Highly Stable Lignin-Based Magnetic Composites for Efficient Removal of Pb(II) from Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of AFe-Fe2O3
2.3. Synthesis of AFe-Fe2O3@PL
2.4. Adsorption Experiments
2.4.1. Standard Curve of Pb(II)
2.4.2. Adsorption of Pb(II)
2.5. Characterizations
3. Results and Discussion
3.1. Characterization
3.1.1. FT-IR
3.1.2. XRD
3.1.3. XPS
3.1.4. VSM
3.1.5. SEM and TEM
3.1.6. BET
3.2. Adsorption Behavior Analysis
3.2.1. The Influence of Solution pH on Adsorption
3.2.2. The Influence of Adsorption Time and Kinetics
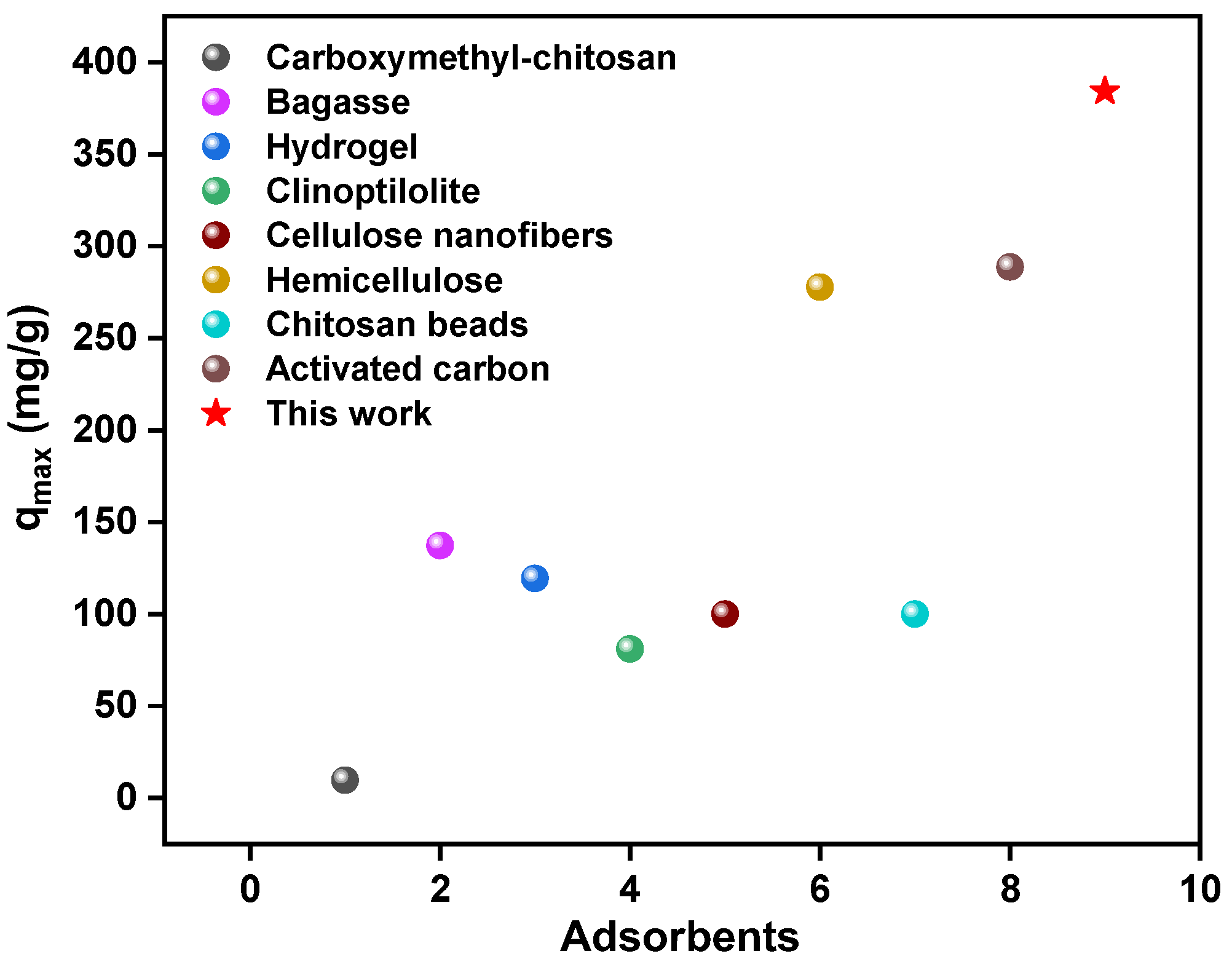
3.2.3. The Influence of Adsorption Concentration and Adsorption Isotherms
3.2.4. Stability and Recyclability of Adsorbents
3.2.5. Adsorption Mechanism Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sathish, K.V.; Manjunatha, H.C.; Vidya, Y.S.; Sankarshan, B.M.; Damodara Gupta, P.S.; Seenappa, L.; Sridhar, K.N.; Raj, A.C. Investigation on shielding properties of lead based alloys. Prog. Nucl. Energy 2021, 137, 103788. [Google Scholar] [CrossRef]
- Gao, H.; Wei, P.; Liu, H.; Long, M.; Fu, H.; Qu, X. Sunlight-Mediated Lead and Chromium Release from Commercial Lead Chromate Pigments in Aqueous Phase. Environ. Sci. Technol. 2019, 53, 4931–4939. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lin, N.; Zhang, W.; Lin, Z.; Zhang, Z.; Wang, Y.; Shi, J.; Bao, J.; Lin, H. Highly reversible lead-carbon battery anode with lead grafting on the carbon surface. J. Energy Chem. 2018, 27, 1674–1683. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, X.-k.; Yang, L.-Y. Adsorption of Pb(II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J. Mol. Liq. 2020, 322, 114523. [Google Scholar] [CrossRef]
- Shi, S.; Xu, C.; Dong, Q.; Wang, Y.; Zhu, S.; Zhang, X.; Chow, Y.T.; Wang, X.; Zhu, L.; Zhang, G.; et al. High saturation magnetization MnO2/PDA/Fe3O4 fibers for efficient Pb(II) adsorption and rapid magnetic separation. Appl. Surf. Sci. 2021, 541, 148379. [Google Scholar] [CrossRef]
- Vinay, K.; Dwivedi, S.K.; Seungdae, O. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Process Eng. 2021, 45, 102518. [Google Scholar] [CrossRef]
- Wu, F.; Chen, L.; Hu, P.; Wang, Y.; Deng, J.; Mi, B. Industrial alkali lignin-derived biochar as highly efficient and low-cost adsorption material for Pb(II) from aquatic environment. Bioresour. Technol. 2020, 322, 124539. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-Q.; Zhu, J.; Han, H.; Zhang, J.-Z.; Wu, F.-F.; Qin, X.-H.; Yu, J.-Y. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: In vitro and in vivo study. Acta Biomater. 2017, 65, 305–316. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Awual, M.R.; Islam, A.; Hasan, M.M.; Rahman, M.M.; Asiri, A.M.; Khaleque, M.A.; Sheikh, M.C. Introducing an alternate conjugated material for enhanced lead(II) capturing from wastewater. J. Clean. Prod. 2019, 224, 920–929. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Ma, X.; Qiao, W.; Wang, Z.; Hao, X.; Guan, G. Electrochemical redox induced rapid uptake/release of Pb(II) ions with high selectivity using a novel porous electroactive HZSM-5@PANI/PSS composite film. Electrochim. Acta 2018, 282, 384–394. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpää, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 2011, 266, 213–217. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Y.; Yang, X.; Lu, X.; Zhao, X.; Chen, Z.; Duan, W.; Li, J.; Zhao, M.; Yin, Q. Preparation of esterified biomass waste hydrogels and their removal of Pb2+, Cu2+ and Cd2+ from aqueous solution. Environ. Sci. Pollut. Res. 2023, 30, 56580–56593. [Google Scholar] [CrossRef] [PubMed]
- Youssif, M.M.; El-Attar, H.G.; Hessel, V.; Wojnicki, M. Recent Developments in the Adsorption of Heavy Metal Ions from Aqueous Solutions Using Various Nanomaterials. Materials 2024, 17, 5141. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bao, L.; Zhong, Y.; Hao, C.; Chen, J.; Wu, J.; Wang, X. Fabrication of in situ metal-organic framework grown on sodium lignosulphonate hydrogel for removal of Pb2+, methylene blue and crystal violet from aqueous solution. J. Clean. Prod. 2023, 434, 139831. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, J.; Kou, J.; Yang, P.; Li, M.; Tang, C.; Zhuang, W.; Ying, H.; Wu, J. Tunable synthesis of polyethylene polyamine modified lignin and application for efficient adsorption of Fe2+ in super acid system. Sep. Purif. Technol. 2021, 272, 118950. [Google Scholar] [CrossRef]
- Ye, W.; Li, X.; Luo, J.; Wang, X.; Sun, R. Lignin as a green reductant and morphology directing agent in the fabrication of 3D graphene-based composites for high-performance supercapacitors. Ind. Crop. Prod. 2017, 109, 410–419. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.O.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, M.; Wang, J.; Hou, D.; Lu, Y.; Yang, F.; Liu, C.; Lin, X.; Zheng, Z.; Zheng, Y. In-depth understanding of the synergistic effect in catalytic copyrolysis of lignin-plastic mixtures with lignin-tailored hierarchical HZSM-5 catalysts. Fuel 2024, 368, 131623. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Diao, X.; Ji, N.; Li, T.; Jia, Z.; Jiang, S.; Wang, Z.; Song, C.; Liu, C.; Lu, X.; Liu, Q. Rational design of oligomeric MoO3 in SnO2 lattices for selective hydrodeoxygenation of lignin derivatives into monophenols. J. Catal. 2021, 401, 234–251. [Google Scholar] [CrossRef]
- Lin, X.; Tao, S.; Minghui, L.; Jingwen, S.; Wei, Z.; Ming, L.; Hong, X.; Chenjie, Z.; Hanjie, Y.; Pingkai, O. Synthesis, characterization, and utilization of poly-amino acid-functionalized lignin for efficient and selective removal of lead ion from aqueous solution. J. Clean. Prod. 2022, 347, 131219. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Q.; Xiao, L.-P.; Li, X.-Y.; Xiao, X.; Li, M.-X.; Lin, M.-R.; Zhao, Y.-M.; Sun, R.-C. Metal–organic framework-derived CuO catalysts for the efficient hydrogenolysis of hardwood lignin into phenolic monomers. J. Mater. Chem. A 2023, 11, 23809–23820. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Wang, S.; Li, H.; Li, Z.; Shi, Z.-J.; Xiao, L.; Sun, R.-C.; Fang, Y.; Song, G. Catalytic Hydrogenolysis of Lignins into Phenolic Compounds over Carbon Nanotube Supported Molybdenum Oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Khodavandegar, S.; Fatehi, P. Phytic acid derivatized lignin as a thermally stable and flame retardant material. Green Chem. 2024, 26, 10070–10086. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Q.; Xu, W.; Qin, M.; Fu, Y.; Wang, Z.; Willför, S.; Xu, C. Revealing the structure of bamboo lignin obtained by formic acid delignification at different pressure levels. Ind. Crop. Prod. 2017, 108, 864–871. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, M.; Xu, W.; Fu, Y.; Wang, Z.; Li, Z.; Willför, S.; Xu, C.; Hou, Q. Structural changes of bamboo-derived lignin in an integrated process of autohydrolysis and formic acid inducing rapid delignification. Ind. Crop. Prod. 2018, 115, 194–201. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.R.; Fatehi, P. Water soluble kraft lignin-acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, D.; Yu, D.; Wu, C. Lignin-based adsorbents for heavy metals. Ind. Crop. Prod. 2022, 193, 116119. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Xu, Q.; Bai, X.; Wang, Z.; Cui, S. Stimuli-responsive adsorption behavior toward heavy metal ions based on comb polymer functionalized magnetic nanoparticles. J. Clean. Prod. 2019, 253, 119915. [Google Scholar] [CrossRef]
- Gan, W.; Gao, L.; Zhan, X.; Li, J. Preparation of thiol-functionalized magnetic sawdust composites as an adsorbent to remove heavy metal ions. RSC Adv. 2016, 6, 37600–37609. [Google Scholar] [CrossRef]
- Rozumová, L.; Životský, O.; Seidlerová, J.; Motyka, O.; Šafařík, I.; Šafaříková, M. Magnetically modified peanut husks as an effective sorbent of heavy metals. J. Environ. Chem. Eng. 2016, 4, 549–555. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Liao, C.; Li, Z. Facile fabrication of green geopolymer/alginate hybrid spheres for efficient removal of Cu(II) in water: Batch and column studies. Chem. Eng. J. 2016, 311, 126–134. [Google Scholar] [CrossRef]
- Wang, K.; Fu, J.; Wang, S.; Gao, M.; Zhu, J.; Wang, Z.; Xu, Q. Polydopamine-coated magnetic nanochains as efficient dye adsorbent with good recyclability and magnetic separability. J. Colloid Interface Sci. 2018, 516, 263–273. [Google Scholar] [CrossRef]
- Feng, T.; Xu, J.; Yu, C.; Cheng, K.; Wu, Y.; Wang, Y.; Li, F. Graphene oxide wrapped melamine sponge as an efficient and recoverable adsorbent for Pb(II) removal from fly ash leachate. J. Hazard. Mater. 2018, 367, 26–34. [Google Scholar] [CrossRef]
- He, H.; Meng, X.; Yue, Q.; Yin, W.; Gao, Y.; Fang, P.; Shen, L. Thiol-ene click chemistry synthesis of a novel magnetic mesoporous silica/chitosan composite for selective Hg(II) capture and high catalytic activity of spent Hg(II) adsorbent. Chem. Eng. J. 2020, 405, 126743. [Google Scholar] [CrossRef]
- He, W.; Cao, J.; Guo, F.; Guo, Z.; Zhou, P.; Wang, R.; Liang, S.; Pang, Q.; Wei, B.; Jiao, Y.; et al. Nanostructured carboxylated-wood aerogel membrane for high-efficiency removal of Cu(II) ions from wastewater. Chem. Eng. J. 2023, 468, 143747. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, S.; Cheng, W.; Zhang, L.; Meng, P.; Zhang, T.; Yu, H.; Peng, D. Theoretical calculations, molecular dynamics simulations and experimental investigation of the adsorption of cadmium(ii) on amidoxime-chelating cellulose. J. Mater. Chem. A 2019, 7, 13714–13726. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.-L.; Shi, C.; Yu, H.-Q.; Sheng, G.-P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef]
- Tang, C.; Brodie, P.; Li, Y.; Grishkewich, N.J.; Brunsting, M.; Tam, K.C. Shape Recoverable and Mechanically Robust Cellulose Aerogel Beads for Efficient Removal of Copper ions. Chem. Eng. J. 2020, 392, 124821. [Google Scholar] [CrossRef]
- Patel, P.K.; Pandey, L.M.; Uppaluri, R.V.S. Synthesized carboxymethyl-chitosan variant composites for cyclic adsorption-desorption based removal of Fe, Pb, and Cu. Chemosphere 2023, 340, 139780. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, R.; Yu, J.; Guo, L.; Li, X.; Xiao, C.; Hou, H.; Chi, R.; Feng, G. Simultaneous removal of lead, manganese, and copper released from the copper tailings by a novel magnetic modified biosorbent. J. Environ. Manag. 2022, 322, 116157. [Google Scholar] [CrossRef] [PubMed]
- Minale, M.; Gu, Z.; Guadie, A.; Li, Y.; Wang, Y.; Meng, Y.; Wang, X. Hydrous manganese dioxide modified poly(sodium acrylate) hydrogel composite as a novel adsorbent for enhanced removal of tetracycline and lead from water. Chemosphere 2021, 272, 129902. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, R.E.; Kamal, K.H.; Abd El-Aziz, M.E.; Morsi, S.M.; Kamel, S. Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions. Int. J. Biol. Macromol. 2020, 167, 1091–1101. [Google Scholar] [CrossRef]
- Mohammadabadi, S.I.; Javanbakht, V. Ultrasonic assisted hydrolysis of barley straw biowastes into construction of a novel hemicellulose-based adsorbent and its adsorption properties for lead ions from aqueous solutions. Renew. Energy 2020, 161, 893–906. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A. Dye removal using waste beads: Efficient utilization of surface-modified chitosan beads generated after lead adsorption process. J. Water Process Eng. 2019, 31, 100882. [Google Scholar] [CrossRef]
- Kavand, M.; Eslami, P.; Razeh, L. The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems. J. Water Process Eng. 2020, 34, 101151. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Yu, Z.; Zeng, G.; Luo, Y.; Jiang, L.; Yang, Z.; Qian, Y.; Wu, H. Amorphous MnO2 Modified Biochar Derived from Aerobically Composted Swine Manure for Adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]



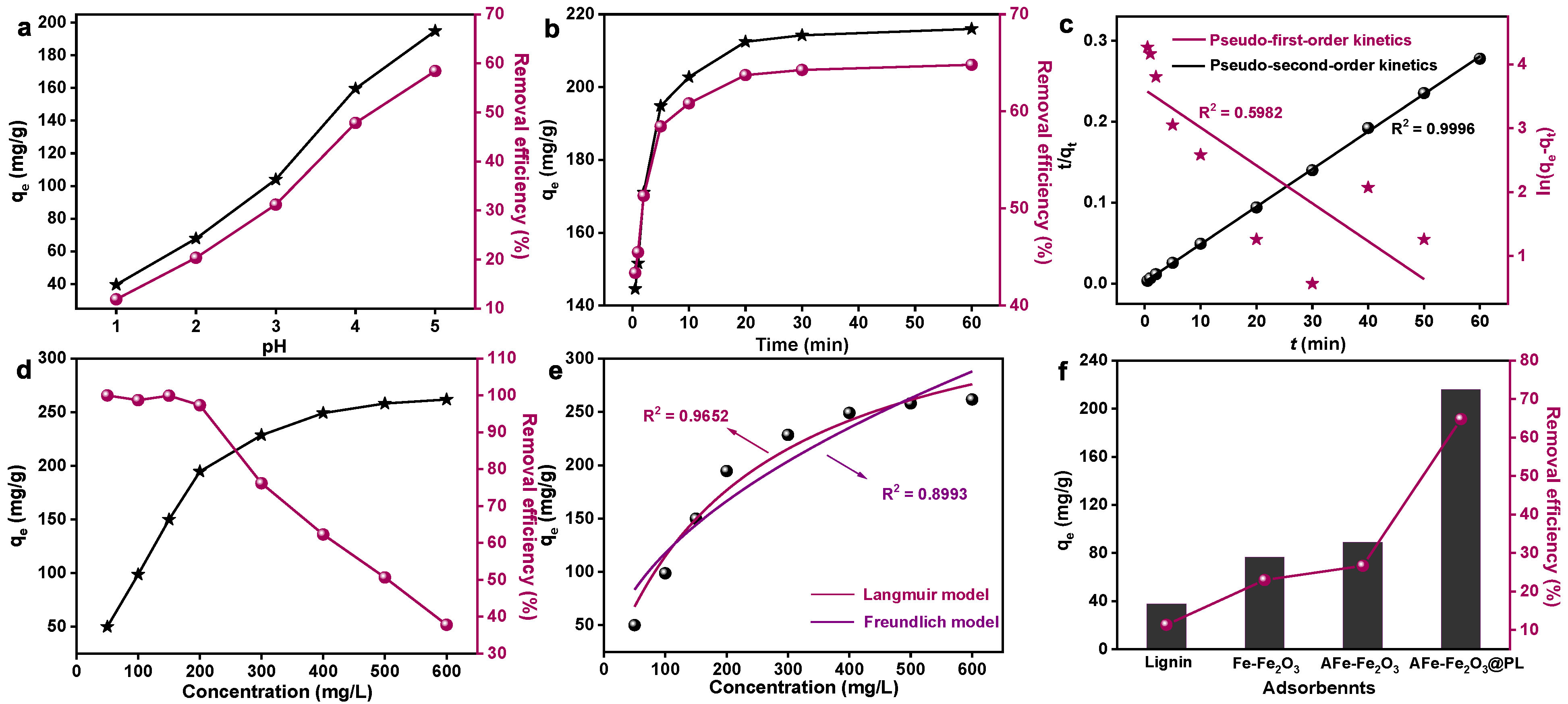

| Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|
| k1 (min−1) | qe (mg/g) | R2 | k2 (g/mg min) | qe (mg/g) | R2 |
| 0.0590 | 36.76 | 0.5982 | 0.0097 | 215.52 | 0.9996 |
| Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | KF (L/g) | 1/n | R2 |
| 384.24 | 0.0043 | 0.9652 | 11.81 | 0.50 | 0.8993 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Z.-H.; Li, X.-Y.; Zhao, Y.-Q.; Li, Y.-S.; Wang, Q.; Jia, J.-P.; Sánchez, J.; Zhu, K.-R.; Zhai, S.; Xiao, L.-P.; et al. Highly Stable Lignin-Based Magnetic Composites for Efficient Removal of Pb(II) from Wastewater. J. Compos. Sci. 2025, 9, 223. https://doi.org/10.3390/jcs9050223
Ren Z-H, Li X-Y, Zhao Y-Q, Li Y-S, Wang Q, Jia J-P, Sánchez J, Zhu K-R, Zhai S, Xiao L-P, et al. Highly Stable Lignin-Based Magnetic Composites for Efficient Removal of Pb(II) from Wastewater. Journal of Composites Science. 2025; 9(5):223. https://doi.org/10.3390/jcs9050223
Chicago/Turabian StyleRen, Zhi-Hong, Xiao-Ying Li, Yan-Qing Zhao, Yong-Sheng Li, Qiang Wang, Jie-Ping Jia, Julio Sánchez, Kai-Ruo Zhu, Shangru Zhai, Ling-Ping Xiao, and et al. 2025. "Highly Stable Lignin-Based Magnetic Composites for Efficient Removal of Pb(II) from Wastewater" Journal of Composites Science 9, no. 5: 223. https://doi.org/10.3390/jcs9050223
APA StyleRen, Z.-H., Li, X.-Y., Zhao, Y.-Q., Li, Y.-S., Wang, Q., Jia, J.-P., Sánchez, J., Zhu, K.-R., Zhai, S., Xiao, L.-P., & Sun, R.-C. (2025). Highly Stable Lignin-Based Magnetic Composites for Efficient Removal of Pb(II) from Wastewater. Journal of Composites Science, 9(5), 223. https://doi.org/10.3390/jcs9050223








