Abstract
In this study, a novel lignin-based magnetic composite with a shell-and-core structure and high saturated magnetic strength has been developed for the efficient removal of Pb(II) from wastewater. The adsorbent was fabricated through the introduction of silica–amino groups and a cross-linking complex with lignin, utilizing Fe-Fe2O3 as a magnetic source. The paramagnetic characteristics enabled its rapid separation from the aqueous solution within merely 15 s. Batch adsorption experiments demonstrated that the adsorbents could reach equilibrium for Pb(II) adsorption within 30 min. When the concentration of Pb(II) is in the low range of 0 to 200 mg/L, the removal rate of Pb(II) approaches 100%, and the theoretical maximum adsorption capacity is as high as 384.2 mg/g. The mechanism analysis indicated that the adsorption process was primarily characterized as monolayer chemisorption. Notably, the resultant bio-composites demonstrated a high level of stability even after eight consecutive adsorption and desorption cycles, with the removal rate of Pb(II) still reaching 82.3%. This work outlines a novel approach for designing highly efficient lignin-derived adsorbents toward wastewater treatment.
1. Introduction
The distinctive characteristics of Pb make it a highly versatile material that is employed in a multitude of industrial sectors, including alloys, pigments, cables, and storage battery manufacturing [1,2,3]. Concurrently, the discharge of industrial wastewater contains a considerable quantity of Pb(II) residues, which represent one of the most significant pollutants in the ecological environment [4,5,6]. If they enter the ecosystem through soil, air, water, and other pathways, they can inflict serious harm on the human nervous system and kidneys, thereby posing a substantial menace to human health [7,8,9,10]. As early as 2017, the International Agency for Research on Cancer (IARC) has designated Pb as a carcinogen belonging to Class 2B, indicating its toxicity and harmful effects on aquatic ecosystems. Moreover, Pb has the capacity to be transported within the food chain. Once introduced into the environment, Pb is capable of contaminating soil and water sources. Plants absorb Pb from the soil, and subsequently, through the consumption process, Pb makes its way into the food chain, ultimately presenting a health threat to both humans and animals at various trophic levels. Therefore, it is imperative that measurements be taken to address the issue of lead ions in water pollutants. Common techniques for the elimination of heavy metal ions from water consist of electrolysis [11], membrane separation [12], precipitation [13], and adsorption [14,15,16]. Of these, adsorption is the most widely used, which was due to its simplicity of operation, mild operation conditions and strong adsorption capacity in comparison with other methods [17,18]. However, there are some issues associated with the preparation of adsorbents, including high production costs, separation difficulties, low reuse efficiency, and the potential for secondary pollution [19]. Accordingly, it is necessary to develop economical, environmentally friendly materials toward efficient removal of heavy metals from wastewater.
Lignin represents the second largest bioresource in the plant kingdom, surpassed in reserves only by cellulose [20,21,22]. Among aromatic compounds, it is one of the few renewable resources [23,24,25]. Annually, approximately 70 million tons of industrial lignin is utilized, predominantly in the form of fuel, which represents a significant waste of resources [26,27]. Furthermore, lignin is rich in aromatic rings, aliphatic and aromatic hydroxyl and carboxyl groups, and other active groups [28]. These functional groups possess the capacity to form specific interactions with metal ions, thereby facilitating the process of adsorption [29,30]. Moreover, single lignin adsorbents have the disadvantages of few active sites, poor stability and poor cycling performance. Hence, functionalizing lignin is essential for enhancing its adsorption capabilities [31,32]. The distinctive physicochemical and characteristics of magnetic nanoparticles have resulted in their extensive application across various domains, such as biosensing, drug delivery, and magnetic resonance imaging [33]. In light of this, numerous studies have employed them in the synthesis of adsorbents. Gan et al. [34] synthesized a thiol-functionalized magnetic sawdust exhibiting a magnetization strength of 7.28 emu/g and an adsorption capacity of 12.5 mg/g for Pb(II). Rozumová et al. [35] developed a magnetic adsorbent derived from peanut shells, which demonstrated a magnetization strength of 2.14 emu/g and an adsorption capacity of Pb(II) up to 28.3 mg/g. However, most of the investigated magnetic adsorbents face a number of challenges, such as non-degradability, high cost, insufficient adsorption sites, and oxidation of the magnetic material.
Herein, we have developed a novel lignin-based magnetic adsorbent toward efficient removal of Pb(II) for wastewater treatment. To enhance the active sites on the adsorbent’s surface, we introduced silica amine functional groups on the magnetic nano-chains (Fe-Fe2O3). The silica encapsulation provided Fe-Fe2O3 with enhanced stability in aqueous solution. Subsequently, a novel shell-and-core structured magnetic adsorbent (AFe-Fe2O3@PL) exhibiting high saturated magnetic strength was synthesized by cross-linking the composite with lignin. The adsorbent’s structure and morphology were thoroughly characterized using FT-IR, XRD, SEM and TEM analytical techniques. The magnetic properties of AFe-Fe2O3@PL were tested by vibrating sample magnetometer (VSM). Additionally, the kinetic and isothermal models of the adsorption process were further analyzed by exploring the effects of AFe-Fe2O3@PL on the adsorption characteristics of Pb(II) under various circumstances. Batch cycling experiments and XPS tests were analyzed to reveal the stability and possible adsorption mechanism of AFe-Fe2O3. This work presents the preparation of an efficient and stable lignin-based magnetic adsorbent, which offers a novel avenue for the reuse of lignin and facilitates large-scale industrial applications for wastewater treatment.
2. Materials and Methods
2.1. Materials
Industrial pre-hydrolyzed lignin (PL) was supplied by Jining Minsheng New Materials Co., Ltd., Jining, China. Tetraethyl orthosilicate (TEOS), iron (III) chloride, poly ethylene glycol, sodium borohydride (NaBH4), sodium dodecyl benzene sulfonate (SDBS), (3-aminopropyl) trimethoxy silane (APTMS), and ethanol (99.7%) were purchased from Macklin Biochemical Co, Ltd., Shanghai, China. Paraffin liquid, lead nitrate (Pb(NO3)2), epichlorohydrin (ECH), cyclohexane, and sodium alginate were obtained from Aladdin Reagent Co., Ltd., Shanghai, China. All the above reagents were of analytical grade and were used directly. The solutions for the experimental procedures were prepared using deionized (DI) water.
2.2. Synthesis of AFe-Fe2O3
Initially, 0.04 mol of NaBH4 was distributed in 100 mL of cyclohexane while being mechanically stirred in an ice bath at 0 °C. The resulting solution was then subjected to ultrasonic treatment to enhance dispersion. Subsequently, the solution was transferred to an ice bath, and 50 mL of 0.1 M FeCl3 solution was slowly added to it while vigorously stirring for 30 min. The resultant mixture underwent multiple washings with DI water and ethanol. After that, it was dried in an oven at 40 °C for 1 h, yielding the desired fluffy Fe-Fe2O3 magnetic nanocrystalline chains.
The prepared Fe-Fe2O3 magnetic nano-chains (1.2 g) were dispersed in a solution of ethanol and water (400/40 mL) and then underwent sonication for 20 min. Subsequently, TEOS (0.5 g) was introduced after 2 h of stirring at 25 °C, followed by the gradual addition of APTMS (0.5 g) and stirring overnight. The resulting solids of amine-functionalized magnetic nanoparticles were isolated via filtration and lyophilization, and finally named as AFe-Fe2O3.
2.3. Synthesis of AFe-Fe2O3@PL
The AFe-Fe2O3@PL adsorbent with a core-shell structure was synthesized via reversed-phase suspension polymerization (Figure 1). Initially, PL (0.8 g) was dispersed in 20 mL of DI water and agitated for 10 min at 180 rpm. Subsequently, AFe-Fe2O3 (0.4 g), PEG (0.16 g), SDBS (0.3 g), and a 1.5 wt% sodium alginate solution (30 mL) were sequentially added and thoroughly mixed using a shaker. The resulting solution was emulsified dropwise into 120 mL of liquid paraffin under stirring conditions. Afterward, 15 mL of the crosslinker ECH was incrementally added to the emulsion under stirring and copolymerized for 2 h to form microspheres. The resulting samples were washed with ethanol and petroleum ether and then dried at 50 °C overnight. Finally, the prepared adsorbent was obtained and designated as AFe-Fe2O3@PL. For more detailed characterizations, please see the Supplementary Material.
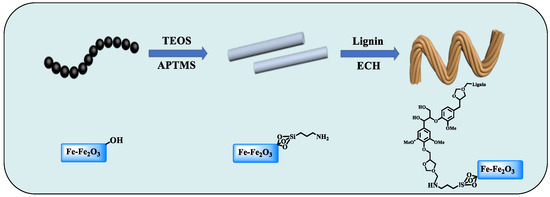
Figure 1.
Preparation of the adsorbent AFe-Fe2O3@PL with shell and core structure.
2.4. Adsorption Experiments
2.4.1. Standard Curve of Pb(II)
A certain amount of Pb(NO3)2 was weighed and prepared into a solution of 100 mg/L, followed by taking 10 mL of the above solution and diluting it to 100 mL to make a mother liquor of 10 mg/L. A volume of 0, 1, 2, 3, 4, or 5 mL of the mother liquor was pipetted into a 10 mL volumetric flask, 0.5 mL of color developer and 1 mL of buffer solution were added, and the volume was adjusted to 10 mL with water. The absorbance of the corresponding concentration was measured at 575 nm using an ultraviolet spectrophotometer. By linearly fitting the absorbance values at different Pb(II) concentrations, the equation: y = 0.063x + 0.2129 was obtained, and the fitting correlation coefficient R2 was 0.9996. The concentration of Pb(II) in the solution to be tested could be calculated by measuring the absorbance of the solution to be tested by UV spectrophotometry.
2.4.2. Adsorption of Pb(II)
The experimental procedure was carried out using static adsorption conditions. Initially, a conical flask was charged with a specified concentration of Pb(NO3)2 solution, into which a predetermined mass of the adsorbent AFe-Fe2O3@PL was introduced. Subsequently, the flask was positioned on a shaker and agitated for a defined duration at 25 °C and 180 rpm. Following agitation, the supernatant was filtered and diluted to a concentration range that corresponded to that of the standard curve. Subsequently, the absorbance at 575 nm was determined by a UV spectrophotometer. Adsorption and removal efficiencies were determined by calculations using Formulas (S1) and (S2). All data were averaged by repeating the experiments three times.
2.5. Characterizations
The residual amounts of Pb(II) in the solution were measured employing a UV spectrometer. The chemical structure of the adsorbent was analyzed by Fourier transform infrared spectroscopy (FT-IR). The crystal structure of the samples was analyzed using X-ray diffraction (XRD). The morphological features and elemental distribution of the samples were observed by scanning electron microscopy (SEM) and high-resolution transmission electron microscopy (HR-TEM) coupled with energy dispersive spectroscopy (EDS). Surface chemical composition and elemental chemical states were analyzed before and after adsorption using X-ray photoelectron spectroscopy (XPS). The magnetic properties of the nanocomposites were investigated using a Vibrating Sample Magnetometer (VSM).
3. Results and Discussion
3.1. Characterization
3.1.1. FT-IR
The chemical structures of Fe-Fe2O3, AFe-Fe2O3, AFe-Fe2O3@PL, and PL were analyzed using FT-IR (Figure 2a) to elucidate the preparation process. The vibrational peak at 3425 cm−1 corresponding to the O-H bond in AFe-Fe2O3 exhibits a reduction as compared to the unmodified Fe-Fe2O3. Furthermore, the peak at 557 cm−1 corresponds to the Fe-O bond, and the strong absorbance peaks at 815 and 1050 cm−1 are associated with the absorbance peaks of the Si-O-Si bond, signifying the chemical encapsulation of amine silica on the surface of Fe-Fe2O3 [36]. Moreover, the absorbance peaks at 1605, 1511, and 1420 cm−1 primarily represent vibrations of the aromatic skeleton, confirming the aromatic structure of both PL and AFe-Fe2O3@PL. The most noteworthy difference between PL and AFe-Fe2O3@PL was the emergence of a new absorbance peak at 1414 cm−1, attributing to the C-N stretching vibration. Notably, the absence of the C–O absorbance peaks at 1120 cm−1 for primary alcohols and phenols suggested the successful copolymerization of lignin with AFe-Fe2O3 and ECH.
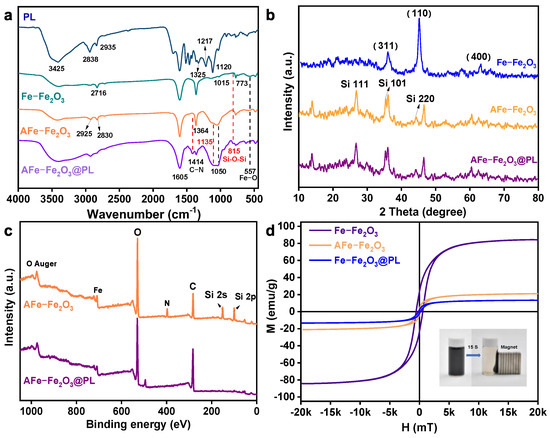
Figure 2.
(a) FT-IR spectra and (b) XRD patterns of the fabricated materials. (c) XPS wide spectra of the AFe-Fe2O3 and the AFe-Fe2O3@PL magnetic nanochains. (d) Magnetization curve of the Fe-Fe2O3, AFe-Fe2O3, and the AFe-Fe2O3@PL magnetic nanochains.
3.1.2. XRD
The XRD patterns of the Fe-Fe2O3, AFe-Fe2O3, and AFe-Fe2O3@PL magnetic nano strands are illustrated in Figure 2b. The prominent diffraction peak at 2θ = 44.8° is ascribed to the (110) crystallographic plane of α-Fe. Additionally, the two minor absorption peaks at 2θ = 35.7° and 62.8° are ascribed to the (311) and (400) crystallographic planes of the magnetic hyperfine phase γ-Fe2O3. In AFe-Fe2O3, the peaks at 2θ = 26.8°, 35.5°, and 44.2° correspond to the (111), (101), and (220) crystal planes of Si-O-Si bonding, indicating the chemical encapsulation of amine silica on the Fe-Fe2O3 surface [37]. The average grain sizes of Fe and Fe2O3 were calculated using the Scherrer formula (Formula (S7)) based on the XRD data. Specifically, the average grain size of Fe was determined to be 0.55 nm, while that of Fe2O3 was 1.34 nm. Moreover, the XRD pattern of AFe-Fe2O3@PL revealed that the adsorbent, post cross-linking with lignin, maintained its crystal structure without significant disruption.
3.1.3. XPS
The XPS spectra of the AFe-Fe2O3 and AFe-Fe2O3@PL magnetic nanochains are depicted in Figure 2c, revealing peaks corresponding to N 1s at 398.00 eV and Si 2s and Si 2p at 151 eV and 100 eV, respectively. These findings provided additional evidence supporting the chemical coating of amine silica on the surfaces of Fe-Fe2O3 [38]. A notable decrease in the levels of Fe, N, and Si elements in AFe-Fe2O3@PL magnetic nanochains indicated the successful coverage of the AFe-Fe2O3 nanochains by PL. These observations aligned well with the results from the XRD and FT-IR analyses. The high-resolution Fe 2p spectrum exhibits two intense peaks at 709.11 eV and 723.04 eV (Figure S4), corresponding to the Fe 2p3/2 and Fe 2p1/2 spin-orbit doublets, respectively. The main peaks of Fe 2p3/2 at 710.3 eV and Fe 2p1/2 at 723.9 eV are characteristic of Fe2O3, accompanied by distinct satellite peaks (718.1 eV). While the signals at 707.9 eV and 721 eV can be attributed to metallic Fe, consistent with the phase identification by XRD analysis.
3.1.4. VSM
A hysteresis loop depicts the relationship between the magnetic field strength H with a substance and the induced magnetization strength M. The saturation points of the hysteresis curve, which referred to the Ms value, reflect the magnetic characteristics of the prepared materials. The hysteresis curves of Fe-Fe2O3, AFe-Fe2O3, and AFe-Fe2O3@PL magnetic nanoclusters are depicted in Figure 2d with their respective Ms values measured at 84.17, 20.99, and 13.35 emu/g. These values indicated the favorable paramagnetic properties of the materials. The reduction in saturation magnetization intensity of AFe-Fe2O3 as compared to Fe-Fe2O3 could be attributed to the introduction of non-magnetic amine structures on the surface of Fe-Fe2O3 nanochains. Furthermore, the decrease in the Ms value of AFe-Fe2O3@PL magnetic nanoclusters was due to the successful coating of the non-magnetic lignin components on the Fe-Fe2O3 nanoclusters, forming a core-shell structured material. Despite the diminished magnetization properties observed in AFe-Fe2O3@PL magnetic nano sorbents, they still exhibited rapid separation capabilities in aqueous solutions under an applied magnetic field. Furthermore, SEM and TEM examinations were conducted to further validate the successful fabrication of AFe-Fe2O3@PL core-shell magnetic nanochains.
3.1.5. SEM and TEM
The macroscopic morphologies of PL, Fe-Fe2O3, AFe-Fe2O3, and AFe-Fe2O3@PL are illustrated in Figure S1. To characterize the microstructures of Fe-Fe2O3, AFe-Fe2O3, and AFe-Fe2O3@PL magnetic nanoclusters, SEM and TEM analyses were conducted (Figure 3). The SEM images revealed that Fe-Fe2O3 exhibited a chain-like structure composed of interconnected nanoparticles. AFe-Fe2O3 and AFe-Fe2O3@PL maintained this chain-like morphology, whereas AFe-Fe2O3@PL showed a twisted and entangled structure, which further confirmed that AFe-Fe2O3@PL was a complex of lignin cross-linked with AFe-Fe2O3. The EDS mapping images showed that AFe-Fe2O3@PL was primarily composed of a complex mixture of C, O, N, and Fe elements (Figure S2). The TEM image of AFe-Fe2O3@PL displayed a distinct shell-core architecture, where the inner layer corresponded to AFe-Fe2O3 and the outer shell indicated the successful encapsulation of lignin.
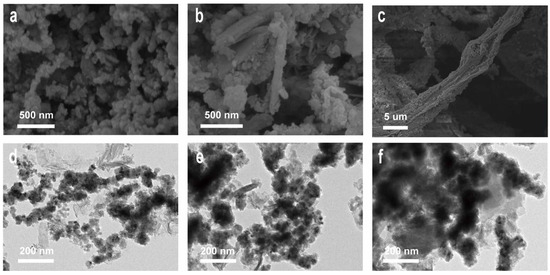
Figure 3.
SEM (a–c) and TEM (d–f) images of Fe-Fe2O3, AFe-Fe2O3, and AFe-Fe2O3@PL.
3.1.6. BET
The specific surface area and porosity of PL, AFe-Fe2O3, and AFe-Fe2O3@PL were determined by BET. From the data (Figure S3 and Table S1), it can be seen that both AFe-Fe2O3 and AFe-Fe2O3 show limited pore volume with values lower than 0.13 cm3/g. Furthermore, although the specific surface area of PL was 1.2279 m2/g, the AFe-Fe2O3@PL (19.1163 m2/g) composite shows a substantial increase in the specific surface area when compared to AFe-Fe2O3 (11.4340 m2/g). This is mainly due to the fact that AFe-Fe2O3 is encapsulated by PL, which gives it a larger specific surface area that not only makes it easy to expose more active sites but also favors the protection of magnetic AFe-Fe2O3.
3.2. Adsorption Behavior Analysis
3.2.1. The Influence of Solution pH on Adsorption
The pH level significantly affected the effectiveness of the adsorbent by impacting its surface charge and the chemical speciation of the heavy metal ions in the wastewater. Hence, it was of great importance to investigate the impact of pH on the adsorption of Pb (II) by AFe-Fe2O3@PL in a Pb(NO3)2 solution. The results demonstrated that under acidic conditions, the adsorption capacity of Pb(II) significantly increased with rising pH (Figure 4a), owing to the reduced presence of protonated amine and hydroxyl groups, which were less conducive to Pb(II) adsorption. Furthermore, Pb(II) tended to precipitate when the pH of the Pb(NO3)2 solution reached 6.0 or higher [39]. Consequently, all adsorption experiments in this study were conducted at a pH of 5.0.
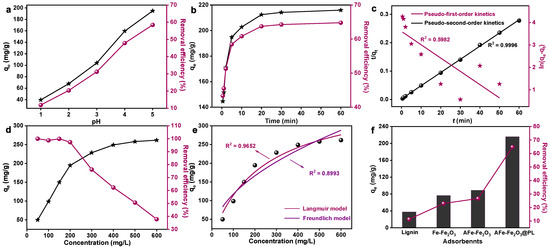
Figure 4.
Effects of pH (a), time (b), and concentration (d) on Pb(II) adsorption by AFe-Fe2O3@PL. Pseudo-first-order and Pseudo-second-order kinetics models of Pb(II) uptake by AFe-Fe2O3@PL (c). Adsorption isotherms of Pb(II) uptake by AFe-Fe2O3@PL (e). Comparison of the adsorption capacities of PL, Fe-Fe2O3, AFe-Fe2O3, and AFe-Fe2O3 (f).
3.2.2. The Influence of Adsorption Time and Kinetics
This work investigated the intermittent adsorption kinetics of Pb(II) by AFe-Fe2O3@PL across various contact times. As depicted in Figure 4b, the adsorption capacity of AFe-Fe2O3@PL for Pb(II) rapidly increased during the first 10 min. This phenomenon was attributed to the abundant adsorption sites on the adsorbent, thus facilitating mass transfer and efficiently capturing free Pb(II). As reaction time increased, the adsorption rate gradually diminished, eventually reaching equilibrium at 30 min. This indicated that the adsorption of Pb(II) decelerated once the primary adsorption sites on the adsorbent became filled. In light of this dataset, the kinetic behavior of Pb(II) adsorption by AFe-Fe2O3@PL was fitted to the pseudo-first-order [40] and pseudo-second-order kinetic models [41] (Figure 4c) using Equations (S3) and (S4).
As shown in Table 1, the correlation coefficient (R2) of the proposed secondary kinetic model of AFe-Fe2O3@PL (R2 = 0.9996) was much higher than that of the proposed primary kinetic model (R2 = 0.5982). Moreover, the qe value (215.52 mg/g) obtained from the fitting of the proposed secondary kinetic model coincided with the experimental measurement (212.5 mg/g). Therefore, the adsorption behavior of AFe-Fe2O3@PL on Pb(II) aligned with the second-order kinetic model, suggesting a typical chemisorption process between the Pb(II) ions and the adsorbent.

Table 1.
Fitting parameters of the pseudo-first-order and pseudo-second-order kinetic models for Pb(II) adsorption onto AFe-Fe2O3@PL.
3.2.3. The Influence of Adsorption Concentration and Adsorption Isotherms
For determining the maximum adsorption capacity of Pb(II) by AFe-Fe2O3@PL in the present work, the adsorption isotherm of Pb(II) onto AFe-Fe2O3@PL was experimentally investigated at various initial concentrations (50~600 mg/g) under pH 5.0 and a temperature of 25 °C (Figure 4d). The results illustrated a gradual increase in the adsorption of the adsorbent with rising initial concentrations until saturation was reached. Notably, a sharp increase in adsorption was observed at the lower initial concentrations. This was primarily because of the abundant active sites on AFe-Fe2O3@PL, leading to a robust capacity for Pb(II) adsorption. As the initial concentration further increased, the adsorbed Pb(II) on the surface of AFe-Fe2O3@PL began to repel free Pb(II) in the solution. Additionally, as equilibrium was approached, the adsorption of Pb(II) on AFe-Fe2O3@PL slowed down because the already adsorbed Pb(II) occupied a portion of the pores on AFe-Fe2O3@PL, impeding further mass transfer.
The isothermal adsorption models were mathematical models employed to depict the process of a solute being adsorbed on solid surfaces, which in turn helped to understand and predict the characteristics and mechanisms of the adsorption process. Accordingly, adsorption data at different concentrations were fitted using the Langmuir [42] and Freundlich [43] isothermal models, which were determined by Equations (S5) and (S6), respectively.
The adsorption isotherms of Pb(II) on AFe-Fe2O3@PL were obtained through nonlinear fitting (Figure 4e), with the relevant parameters summarized in Table 2. The findings demonstrated that the Langmuir model (R2 = 0.9652) offered a superior fit in comparison with the Freundlich model (R2 = 0.8993) as indicated by a higher correlation coefficient. The higher fit supported the conclusion that Pb(II) adsorption occurred as a monolayer on AFe-Fe2O3@PL. Furthermore, the maximum adsorption of Pb(II) on AFe-Fe2O3@PL, as derived from the Langmuir model, reached 384.24 mg/g. This strong adsorption performance of the lignin-based magnetic adsorbent could be primarily attributed to its core-shell structure and the presence of surface-active sites.

Table 2.
Fitting parameters of the adsorption isotherms for Pb(II) adsorption onto AFe-Fe2O3@PL.
3.2.4. Stability and Recyclability of Adsorbents
By comparing the adsorption properties with the raw materials PL, Fe-Fe2O3, AFe-Fe2O3, and AFe-Fe2O3@PL (Figure 4f), the results showed that the adsorption properties of lignin-based magnetic adsorbents far exceeded those of the raw materials. This could be ascribed to the presence of N and O groups on the adsorbent’s structure, which favored the chelation of Pb(II). Additionally, the good distribution of Fe-Fe2O3 particles on the lignin polymer network facilitated the adsorption of Pb(II). In addition, the adsorption capacity of AFe-Fe2O3@PL composites for Pb(II) was significantly superior to that of other commonly used adsorbent materials, as depicted in Figure 5. This apparent adsorption performance advantage highlighted the potential application of AFe-Fe2O3@PL as an efficient and promising candidate for the removal of Pb(II) for wastewater treatment.
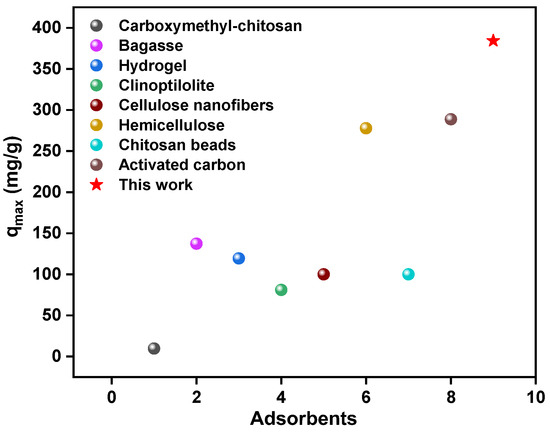
Figure 5.
Comparison of the maximum adsorption property (qmax) of AFe-Fe2O3@PL with other adsorbents [44,45,46,47,48,49,50,51].
The ability to reuse an adsorbent is crucial for cost-saving and supporting sustainable development. Therefore, the recyclability of the adsorbent was thoroughly examined in this study (Figure 6a). Remarkably, the results demonstrated that even after undergoing eight adsorption–desorption cycles, the adsorbent retained a significant adsorption capacity of 82.3% with only a marginal decrease of 16.6%. This finding underscored the outstanding stability and excellent reusability of the adsorbent, highlighting its potential for long-term application.
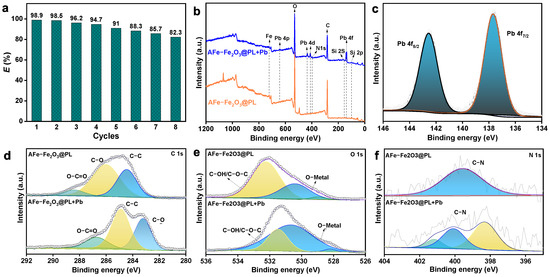
Figure 6.
(a) Reusability of AFe-Fe2O3@PL, (b) XPS spectra of AFe-Fe2O3@PL and (c) AFe-Fe2O3@PL + Pb, (d–f) deconvoluted of C 1s, O 1s, and N 1s XPS spectra of AFe-Fe2O3@PL and AFe-Fe2O3@PL + Pb.
3.2.5. Adsorption Mechanism Study
In order to explore the adsorption mechanism, XPS was utilized to analyze the N- and O-containing groups present before and after Pb(II) adsorption on AFe-Fe2O3@PL. As depicted in Figure 6b, the XPS spectra revealed an increase in the proportion of C and O elements in AFe-Fe2O3@PL + Pb, accompanied by the observation of absorption peaks of Pb in the Pb fine spectrum (Figure 6c). This observation further corroborated the adsorption of Pb(II) by the lignin-based magnetic adsorbent AFe-Fe2O3@PL. As illustrated in Figure 6d, the C 1s spectra of AFe-Fe2O3@PL depicted three peaks at 288.4, 286.1, and 284.4 eV, corresponding to the O-C=O, C-O, and C-C bonds, respectively. Upon adsorption of Pb, the binding energies of these peaks changed and shifted to 286.8, 283.2, and 284.9 eV, respectively. Moreover, the N 1s spectrum of AFe-Fe2O3@PL displayed a shift in the C-N peaks following adsorption (Figure 6f), suggesting that the adsorption of Pb(II) impacted the displacement of the C-N peaks. Concurrently, the O 1s spectrum of AFe-Fe2O3@PL exhibited absorption peaks at 531.62 eV attributable to C-OH and C-O-C (comprising 63.8%) and at 530.20 eV ascribed to O-Metal (comprising 36.2%) (Figure 6e). Notably, both peaks underwent migration following the adsorption of Pb(II) with the proportion of C-OH and C-O-C decreasing to 12.4%, while the O-Metal content rose to 87.6%. This shift suggested the existence of chelation or complexation between the O groups and Pb(II) [52]. The XPS analysis firmly indicated that chemical interactions were vital for enhancing the adsorption of Pb(II) on the magnetic adsorbent surface.
4. Conclusions
In summary, a novel core-shell structured AFe-Fe2O3@PL magnetic adsorbent was successfully synthesized through the crosslinking of amine-functionalized Fe-Fe2O3 with lignin. Batch experiments demonstrated that AFe-Fe2O3@PL could rapidly reach the adsorption equilibrium for Pb(II) within 30 min, highlighting its highly efficient kinetic performance. The theoretical maximum adsorption capacity reaches 384.2 mg/g, and it has been convincingly verified by the pseudo-second-order kinetic model and the Langmuir model that the adsorption process conforms to the mechanism of monolayer chemisorption. Its favorable paramagnetic property enables it to be rapidly separated from the aqueous solution within 30 s, simplifying the practical application process. Notably, the removal rate can still be maintained at 82.3% after eight regeneration cycles, indicating that AFe-Fe2O3@PL possesses excellent stability and cost-effective cyclic performance. This work has established a paradigm for the design of multifunctional lignocellulosic materials, addressing key challenges such as the recyclability of adsorbents and the realization of energy-efficient separation in large-scale water purification applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcs9050223/s1, Figure S1: Images of PL, Fe-Fe2O3, AFe-Fe2O3 and AFe-Fe2O3@PL materials; Figure S2: EDS mapping of AFe-Fe2O3@PL; Figure S3: (a) The isotherms of N2 adsorption-desorption and (b) the pore width distribution curves of PL, AFe-Fe2O3 and AFe-Fe2O3@PL; Figure S4. The High-resolution XPS spectra of Fe 2p; Table S1: Pore size characteristic data of PL, AFe-Fe2O3 and AFe-Fe2O3@PL.
Author Contributions
Conceptualization, Q.W. and L.-P.X., methodology, Q.W., Z.-H.R. and X.-Y.L.; validation, J.-P.J., J.S., K.-R.Z. and S.Z.; formal analysis, Z.-H.R. and X.-Y.L.; investigation, Z.-H.R. and X.-Y.L.; resources, L.-P.X.; data curation, Z.-H.R., X.-Y.L., Y.-Q.Z. and Y.-S.L.; writing—original draft preparation, Z.-H.R.; writing—review and editing, L.-P.X. and R.-C.S.; supervision, L.-P.X.; project administration, L.-P.X. and R.-C.S.; and funding acquisition, L.-P.X. and R.-C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (22278049, U24A20559, and 51961125207) and the Dalian High-Level Talent Innovation Program (2024RJ017).
Data Availability Statement
Data is contained within the article or supplementary material.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sathish, K.V.; Manjunatha, H.C.; Vidya, Y.S.; Sankarshan, B.M.; Damodara Gupta, P.S.; Seenappa, L.; Sridhar, K.N.; Raj, A.C. Investigation on shielding properties of lead based alloys. Prog. Nucl. Energy 2021, 137, 103788. [Google Scholar] [CrossRef]
- Gao, H.; Wei, P.; Liu, H.; Long, M.; Fu, H.; Qu, X. Sunlight-Mediated Lead and Chromium Release from Commercial Lead Chromate Pigments in Aqueous Phase. Environ. Sci. Technol. 2019, 53, 4931–4939. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Lin, N.; Zhang, W.; Lin, Z.; Zhang, Z.; Wang, Y.; Shi, J.; Bao, J.; Lin, H. Highly reversible lead-carbon battery anode with lead grafting on the carbon surface. J. Energy Chem. 2018, 27, 1674–1683. [Google Scholar] [CrossRef]
- Xu, X.; Ouyang, X.-k.; Yang, L.-Y. Adsorption of Pb(II) from aqueous solutions using crosslinked carboxylated chitosan/carboxylated nanocellulose hydrogel beads. J. Mol. Liq. 2020, 322, 114523. [Google Scholar] [CrossRef]
- Shi, S.; Xu, C.; Dong, Q.; Wang, Y.; Zhu, S.; Zhang, X.; Chow, Y.T.; Wang, X.; Zhu, L.; Zhang, G.; et al. High saturation magnetization MnO2/PDA/Fe3O4 fibers for efficient Pb(II) adsorption and rapid magnetic separation. Appl. Surf. Sci. 2021, 541, 148379. [Google Scholar] [CrossRef]
- Vinay, K.; Dwivedi, S.K.; Seungdae, O. A critical review on lead removal from industrial wastewater: Recent advances and future outlook. J. Water Process Eng. 2021, 45, 102518. [Google Scholar] [CrossRef]
- Wu, F.; Chen, L.; Hu, P.; Wang, Y.; Deng, J.; Mi, B. Industrial alkali lignin-derived biochar as highly efficient and low-cost adsorption material for Pb(II) from aquatic environment. Bioresour. Technol. 2020, 322, 124539. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-Q.; Zhu, J.; Han, H.; Zhang, J.-Z.; Wu, F.-F.; Qin, X.-H.; Yu, J.-Y. Synthesis and characterization of arginine-NIPAAm hybrid hydrogel as wound dressing: In vitro and in vivo study. Acta Biomater. 2017, 65, 305–316. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of Lignin and Its Derivatives in Adsorption of Heavy Metal Ions in Water: A Review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Awual, M.R.; Islam, A.; Hasan, M.M.; Rahman, M.M.; Asiri, A.M.; Khaleque, M.A.; Sheikh, M.C. Introducing an alternate conjugated material for enhanced lead(II) capturing from wastewater. J. Clean. Prod. 2019, 224, 920–929. [Google Scholar] [CrossRef]
- Du, X.; Zhang, D.; Ma, X.; Qiao, W.; Wang, Z.; Hao, X.; Guan, G. Electrochemical redox induced rapid uptake/release of Pb(II) ions with high selectivity using a novel porous electroactive HZSM-5@PANI/PSS composite film. Electrochim. Acta 2018, 282, 384–394. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.-J.; Sillanpää, M.; Zhao, F. Recent advances in membrane filtration for heavy metal removal from wastewater: A mini review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Ghosh, P.; Samanta, A.N.; Ray, S. Reduction of COD and removal of Zn2+ from rayon industry wastewater by combined electro-Fenton treatment and chemical precipitation. Desalination 2011, 266, 213–217. [Google Scholar] [CrossRef]
- Pham, T.D.; Tran, T.T.; Le, V.A.; Pham, T.T.; Dao, T.H.; Le, T.S. Adsorption characteristics of molecular oxytetracycline onto alumina particles: The role of surface modification with an anionic surfactant. J. Mol. Liq. 2019, 287, 110900. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Y.; Yang, X.; Lu, X.; Zhao, X.; Chen, Z.; Duan, W.; Li, J.; Zhao, M.; Yin, Q. Preparation of esterified biomass waste hydrogels and their removal of Pb2+, Cu2+ and Cd2+ from aqueous solution. Environ. Sci. Pollut. Res. 2023, 30, 56580–56593. [Google Scholar] [CrossRef] [PubMed]
- Youssif, M.M.; El-Attar, H.G.; Hessel, V.; Wojnicki, M. Recent Developments in the Adsorption of Heavy Metal Ions from Aqueous Solutions Using Various Nanomaterials. Materials 2024, 17, 5141. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Bao, L.; Zhong, Y.; Hao, C.; Chen, J.; Wu, J.; Wang, X. Fabrication of in situ metal-organic framework grown on sodium lignosulphonate hydrogel for removal of Pb2+, methylene blue and crystal violet from aqueous solution. J. Clean. Prod. 2023, 434, 139831. [Google Scholar] [CrossRef]
- Qi, X.; Tong, X.; Pan, W.; Zeng, Q.; You, S.; Shen, J. Recent advances in polysaccharide-based adsorbents for wastewater treatment. J. Clean. Prod. 2021, 315, 128221. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, J.; Kou, J.; Yang, P.; Li, M.; Tang, C.; Zhuang, W.; Ying, H.; Wu, J. Tunable synthesis of polyethylene polyamine modified lignin and application for efficient adsorption of Fe2+ in super acid system. Sep. Purif. Technol. 2021, 272, 118950. [Google Scholar] [CrossRef]
- Ye, W.; Li, X.; Luo, J.; Wang, X.; Sun, R. Lignin as a green reductant and morphology directing agent in the fabrication of 3D graphene-based composites for high-performance supercapacitors. Ind. Crop. Prod. 2017, 109, 410–419. [Google Scholar] [CrossRef]
- Torres, L.A.Z.; Woiciechowski, A.L.; de Andrade Tanobe, V.O.; Karp, S.G.; Lorenci, L.C.G.; Faulds, C.; Soccol, C.R. Lignin as a potential source of high-added value compounds: A review. J. Clean. Prod. 2020, 263, 121499. [Google Scholar] [CrossRef]
- Chen, Y.; Fu, M.; Wang, J.; Hou, D.; Lu, Y.; Yang, F.; Liu, C.; Lin, X.; Zheng, Z.; Zheng, Y. In-depth understanding of the synergistic effect in catalytic copyrolysis of lignin-plastic mixtures with lignin-tailored hierarchical HZSM-5 catalysts. Fuel 2024, 368, 131623. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Diao, X.; Ji, N.; Li, T.; Jia, Z.; Jiang, S.; Wang, Z.; Song, C.; Liu, C.; Lu, X.; Liu, Q. Rational design of oligomeric MoO3 in SnO2 lattices for selective hydrodeoxygenation of lignin derivatives into monophenols. J. Catal. 2021, 401, 234–251. [Google Scholar] [CrossRef]
- Lin, X.; Tao, S.; Minghui, L.; Jingwen, S.; Wei, Z.; Ming, L.; Hong, X.; Chenjie, Z.; Hanjie, Y.; Pingkai, O. Synthesis, characterization, and utilization of poly-amino acid-functionalized lignin for efficient and selective removal of lead ion from aqueous solution. J. Clean. Prod. 2022, 347, 131219. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, Q.; Xiao, L.-P.; Li, X.-Y.; Xiao, X.; Li, M.-X.; Lin, M.-R.; Zhao, Y.-M.; Sun, R.-C. Metal–organic framework-derived CuO catalysts for the efficient hydrogenolysis of hardwood lignin into phenolic monomers. J. Mater. Chem. A 2023, 11, 23809–23820. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Wang, S.; Li, H.; Li, Z.; Shi, Z.-J.; Xiao, L.; Sun, R.-C.; Fang, Y.; Song, G. Catalytic Hydrogenolysis of Lignins into Phenolic Compounds over Carbon Nanotube Supported Molybdenum Oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Khodavandegar, S.; Fatehi, P. Phytic acid derivatized lignin as a thermally stable and flame retardant material. Green Chem. 2024, 26, 10070–10086. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Q.; Xu, W.; Qin, M.; Fu, Y.; Wang, Z.; Willför, S.; Xu, C. Revealing the structure of bamboo lignin obtained by formic acid delignification at different pressure levels. Ind. Crop. Prod. 2017, 108, 864–871. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, M.; Xu, W.; Fu, Y.; Wang, Z.; Li, Z.; Willför, S.; Xu, C.; Hou, Q. Structural changes of bamboo-derived lignin in an integrated process of autohydrolysis and formic acid inducing rapid delignification. Ind. Crop. Prod. 2018, 115, 194–201. [Google Scholar] [CrossRef]
- Kong, F.; Wang, S.; Price, J.T.; Konduri, M.K.R.; Fatehi, P. Water soluble kraft lignin-acrylic acid copolymer: Synthesis and characterization. Green Chem. 2015, 17, 4355–4366. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, D.; Yu, D.; Wu, C. Lignin-based adsorbents for heavy metals. Ind. Crop. Prod. 2022, 193, 116119. [Google Scholar] [CrossRef]
- Liu, X.; Guan, J.; Lai, G.; Xu, Q.; Bai, X.; Wang, Z.; Cui, S. Stimuli-responsive adsorption behavior toward heavy metal ions based on comb polymer functionalized magnetic nanoparticles. J. Clean. Prod. 2019, 253, 119915. [Google Scholar] [CrossRef]
- Gan, W.; Gao, L.; Zhan, X.; Li, J. Preparation of thiol-functionalized magnetic sawdust composites as an adsorbent to remove heavy metal ions. RSC Adv. 2016, 6, 37600–37609. [Google Scholar] [CrossRef]
- Rozumová, L.; Životský, O.; Seidlerová, J.; Motyka, O.; Šafařík, I.; Šafaříková, M. Magnetically modified peanut husks as an effective sorbent of heavy metals. J. Environ. Chem. Eng. 2016, 4, 549–555. [Google Scholar] [CrossRef]
- Ge, Y.; Cui, X.; Liao, C.; Li, Z. Facile fabrication of green geopolymer/alginate hybrid spheres for efficient removal of Cu(II) in water: Batch and column studies. Chem. Eng. J. 2016, 311, 126–134. [Google Scholar] [CrossRef]
- Wang, K.; Fu, J.; Wang, S.; Gao, M.; Zhu, J.; Wang, Z.; Xu, Q. Polydopamine-coated magnetic nanochains as efficient dye adsorbent with good recyclability and magnetic separability. J. Colloid Interface Sci. 2018, 516, 263–273. [Google Scholar] [CrossRef]
- Feng, T.; Xu, J.; Yu, C.; Cheng, K.; Wu, Y.; Wang, Y.; Li, F. Graphene oxide wrapped melamine sponge as an efficient and recoverable adsorbent for Pb(II) removal from fly ash leachate. J. Hazard. Mater. 2018, 367, 26–34. [Google Scholar] [CrossRef]
- He, H.; Meng, X.; Yue, Q.; Yin, W.; Gao, Y.; Fang, P.; Shen, L. Thiol-ene click chemistry synthesis of a novel magnetic mesoporous silica/chitosan composite for selective Hg(II) capture and high catalytic activity of spent Hg(II) adsorbent. Chem. Eng. J. 2020, 405, 126743. [Google Scholar] [CrossRef]
- He, W.; Cao, J.; Guo, F.; Guo, Z.; Zhou, P.; Wang, R.; Liang, S.; Pang, Q.; Wei, B.; Jiao, Y.; et al. Nanostructured carboxylated-wood aerogel membrane for high-efficiency removal of Cu(II) ions from wastewater. Chem. Eng. J. 2023, 468, 143747. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, S.; Cheng, W.; Zhang, L.; Meng, P.; Zhang, T.; Yu, H.; Peng, D. Theoretical calculations, molecular dynamics simulations and experimental investigation of the adsorption of cadmium(ii) on amidoxime-chelating cellulose. J. Mater. Chem. A 2019, 7, 13714–13726. [Google Scholar] [CrossRef]
- Zhang, N.; Zang, G.-L.; Shi, C.; Yu, H.-Q.; Sheng, G.-P. A novel adsorbent TEMPO-mediated oxidized cellulose nanofibrils modified with PEI: Preparation, characterization, and application for Cu(II) removal. J. Hazard. Mater. 2016, 316, 11–18. [Google Scholar] [CrossRef]
- Tang, C.; Brodie, P.; Li, Y.; Grishkewich, N.J.; Brunsting, M.; Tam, K.C. Shape Recoverable and Mechanically Robust Cellulose Aerogel Beads for Efficient Removal of Copper ions. Chem. Eng. J. 2020, 392, 124821. [Google Scholar] [CrossRef]
- Patel, P.K.; Pandey, L.M.; Uppaluri, R.V.S. Synthesized carboxymethyl-chitosan variant composites for cyclic adsorption-desorption based removal of Fe, Pb, and Cu. Chemosphere 2023, 340, 139780. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhou, R.; Yu, J.; Guo, L.; Li, X.; Xiao, C.; Hou, H.; Chi, R.; Feng, G. Simultaneous removal of lead, manganese, and copper released from the copper tailings by a novel magnetic modified biosorbent. J. Environ. Manag. 2022, 322, 116157. [Google Scholar] [CrossRef] [PubMed]
- Minale, M.; Gu, Z.; Guadie, A.; Li, Y.; Wang, Y.; Meng, Y.; Wang, X. Hydrous manganese dioxide modified poly(sodium acrylate) hydrogel composite as a novel adsorbent for enhanced removal of tetracycline and lead from water. Chemosphere 2021, 272, 129902. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, I. Lead removal from aqueous solution by natural and pretreated clinoptilolite: Adsorption equilibrium and kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Abou-Zeid, R.E.; Kamal, K.H.; Abd El-Aziz, M.E.; Morsi, S.M.; Kamel, S. Grafted TEMPO-oxidized cellulose nanofiber embedded with modified magnetite for effective adsorption of lead ions. Int. J. Biol. Macromol. 2020, 167, 1091–1101. [Google Scholar] [CrossRef]
- Mohammadabadi, S.I.; Javanbakht, V. Ultrasonic assisted hydrolysis of barley straw biowastes into construction of a novel hemicellulose-based adsorbent and its adsorption properties for lead ions from aqueous solutions. Renew. Energy 2020, 161, 893–906. [Google Scholar] [CrossRef]
- Pal, P.; Pal, A. Dye removal using waste beads: Efficient utilization of surface-modified chitosan beads generated after lead adsorption process. J. Water Process Eng. 2019, 31, 100882. [Google Scholar] [CrossRef]
- Kavand, M.; Eslami, P.; Razeh, L. The adsorption of cadmium and lead ions from the synthesis wastewater with the activated carbon: Optimization of the single and binary systems. J. Water Process Eng. 2020, 34, 101151. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Yu, Z.; Zeng, G.; Luo, Y.; Jiang, L.; Yang, Z.; Qian, Y.; Wu, H. Amorphous MnO2 Modified Biochar Derived from Aerobically Composted Swine Manure for Adsorption of Pb(II) and Cd(II). ACS Sustain. Chem. Eng. 2017, 5, 5049–5058. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).