Application of DFT and Experimental Tests for the Study of Compost Formation Between Chitosan-1,3-dichloroketone with Uses for the Removal of Heavy Metals in Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Computational Details
2.2. Experimental Part
2.2.1. Synthesis of Shrimp Chitosan
2.2.2. Determination and Characterization of the Physicochemical Properties of Chitosan
2.2.3. Chitosan Solubility
2.2.4. Moisture Content
2.2.5. Ash Content
2.2.6. Degree of Deacetylation (DDA)
2.2.7. Development of Calibration Standards for AAS
2.2.8. Synthesis of Shrimp Chitosan Derivatives
3. Results and Discussion
3.1. Method Selection
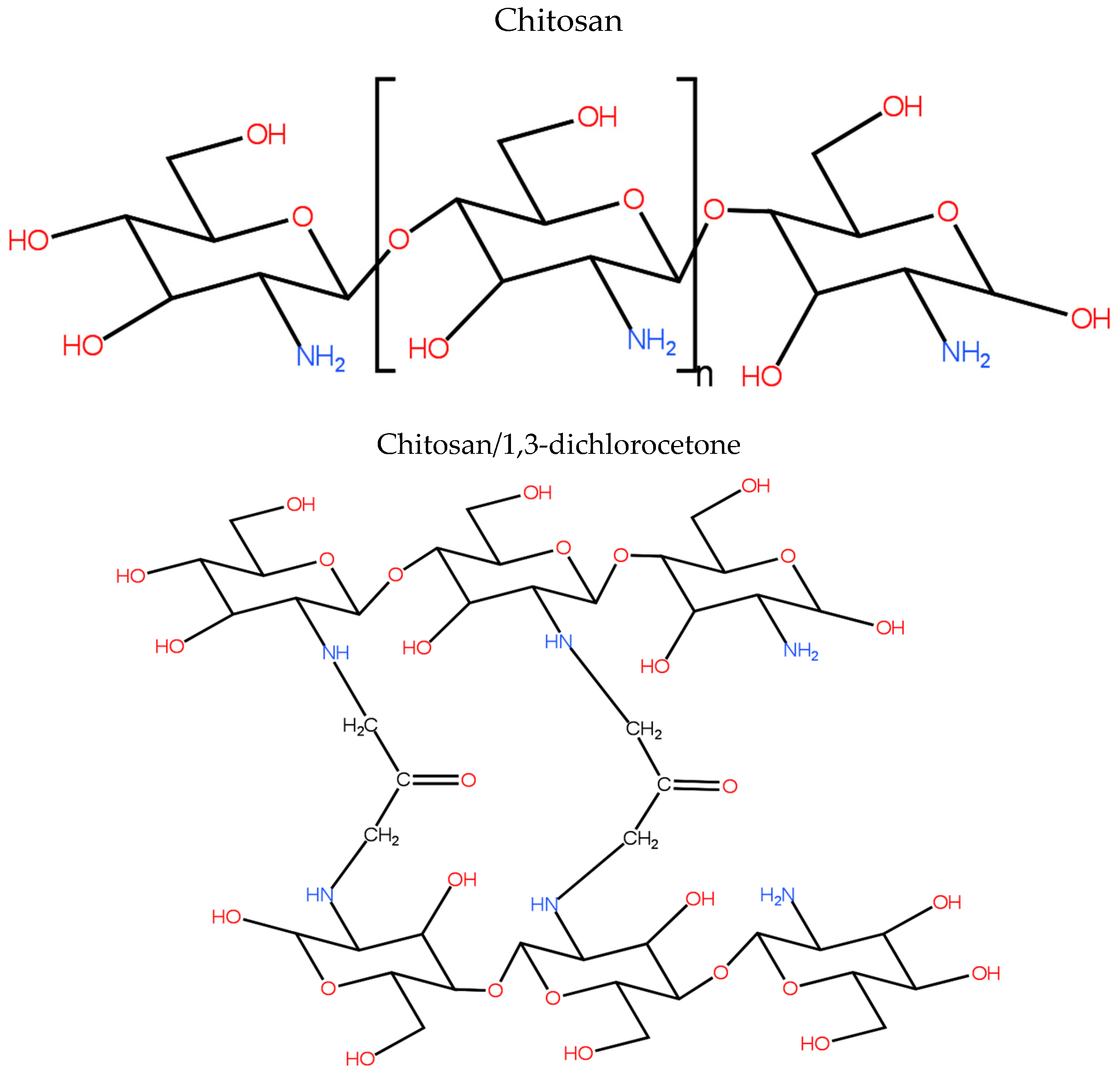
3.2. Model Structure
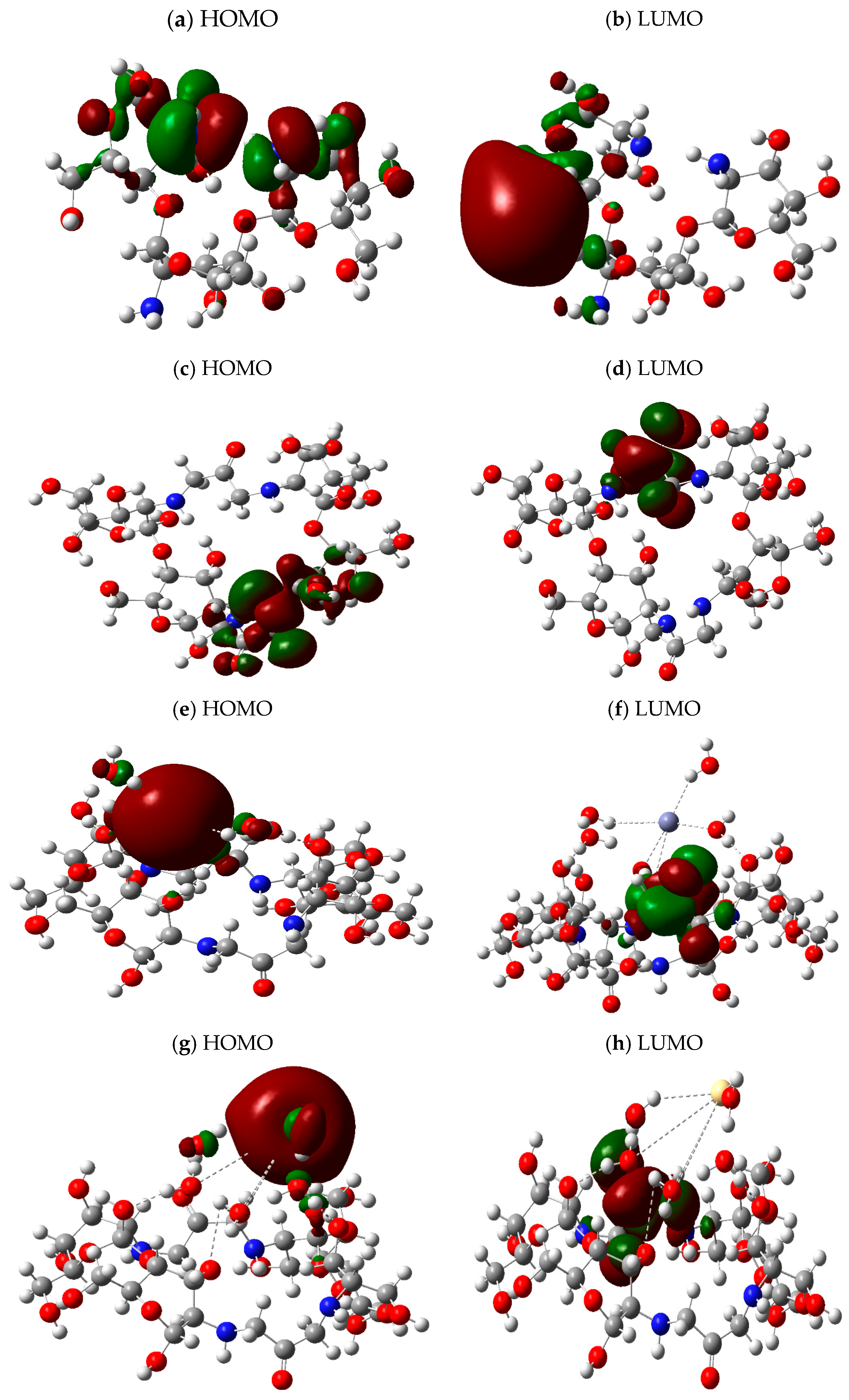
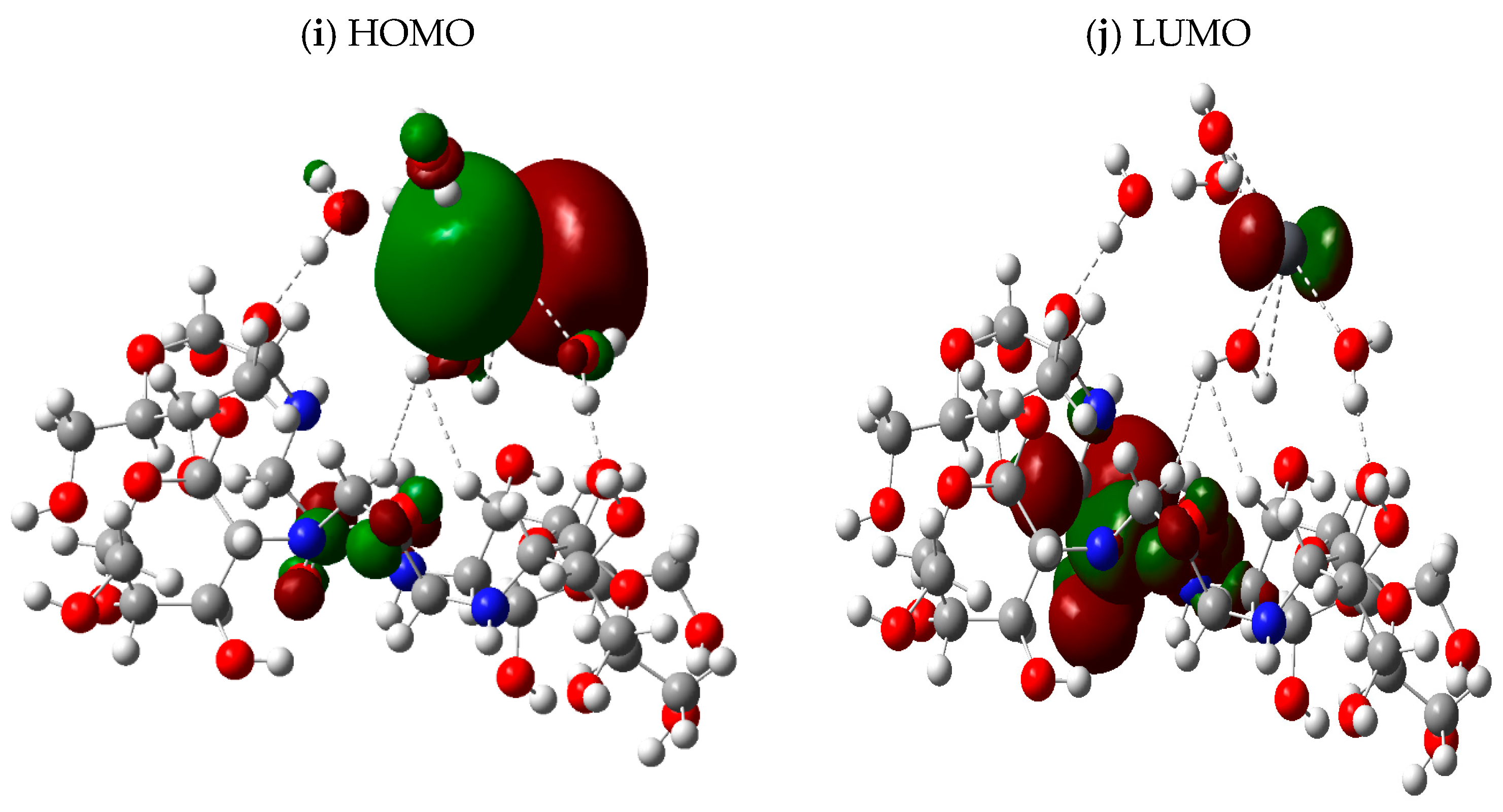
3.3. HOMO–LUMO Calculations
3.4. Global Descriptors
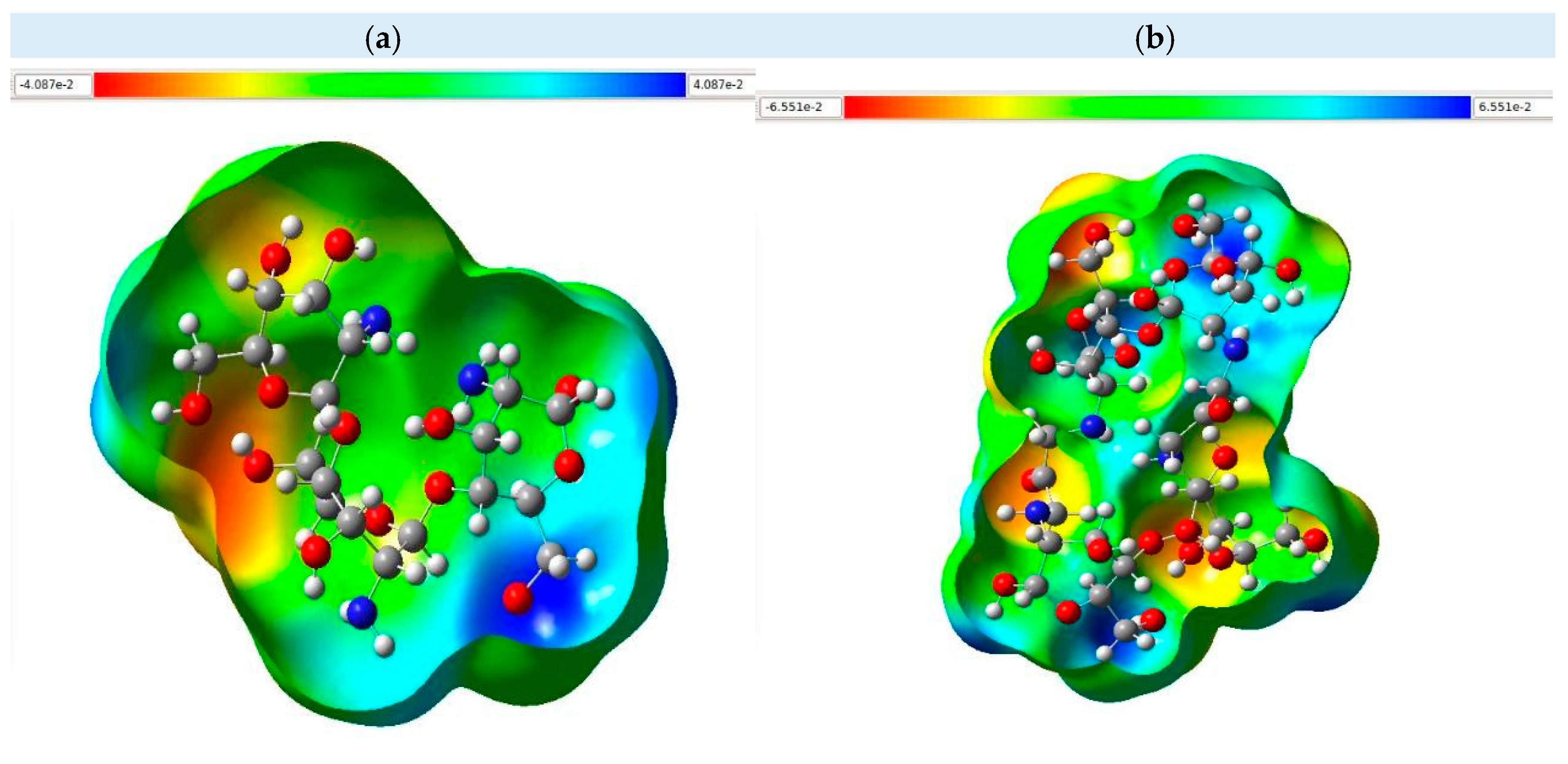
3.5. Molecular Electrostatic Potential Map
3.6. Experimental Results
3.6.1. Performance
3.6.2. Chitosan Solubility
3.6.3. Moisture Content
3.6.4. Ash Content
3.6.5. Degree of Deacetylation (DDA)
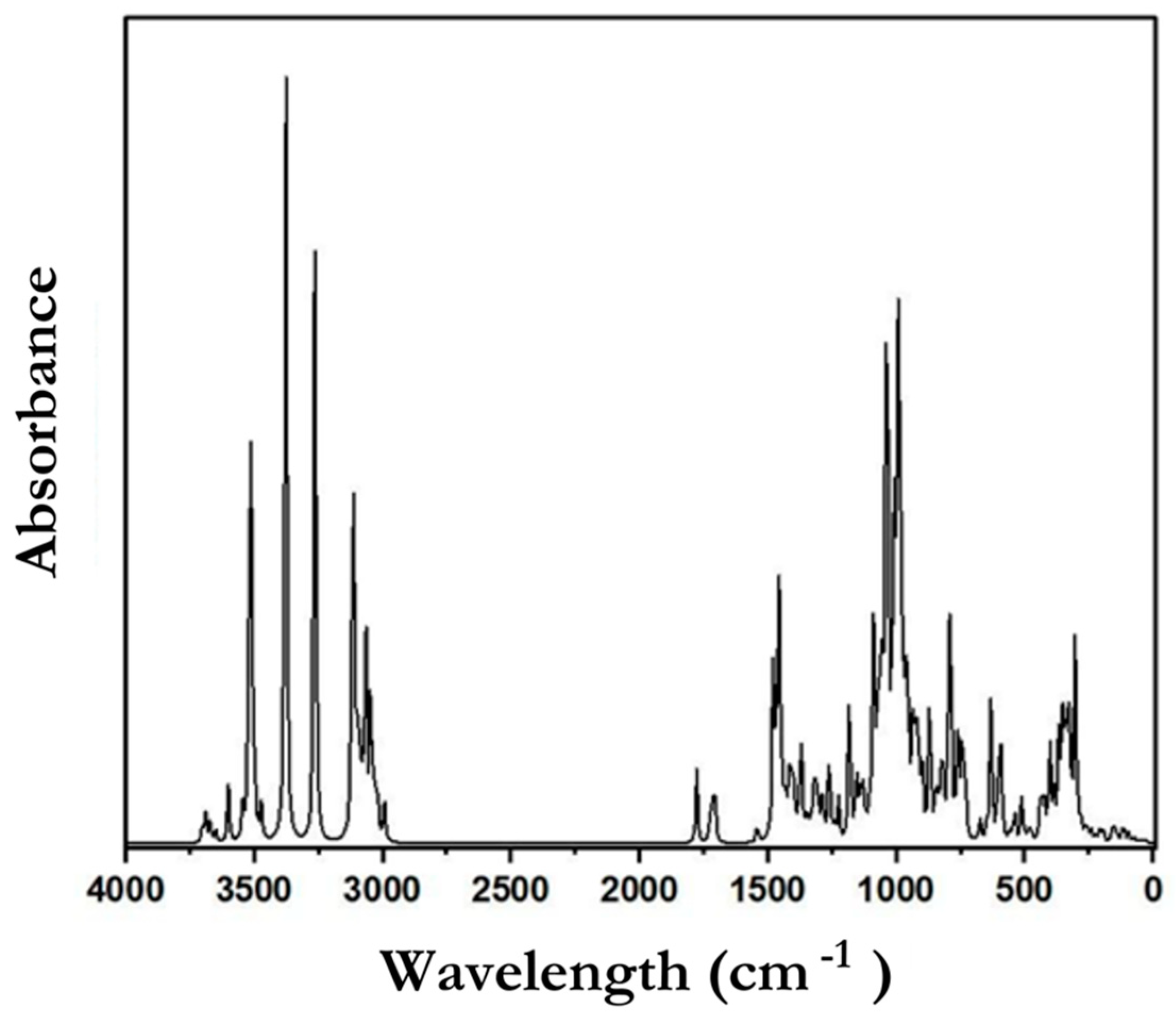
3.6.6. FTIR Analysis
3.6.7. Diffractometry (XRD)
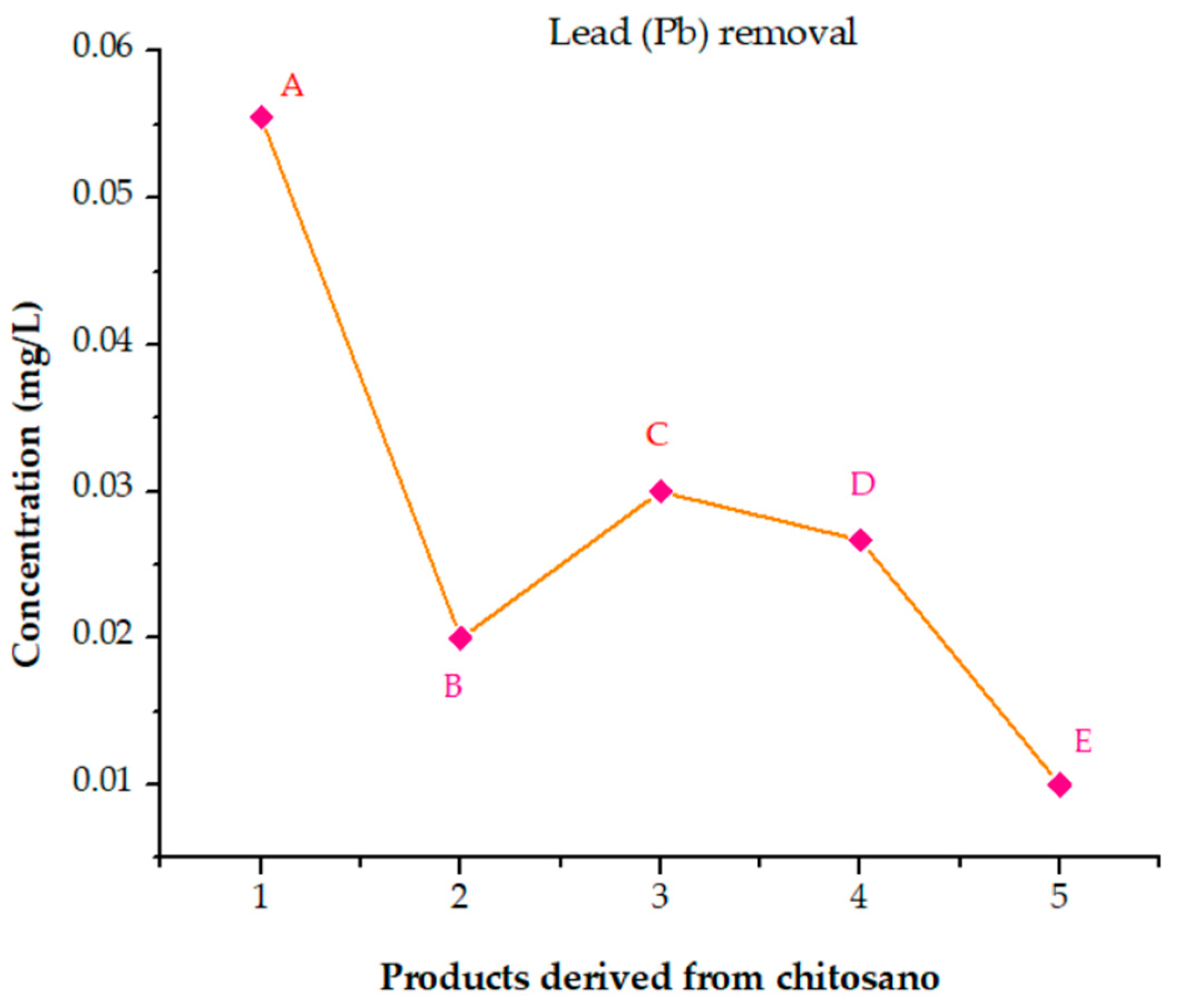
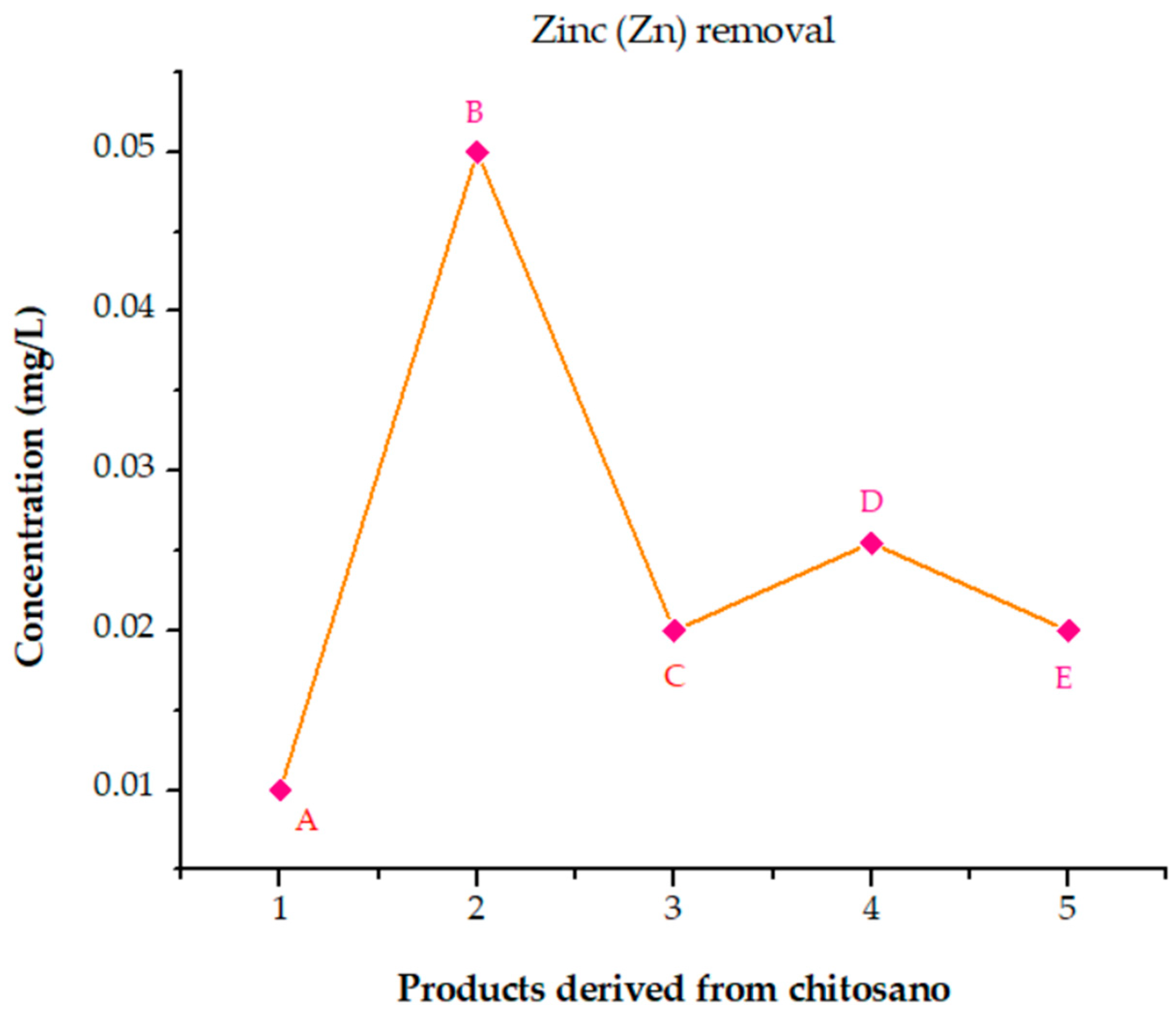
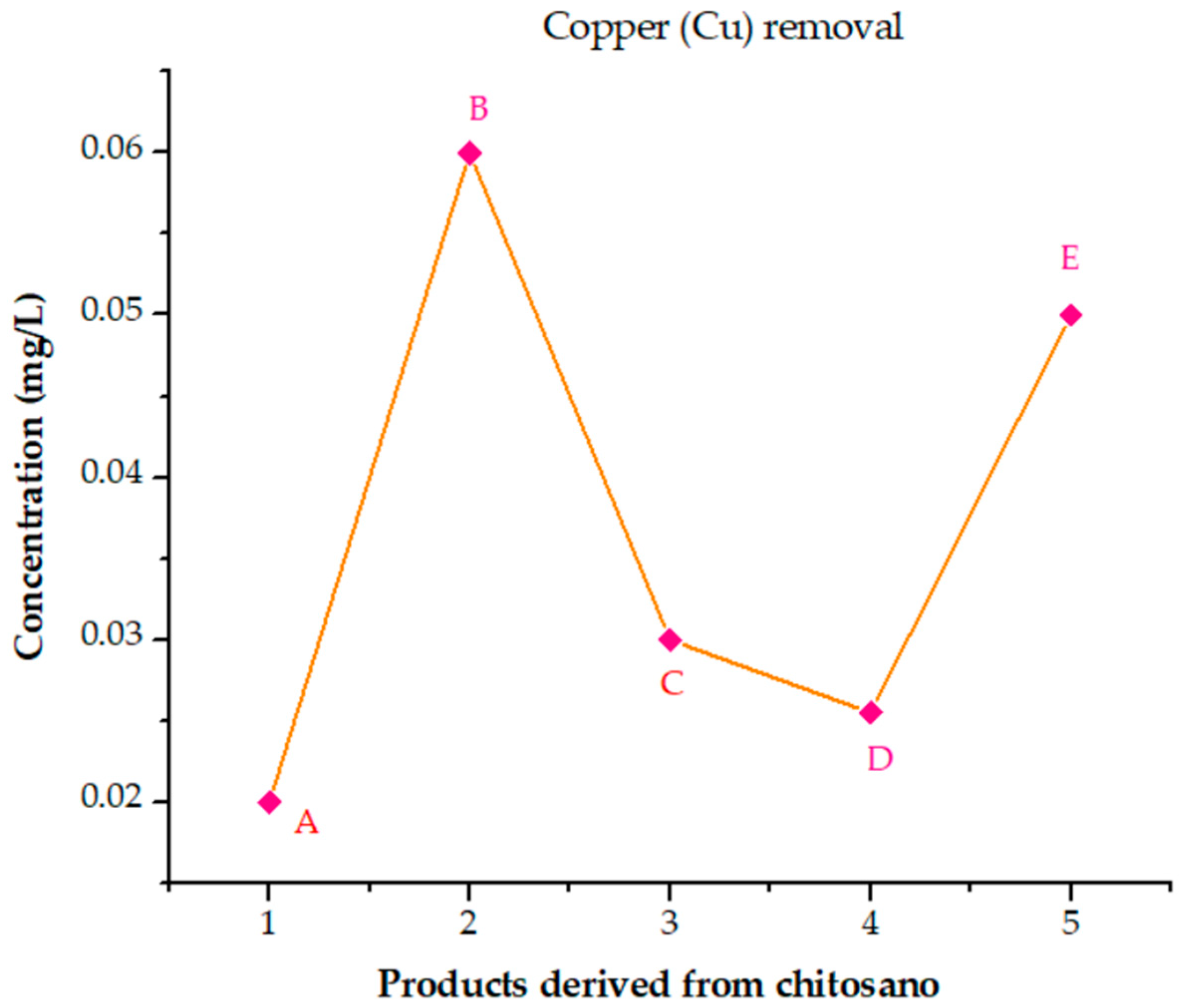
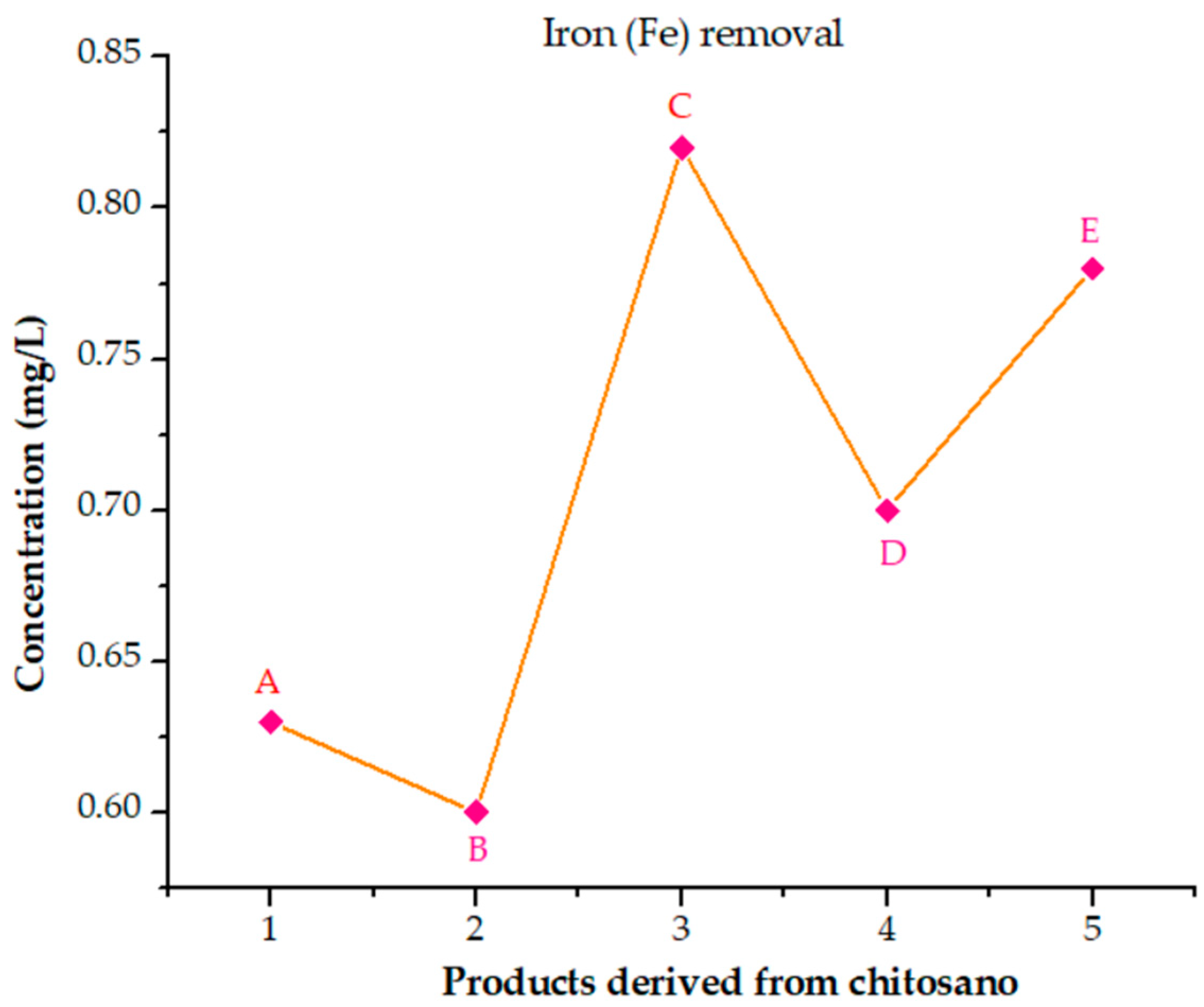
3.6.8. Analysis of the Performance of Cross-Linked Chitosan Products in Removing Elements from Poultry Wastewater
- (A)
- Glutaraldehyde;
- (B)
- Epichlorohydrin;
- (C)
- S-methylbutylamine;
- (D)
- p-Benzoquinone;
- (E)
- 1,3-Dichloroaceton.
4. Conclusions
5. Supplementary Information
- -
- Chemical potential (μ): This descriptor reflects the molecule’s tendency to undergo electron gain or loss and is related to the energetic stability of the system. Low values of μ indicate a greater probability that the molecule will give up electrons, while high values suggest a greater propensity to accept them [37].
- -
- -
- -
- Electronegativity (χ): This descriptor, introduced by Pauling, measures the tendency of an atom or molecule to draw electrons towards itself in a chemical interaction. In global terms, high electronegativity implies more significant electron attraction, which influences the polarity of molecular interactions [39,55].
- -
- Chemical hardness (η): Related to a molecule’s resistance to changes in its electron density, hardness is a key parameter for assessing chemical stability. Higher hardness implies lower reactivity to external agents, while low hardness indicates greater susceptibility to chemical interactions [37,38].
- -
- Electrophilicity (ω): This descriptor combines electronegativity and hardness to quantify a molecule’s ability to accept electrons during a chemical interaction. It is beneficial for identifying highly reactive systems in electron transfer processes or nucleophilic reactions [56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71].
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suba, V.; Rathika, G. Novel Adsorbents for the Removal of Dyes and Metals from Aqueous Solution—A Review. J. Adv. Phys. 2016, 5, 277–294. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.; Ejiogu, I.K. Toxicity and Bioremediation of Heavy Metals Contaminated Ecosystem from Tannery Wastewater: A Review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [PubMed]
- Achary, M.S.; Satpathy, K.; Panigrahi, S.; Mohanty, A.K.; Padhi, R.; Biswas, S.; Prabhu, R.K.; Vijayalakshmi, S.; Panigrahy, R.C. Concentration of heavy metals in the {food chain components of the nearshore coastal waters of Kalpakkam, southeast coast of India. Food Control 2017, 72, 232–243. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Khajeh, A.; Mesbah, M. Membrane filtration of wastewater from gas and oil production. Environ. Chem. Lett. 2017, 16, 367–388. [Google Scholar] [CrossRef]
- Şahinkaya, E.; Şahin, A.Z.; Yurtsever, A.; Kitiş, M. Concentrate minimization and water recovery enhancement using pellet precipitator in a reverse osmosis process treating textile wastewater. J. Environ. Manag. 2018, 222, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zhang, X.; Prabaharan, K.; Dai, Y. Separation of cesium from wastewater with copper hexacyanoferrate film in an electrochemical system driven by microbial fuel cells. Bioresour. Technol. 2019, 278, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.M.; Menazea, A. Laser-assisted for preparation ZnO/CdO thin film prepared by pulsed laser deposition for catalytic degradation. Radiat. Phys. Chem. 2020, 176, 109020. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Mwafy, E.A. Synthesis of ZnO/CdO thin film for catalytic degradation of 4-nitrophenol. J. Mol. Struct. 2020, 1221, 128872. [Google Scholar] [CrossRef]
- Zakaria, M.A.; Menazea, A.; Mostafa, A.M.; Al-Ashkar, E.A. Ultra-thin silver nanoparticles film prepared via pulsed laser deposition: Synthesis, characterization, and its catalytic activity on reduction of 4-nitrophenol. Surf. Interfaces 2020, 19, 100438. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palácio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Rae, I.B.; Pap, S.; Svobodová, D.; Gibb, S.W. Comparison of sustainable biosorbents and ion-exchange resins to remove Sr2+ from simulant nuclear wastewater: Batch, dynamic and mechanism studies. Sci. Total Environ. 2019, 650, 2411–2422. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. Fiber-in-tube solid-phase microextraction of caffeine as a molecular tracer in wastewater by electrochemically deposited layered double hydroxide. J. Sep. Sci. 2018, 41, 2393–2400. [Google Scholar] [CrossRef]
- Mostafa, A.M.; Yousef, S.A.; Eisa, W.H.; Ewaida, M.A.; Al-Ashkar, E.A. WO3 quantum dot: Synthesis, characterization and catalytic activity. J. Mol. Struct. 2019, 1185, 351–356. [Google Scholar] [CrossRef]
- Alghool, S.; El-Halim, H.F.A.; Mostafa, A.M. An Eco-friendly Synthesis of V2O5 Nanoparticles and Their Catalytic Activity for the Degradation of 4-Nitrophrnol. J. Inorg. Organomet. Polym. Mater. 2019, 29, 1324–1330. [Google Scholar] [CrossRef]
- Yu, H.; Hu, J.; Liu, Z.; Ju, X.; Xie, R.; Wang, W.; Chu, L. Ion-recognizable hydrogels for efficient removal of cesium ions from aqueous environment. J. Hazard. Mater. 2017, 323, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Poshina, D.N.; Raik, S.V.; Poshin, A.N.; Skorik, Y.A. Accessibility of chitin and chitosan in enzymatic hydrolysis: A review. Polym. Degrad. Stab. 2018, 156, 269–278. [Google Scholar] [CrossRef]
- Zhang, L.; Zeng, Y.; Cheng, Z. Removal of heavy metal ions using chitosan and modified chitosan: A review. J. Mol. Liq. 2016, 214, 175–191. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Pang, Y.; Chen, D.; Kong, L.; Mehmood, S.; Subbaiah, M.V.; Rao, D.S.; Pavuluri, C.M.; Wen, J.; Reddy, G.M. Modification of chitosan macromolecule and its mechanism for the removal of Pb(II) ions from aqueous environment. Int. J. Biol. Macromol. 2019, 136, 177–188. [Google Scholar] [CrossRef]
- Lee, M.; Hong, K.; Kajiuchi, T.; Yang, J. Synthesis of chitosan-based polymeric surfactants and their adsorption properties for heavy metals and fatty acids. Int. J. Biol. Macromol. 2005, 36, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, N.H.; Shameli, K.; Abdullah, E.C.; Abdullah, L.C. Solid matrices for fabrication of magnetic iron oxide nanocomposites: Synthesis, properties, and application for the adsorption of heavy metal ions and dyes. Compos. Part B Eng. 2019, 162, 538–568. [Google Scholar] [CrossRef]
- Sutirman, Z.A.; Sanagi, M.M.; Karim, K.J.A.; Naim, A.A.; Ibrahim, W.A.W. Chitosan-based adsorbents for the removal of metal ions from aqueous solutions. Malays. J. Anal. Sci. 2018, 22. [Google Scholar] [CrossRef]
- Tommalieh, M.; Ibrahium, H.A.; Awwad, N.S.; Menazea, A. Gold nanoparticles doped Polyvinyl Alcohol/Chitosan blend via laser ablation for electrical conductivity enhancement. J. Mol. Struct. 2020, 1221, 128814. [Google Scholar] [CrossRef]
- Viyapuri, R.; Ward, T.A.; Chee, C.Y.; Nair, P. Physical and chemical reinforcement of chitosan film using nanocrystalline cellulose and tannic acid. Cellulose 2015, 22, 2529–2541. [Google Scholar] [CrossRef]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects-an update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M.; Sivashankari, P. Prospects of Bioactive Chitosan-Based Scaffolds in Tissue Engineering and Regenerative Medicine. In Chitin and Chitosan for Regenerative Medicine; En Springer Series on Polymer and Composite Materials; Springer: Berlin/Heidelberg, Germany, 2015; pp. 41–59. [Google Scholar] [CrossRef]
- Xiao, G.; Su, H.; Tan, T. Synthesis of core–shell bioaffinity chitosan–TiO2 composite and its environmental applications. J. Hazard. Mater. 2015, 283, 888–896. [Google Scholar] [CrossRef] [PubMed]
- Gang, D.D.; Deng, B.; Lin, L. As(III) removal using an iron-impregnated chitosan sorbent. J. Hazard. Mater. 2010, 182, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, W.; Chen, L.; Huang, X.; Liu, J. Preparation and evaluation of magnetic nanoparticles impregnated chitosan beads for arsenic removal from water. Chem. Eng. J. 2014, 251, 25–34. [Google Scholar] [CrossRef]
- Liu, B.; Lv, X.; Meng, X.; Yu, G.; Wang, D. Removal of Pb(II) from aqueous solution using dithiocarbamate modified chitosan beads with Pb(II) as imprinted ions. Chem. Eng. J. 2013, 220, 412–419. [Google Scholar] [CrossRef]
- Zhu, Y.; Hu, J.; Wang, J. Competitive adsorption of Pb(II), Cu(II) and Zn(II) onto xanthate-modified magnetic chitosan. J. Hazard. Mater. 2012, 221–222, 155–161. [Google Scholar] [CrossRef]
- Ngah, W.S.W.; Teong, L.C.; Toh, R.; Hanafiah, M.A.K.M. Comparative study on adsorption and desorption of Cu(II) ions by three types of chitosan–zeolite composites. Chem. Eng. J. 2013, 223, 231–238. [Google Scholar] [CrossRef]
- Negm, N.A.; Sheikh, R.E.; El-Farargy, A.F.; Hefni, H.H.; Bekhit, M. Treatment of industrial wastewater containing copper and cobalt ions using modified chitosan. J. Ind. Eng. Chem. 2015, 21, 526–534. [Google Scholar] [CrossRef]
- Monier, M.; Abdel-Latif, D. Preparation of cross-linked magnetic chitosan-phenylthiourea resin for adsorption of Hg(II), Cd(II) and Zn(II) ions from aqueous solutions. J. Hazard. Mater. 2012, 209, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Sıafaka, P.I.; Lambropoulou, D.A.; Lazaridis, N.K.; Bikiaris, D.N. Poly(itaconic acid)-Grafted Chitosan Adsorbents with Different Cross-Linking for Pb(II) and Cd(II) Uptake. Langmuir 2014, 30, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Tirtom, V.N.; Dinçer, A.; Becerik, S.; Aydemir, T.; Çelik, A. Comparative adsorption of Ni(II) and Cd(II) ions on epichlorohydrin crosslinked chitosan–clay composite beads in aqueous solution. Chem. Eng. J. 2012, 197, 379–386. [Google Scholar] [CrossRef]
- Aliabadi, M.; Irani, M.; Ismaeili, J.; Piri, H.; Parnian, M.J. Electrospun nanofiber membrane of PEO/Chitosan for the adsorption of nickel, cadmium, lead and copper ions from aqueous solution. Chem. Eng. J. 2013, 220, 237–243. [Google Scholar] [CrossRef]
- Vieira, C.L.; Sanches-Neto, F.O.; Carvalho-Silva, V.H.; Signini, R. Design of apolar chitosan-type adsorbent for removal of Cu(II) and Pb(II): An experimental and DFT viewpoint of the complexation process. J. Environ. Chem. Eng. 2019, 7, 103070. [Google Scholar] [CrossRef]
- Ezzat, H.A.; Menazea, A.; Omara, W.; Basyouni, O.H.; Helmy, S.; Mohamed, A.; Tawfik, W. DFT: B3LYP/ LANL2DZ Study for the Removal of Fe, Ni, Cu, As, Cd and Pb with Chitosan. Biointerface Res. Appl. Chem. 2020, 10, 7002–7010. [Google Scholar] [CrossRef]
- Menazea, A.; Ezzat, H.A.; Omara, W.; Basyouni, O.H.; Ibrahim, S.; Mohamed, A.A.; Tawfik, W.; Ibrahim, M. Chitosan/graphene oxide composite as an effective removal of Ni, Cu, As, Cd and Pb from wastewater. Comput. Theor. Chem. 2020, 1189, 112980. [Google Scholar] [CrossRef]
- Mardani, H.R.; Ravari, F.; Kalaki, A.; Hokmabadi, L. New Schiff-Base’s Modified Chitosan: Synthesis, Characterization, Computational, Thermal Study and Comparison on Adsorption of Copper(II) and Nickel(II) Metal Ions in Aqueous. J. Polym. Environ. 2020, 28, 2523–2538. [Google Scholar] [CrossRef]
- Frisch, M.J. Revisión B. 01; Gaussiano. Inc.: Wallingford CT, USA, 2018. [Google Scholar]
- Giroday, T.; Montero-Campillo, M.M.; Mora-Diez, N. Thermodynamic stability of PFOS: M06-2X and B3LYP comparison. Comput. Theor. Chem. 2014, 1046, 81–92. [Google Scholar] [CrossRef]
- Miehlich, B.; Savin, A.; Stoll, H.; Preuß, H. Results obtained with the correlation energy density functionals of becke and Lee, Yang and Parr. Chem. Phys. Lett. 1989, 157, 200–206. [Google Scholar] [CrossRef]
- Alabaraoye, E.; Achilonu, M.; Hester, R. Biopolymer (Chitin) from Various Marine Seashell Wastes: Isolation and Characterization. J. Polym. Environ. 2017, 26, 2207–2218. [Google Scholar] [CrossRef]
- Horwitz, W. Official methods of analysis of the Association of Official Analytical Chemists; Association of Official Agricultural Chemists, Inc.: Gaithersburg, MD, USA, 1990; Volume 1, Available online: https://law.resource.org/pub/us/cfr/ibr/002/aoac.methods.1.1990.pdf (accessed on 20 January 2025).
- Black, C.A.; Evans, D.D.; Ensminger, L.; White, J.; Clark, F. Methods of Soil Analysis. Part 1. Physical and Mineralogical Properties, Including Statistics of Measurement and Sampling; Agronomy Monograph 9.1; American Society of Agronomy (ASA). Inc. Publisher: Madison, WI, USA, 1965. [Google Scholar]
- Jiao, T.F.; Zhou, J.; Zhou, J.; Gao, L.; Xing, Y.; Li, X. Synthesis and characterization of chitosan-based schiff base compounds with aromatic substituent groups. Iran. Polym. J. 2011, 20, 123–136. [Google Scholar]
- Atangana, E.; Chiweshe, T.T.; Roberts, H. Modification of Novel Chitosan-Starch Cross-Linked Derivatives Polymers: Synthesis and Characterization. J. Polym. Environ. 2019, 27, 979–995. [Google Scholar] [CrossRef]
- Petry, R.; Focassio, B.; Schleder, G.; Martinez, D.; Fazzio, A. Análisis conformacional del ácido tánico: Efectos del entorno en las propiedades electrónicas y de reactividad. J. Chem. Phys. 2021, 154, 224102. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, R.; Subramanian, V.; Chattaraj, P. Comparison of Global Reactivity Descriptors Calculated Using Various Density Functionals: A QSAR Perspective. J. Chem. Theory Comput. 2009, 5, 2744–2753. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Kurup, S.; Joshi, K. PyGlobal: A toolkit for automated compilation of DFT-based descriptors. J. Comput. Chem. 2016, 37, 1505–1510. [Google Scholar] [CrossRef]
- Stefaniu, A.; Lucia, P. Molecular Descriptors and Properties of Organic Molecules. In Symmetry (Group Theory) and Mathematical Treatment in Chemistry; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Li, Q.; Dunn, E.; Grandmaison, E.; Goosen, M. Applications and Properties of Chitosan. J. Bioact. Compat. Polym. 1992, 7, 370–397. [Google Scholar] [CrossRef]
- Atangana, E.; Oberholster, P.J. Modified Biopolymer (Chitin–Chitosan Derivatives) for the Removal of Heavy Metals in Poultry Wastewater. J. Polym. Environ. 2019, 28, 388–398. [Google Scholar] [CrossRef]
- Baerends, E. Chemical potential, derivative discontinuity, fractional electrons, jump of the Kohn-Sham potential, atoms as thermodynamic open systems, and other (mis)conceptions of the density functional theory of electrons in molecules. Phys. Chem. Chem. Phys. 2022, 24, 12745–12766. [Google Scholar] [CrossRef] [PubMed]
- Robles, J.; Manzanilla, B. Conceptual DFT Reactivity Descriptors Computational Study of Graphene and Derivatives Flakes: Doped Graphene, Graphane, Fluorographene, Graphene Oxide, Graphyne, and Graphdiyne. Rev. Soc. Química Mex. 2020, 64, 238–252. [Google Scholar] [CrossRef]
- Poier, P.; Jensen, F. Describing Molecular Polarizability by a Bond Capacity Model. J. Chem. Theory Comput. 2019, 15, 3093–3107. [Google Scholar] [CrossRef] [PubMed]
- Allgäuer, D.; Jangra, H.; Asahara, H.; Li, Z.; Chen, Q.; Zipse, H.; Ofial, A.; Mayr, H. Quantification and Theoretical Analysis of the Electrophilicities of Michael Acceptors. J. Am. Chem. Soc. 2017, 139, 13318–13329. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Pei, Z.; Gong, C.; Mo, H.; Yang, K.; Qu, L.; Wei, D.; Song, J.; Li, S.; Lan, Y. Is the reaction sequence in phosphine-catalyzed [8+2] cycloaddition controlled by electrophilicity? Chem. Commun. 2020, 57, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernandez, J.; Rodríguez, E. Determination of phenolic antioxidants additives in industrial wastewater from polypropylene production using solid phase extraction with high-performance liquid chromatography. J. Chromatogr. A 2019, 1607, 460442. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano, H.; Aldas, M. Impact of Traces of Hydrogen Sulfide on the Efficiency of Ziegler–Natta Catalyst on the Final Properties of Polypropylene. Polymers 2022, 14, 3910. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Guerra, Y.; Espinosa, E. Development and Application of a Principal Component Analysis Model to Quantify the Green Ethylene Content in Virgin Impact Copolymer Resins During Their Synthesis on an Industrial Scale. J. Polym. Env. 2022, 30, 4800–4808. [Google Scholar] [CrossRef]
- Chaco, H.; Cano, H.; Hernandez-Fernandez, J.; Guerra, Y.; Puello-Polo, E.; Rios-Rojas, J.; Ruiz, Y. Effect of Addition of Polyurea as an Aggregate in Mortars: Analysis of Microstructure and Strength. Polymers 2022, 14, 1753. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Ortega-Toro, R.; López-Martinez, J. A New Route of Valorization of Petrochemical Wastewater: Recovery of 1,3,5-Tris (4-tert-butyl-3-hydroxy-2,6-dimethyl benzyl)–1,3,5-triazine-2,4,6-(1H,3H,5H)-trione (Cyanox 1790) and Its Subsequent Application in a PP Matrix to Improve Its Thermal Stability. Molecules 2023, 28, 2003. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.H.; Cano, H.; Guerra, Y.; Polo, E.P.; Ríos-Rojas, J.F.; Vivas-Reyes, R.; Oviedo, J. Identification and Quantification of Microplastics in Effluents of Wastewater Treatment Plant by Differential Scanning Calorimetry (DSC). Sustainability 2022, 14, 4920. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Lopez-Martinez, J.; Barceló, D. Development and validation of a methodology for quantifying partsper-billion levels of arsine and phosphine in nitrogen, hydrogen, and liquefied petroleum gas using a variable pressure sampler coupled to gas chromatography-mass spectrometry. J. Chromatogr. A 2021, 1637, 461833. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Castro-Suarez, J.R.; Toloza, C.A.T. Iron Oxide Powder as Responsible for the Generation of Industrial PolypropyleneWaste and as a Co-Catalyst for the Pyrolysis of Non-Additive Resins. Int. J. Mol. Sci. 2022, 23, 11708. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Fernandez, J.; Cano, H.; Guerra, Y. Detection of Bisphenol A and Four Analogues in Atmospheric Emissions in Petrochemical Complexes Producing Polypropylene in South America. Molecules 2022, 27, 4832. [Google Scholar] [CrossRef]
- Hernández-Fernández, J.; Cano, H.; Reyes, A.F. Valuation of the Synthetic Antioxidant Tris-(Diterbutyl-Phenol)-Phosphite (Irgafos P-168) from Industrial Wastewater and Application in Polypropylene Matrices to Minimize Its Thermal Degradation. Molecules 2023, 28, 3163. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; Vivas-Reyes, R.; Toloza, C.A.T. Experimental Study of the Impact of Trace Amounts of Acetylene and Methylacetylene on the Synthesis, Mechanical and Thermal Properties of Polypropylene. Int. J. Mol. Sci. 2022, 23, 12148. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fernández, J.; González-Cuello, R.; Ortega-Toro, R. Parts per Million of Propanol and Arsine as Responsible for the Poisoning of the Propylene Polymerization Reaction. Polymers 2023, 15, 3619. [Google Scholar] [CrossRef]




| Parameters | Percentages |
|---|---|
| Performance | 85 |
| Solubility | Soluble in acetic acid (1.5%) Medium soluble in H2O Insoluble in NaOH |
| Ash content | 2.4 |
| Moisture content | 7.3 |
| Degree of deacetylation | 73 |
| Chitosan | Electronic Energy (Hartree) | Total Dipole Moment (TDM) (Debye) |
| B3LYP/LANLD2Z | −1849.47570 | 5.39787 |
| M06-2X/LANLD2Z | −1848.96588 | 2.17032 |
| M05-2X/LANLD2Z | −1849.17438 | 2.88629 |
| Chitosan/1,3-dichlorocetone | Electronic Energy (Hartree) | Total Dipole Moment (TDM) (Debye) |
| B3LYP/LANLD2Z | −3049.37516 | 5.40473 |
| M06-2X/LANLD2Z | −3048.12123 | 7.02216 |
| M05-2X/LANLD2Z | −3048.89902 | 5.90192 |
| Structure | Total Dipole Moment (TDM) (Debye) | HOMO eV | LUMO eV | ∆E eV |
|---|---|---|---|---|
| Chitosan | 5.397866 | −0.24324 | −0.02965 | 0.21359 |
| Chitosan/1,3-dichlorocetone | 5.404731 | −0.23983 | −0.04618 | 0.19365 |
| Chitosan/1,3-dichlorocetone + Zn | 14.693271 | −0.16098 | −0.06345 | 0.09753 |
| Chitosan/1,3-dichlorocetone + Cd | 4.515224 | −0.17554 | −0.06478 | 0.11076 |
| Chitosan/1,3-dichlorocetone + Pb | 7.448823 | −0.05993 | −0.04508 | 0.01485 |
| Name | Chemical Potential (μ) | Ionization Potential (I) | Electronegativity (χ) | Electronic Affinity (A) | Electrophilicity (ω) | Hardness (η) |
|---|---|---|---|---|---|---|
| Chitosan | −0.21359 | 0.24324 | 0.13645 | 0.02965 | 0.00244 | 0.10680 |
| Chitosan/1,3-dichlorocetone | −0.19365 | 0.23983 | 0.14301 | 0.04618 | 0.00182 | 0.09683 |
| Chitosan/1,3-dichlorocetone + Zn | −0.09753 | 0.16098 | 0.11222 | 0.06345 | 0.00023 | 0.04877 |
| Chitosan/1,3-dichlorocetone + Cd | −0.11076 | 0.17554 | 0.12016 | 0.06478 | 0.00034 | 0.05538 |
| Chitosan/1,3-dichlorocetone + Pb | −0.01485 | 0.05993 | 0.05251 | 0.04508 | 0.00000 | 0.00743 |
| Metal | Concentration Range (mg/L) | qmax (mg/g) | KL (L/mg) | % Removal |
|---|---|---|---|---|
| Pb | 0.01–0.05 | 0.05 | 0.45 | 72 |
| Cr | 0.01–0.02 | 0.02 | 0.38 | 68 |
| Cu | 0.011–0.05 | 0.05 | 0.42 | 70 |
| Zn | 0.017–0.09 | 0.09 | 0.35 | 63 |
| Fe | 0.50–0.85 | 0.85 | 0.30 | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández Fernández, J.A.; Prieto Palomo, J.A.; Ortega-Toro, R. Application of DFT and Experimental Tests for the Study of Compost Formation Between Chitosan-1,3-dichloroketone with Uses for the Removal of Heavy Metals in Wastewater. J. Compos. Sci. 2025, 9, 91. https://doi.org/10.3390/jcs9020091
Hernández Fernández JA, Prieto Palomo JA, Ortega-Toro R. Application of DFT and Experimental Tests for the Study of Compost Formation Between Chitosan-1,3-dichloroketone with Uses for the Removal of Heavy Metals in Wastewater. Journal of Composites Science. 2025; 9(2):91. https://doi.org/10.3390/jcs9020091
Chicago/Turabian StyleHernández Fernández, Joaquín Alejandro, Jose Alfonso Prieto Palomo, and Rodrigo Ortega-Toro. 2025. "Application of DFT and Experimental Tests for the Study of Compost Formation Between Chitosan-1,3-dichloroketone with Uses for the Removal of Heavy Metals in Wastewater" Journal of Composites Science 9, no. 2: 91. https://doi.org/10.3390/jcs9020091
APA StyleHernández Fernández, J. A., Prieto Palomo, J. A., & Ortega-Toro, R. (2025). Application of DFT and Experimental Tests for the Study of Compost Formation Between Chitosan-1,3-dichloroketone with Uses for the Removal of Heavy Metals in Wastewater. Journal of Composites Science, 9(2), 91. https://doi.org/10.3390/jcs9020091








