Abstract
Bio-nanocomposite films were prepared using chitosan, gelatin, and varying concentrations (0, 0.5, 1.0, 2.0, and 5.0 wt%) of titanium dioxide (TiO2) nanoparticles in acetic acid via a casting method. The incorporation of TiO2 nanoparticles into the bio-chitosan matrix enhanced ultraviolet (UV) absorption and improved the films’ physical, mechanical, and electrical properties. Additionally, the TiO2-loaded films exhibited antimicrobial activity, contributing to the extended preservation of packaged products by inhibiting microbial growth. Notably, the bio-nanocomposite films containing 1.0 wt% TiO2 exhibited an electroactive response, bending under relatively low electric field strength (250 V/mm), whereas the control film without TiO2 required higher field strength (550 V/mm) to achieve bending. This indicates potential applications in electroactive actuators requiring precise movement control. Among the tested concentrations, films containing 0.5 wt% and 1.0 wt% TiO2 (Formulas 7 and 8) demonstrated optimal performance. These films presented a visually appealing appearance with no tear marks, low bulk density (0.91 ± 0.04 and 0.85 ± 0.18 g/cm3), a satisfactory electromechanical response at 250 V/m (17.85 ± 2.58 and 61.48 ± 6.97), low shrinkage percentages (59.95 ± 3.59 and 54.17 ± 9.28), high dielectric constant (1.80 ± 0.07 and 8.10 ± 0.73), and superior UV absorption compared with pure bio-chitosan films, without and with gelatin (Formulas 1 and 6).
1. Introduction
Chitosan is a biodegradable, natural polymer composed of N-acetyl-2-amino-2-deoxy-D-glucopyranose (acetylated unit) and 2-amino-2-deoxy-D-glucopyranose (deacetylated unit), with repeating units linked via β-(1→4)-glycosidic bonds [1,2]. It shares structural similarities with hyaluronic acid and glycosaminoglycans (GAGs), components of the extracellular matrix (ECM) in cartilage [3]. Chitosan exhibits a linear polymeric structure and contains amino groups in its polysaccharide backbone. It is derived from the deacetylation of chitin, the second most abundant natural polymer after cellulose [4,5]. Due to the presence of free amino groups, chitosan carries a positive charge under acidic conditions (pH 2–6), functioning as a polyelectrolyte [6,7]. The molecular structure of chitosan features functional groups such as amino (–NH2), hydroxyl (–OH), and acetyl (–COCH3). According to Kumar et al. [7], the amino groups become protonated at pH 6 or below, forming cationic -NH3+ groups. This protonation enhances intermolecular electrostatic repulsion, increasing solubility and conferring polycationic behavior. Several mechanisms have been proposed to explain chitosan’s antimicrobial activity, with the most widely accepted including the following:
- Electrostatic interaction with microbial membranes: Positively charged amino groups interact with negatively charged microbial cell membranes, disrupting membrane integrity and causing the leakage of intracellular contents, ultimately leading to cell death [8].
- Chelation of metal ions: Chitosan’s chelating properties enable it to bind selectively to metal ions, interfering with microbial enzyme activity and inhibiting metabolic processes.
- Influence of molecular weight: The antimicrobial effectiveness of chitosan is influenced by its molecular weight. High-molecular-weight chitosan forms impermeable layers on cell surfaces, impeding nutrient uptake, while low-molecular-weight chitosan penetrates the cytoplasm, binds to DNA, and disrupts mRNA and protein synthesis [8,9].
Alderman et al. [10] describe chitosan as a linear, semicrystalline polysaccharide with improved solubility relative to chitin due to its deacetylated amino groups. The degree of deacetylation (DD), representing the proportion of free amino groups, is a key metric distinguishing chitosan from chitin [8]. Although chitosan is valued for its non-toxicity, biocompatibility, and biodegradability, its high solution viscosity can hinder electrospinning. Effective fiber formation requires control over polymer chain interactions—such as entanglement, hydrogen bonding, and hydrophobic effects—to achieve nanoscale fiber diameters. Chitosan’s hydrophilic nature promotes cellular interaction, and it exhibits diverse biological activities, including antiviral, antioxidant, antibacterial, and anticancer effects [8,9,10,11,12,13]. These properties underpin its use in food packaging, cosmetics, agriculture, tissue engineering, wound dressings, facial masks, and drug delivery systems.
Titanium dioxide (TiO2), particularly in its anatase crystalline form, is widely utilized due to its superior photocatalytic activity compared with its rutile and brookite counterparts [10,11,12,13,14,15]. The anatase phase features a body-centered tetragonal crystal structure, while rutile has a primitive tetragonal structure and brookite an orthorhombic one [16]. As a metal oxide, TiO2 exhibits remarkable physicochemical and optical properties, making it suitable for a range of industrial applications [17,18,19,20,21,22].
Despite chitosan’s structural similarity to cellulose―both contain hydroxyl (–OH) and amino (–NH2) groups―it exhibits unique characteristics, such as polyelectrolyte behavior, distinctive optical and physicochemical properties, nanocrystallinity, and specific degradation pathways [22]. The incorporation of TiO2 nanoparticles into bio-chitosan nanocrystal films enhances crystallinity, thereby improving mechanical, thermal, optical, and morphological properties. Additionally, TiO2 contributes to better gas and solvent barrier performance, increased biocompatibility, photocatalytic activity, and UV light absorption. Its non-toxic, eco-friendly, and biologically inert nature makes it suitable for a wide range of applications, including electronics, cosmetics, wound dressings, food preservation, agricultural, healthcare, environmental remediation, and smart packaging [23,24,25,26,27]. The combination of these functional properties makes TiO2 an exceptionally versatile material across industries such as cosmetics, healthcare, electronics, environmental remediation, and antimicrobial applications [28,29,30,31,32]. Assessing cytotoxicity and genotoxicity is essential to ensuring its safety in biomedical and food-related applications [33,34,35,36,37,38]. Numerous studies have investigated TiO2 cytotoxicity in various cell lines. For example, Gea et al. [38] reported low cytotoxicity in a non-tumorigenic lung epithelial cell line (BEAS-2B) using the WST-1 assay, indicating minimal adverse effects on cell viability. Similarly, Proquin et al. [39] observed no cytotoxic effects on colorectal cancer cell lines exposed to TiO2 nanoparticles at concentrations of up to 100 μg/cm2. Owing to its favorable properties—such as biocompatibility, antimicrobial activity, and photocatalytic behavior—TiO2 has been widely employed as an additive in biopolymer-based films. It has been successfully incorporated into various biopolymer matrices, including chitosan [17], starch [40], hydroxypropyl methylcellulose [41], gelatin [42], and whey protein [43], resulting in composite films with enhanced functionality. When incorporated into chitosan nanocrystal films, TiO2 enhances physical strength and gas barrier properties. In smart packaging applications, it not only extends the shelf life of products but also serves as a biosensor for monitoring the freshness of harvested fruits [44,45].
Gelatin, a biopolymer that functions as both a gelling and crosslinking agent, is commonly extracted through the partial or complete hydrolysis of collagen. It is a cost-effective and widely available material, typically derived from various animal by-products, such as pig skin, fish skin, pig and cattle bones, and bovine hides [46]. Owing to its favorable mechanical, thermal, and barrier properties, as well as its biocompatibility and non-toxicity, gelatin is well-suited for use in film packaging across the food, agricultural, and medical industries [47,48]. Despite its water-binding capacity, good film- and foam-forming abilities through the casting process, and emulsifying properties, the application of gelatin is limited by its sensitivity to moisture, rigidity, brittleness, and propensity to swell and disintegrate. To overcome these limitations, gelatin is frequently blended with other biopolymers to enhance the elasticity, flexibility, and mechanical properties of biofilms [49].
Bio-nanocomposite films combining chitosan, gelatin, and TiO2 nanoparticles can be fabricated through methods such as casting, coating, electrospinning, and encapsulation [45,46,50,51,52]. In the casting method, chitosan and gelatin are dissolved in an appropriate solvent, and TiO2 nanoparticles are dispersed into this solution to create a film-forming colloid. This mixture is poured into molds and subjected to sol–gel reactions—specifically hydrolysis and condensation—leading to semi-rigid-film formation. The casting process offers simplicity and control over film thickness and composition through adjustments in biopolymer and nanoparticle concentrations. However, alternative methods like electrospinning and coating may offer advantages depending on specific application goals and desired film properties.
This study aims to develop bio-nanocomposite films by incorporating TiO2 nanoparticles into chitosan dissolved in acetic acid, with gelatin as a supplementary component. The films were fabricated using a combination of casting and sol–gel techniques. Their physical, mechanical, and electrical properties were systematically compared with those of pure chitosan films. Characterization techniques included X-ray diffraction (XRD), contact angle measurements, tensile strength testing, and impedance spectroscopy. The results are presented and discussed in detail in this report.
2. Experimental Methods
2.1. Materials
Chitosan powder, also known as poly(β-(1,4)-D-glucosamine), with a purity greater than 98%, was obtained from Sandee Co. Ltd., Bangkok, Thailand. This chitosan, produced via the deacetylation of chitin, appears as a creamy white, odorless powder composed of fine, agglomerated particles approximately 0.5 mm in size. Food-grade gelatin, used as an additive and gelling agent, was purchased from Imperial Co. Ltd., Bangkok, Thailand. It appears as a fine powder with a particle size of 30 μm, exhibiting a white-yellow color and a characteristic fishy odor. Purified titanium dioxide nanoparticles (100% TiO2) were supplied by Ajax Finechem Co. Ltd., Nonthaburi, Thailand. These particles are white, odorless, and less than 10 nm in diameter. Acetic acid, with a purity of 99.85% and of food-grade quality, was sourced from Nippon Co. Ltd., Bangkok, Thailand.
2.2. Instruments
An X-ray diffractometer (XRD; Philips, Eindhoven, The Netherlands, X’Pert) equipped with a VANTEC-1 detector and double-crystal wide-angle goniometer was employed to analyze the crystalline structure and phase composition of raw materials (chitosan, gelatin, and titanium dioxide powders) and bio-chitosan composite films. Data were collected at a scan speed of 2° 2θ/min with increments of either 0.05° or 0.03° across a scanning range of 0° to 80°. CuKα radiation (λ = 0.15406 nm) served as the X-ray source. The resulting diffraction patterns were compared with standard data from the International Center for Diffraction Data (Joint Committee on Powder Diffraction Standards, JCPDS) to identify crystalline phases in both powder and bio-nanocomposite films.
Fourier-transform infrared (FTIR) spectroscopy (PerkinElmer, Waltham, USA; Bruker Alpha E, Billerica, USA) was used to identify chemical functional groups in chitosan and gelatin powders, as well as in the bio-nanocomposite films. FTIR spectra were recorded in the range of 500–4000 cm−1 using Attenuated Total Reflectance (ATR) mode. A bio-chitosan film without gelatin or TiO2 served as the reference for comparison.
A UV–visible spectrophotometer (SHIMADZU, Kyoto, Japan; UV-1700 PharmaSpec) was used to assess the UV transmission characteristics of the bio-chitosan nanocomposite films. Measurements were taken across the 200–1100 nm wavelength range to determine both absorbance and transmittance.
A Universal Testing Machine (UTM; HOUNSFIELD, Brighton, UK; H50KS) was employed to evaluate the mechanical properties of bio-chitosan nanocomposite films, both with and without the addition of titanium dioxide nanoparticles. The properties assessed included stress, strain, Young’s modulus, and tensile strength. The machine applied a maximum load of 900 N at a constant crosshead speed of 50 mm/min, with a gauge length set to 50 mm. Each sample formulation was tested in triplicate, and the results are reported as mean values with standard deviations.
Crosslink density was measured by immersing film samples in toluene at room temperature, following ASTM D-6814-02. Square specimens (1 cm × 3–5 mm) were weighed before and after 72 h of swelling. Crosslink density was calculated using the Flory–Rehner equation. Three replicates were analyzed for each formulation.
An Impedance Analyzer (KEYSIGHT, Santa Rosa, USA; E4980AL) or LCR meter was used to measure the electrical properties of the materials, including capacitance, electrical conductivity, dielectric constant, dielectric loss, and electromechanical response. Measurements were conducted over a frequency range of 500–20,000 Hz. Each formulation was tested in triplicate, and the results are reported as mean values with standard deviations.
A Contact Angle Analyzer (KYOWA, Saitama, Japan; DM-CE 1) was used to evaluate the surface wettability of the samples. The contact angle between a water droplet and the sample surface was measured within 2000 ms, providing insights into surface hydrophobicity or hydrophilicity. Each test was performed in triplicate, and the results are reported as mean values with standard deviations.
A Scanning Electron Microscope (SEM; HITACHI, Tokyo, Japan; SU3500) was employed to observe and characterize the micro- and nanostructures of bio-chitosan fiber and membrane samples. Prior to imaging, the samples were coated with a thin layer of gold by using a sputter coater (EDWARDS, UK; Pirani 501) to enhance electrical conductivity. SEM images were acquired at an accelerating voltage of 10 kV and magnifications of 15,000× and 50,000×.
Deflection measurements were conducted to investigate the electromechanical response of chitosan composite films under an applied electric field. Deflections were recorded using photographic and video analyses with a digital image analyzer (Panasonic, Osaka, Japan; M3000) at approximately 25 °C. The films were vertically mounted between parallel copper electrodes (40 mm × 30 mm × 1 mm) and immersed in a silicone oil bath, as illustrated in Scheme 1 and Scheme 2. A high DC voltage was applied in non-contact mode, maintaining an electrode gap of 30 mm. Key parameters included the displacement of the film’s free end (denoted by d), the film length (l), and the bending angle (θ), which was calculated using the equation θ = arctan(d/l) across electric field strengths ranging from 0 to 600 V/mm. All measurements were performed at room temperature (25 °C).
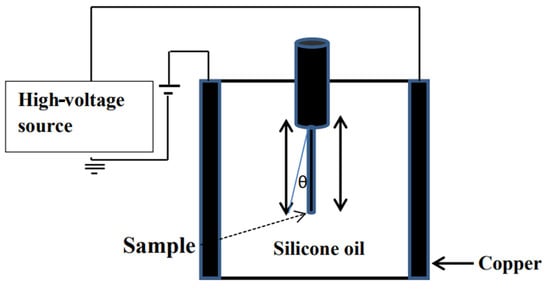
Scheme 1.
Deflection measurement setup.
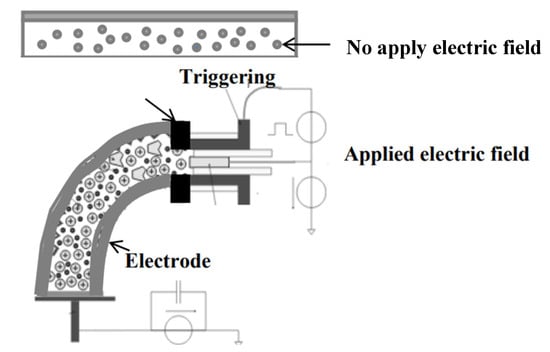
Scheme 2.
A schematic representation of charge polarization in titanium dioxide (TiO2) within bio-chitosan composite films, illustrating their function as electroactive actuators or compliant electrodes before and after the application of an electric field.
2.3. Preparation of Bio-Chitosan Composite Films with and Without Gelatin Powder and Titanium Dioxide (TiO2) Filler for Hydrophobic, Hydrophilic, and Photoelectrical Conductivity Applications
Ten different formulations of bio-chitosan composite films were prepared, as summarized in Table 1. Formulas 1 and 6 served as reference films—with and without gelatin powder, respectively—for comparison with the other formulations. Chitosan films were prepared by dissolving chitosan powder in an acetic acid solution and casting the resulting mixture by using a sol–gel process, which involves the transition from sol to gel within a silicone mold, as illustrated in Scheme 3. The key chemical processes in sol–gel formation are hydrolysis and condensation, which are influenced by the composition of raw materials, aging time and temperature, pH, and the presence of a catalyst. Formulas 2, 3, 4, and 5 consisted of chitosan composite films containing 0.5, 1.0, 2.0, and 5.0 wt% titanium dioxide (TiO2) nanoparticles, respectively, without the inclusion of gelatin (Formulas 1–5). Formula 6, which contained chitosan and gelatin but no TiO2, served as the reference for Formulas 7–10, which combined gelatin with increasing concentrations of titanium dioxide (0.5, 1.0, 2.0, and 5.0 wt%, respectively). The objective of using Formula 6 as a reference was to assess the influence of titanium dioxide nanopowder on the properties of the chitosan–gelatin composite films (Formulas 7–10). Notably, attempts to fabricate composite films without gelatin but with higher TiO2 concentrations (2.0 and 5.0 wt%) were unsuccessful due to phase separation, attributed to excessive TiO2 filler content. Although Formulas 9 and 10 (which included both gelatin and higher concentrations of TiO2) yielded films with acceptable overall appearance, minor surface defects such as tear marks were observed as a result of the excess filler.

Table 1.
Formulations of bio-nanocomposite films.
Scheme 3.
Schematic illustration of preparation process of bio-chitosan–gelatin–TiO2 nanocomposite films.
2.4. Measurement of Crosslink Density in Bio-Chitosan Composite Films with Titanium Dioxide Filler for Hydrophobic, Hydrophilic, and Photoelectrical Conductivity Applications
To determine the crosslink density of the bio-chitosan composite films, the toluene swelling method was employed in conjunction with the Flory–Rehner equation. It should be noted that the Flory–Rehner equation assumes ideal behavior and therefore provides only an approximate estimation of crosslink density. For accurate and standardized measurements, adherence to the guidelines outlined in ASTM D-6814-02 is recommended. Bio-chitosan composite film specimens were cut into small pieces measuring 1 × 1 cm, with thicknesses ranging from 0.03 to 0.20 cm. Each specimen was individually weighed using an analytical balance prior to swelling. The pre-weighed specimens were then placed in appropriate containers filled with toluene and allowed to swell at room temperature for 72 h to achieve equilibrium. The crosslink density was subsequently calculated using Flory–Rehner Equations (1) and (2):
where Ve is the effective number of polymer chains in the network per unit volume (mol/cm3), Vr is the polymer volume fraction in the swollen network at equilibrium, χ1 is the Flory–Huggins interaction parameter for toluene in chitosan (17.92 at 25 °C), and V1 is the molar volume of the solvent (toluene), approximately 106.3 cm3/mol.
3. Results and Discussion
3.1. Characteristics and Physical Properties of Chitosan Powder Embedded with Titanium Dioxide for Preparation of Bio-Chitosan Nanocomposite Films
Biocomposite films composed of chitosan—with and without the addition of titanium dioxide (TiO2) nanopowder at concentrations of 0, 0.5, 1.0, 2.0, and 5.0 wt%—and gelatin powder as a binder exhibited varying physical appearances, as shown in Figure 1 and summarized in Table 1. Formula 1, a pure chitosan biofilm without gelatin, served as the reference sample and was characterized by a transparent and aesthetically pleasing appearance. Similarly, Formula 6, which incorporated gelatin as a binder and crosslinking agent, also yielded a clear and visually appealing film.
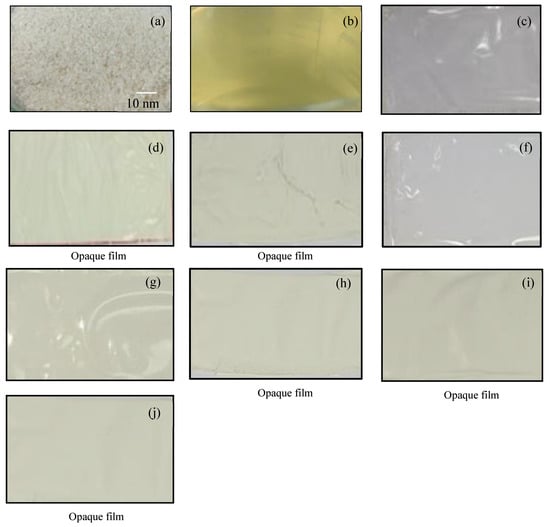
Figure 1.
Visual appearance of chitosan powder and bio-chitosan composite films with and without the addition of TiO2 powder: (a) chitosan powder; (b) chitosan solution used for sol–gel film casting; (c) Formula 1 (bio-chitosan film without additives); (d) Formula 2 (bio-chitosan film with 0.5 wt% TiO2); (e) Formula 3 (bio-chitosan film with 1.0 wt% TiO2); (f) Formula 6 (bio-chitosan film with gelatin powder and 0 wt% TiO2); (g) Formula 7 (with gelatin powder and 0.5 wt% TiO2); (h) Formula 8 (with gelatin powder and 1.0 wt% TiO2); (i) Formula 9 (with gelatin powder and 2.0 wt% TiO2); and (j) Formula 10 (with gelatin powder and 5.0 wt% TiO2). Note: Formulas 4 and 5 did not form biofilms due to excessive brittleness.
While both Formulas 1 and 6 demonstrated transparency and good film quality, Formulas 2, 3, 4, and 5 (chitosan films with increasing TiO2 content but no gelatin), as well as Formulas 7, 8, 9, and 10 (films containing both TiO2 and gelatin), resulted in opaque films. This is evident in Figure 1d–j. Notably, Formulas 4, 5, 9, and 10—containing higher TiO2 concentrations (2.0 and 5.0 wt%)—exhibited film formation issues, including brittleness, cracking, and phase segregation, which makes them unsuitable for use as biofilms.
All bio-chitosan composite films were characterized for their physical properties, including water and ethanol swelling behavior, percentage shrinkage, bulk density, and visual appearance, as presented in Table 2. Films derived from Formulas 1 to 6 showed good swelling in water, indicating effective water absorption. Kumar et al. [51] reported a swelling index of 104% for chitosan in water, supporting these findings. In contrast, Formulas 7 and 8 displayed moderate swelling, likely due to the presence of TiO2, which is water-insoluble. TiO2 is a white nanopowder with a refractive index of 2.49 and typically adopts an anatase crystal structure (tetragonal phase), properties that are relevant to its interaction within the bio-chitosan matrix. None of the films exhibited swelling in ethanol, indicating stability and resistance to disintegration in this solvent. The incorporation of TiO2 reduced the percentage of shrinkage in the films, suggesting enhanced dimensional stability. However, at higher concentrations (2.0 and 5.0 wt%), slight tear marks appeared, implying that excessive TiO2 compromised film integrity and led to surface defects. The shrinkage percentage was inversely proportional to the amount of TiO2 added, consistent with the mixture rule and supported by SEM observations shown in Figure 2. The reference films (Formulas 1 and 6) remained clear and transparent, whereas formulations with TiO2 (Formulas 2, 3, 7, 8, 9, and 10) displayed visible particle distribution within the matrix. SEM analysis revealed an increasing degree of TiO2 dispersion as the TiO2 content increased. However, excessive TiO2 led to poor dispersion, brittleness, and surface cracking, particularly in Formulas 4 and 5. In contrast, Formulas 7 and 8 exhibited smooth, intact surfaces, while Formulas 9 and 10 showed minor surface cracks, fractures, and slight porosity. Biocomposite films from Formulas 9 and 10 exhibited small cracks, surface fractures, and slight porosity. SEM images at magnifications of 15,000× and 50,000× revealed the adhesion and distribution of TiO2 nanoparticles within the bio-chitosan matrix, consistent with the SEM findings reported by Halawa et al. [53].

Table 2.
Characteristics and physical properties of bio-chitosan composite films, presented as mean values ± standard deviations (n = 3).
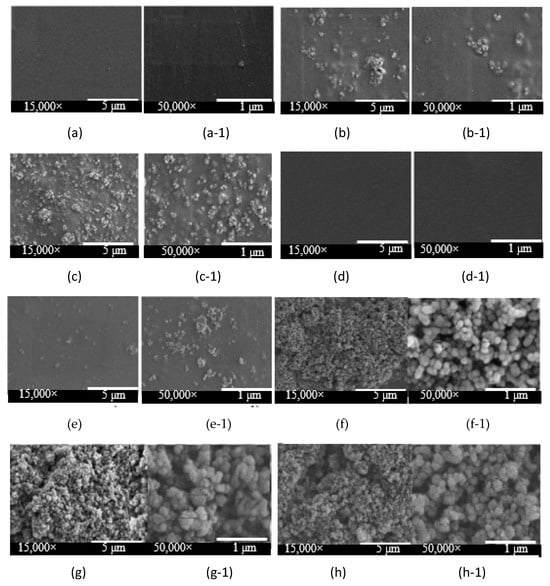
Figure 2.
SEM micrographs of bio-chitosan composite films at magnifications of 15,000× and 50,000× for the following formulations: (a,a-1) Formula 1; (b,b-1) Formula 2; (c,c-1) Formula 3; (d,d-1) Formula 6; (e,e-1) Formula 7; (f,f-1) Formula 8; (g,g-1) Formula 9; and (h,h-1) Formula 10. Note: Formulas 4 and 5 were unable to form biofilms due to excessive brittleness.
The FTIR spectra of chitosan, gelatin, and bio-chitosan composite films were recorded in the wavenumber range of 500–4000 cm−1 (Figure 3). The FTIR spectrum of raw chitosan exhibited characteristic peaks corresponding to various chemical functional groups, including O–H (hydroxyl), C–H (alkyl), N-H (amine), and C–O–C (ether linkages), observed at approximately 3250, 2857–2922, 1585–1647, and 1063 cm−1, respectively. Gelatin, acting as a binder and crosslinking agent, showed peaks associated with O–H, C–H, N–H, C–O, and C–N functional groups at 3279, 2854–2922, 1524–1629, 1403, and 1231 cm−1, respectively. The FTIR spectrum of the bio-chitosan film without gelatin and TiO2 (Formula 1) displayed functional groups similar to those of pure chitosan, including O–H, –CH2, –CH3, N–H bending, and C–O–C, with characteristic peaks at 3183, 2876, 2920, 1537–1633, 1403, 1016, and 647 cm−1, respectively. These findings are consistent with previous studies by Knidri et al. [54], Laaraibi et al. [55], and Teli et al. [56]. In Formula 6, the bio-chitosan film exhibited an additional peak around 1628 cm−1, attributed to the amide I band. Peaks near 1528 cm−1 corresponded to the N-H bending of the amide II bands, while signals at 1402 and 1240 cm−1 were related to the C–H bending vibrations of –CH2 groups. The amide III band, representing contributions from C–O–C, C–N, and N-H bonds, appeared between 1021 and 1068 cm−1, indicating peptide bond interactions between chitosan and gelatin [57,58,59]. The FTIR spectra of bio-chitosan films containing TiO2 (Formulas 2, 3, 4, 5, 7, 8, 9, and 10), both with and without gelatin, exhibited small peaks corresponding to Ti-O–Ti stretching vibrations. These peaks were observed at lower wavenumbers, around 504–523 cm−1 and 680 cm−1, consistent with previously reported FTIR data by Halawa et al. [53] and Spoială et al. [60]. Upon exposure to light and UV radiation, TiO2 nanoparticles embedded in the chitosan matrix undergo structural changes similar to those observed under photocatalytic conditions. These changes promote charge separation between O2−1 and Ti4+, leading to the formation of reactive species such as •O–Ti3+ [60,61]. The generated oxygen radicals attack chemical bonds including C–H, C–C, C–O, C–N, and N–H in the chitosan structure, resulting in the regeneration and activation of new functional groups. These include Ti–O–CH3, Ti–O–C, Ti–O=CH2, Ti–O–N, and Ti3+–C bonds, formed through photoexcitation processes at the film surface. The electrons are transferred from the Ti sites through the conduction band of TiO2, reaching an intermediate energy state, as supported by FTIR evidence. The efficiency of TiO2 interaction with chitosan is influenced by particle size, morphology, and the availability of active Ti sites. The proposed reaction mechanism underlying the formation of CH3–O–Ti, C–O–Ti, CH2=O–Ti, and N–O–Ti bonds involves both photolumination and water oxidation processes. These results suggest that the incorporation of gelatin contributes to the formation of a homogeneous and well-integrated biofilm, as indicated by the shared FTIR absorption bands in chitosan and gelatin. Furthermore, the presence of TiO2 in the composite films introduces distinct peaks related to Ti–O–Ti and other Ti–O bonding configurations.
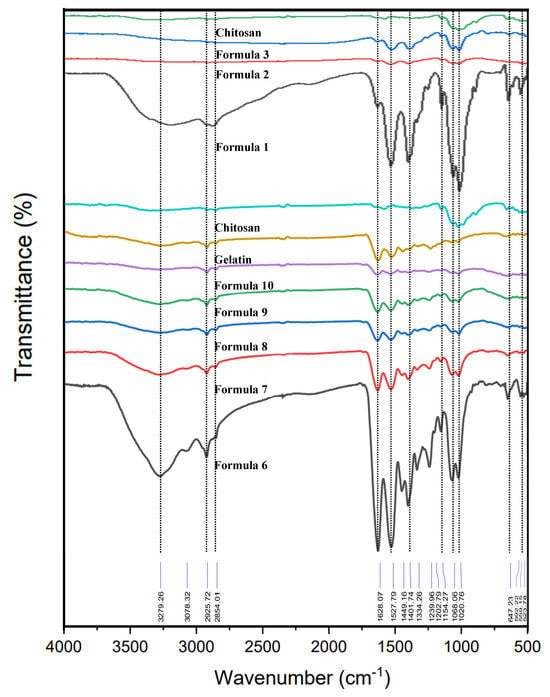
Figure 3.
FTIR spectra of raw materials (chitosan and gelatin powders) and bio-chitosan composite films, recorded over the wavenumber range of 500–4000 cm−1. Note: Formulas 4 and 5 were unable to form biofilms due to excessive brittleness.
The XRD patterns of the raw materials (chitosan, gelatin, and TiO2) and bio-chitosan composite films—with and without gelatin and TiO2—are presented in Figure 4. The bio-chitosan film exhibited a semicrystalline structure, with two characteristic crystalline peaks at 2θ angles of approximately 9.5° and 20°, consistent with the results reported by Knidri et al. [54], Küçükgülmez et al. [62], and Naghibzadeh et al. [63]. Gelatin also demonstrated a semicrystalline nature, characterized by a broad amorphous peak around 19.5° and a sharp crystalline peak at 8.0°, in agreement with the findings obtained by Islam et al. [64]. The XRD pattern of TiO2 confirmed its crystalline nature, particularly the anatase phase, as verified by JCPDS reference pattern No. 01-083-5914. The bio-chitosan composite films—specifically Formulas 1 (chitosan only), 2 (chitosan with 0.5 wt% TiO2), and 6 (chitosan with gelatin)—exhibited peaks at 2θ angles of 9°, 12°, and 19°, which correspond to JCPDS No. 96-210-1328. These peaks indicate that the films predominantly consist of organic compounds such as carbon, hydrogen, nitrogen, and oxygen. In contrast, the bio-chitosan films in Formulas 3, 7, 8, 9, and 10 exhibited sharp diffraction peaks at different positions—specifically at 2θ values of 10°, 25°, 39°, and 48°—which align with the crystalline pattern of anatase-phase TiO2, as referenced by JCPDS No. 01-083-5914. These observations confirm the incorporation and crystallinity of TiO2 within the film matrices.
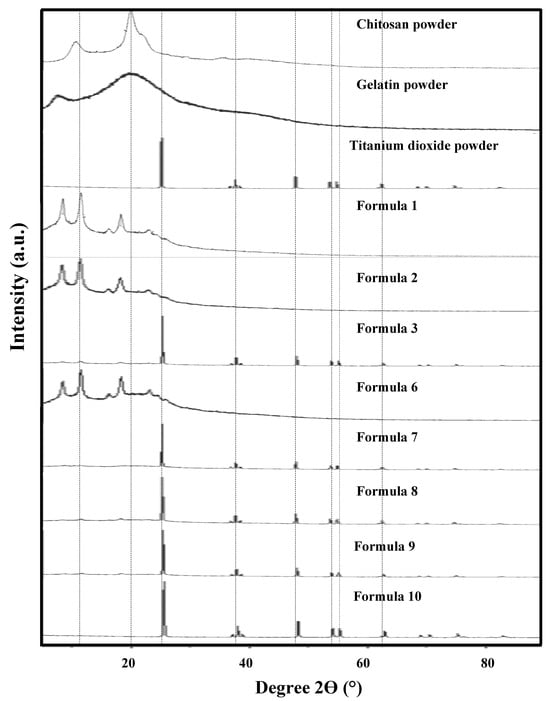
Figure 4.
XRD patterns of raw materials and bio-chitosan composite films. Note: Formulas 4 and 5 were unable to form biofilms due to brittleness and surface cracking.
A study was conducted to investigate the wetting behavior of bio-chitosan composite films containing varying concentrations of titanium dioxide (TiO2). The water contact angle on the surface of these films was measured at different time intervals, from the initial time point (t0) to 2000 milliseconds (t2000), as shown in Figure 5 and summarized in Table 3. The contact angle serves as an indicator of surface wettability, which is influenced by factors such as the zeta potential and surface charge of the TiO2 particles embedded in the biofilms as shown in Figure 5. In general, higher contact angles correspond to lower wettability (i.e., more hydrophobic surfaces), while lower contact angles indicate increased wettability (i.e., more hydrophilic surfaces). The results demonstrated that increasing the TiO2 content in the bio-chitosan films led to a decrease in the contact angle, indicating enhanced hydrophilicity. This suggests that TiO2 incorporation alters key physical properties of the films, including transparency, opacity, crystallinity, surface smoothness, porosity, and hydrophilicity. These modifications in surface wettability have important implications for applications such as food packaging, wound dressings, cosmetics, and drug delivery systems, where specific hydrophobic or hydrophilic characteristics are essential. At t0, the highest contact angle measured was 103.98° ± 6.35°, while at t2000, the maximum value recorded was 92.77° ± 4.54°. In contrast, Formula 8 (containing 5.0 wt% TiO2) exhibited the lowest contact angles at both time points: 69.72° ± 6.54° at t0 and 40.56° ± 4.17° at t2000. These findings align with the results reported by Cui et al. [65], who observed that pure chitosan films typically exhibit contact angles ranging from 76° to 104°, reflecting the inherent hydrophobicity of the chitosan backbone. This hydrophobic nature makes pure chitosan suitable for applications requiring water resistance, such as coatings, packaging, drug release systems, and biofilms. The observed reduction in contact angle with the increase in TiO2 content can be attributed to changes in surface roughness, crystallinity, and opacity, as also supported by SEM data and the findings obtained by Ling et al. [66]. Overall, this study underscores the potential of tailoring the hydrophilic or hydrophobic properties of bio-chitosan composite films through the controlled incorporation of TiO2, enabling their optimization for specific functional applications.
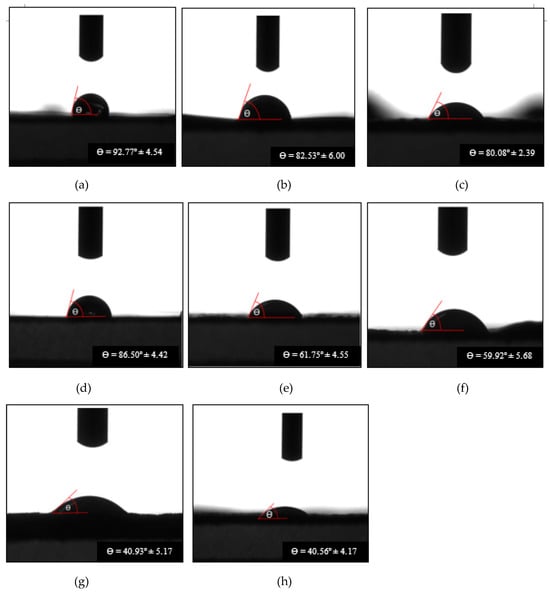
Figure 5.
Contact angles of water droplets on bio-chitosan composite films after 2000 ms: (a) Formula 1; (b) Formula 2; (c) Formula 3; (d) Formula 6; (e) Formula 7; (f) Formula 8; (g) Formula 9; and (h) Formula 10. Note: Formulas 4 and 5 were unable to form biofilms due to cracking and brittleness.

Table 3.
Water contact angles on the surface of bio-chitosan composite films, presented as mean values ± standard deviations (measured in triplicate).
The crosslink density of chitosan composite films, measured in toluene solution at 27 °C, is presented in Table 4. This study also includes calculations of the crosslink density (Ve) and the volume fraction of polymer in the swollen network at equilibrium with the pure solvent (Vr), using Equations (1) and (2), respectively. According to the findings, the incorporation of TiO2 and gelatin into bio-chitosan films resulted in improved adhesion and increased crosslink density. This enhancement is attributed to the distinctive properties of TiO2 nanoparticles, including their small size, spherical shape, mechanical strength, UV light barrier capabilities, and thermal, optical, and antimicrobial characteristics, as referenced in [66,67]. As the concentration of TiO2 in the bio-chitosan films increased, the crosslink density values also rose. In contrast, the pure bio-chitosan films exhibited the lowest crosslink density, reported as 2.263 × 10−2 mol/cm3. This study also examined the percentage of shrinkage, which was approximately 88.25 ± 2.15% for most formulations, with Formula 10 showing the lowest shrinkage at 28.50 ± 2.35%. Overall, the findings indicate that the incorporation of TiO2 nanoparticles into bio-chitosan films not only enhances adhesion and crosslink density but also reduces shrinkage and swelling. These results underscore the potential of TiO2-based composite films for a range of applications due to their superior performance characteristics.

Table 4.
Crosslink density of bio-chitosan composite films measured in toluene solution at 27 °C.
Crosslink density was determined using the toluene swelling method at room temperature, following ASTM D6814-02. The samples were cut into squares approximately 1 cm in width and 3–5 mm in thickness. The weight of each specimen was recorded before and after immersion in toluene for 72 h. Crosslink density was calculated using the Flory–Rehner Equations (1) and (2).
3.2. Mechanical, Optical, and Electrical Properties of Bio-Chitosan Composite Films
The mechanical properties of the bio-chitosan films, including maximum load, tensile strength, and Young’s modulus—were evaluated and are presented in Figure 6 and Figure 7. The results indicate that the incorporation of TiO2 nanoparticles significantly enhances these mechanical properties compared with pure bio-chitosan films. This improvement is consistent with other observed physical characteristics, including nanostructure, SEM imaging, contact angle measurements, crosslink density, and shrinkage percentage. These findings are in agreement with previous studies by Roy et al. [67] and Vilela et al. [68], which similarly reported enhanced mechanical performance in chitosan-based films reinforced with TiO2 nanoparticles. The integration of TiO2 contributes positively to the mechanical integrity of the films, as supported by both experimental data and the literature. Among the tested formulations, Formula 8 exhibited the highest tensile strength (0.43 MPa), while Formula 1 demonstrated the lowest value (0.10 MPa). However, it is important to note that excessive addition of TiO2 can result in brittleness and cracking, primarily due to nanoparticle agglomeration and phase segregation within the polymer matrix. These results suggest that TiO2-reinforced bio-chitosan composite films possess superior mechanical properties and may be suitable for a wide range of applications where enhanced strength and durability are required.
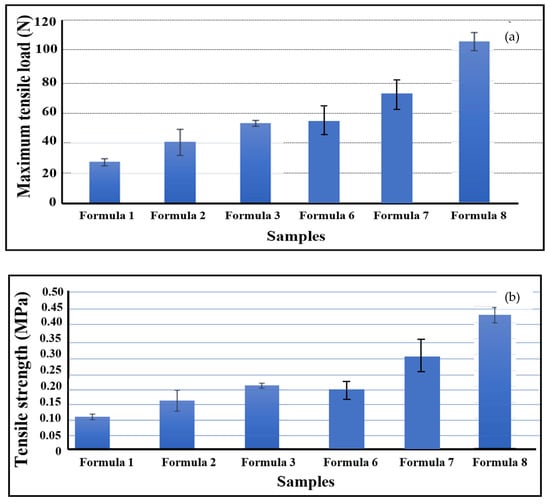
Figure 6.
Mechanical properties of bio-chitosan composite films: (a) maximum tensile load and (b) tensile strength. Values are presented as means ± standard deviations (SDs). Note: Formulas 4 and 5 did not form biofilms due to cracking and brittleness.
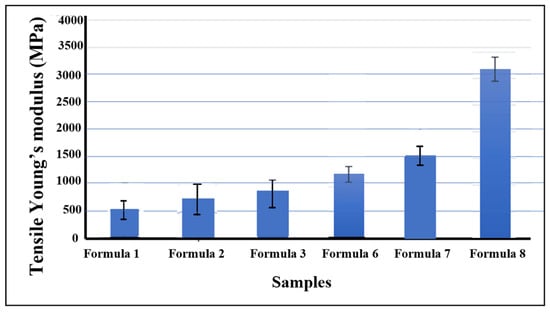
Figure 7.
Tensile Young’s modulus of bio-chitosan composite films presented as means ± standard deviations (SDs). Note: Formulas 4, 5, 9, and 10 could not form biofilms or be measured due to cracking and brittleness.
The optical properties of bio-chitosan composite films, with and without the addition of TiO2, were measured and are presented in Figure 8. Formulas 1 and 6, which represent biofilms without TiO2, exhibited high UV transmittance, reaching up to 90% in the wavelength range of 200–1000 nm. In contrast, the incorporation of TiO2 nanoparticles drastically reduced UV transmittance to below 1.0%. This significant reduction is attributed to the strong UV light absorption capabilities of TiO2 nanoparticles. As a result, bio-chitosan composite films containing TiO2 are well-suited for a wide range of applications, including cosmetics, biomedical products, food packaging, drug delivery, agricultural packaging (smart packaging), environmental materials, photoactivated films, photocatalysts, and UV-protective coatings. Furthermore, TiO2 nanoparticles confer antibacterial properties through chemical mechanisms activated by UV light, making these films beneficial in medical and dental applications, including surgical instruments, where sterility and bacterial inhibition are critical (as noted in reference [68]). Overall, the incorporation of TiO2 nanoparticles significantly enhances the functional properties of bio-chitosan composite films by reducing UV transmittance and imparting photocatalytic and antibacterial capabilities under UV exposure.
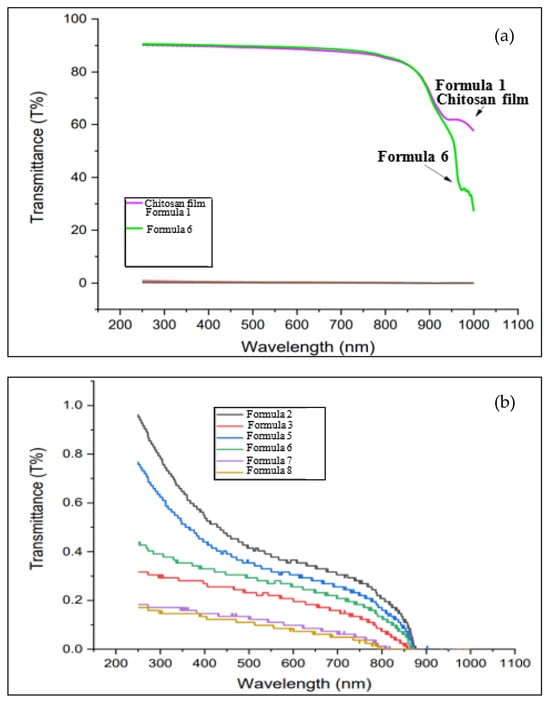
Figure 8.
UV transmittance of bio-chitosan composite films: (a) High-transmittance films. (b) Low-transmittance films.
The electrical properties of bio-chitosan composite films—specifically capacitance, dielectric constant, electroactive response, and bending behavior—are presented in Figure 9 and Figure 10 and summarized in Table 5 and Table 6, respectively. The capacitance and dielectric constant of bio-chitosan composite films containing TiO2 decrease slightly with the increase in frequency, which can be attributed to the polarization of Ti4+ and O2− ions on the surface of the composite films, as illustrated in Scheme 3. These findings are consistent with those reported by Gaabour, L.H. [69]. As the TiO2 content in the bio-chitosan films increases, both capacitance and dielectric constant also increase. TiO2 has reported capacitance values of approximately 90 F [70], 392 F [71], and 810 F [72], while chitosan exhibits a high capacitance value of approximately 542.63 F, as reported by Anandhavelu et al. [70]. The incorporation of TiO2 modifies the optical, mechanical, antimicrobial, and electrical properties of the bio-chitosan films, in accordance with the rule of mixtures [73,74]. Moreover, the polarization of Ti4+ and O2− ions at the film surface enhances the electromechanical response of the composite films. Under an electric field ranging from 0 to 550 V/mm at room temperature (27 °C), the films demonstrate a significantly higher electromechanical response compared with bio-chitosan films without TiO2, as shown in Figure 10 and detailed in Table 6. This enhancement becomes more pronounced with the increase in TiO2 content, resulting in a reduction in the required electric field strength by approximately four orders of magnitude compared with pure bio-chitosan films. These results indicate that TiO2 incorporation substantially improves the electromechanical performance of bio-chitosan composite films under an applied electric field.
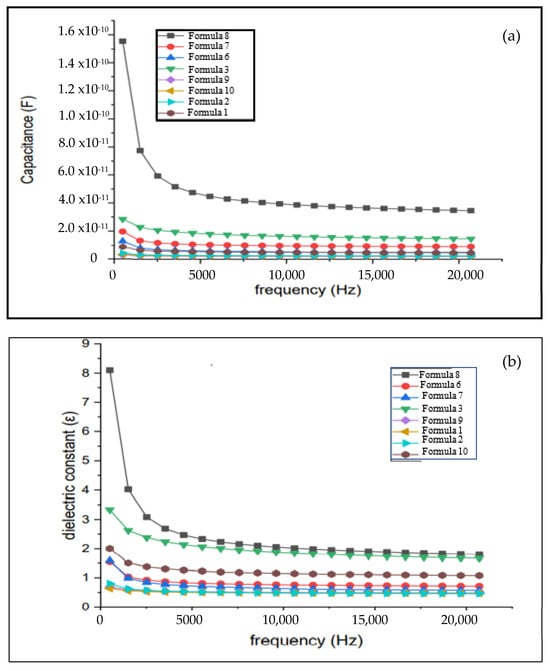
Figure 9.
Electrical properties of bio-chitosan composite films: (a) Capacitance vs. frequency (b) Dielectric constant vs. frequency. Note: Formulas 4 and 5 did not form films due to cracking and brittleness.

Figure 10.
Electromechanical response of Formulas 1, 2, 3, 6, 7, and 8 in bio-chitosan composite films, measured under electric field strengths ranging from 0 to 550 V/mm: (a) at 0 V/mm and (b) at the onset of electromechanical response. Note: Formulas 4, 5, 9, and 10 did not exhibit electroactive responses due to excessive TiO2 content, which caused brittleness and cracking.

Table 5.
Electrical properties and dielectric constant of bio-chitosan composite films measured at 27 °C (n = 3; mean ± standard deviation) at a frequency of 500 Hz.

Table 6.
Electromechanical response properties of bio-chitosan composite films measured at 27 °C (n = 3; mean ± standard deviation) under an applied electric field ranging from 0 to 550 V/mm.
The electromechanical response of bio-chitosan composite films under electric field strengths ranging from 0 to 550 V/mm varies across different formulations. Formulas 1 and 6—without and with gelatin, respectively—exhibited the lowest electromechanical responses, with bending angles of 22.03° ± 5.34° and 23.81° ± 4.51° at 550 V/mm, respectively. In contrast, Formulas 2, 3, 7, and 8, which incorporated TiO2 nanopowder, demonstrated significantly higher electromechanical responses, with bending angles of 68.35° ± 2.33°, 64.04° ± 2.46°, 70.03° ± 1.53°, and 81.29° ± 0.75° at electric field strengths of 550, 500, 350, and 250 V/mm, respectively. Notably, Formula 8, which contained high TiO2 content, exhibited the most pronounced electromechanical response at a relatively low electric field strength of 250 V/mm, achieving a bending angle of 61.48° ± 6.97°. In summary, the incorporation of TiO2 nanoparticles into bio-chitosan composite films significantly affects their electrical properties, including capacitance, dielectric constant, and electromechanical response. These results highlight the potential for tailoring and enhancing the optical absorption, mechanical strength, antimicrobial activity, and electrical characteristics of bio-chitosan films for diverse applications.
4. Conclusions
The integration of titanium dioxide (TiO2) into chitosan powder imparts desirable characteristics and enhances the physical properties of the resulting bio-chitosan nanocomposite films. A comprehensive analysis of their morphological, structural, and mechanical features provides a robust foundation for understanding the potential applications of these advanced materials. These findings contribute to ongoing efforts in developing sustainable, high-performance biomaterials for diverse industrial and biomedical applications. Bio-chitosan composite films can be fabricated by incorporating chitosan with gelatin and TiO2 nanoparticles. These films exhibit favorable physical, mechanical, optical, and electrical properties. They possess an aesthetically appealing appearance, minimal tear marks, improved wetting behavior, and a low degree of shrinkage. Additionally, the biocomposite films demonstrate high crosslink density, excellent UV absorption, and enhanced tensile strength and Young’s modulus. However, it is important to note that the excessive incorporation of TiO2 may have adverse effects. The intercalation of TiO2 nanoparticles within the chitosan matrix can lead to particle agglomeration, resulting in increased hardness and brittleness and the appearance of minor tear marks. Nevertheless, the presence of Ti4+ and O2− charge polarization contributes to improved resistance to deformation, thereby providing good electrical stability and a reduced electromechanical response under an applied electric field. Based on the results obtained, bio-chitosan composite films show promise for a wide range of applications, including use as biocomposite films or coatings in the food, medical, pharmaceutical, agricultural, and petrochemical packaging industries. Furthermore, the antimicrobial properties of TiO2 contribute to environmental protection against microbial growth, owing to its photocatalytic activity—particularly under ultraviolet (UV) irradiation in both the UV-A and UV-B ranges.
Author Contributions
N.T. contributed to conceptualization, validation, formal analysis, investigation, and data curation. She was responsible for drafting the original manuscript, reviewing and editing the content, and supervising the overall project. She also managed project administration and secured funding. N.M. and G.W. contributed to the methodology, validation, formal analysis, and data curation and were also involved in data visualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. Analytical and testing services were supported by the Department of Materials Engineering, Faculty of Engineering, Kasetsart University.
Institutional Review Board Statement
The authors confirm that all research was conducted in accordance with accepted ethical standards. Informed consent was obtained from all study participants, who were made aware of who would have access to the collected data and how the data would be used. Participant confidentiality was respected, and transparency in data handling was maintained throughout the study.
Informed Consent Statement
Upon acceptance of the manuscript, the authors grant the publisher the copyright license, providing exclusive rights to publish the work.
Data Availability Statement
All data related to this study are available to the organization, its partners, or end-users upon request, as needed.
Acknowledgments
The authors would like to express their sincere gratitude to the Materials Engineering Department at Kasetsart University, Bangkok, Thailand, for providing access to the analytical equipment used in this research study. They also acknowledge the support provided to the Undergraduate student by the Faculty of Engineering at Kasetsart University, Bangkok, Thailand.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the research, publication, or any other aspect of this work.
References
- Paulino, A.T.; Simionato, J.I.; Garcia, J.C.; Nozaki, J. Characterization of chitosan and chitin produced from silkworm crysalides. Carbohydr. Polym. 2006, 64, 98. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, X.; Yang, F.; Wang, T.; Ni, M.; Chen, Y.; Yang, F.; Huang, D.; Fu, C.; Wang, S. Preparation and Characterization of Chitosan-Based Ternary Blend Edible Films with Efficient Antimicrobial Activities for Food Packaging Applications. J. Food Sci. 2019, 84, 1411. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.H.Y.; Kade, J.; Jeiranikhameneh, A.; Yue, Z.; Mukherjee, P.; Wallace, G.G. A Bioprinting printing approach to regenerate cartilage for microtia treatment. Bioprinting 2018, 12, e00031. [Google Scholar] [CrossRef]
- Li, H.; Hu, C.; Yu, H.; Chen, C. Chitosan composite scaffolds for articular cartilage defect repair: A review. RSC Adv. 2018, 8, 3736. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ali, A.; Sheikh, J. A review on chitosan centred scaffolds and their applications in tissue engineering. Int. J. Biol. Macromol. 2018, 116, 849. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Wu, J.; Le, X. Molecular interactions, characterization and antimicrobial activity of curcumin–chitosan blend films. Food Hydrocoll. 2016, 52, 564. [Google Scholar] [CrossRef]
- Kumar, S.; Mukherjee, A.; Dutta, J. Chitosan based nanocomposite films and coatings: Emerging antimicrobial food packaging alternatives. Trends Food Sci. Technol. 2020, 97, 196. [Google Scholar] [CrossRef]
- Hoseinnejad, M.; Jafari, S.M.; Katouzian, I. Review on chitosan-based nanoparticles and their applications in food and agriculture. Critic. Rev. Microbiol. 2018, 44, 161. [Google Scholar]
- Zheng, L.Y.; Zhu, J.F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Alderman, O.L.G.; Skinner, L.B.; Benmore, C.J.; Tamalonis, A.; Weber, J.K.R. Structural study of amorphous chitosan materials. Phys. Rev. B 2014, 90, 094204. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, M.; Winter, J.; Gallert, C. Chitosan production and applications in wastewater treatment. Biochem. Eng. J. 2011, 56, 51. [Google Scholar]
- Wang, H.; Gong, X.; Miao, Y.; Guo, X.; Liu, C.; Fan, Y.Y.; Zhang, J.; Niu, B.; Li, W. Development of chitosan-based active food packaging materials. Food Chem. 2019, 283, 397. [Google Scholar] [CrossRef] [PubMed]
- Bahal, M.; Kaur, N.; Sharotri, N.; Sud, D. Recent advancements in chitosan-based biopolymer composites. Adv. Polym. Technol. 2019, 1, 12. [Google Scholar]
- Roy, S.J.-W. Applications of chitosan in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2020, 148, 666. [Google Scholar]
- Higashimoto, S.; Azuma, M. Chitosan-based catalysts and their applications. Appl. Catal. B Environ. 2009, 89, 557. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.W. Chitosan-based biodegradable films for food packaging. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Mesgari, M.; Aalami, A.H.; Sahebkar, A. Biomedical applications of chitosan and its derivatives. Int. J. Biol. Macromol. 2021, 176, 530. [Google Scholar]
- Awwad, A.M.; Amer, M.W.; Salem, N.M.; Abdeen, A.O. Synthesis and characterization of chitosan-based hydrogels for biomedical applications. Chem. Int. 2020, 6, 151. [Google Scholar]
- Alsohaimi, H.I.; Nassar, A.M.; Elnasr, T.A.S.; Cheba, B.A. Chitosan composites for water purification. J. Clean. Prod. 2020, 248, 119274. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Photocatalytic degradation using chitosan-based materials. Photochem. Photobiol. B Biol. 2019, 190, 86. [Google Scholar]
- Hassan, H.; Omoniyi, K.; Okibe, F.; Nuhu, A.; Echioba, E. Environmental applications of chitosan composites. J. Appl. Sci. Environ. Manag. 2019, 23, 1795. [Google Scholar]
- Daghrir, R.; Drogui, P.; Robert, D. Advanced oxidation processes for water treatment using chitosan composites. Ind. Eng. Chem. Res. 2013, 52, 3581. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. Chitosan-based nanomaterials: Synthesis and applications. Int. J. Biol. Macromol. 2018, 109, 273. [Google Scholar]
- Ullattil, S.G.; Narendranath, S.B.; Pillai, S.C.; Periyat, P. Chitosan-based catalysts in chemical engineering applications. Chem. Eng. J. 2018, 343, 708. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Preparation and properties of curcumin-loaded chitosan films. Food Hydrocoll. 2019, 94, 80. [Google Scholar] [CrossRef]
- Shafaee, M.; Goharshadi, E.K.; Mashreghi, M.; Sadeghinia, M.J. Photochemical properties of chitosan nanoparticles. Photochem. Photobiol. A Chem. 2018, 357, 90. [Google Scholar]
- Gumiero, M.; Peressini, D.; Pizzariello, A.; Sensidoni, A.; Iacumin, L.; Comi, G.; Toniolo, R. Effect of chitosan coating on food preservation. Food Chem. 2013, 138, 1633. [Google Scholar] [CrossRef]
- Joy, J.; Mathew, J.; George, S.C. Chitosan-based materials for hydrogen energy storage. Int. J. Hydrogen Energy. 2018, 43, 4804. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Characterization of chitosan films and their antimicrobial activity. Carbohydr. Polym. 2018, 193, 19. [Google Scholar] [CrossRef]
- Jbeli, A.; Ferraria, A.M.; Rego, A.M.B.D.; Boufi, S.; Bouattour, S. Chitosan nanocomposites for biomedical applications. Int. J. Biol. Macromol. 2018, 116, 1098. [Google Scholar]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F. Preparation and characterization of chitosan-based materials. Carbohydr. Polym. 2018, 186, 384. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Tang, J.; Li, X.; Huang, R.; Wu, L.; Xu, Q.; Liu, X.; Bi, X. Antimicrobial activity of chitosan films. J. Food Prot. 2022, 85, 597. [Google Scholar] [CrossRef]
- Lin, B.F.; Luo, Y.G.; Teng, Z.; Zhang, B.C.; Zhou, B.; Wang, Q. Chitosan and its derivatives for food packaging. LWT Food Sci. Technol. 2015, 63, 1206. [Google Scholar] [CrossRef]
- Kraśniewska, K.; Gniewosz, M. Chitosan-based antimicrobial films. J. Nutr. Sci. 2012, 62, 199. [Google Scholar]
- Vejdan, A.; Ojagh, S.M.; Adeli, A.; Abdollahi, M. Edible chitosan films and coatings. LWT Food Sci. Technol. 2016, 71, 88. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Peighambardoust, S.H.; Pournasir, N.M.; Pakdel, P. Shelf life extension using chitosan coatings. Food Packag. Shelf Life 2019, 22, 100420. [Google Scholar] [CrossRef]
- Charles, S.; Jomini, S.; Fessard, V.; Bigorgne-Vizade, E.; Rousselle, C.; Michel, C. Nanotoxicology of chitosan nanoparticles. Nanotoxicology 2018, 12, 357. [Google Scholar] [CrossRef]
- Gea, M.; Bonetta, S.; Iannarelli, L.; Giovannozzi, A.M.; Maurino, V.; Bonetta, S.; Hodoroaba, V.-D.; Armato, C.; Rossi, A.M.; Schilirò, T. Toxicological aspects of chitosan nanomaterials. Food Chem. Toxicol. 2019, 127, 89. [Google Scholar] [CrossRef]
- Proquin, H.; Rodríguez-Ibarra, C.; Moonen, C.G.; Ortega, I.M.U.; Briedé, J.J.; de Kok, T.M.; Van Loveren, H.; Chirino, Y.I. Mutagenicity assessment of chitosan nanoparticles. Mutagenesis 2017, 32, 139. [Google Scholar] [CrossRef]
- de Fonseca, J.M.; Valencia, G.A.; Soares, L.S.; Dotto, M.E.R.; Campos, C.E.M.; de Moreira, R.F.P.M.; Fritz, A.R.M. Hydroxypropyl methylcellulose-TiO2 and gelatin-TiO2 nanocomposite films: Physicochemical and structural properties. Int. J. Biol. Macromol. 2020, 151, 944. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Zhang, Y.; Cai, X.; Wang, S. Toxicity studies of chitosan nanoparticles. Int. J. Biol. Macromol. 2016, 84, 153. [Google Scholar]
- Zolfi, M.; Khodaiyan, F.; Mousavi, M.; Hashemi, M. Development and characterization of the kefiran–whey protein isolate–TiO2 nanocomposite films. Carbohydr. Polym. 2014, 109, 118. [Google Scholar] [CrossRef]
- Mohd, H.R.; Nur, A.I.; Khairul, A.M.A. Recent advances in chitosan composites for biomedical applications. Int. J. Biol. Macromol. 2020, 153, 1117. [Google Scholar]
- Masutani, E.M.; Kinoshita, C.K.; Tanaka, T.T.; Ellison, A.K.D.; Yoza, B.A. Synthesis and characterization of chitosan composites for pharmaceutical applications. Int. J. Biomater. 2014, 979636. [Google Scholar]
- Khodaei, D.; Oltrogge, K.; Hamidi-Esfahani, Z. Chitosan-based polymer composites: Preparation and functional properties. Lebensm. Wiss. Technol. 2020, 117, 108. [Google Scholar]
- Luo, Q.; Hossen, M.A.; Zeng, Y.; Dai, J.; Li, S.; Qin, W.; Liu, Y.J. Efficient removal of contaminants using chitosan-based composites in food engineering. Food Eng. 2022, 313, 110762. [Google Scholar] [CrossRef]
- Duconseille, A.; Astruc, T.; Quintana, N.; Meersman, F.; Sante-Lhoutellier, V. Impact of chitosan coatings on meat properties. Food Hydrocoll. 2015, 43, 360. [Google Scholar] [CrossRef]
- Ahmad, T.; Ismail, A.; Ahmad, S.A.; Khalil, K.A.; Kumar, Y.; Adeyemi, K.D.; Sazili, A.Q. Characterization of edible chitosan-based films with enhanced mechanical properties. Food Hydrocoll. 2017, 63, 85. [Google Scholar] [CrossRef]
- Andreuccetti, C.; Galicia-García, T.; Gonzalez-Nunez, R.; Martínez-Bustos, F.; Grosso, C.R.F. Functional and renewable chitosan polymers for packaging applications. Polym. Renew. Resour. 2017, 8, 11. [Google Scholar]
- Hosseini, S.F.; Rezaei, M.; Zandi, M.; Farahmandghavi, F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocoll. 2015, 44, 172. [Google Scholar] [CrossRef]
- Kumar, B.; Castro, M.; Feller, J.F. Chitosan-based micro- and nanocomposites: A review. J. Mater. Chem. 2012, 22, 10656. [Google Scholar] [CrossRef]
- Halawa, A.A.; Elshopakey, G.E.; Elmetwally, M.A.; El-Adl, M.; Lashen, S.; Shalaby, N.; Eldomany, E.; Farghali, A.; Sayed-Ahmed, M.Z.; Alam, N.; et al. Advanced chitosan-based nanocomposites for biomedical applications. Sci. Rep. 2022, 12, 19667. [Google Scholar]
- Knidri, H.E.L.; Belaabed, R.; Khalfaouy, R.E.L.; Laajeb, A.; Addaou, A.; Lahsin, A.J. Characterization and applications of chitosan composites in environmental science. Mater. Environ. Sci. 2017, 8, 3648. [Google Scholar]
- Laaraibi, A.; Charhouf, I.; Bennamara, A.; Abourriche, A.; Berrada, M.J. Extraction and characterization of chitosan from crab shell waste and its use in removal of heavy metals. Mater. Environ. Sci. 2015, 6, 3511. [Google Scholar]
- Teli, M.D.; Sheikh, J. Preparation and characterization of chitosan nanoparticles using ionic gelation method. Int. J. Biol. Macromol. 2012, 50, 1195. [Google Scholar]
- Bahrami Miyanji, P.; Semnani, D.; Hossein Ravandi, A.; Karbasi, S.; Fakhrali, A.; Mohammadi, S. Fabrication and characterization of chitosan-gelatin/single-walled carbon nanotubes electrospun composite scaffolds for cartilage tissue engineering applications. Polym. Adv. Technol. 2022, 33, 81–95. [Google Scholar] [CrossRef]
- Qian, Y.-F.; Zhang, K.-H.; Chen, F.; Ke, Q.-F.; Mo, X.-M. Fabrication and evaluation of chitosan/gelatin scaffold reinforced by nanoparticles. J. Biomater. Sci. Polym. Ed. 2011, 22, 1099. [Google Scholar]
- Gharaie, S.S.; Habibi, S.; Nazockdast, H.J. Development of chitosan/hemp fiber nonwoven textiles for sustainable applications. Text. Fibrous Mater. 2018, 1, 1. [Google Scholar]
- Spoială, A.; Ilie, C.I.; Dolete, G.; Croitoru, A.M.; Surdu, V.A.; Truşcă, R.D.; Motelica, L.; Oprea, O.C.; Ficai, D.; Ficai, A.; et al. Preparation and Characterization of Chitosan/TiO2 Composite Membranes as Adsorbent Materials for Water Purification. Membranes 2022, 12, 804. [Google Scholar] [CrossRef]
- Li, W.; He, D.; Hu, G.; Li, X.; Banerjee, G.; Li, J.; Lee, S.H.; Dong, Q.; Gao, T.; Brudvig, G.W.; et al. Visible-light-driven photocatalytic hydrogen evolution using chitosan-templated semiconductor nanocrystals. ACS Cent. Sci. 2018, 4, 631. [Google Scholar] [CrossRef] [PubMed]
- Kucukgulmez, A.; Celik, M.; Yanar, Y.; Sen, D.; Polat, H.; Kadak, E. Physicochemical characterization of chitosan extracted from Metapenaeus stebbingi shells. Food Chem. 2011, 126, 1144. [Google Scholar] [CrossRef]
- Naghibzadeh, M.; Amani, A.; Amini, M.; Esmaeilzadeh, E.; Mottaghi-Dastjerdi, N.; Faramarzi, M.A. An insight into the interactions between α-tocopherol and chitosan in ultrasound-prepared nanoparticles. J. Nanomater. 2010, 1, 18717. [Google Scholar] [CrossRef]
- Islam Md, A.A.; Mustafizur Rahman, A.F.M.; Iftekhar, S.; Salem, K.S.; Sultana, N.; Bari, M.L. Morphology, Thermal Stability, Electrical, and Mechanical Properties of Graphene Incorporated Poly (vinyl alcohol)-Gelatin Nanocomposites. J. Compos. Mater. 2016, 6, 172. [Google Scholar]
- Cui, Z.; Beach, E.S.; Anastas, P.T. Evaluation of the greenness of chemical processes for chemical and materials production. Green Chem. Lett. Rev. 2011, 4, 35. [Google Scholar] [CrossRef]
- Ling, Y.; Pan, X.; Wang, X.; Sun, R. Facile fabrication of chitosan active film with xylan via direct immersion. Cellulose 2014, 21, 1873. [Google Scholar]
- Roy, S.; Zhai, L.; Kim, H.C.; Pham, D.H.; Alrobei, H.; Kim, J. Tannic-Acid-Cross-Linked and TiO2-Nanoparticle-Reinforced Chitosan-Based Nanocomposite Film. Polymers 2021, 13, 228. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.B.; Coelho, J.; Domingues, M.R.M.; Daina, S.; Sadocco, P.; Santos, S.A.O.; Freire, C.S.R. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120. [Google Scholar] [CrossRef]
- Gaabour, L.H. Effect of addition of TiO2 nanoparticles on structural and dielectric properties of polystyrene/polyvinyl chloride polymer blend. AIP Adv. 2011, 11, 105120. [Google Scholar] [CrossRef]
- Anandhavelu, S.; Dhansekaran, V.; Sethuraman, V.; Park, H.J. Chitin and Chitosan Based Hybrid Nanocomposites for Super Capacitor Applications. J. Nanosci. Nanotechnol. 2017, 17, 1321. [Google Scholar] [CrossRef]
- Zhang, W.; Rhim, J.-W. Titanium dioxide (TiO2) for the manufacture of multifunctional active food packaging films. Food Packag. 2022, 31, 100806. [Google Scholar] [CrossRef]
- Castillo, B.C.; Prokhorov, E.; GLuna-Bárcenas, G.; Kovalenko, Y. Potential Use of Chitosan-TiO2 Nanocomposites for the Electroanalytical Detection of Imidacloprid. Polymers 2022, 14, 1686. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Gopal, J.; Muthu, M.; Shin, J.; Oh, J.W. Reviewing Chitin/Chitosan Nanofibers and Associated Nanocomposites and Their Attained Medical Milestones. Polymers 2021, 13, 2330. [Google Scholar] [CrossRef] [PubMed]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering, 10th ed.; Wiley: Hoboken, NJ, USA, 2018; ISBN 978-1-119-40549-8. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).