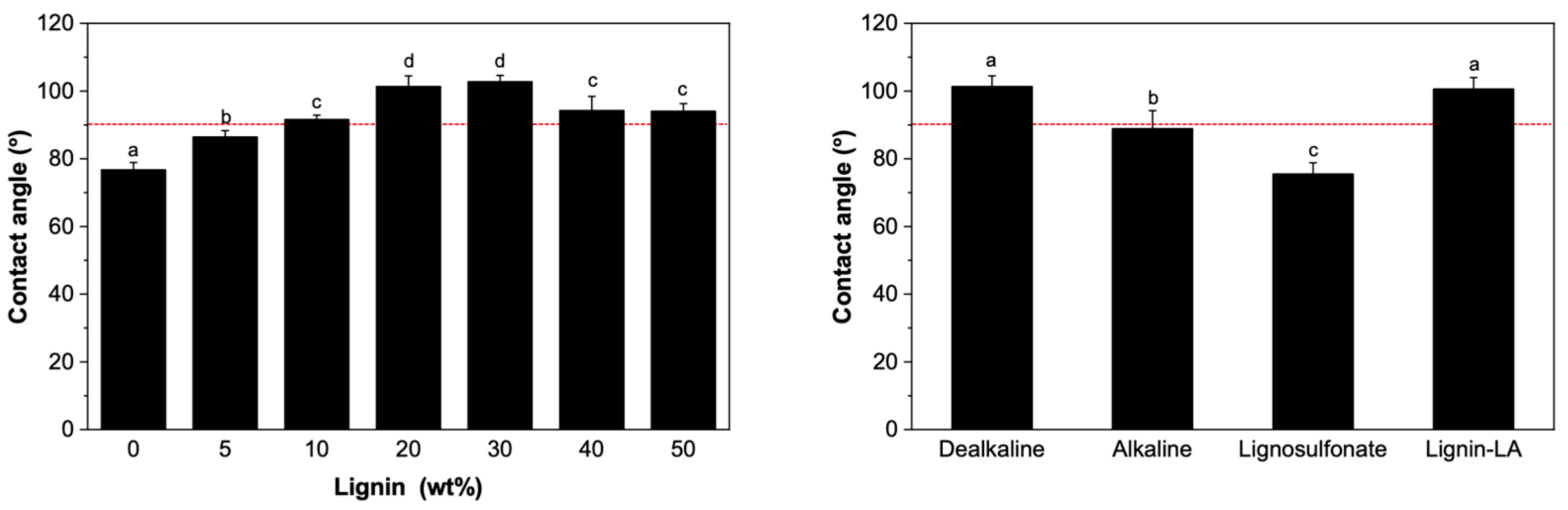
3.3. Surface Properties of PCL–Lignin Blends: Hydrophilicity and Antioxidant Capacity
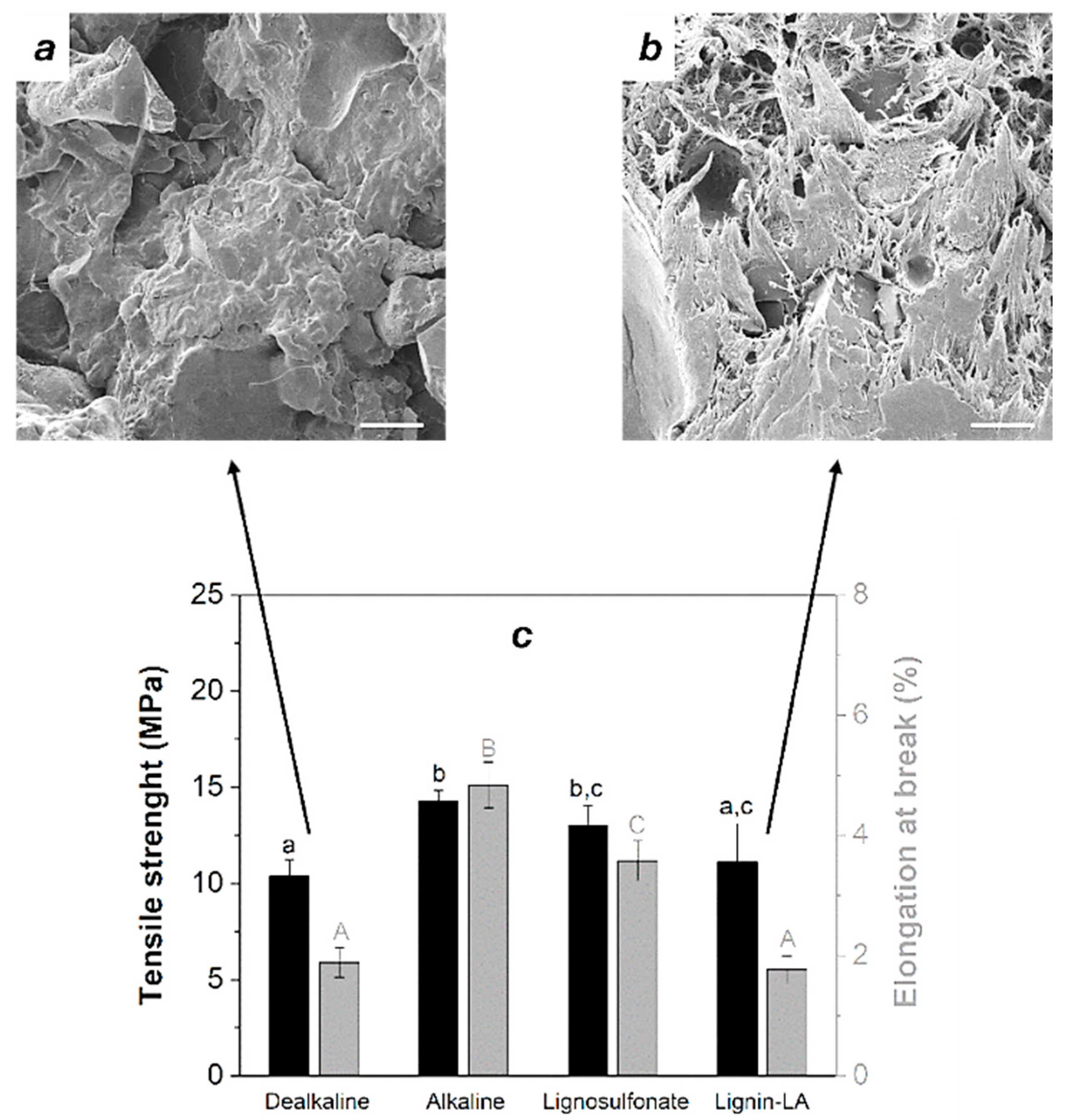
3.4. Mechanical and Morphological Features
3.5. Thermal Stability of PCL–Lignin Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Labet, M.; Thielemans, W. Synthesis of Polycaprolactone: A Review. Chem. Soc. Rev. 2009, 38, 3484–3504. [Google Scholar] [CrossRef]
- Okada, M. Chemical Syntheses of Biodegradable Polymers. Prog. Polym. Sci. 2002, 27, 87–133. [Google Scholar] [CrossRef]
- Nawaz, A.; Hasan, F.; Shah, A.A. Degradation of Poly(ɛ-Caprolactone) (PCL) by a Newly Isolated Brevundimonas Sp. Strain MRL-AN1 from Soil. FEMS Microbiol. Lett. 2015, 362, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical Properties and Cell Cultural Response of Polycaprolactone Scaffolds Designed and Fabricated via Fused Deposition Modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Joy, A.; Unnikrishnan, G.; Megha, M.; Haris, M.; Thomas, J.; Kolanthai, E.; Muthuswamy, S. Polycaprolactone/Graphene Oxide–Silver Nanocomposite: A Multifunctional Agent for Biomedical Applications. J. Inorg. Organomet. Polym. 2022, 32, 912–930. [Google Scholar] [CrossRef]
- Shi, C.; Zhou, A.; Fang, D.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Huang, C.; Li, W. Oregano Essential Oil/β-Cyclodextrin Inclusion Compound Polylactic Acid/Polycaprolactone Electrospun Nanofibers for Active Food Packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation Mechanisms of Polycaprolactone in the Context of Chemistry, Geometry and Environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Melro, E.; Filipe, A.; Sousa, D.; Medronho, B.; Romano, A. Revisiting Lignin: A Tour through Its Structural Features, Characterization Methods and Applications. New J. Chem. 2021, 45, 6986–7013. [Google Scholar] [CrossRef]
- Cao, X.; Huang, J.; He, Y.; Hu, C.; Zhang, Q.; Yin, X.; Wu, W.; Li, R.K.Y. Biodegradable and Renewable UV-Shielding Polylactide Composites Containing Hierarchical Structured POSS Functionalized Lignin. Int. J. Biol. Macromol. 2021, 188, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, J.-Y.; Youn, H.J.; Choi, J.W. Utilization of Lignin Fractions in UV Resistant Lignin-PLA Biocomposites via Lignin-Lactide Grafting. Int. J. Biol. Macromol. 2019, 138, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Kazzaz, A.E.; Feizi, Z.H.; Fatehi, P. Grafting Strategies for Hydroxy Groups of Lignin for Producing Materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef]
- Abdollahi, M.; Bairami Habashi, R.; Mohsenpour, M. Poly(ε-Caprolactone) Chains Grafted from Lignin, Hydroxymethylated Lignin and Silica/Lignin Hybrid Macroinitiators: Synthesis and Characterization of Lignin- Based Thermoplastic Copolymers. Ind. Crops Prod. 2019, 130, 547–557. [Google Scholar] [CrossRef]
- Beniwal, P.; Guliani, D.; Toor, A.P. Influence of Functionalised Lignin on Strength and Antioxidant Properties of Polylactic Acid Films. J. Polym. Res. 2024, 31, 68. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, M.; Liu, G.; Wang, J.; Chen, Y.; Zhang, Z. Alkylated Lignin with Graft Copolymerization for Enhancing Toughness of PLA. J. Mater. Sci. 2022, 57, 8687–8700. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Luo, B.; Ramakrishna, S.; Kai, D.; Loh, X.J.; Yang, I.H.; Deen, G.R.; Mo, X. Engineering PCL/Lignin Nanofibers as an Antioxidant Scaffold for the Growth of Neuron and Schwann Cell. Colloids Surf. B Biointerfaces 2018, 169, 356–365. [Google Scholar] [CrossRef]
- Parit, M.; Jiang, Z. Towards Lignin Derived Thermoplastic Polymers. Int. J. Biol. Macromol. 2020, 165, 3180–3197. [Google Scholar] [CrossRef]
- Adams, B.; Abdelwahab, M.; Misra, M.; Mohanty, A.K. Injection-Molded Bioblends from Lignin and Biodegradable Polymers: Processing and Performance Evaluation. J. Polym. Environ. 2018, 26, 2360–2373. [Google Scholar] [CrossRef]
- Teramoto, Y.; Lee, S.-H.; Endo, T. Phase Structure and Mechanical Property of Blends of Organosolv Lignin Alkyl Esters with Poly(ε-Caprolactone). Polym. J. 2009, 41, 219–227. [Google Scholar] [CrossRef]
- Teramoto, Y.; Lee, S.-H.; Endo, T.; Nishio, Y. Scale of Homogeneous Mixing in Miscible Blends of Organosolv Lignin Esters with Poly(ϵ-Caprolactone). J. Wood Chem. Technol. 2010, 30, 330–347. [Google Scholar] [CrossRef]
- Nitz, H.; Semke, H.; Landers, R.; Mülhaupt, R. Reactive Extrusion of Polycaprolactone Compounds Containing Wood Flour and Lignin. J. Appl. Polym. Sci. 2001, 81, 1972–1984. [Google Scholar] [CrossRef]
- Pucciariello, R.; D’Auria, M.; Villani, V.; Giammarino, G.; Gorrasi, G.; Shulga, G. Lignin/Poly(ε-Caprolactone) Blends with Tuneable Mechanical Properties Prepared by High Energy Ball-Milling. J. Polym. Environ. 2010, 18, 326–334. [Google Scholar] [CrossRef]
- Pucciariello, R.; Villani, V.; Bonini, C.; D’Auria, M.; Vetere, T. Physical Properties of Straw Lignin-Based Polymer Blends. Polymer 2004, 45, 4159–4169. [Google Scholar] [CrossRef]
- Melro, E.; Riddell, A.; Bernin, D.; da Costa, A.M.R.; Valente, A.J.M.; Antunes, F.E.; Romano, A.; Norgren, M.; Medronho, B. Levulinic Acid-Based “Green” Solvents for Lignocellulose Fractionation: On the Superior Extraction Yield and Selectivity toward Lignin. Biomacromolecules 2023, 24, 3094–3104. [Google Scholar] [CrossRef]
- Melro, E.; Duarte, H.; Antunes, F.E.; Valente, A.J.M.; Romano, A.; Norgren, M.; Medronho, B. Engineering Novel Phenolic Foams with Lignin Extracted from Pine Wood Residues via a New Levulinic-Acid Assisted Process. Int. J. Biol. Macromol. 2023, 248, 125947. [Google Scholar] [CrossRef]
- ASTM D570-98; Standard Test Method for Water Absorption of Plastics. ASTM International: New York, NY, USA, 1998. [CrossRef]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; reprint; Clarendon Press: Oxford, UK, 1976; ISBN 978-0-19-853344-3. [Google Scholar]
- Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. [Google Scholar] [CrossRef]
- Melro, E.; Duarte, H.; Eivazi, A.; Costa, C.; Faleiro, M.L.; da Costa, A.M.R.; Antunes, F.E.; Valente, A.J.M.; Romano, A.; Norgren, M.; et al. Poly(Butylene Succinate)-Based Composites with Technical and Extracted Lignins from Wood Residues. ACS Appl. Polym. Mater. 2024, 6, 1169–1181. [Google Scholar] [CrossRef]
- Xie, D.; Pu, Y.; Meng, X.; Bryant, N.D.; Zhang, K.; Wang, W.; Ragauskas, A.J.; Li, M. Effect of the Lignin Structure on the Physicochemical Properties of Lignin-Grafted-Poly(ε-Caprolactone) and Its Application for Water/Oil Separation. ACS Sustain. Chem. Eng. 2022, 10, 16882–16895. [Google Scholar] [CrossRef]
- Palacios Hinestroza, H.; Urena-Saborio, H.; Zurita, F.; Guerrero de León, A.A.; Sundaram, G.; Sulbarán-Rangel, B. Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water. Molecules 2020, 25, 683. [Google Scholar] [CrossRef]
- Janarthanan, G.; Kim, I.G.; Chung, E.-J.; Noh, I. Comparative Studies on Thin Polycaprolactone-Tricalcium Phosphate Composite Scaffolds and Its Interaction with Mesenchymal Stem Cells. Biomater. Res. 2019, 23, 1. [Google Scholar] [CrossRef]
- Kim, B.; Lee, J.; Jang, S.; Park, J.; Choi, J.; Lee, S.; Jung, J.; Park, J. Exploring the Effect of the Polyol Structure and the Incorporation of Lignin on the Properties of Bio-Based Polyurethane. Polymers 2025, 17, 604. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.K.; Kharaghani, D.; Sun, L.; Ullah, S.; Sarwar, M.N.; Ullah, A.; Khatri, M.; Yoshiko, Y.; Gopiraman, M.; Kim, I.S. Synthesized Bioactive Lignin Nanoparticles/Polycaprolactone Nanofibers: A Novel Nanobiocomposite for Bone Tissue Engineering. Biomater. Adv. 2023, 144, 213203. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.M.; Gupta, A.; Owen, P.; David, D.; Yim, E.; Mekonnen, T.H. Compostable Lignin Grafted Poly(ε-Caprolactone) Polyurethane Biomedical Materials: Shape Memory, Foaming Capabilities, and Biocompatibility. Chem. Eng. J. 2024, 485, 149845. [Google Scholar] [CrossRef]
- Karimi, M. Diffusion in Polymer Solids and Solutions. In Mass Transfer in Chemical Engineering Processes; IntechOpen: London, UK, 2011; ISBN 978-953-307-619-5. [Google Scholar]
- Starkova, O.; Aiello, M.A.; Aniskevich, A. Long-Term Moisture Diffusion in Vinylester Resin and CFRP Rebars: A 20-Year Case Study. Compos. Sci. Technol. 2023, 242, 110167. [Google Scholar] [CrossRef]
- Hassanpour, B.; Karbhari, V.M. Characteristics and Models of Moisture Uptake in Fiber-Reinforced Composites: A Topical Review. Polymers 2024, 16, 2265. [Google Scholar] [CrossRef]
- Starkova, O.; Chandrasekaran, S.; Schnoor, T.; Sevcenko, J.; Schulte, K. Anomalous Water Diffusion in Epoxy/Carbon Nanoparticle Composites. Polym. Degrad. Stab. 2019, 164, 127–135. [Google Scholar] [CrossRef]
- Sriamornsak, P.; Sungthongjeeh, S. Modification of Theophylline Release with Alginate Gel Formed in Hard Capsules. AAPS PharmSciTech 2007, 8, 51. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.S.; Aguado, R.; Carta, A.M.M.S.; Bértolo, R.; Murtinho, D.; Valente, A.J.M. Influence of DNA as Additive for Market Pulp on Tissue Paper. Nord. Pulp Pap. Res. J. 2022, 37, 489–496. [Google Scholar] [CrossRef]
- Quintana, J.R.; Valderruten, N.E.; Katime, I. Synthesis and Swelling Kinetics of Poly(Dimethylaminoethyl Acrylate Methyl Chloride Quaternary-Co-Itaconic Acid) Hydrogels. Langmuir 1999, 15, 4728–4730. [Google Scholar] [CrossRef]
- Cova, F.; Murtinho, D.; Pais, A.A.C.C.; Valente, A.J.M.; Utzeri, G. Insights on Macro- and Microscopic Interactions between Confidor and Cyclodextrin-Based Nanosponges. Chem. Eng. J. 2023, 455, 140882. [Google Scholar]
- Dutta, K.; Saikia, A.; Singh, A. Transforming Lignin into Polymer Film with Improved Physiochemical Properties by Modification with Itaconic Acid and Grafting with Polycaprolactone. Int. J. Biol. Macromol. 2025, 305, 141226. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zong, E.; Jiang, J.; Fu, S.; Wang, J.; Xu, B.; Li, W.; Lin, X.; Xu, Y.; Wang, C.; et al. Preparation and Characterization of Lignin-Graft-Poly (ɛ-Caprolactone) Copolymers Based on Lignocellulosic Butanol Residue. Int. J. Biol. Macromol. 2015, 81, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Guiao, K.S.; Tzoganakis, C.; Mekonnen, T.H. Green Mechano-Chemical Processing of Lignocellulosic Biomass for Lignin Recovery. Chemosphere 2022, 293, 133647. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Gu, X.; Shi, Y. A Review on Lignin Antioxidants: Their Sources, Isolations, Antioxidant Activities and Various Applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef]
- Guo, D.; Wu, S.; Lyu, G.; Guo, H. Effect of Molecular Weight on the Pyrolysis Characteristics of Alkali Lignin. Fuel 2017, 193, 45–53. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Jiang, B.; Jiao, H.; Guo, X.; Chen, G.; Guo, J.; Wu, W.; Jin, Y.; Cao, G.; Liang, Z. Lignin-Based Materials for Additive Manufacturing: Chemistry, Processing, Structures, Properties, and Applications. Adv. Sci. 2023, 10, 2206055. [Google Scholar] [CrossRef]
- Tian, J.; Yang, Y.; Song, J. Grafting Polycaprolactone onto Alkaline Lignin for Improved Compatibility and Processability. Int. J. Biol. Macromol. 2019, 141, 919–926. [Google Scholar] [CrossRef]
- Taher, M.A.; Wang, X.; Faridul Hasan, K.M.; Miah, M.R.; Zhu, J.; Chen, J. Lignin Modification for Enhanced Performance of Polymer Composites. ACS Appl. Bio. Mater. 2023, 6, 5169–5192. [Google Scholar] [CrossRef]
- Huang, X.; Yin, H.; Zhang, H.; Mei, N.; Mu, L. Pyrolysis Characteristics, Gas Products, Volatiles, and Thermo–Kinetics of Industrial Lignin via TG/DTG–FTIR/MS and in–Situ Py–PI–TOF/MS. Energy 2022, 259, 125062. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O.S. Chemical and Thermal Properties of Fractionated Bagasse Soda Lignin. Ind. Crops Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef]
- Hatakeyama, T.; Yamashita, S.; Hatakeyama, H. Thermal Properties of Lignin-Based Polycaprolactones. J. Therm. Anal. Calorim. 2021, 143, 203–211. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Synthesis, Thermal Properties, Rheological and Mechanical Behaviors of Lignins-Grafted-Poly(ε-Caprolactone). Polymer 2013, 54, 3882–3890. [Google Scholar] [CrossRef]




| Type of Lignin | Lignin (wt%) | k/(s−n) | n (±s) | MST/(s) |
|---|---|---|---|---|
| Dealkaline | 0 | - | - | - |
| 5 | 4.99 × 10−3 | 0.49 (±0.02) | 16,200 | |
| 20 | 1.80 × 10−2 | 0.46 (±0.03) | 2130 | |
| 30 | 1.64 × 10−2 | 0.53 (±0.01) | 859 | |
| 40 | 7.56 × 10−3 | 0.65 (±0.01) | 719 | |
| 50 | 1.06 × 10−2 | 0.63 (±0.01) | 554 | |
| Alkaline | 20 | 1.71 × 10−2 | n.d. * | n.d. * |
| Lignin-LA | 20 | 8.48 × 10−3 | 0.46 (±0.01) | 11,200 |
| Type of Lignin | MW (Da) |
|---|---|
| Dealkaline | 10,263 |
| Alkali | 6024 |
| Lignosulfonate | 4881 |
| Lignin-LA | 3697 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melro, E.; Duarte, H.; Antunes, F.E.; Valente, A.J.M.; Romano, A.; Medronho, B. Enhancing Polycaprolactone with Levulinic Acid-Extracted Lignin: Toward Sustainable Bio-Based Polymer Blends. J. Compos. Sci. 2025, 9, 366. https://doi.org/10.3390/jcs9070366
Melro E, Duarte H, Antunes FE, Valente AJM, Romano A, Medronho B. Enhancing Polycaprolactone with Levulinic Acid-Extracted Lignin: Toward Sustainable Bio-Based Polymer Blends. Journal of Composites Science. 2025; 9(7):366. https://doi.org/10.3390/jcs9070366
Chicago/Turabian StyleMelro, Elodie, Hugo Duarte, Filipe E. Antunes, Artur J. M. Valente, Anabela Romano, and Bruno Medronho. 2025. "Enhancing Polycaprolactone with Levulinic Acid-Extracted Lignin: Toward Sustainable Bio-Based Polymer Blends" Journal of Composites Science 9, no. 7: 366. https://doi.org/10.3390/jcs9070366
APA StyleMelro, E., Duarte, H., Antunes, F. E., Valente, A. J. M., Romano, A., & Medronho, B. (2025). Enhancing Polycaprolactone with Levulinic Acid-Extracted Lignin: Toward Sustainable Bio-Based Polymer Blends. Journal of Composites Science, 9(7), 366. https://doi.org/10.3390/jcs9070366








