Pineapple-Derived Nanocellulose for Nanocomposites: Extraction, Processing, and Properties
Abstract
1. Introduction
2. Scope and Review Method
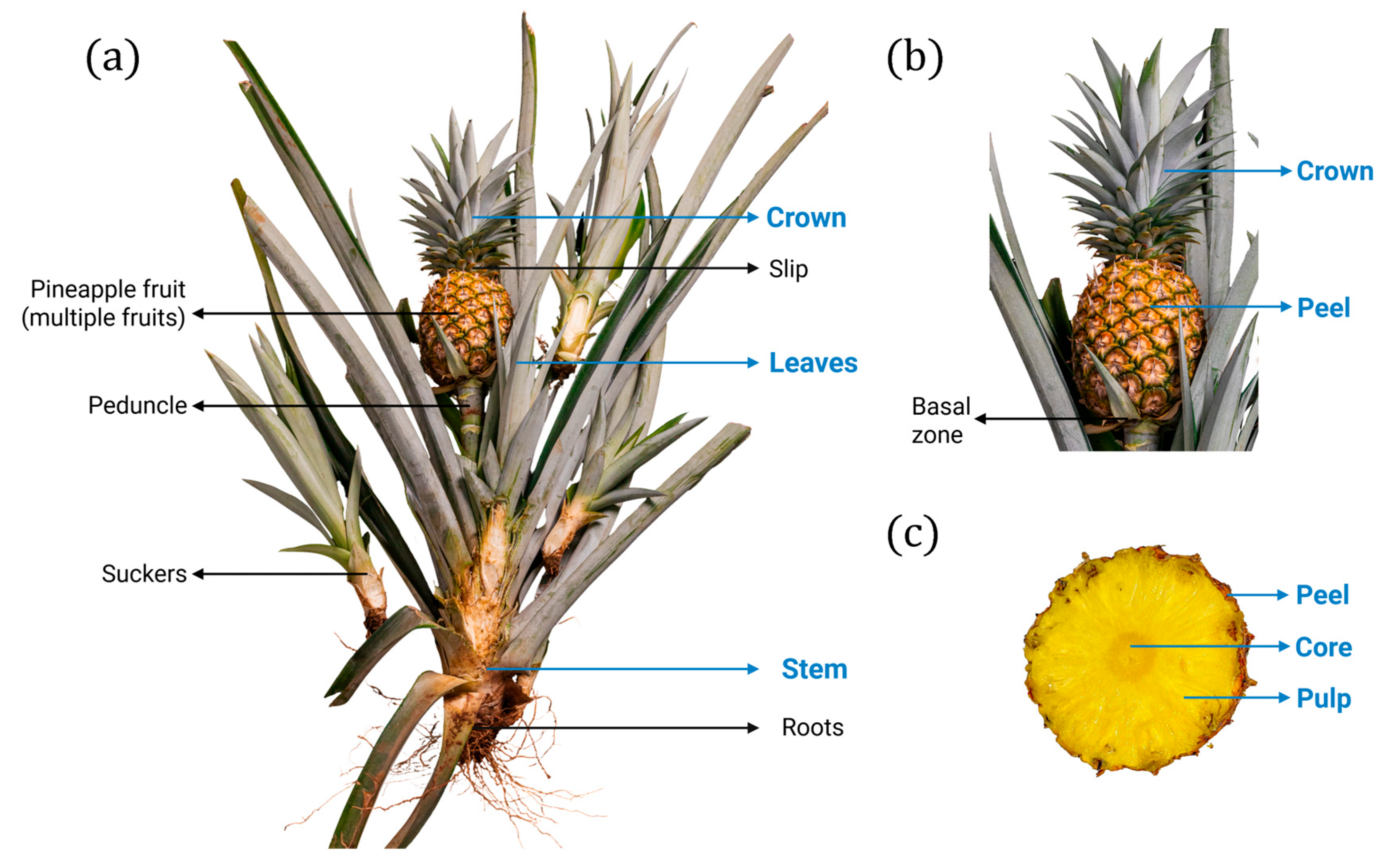
3. Pineapple Cultivation and Biomass Residues
| Pineapple Part | Cellulose * (%) | Hemi Cellulose (%) * | Lignin (%) * | Ash (%) * | Methods Used |
|---|---|---|---|---|---|
| Leaves | 37.25 [41]–81.27 [18] | 12.31 [18]–33.93 [41] | 3.46 [18]–15.9 [42] | 5.32 [41] | ASTM D1103-55T, ASTM D1104-56, ASTM D1106-56, ASTM D4442-92 [18,41,43]; TAPPI T9M-54, TAPPI T13M-54, ASTM D1104-56 [42] |
| Crown leaves | 51.2 [44]–51.4 [45] | 13.3 [44]–13.4 [45] | 12.2 [45]–13.40 [44] | 2.30 [44]–6.73 [45] | TAPPI T 550 om-03, TAPPI T 204 cm-97, TAPPI T 222 om-02, TAPPI T 203 cm-99 [45], Chesson–Datta) [44] |
| Peels | 16.9 [46]–45.52 [41] | 15.8 [46]–39.55 [41] | 15.43 [41]–28.9 [46] | 3.82 [41]–3.92 [46] | ASTM D1103-55T, ASTM D1104-56, ASTM D1106-56, ASTM D4442-92 [41], TAPPI T222 om-88, Browning method [46] |
| Core | 67.86 | 31.64 | 9.31 | 1.70 | ASTM D1103-55T, ASTM D1104-56, ASTM D1106-56, ASTM D4442-92 [41] |
| Pulp | 44.89 | 27.31 | 15.09 | 2.56 | ASTM D1103-55T, ASTM D1104-56, ASTM D1106-56, ASTM D4442-92 [41] |
| Pomace | 31.78 | 60.19 | 3.44 | - | ASTM D1103-55T, ASTM D1104-56, ASTM D1106-56 [47] |
| Stem | 31.86 [48]–37 [49] | 37 [49]–46.15 [48] | 18.60 [48]–20 [49] | 0.40 [48] | Modified Iwamoto et al. [50] method [48], standard methods (numbers not specified) [49] |
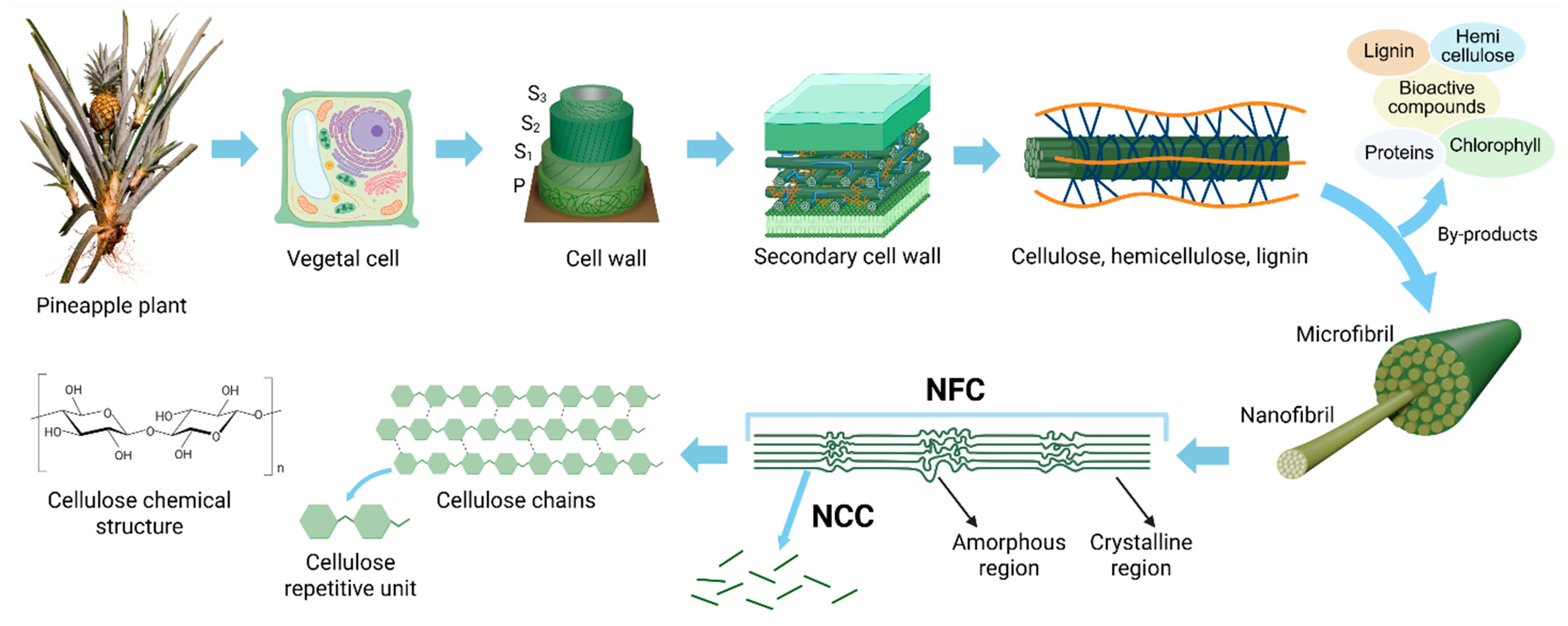
4. Pineapple Nanocellulose
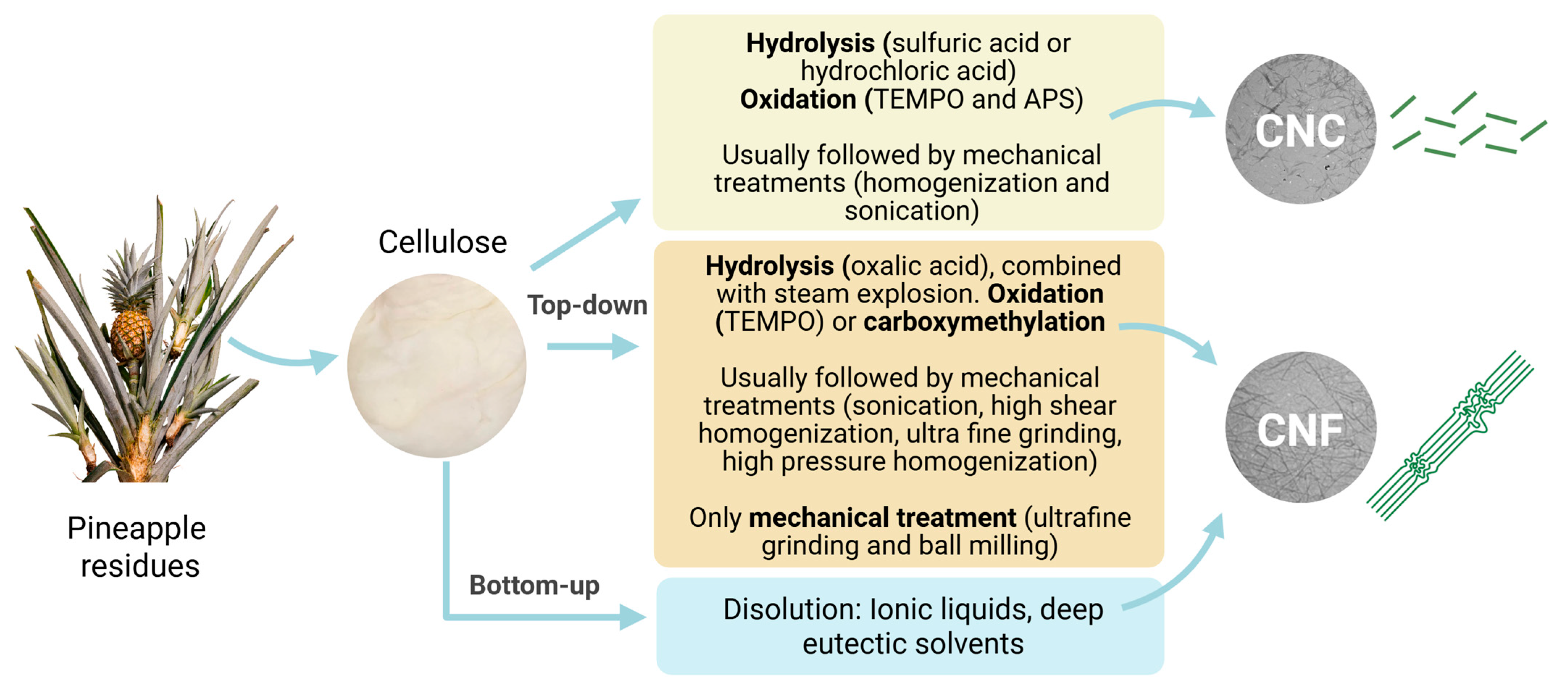
4.1. Extraction Pathways
4.2. General Applications of Pineapple Nanocellulose
4.3. Nanocomposites with Pineapple Nanocellulose
4.3.1. Starch
4.3.2. Chitosan
4.3.3. Polyvinyl Alcohol (PVA)
4.3.4. Whey Protein Isolate (WPI)
4.3.5. Bio-Based Thermoplastic Matrices
4.3.6. Other Proteins (Gelatin)
4.3.7. Polysaccharides
4.3.8. Synthetic Polymers
4.3.9. Natural Rubber (NR)
4.3.10. Epoxy and Urea-Formaldehyde Resins
4.4. Self-Reinforced and Cellulose-Based Nanocomposites
5. Quantitative Benchmarking Across Systems
| Matrix | NC Type, Pineapple Fraction, and Method | Loading (wt.%) and Additives or Treatment and Process | Properties and Trends * |
|---|---|---|---|
| Starch (potato) | CNF (leaves, acid hydrolysis, oxalic acid in autoclave) | 1–4% (optimum 3, relative to starch). Glycerol (30 wt.%, relative to starch). Solution casting. | @ 3%. WVTR: 4.39 → 3.41 g·h−1·m−2 (−22%); SOL: 76.14 → 36.09% (−53%); MA: 16.1 → 11.56% (−28%); DC: 1.59 → 0.087 × 10−4 mm2·s−1 (−95%) RE: 47%; Tonset: ↑; E′ ↑; Tmax ↑; UVB ↑; TP ↔; WCA ↑ [70,78,79] |
| Starch (bengkoang) | CNF (leaves, HCl hydrolysis, high shear homogenization, ultrasonication) | 0.5–2% (optimum 2, relative to starch). Glycerol (20 w/v%, relative to starch), ultrasonication. Solution casting + ultrasonication. | @ 2%. TS: 3.8 → 9.8 MPa (+158%); MA: 20 → 9% (−55%); WVP: 5.6 × 10−11 → 4.5 × 10−11 g·m−1·s−1·Pa−1 (−20%); Tmax: 245 → 310 °C (+27%); CI: 21.71 → 35.17% (+62%); OP: 91.2 → 253.7 AU (+178%, TP: ↓); E: ↑; EB: ↓; [82] |
| Starch (bitter cassava) | CNC (leaves, H2SO4 hydrolysis) | 15% (fixed, relative to starch). Glycerol (20 wt.%). Solution casting. | @ 15%. TS: 1.26 → 1.80 MPa (+43%); E: 4.47 → 11.95 MPa (+167%); EB: 76.77 → 41.75% (−46%); SOL: 6.34 → 4.76% (−25%); WVTR: 2.47 → 2.07 g·h−1·m−2 (−16%); WVP: 4.0 × 10−11 → 3.36 × 10−11 g·m−1·s−1·Pa−1 (−16%); Tmax: 345 → 346 °C (+0.3%); OP: 16.59 → 17.75% (+7%, TP: ↓) [94] |
| PVA | CNC (peel, H2SO4 hydrolysis) | 2–8% (optimum 2%, relative to PVA). Tannic acid (2–8 wt.%, optimum 8%, relative to PVA). Solution casting. | @ 2% + 8% TA. TS: 58.5 → 108.7 MPa (+86%); TP @ 500 nm: 79 → 72% (−9%); EB: ↓; WA: ↓; SOL: ↑ with TA; ↓ with CNC; UVB: ↑; Antimic: S. aureus [77] |
| CNF (leaves, ultra-fine grinder) | 10–50% (optimum 20–40). Glycerol (1 wt.%). Solution casting. | @ 40%. d: 0.68; MC: 6.03 → 5.62% (−7%); Th: 0.11 → 0.02 mm; Tm: 329.54 → 315.27 °C (−4%); CI: 38.3 → 37.8% (−1.3%); WVTR: ↑; TP: ↓ [21] | |
| CNF (leaves, high-shear homogenization + ultrasonication) | 1–5% (optimum 4, relative to PVA). Solution casting. | @ 4%. TS: 22.5 → 28.9 MPa (+28%); EB: 178.4 → 211.4% (+18.5%); CI: 74.8 → 78.8% (+5%); Tmax: 336.3 → 341.8 °C (+1.6%); TP: >75% all films [100] | |
| PVA/chitosan blend (2:1) | CNC (leaves, H2SO4 hydrolysis) | ~0.44% (fixed, relative to total polymer matrix). PEG (2 wt.%). Solution casting. | @ 0.44%. TS: 11.40 → 18.25 MPa (+60%); Tonset: 248 → 274 °C (+11%); SW: 442 → 282% (−36%) [90] |
| Chitosan/Starch (50:50) | CNC (crown leaves, H2SO4 hydrolysis) | 0.3–1.0% (0.7 optimum). Sorbitol (0.5 mL/g starch). Solution casting. | @ 0.7%. E′ (40 °C): 2484 → 4709 MPa (+90%); Tg: 66 → 73 °C (+11%); thermal stability ↔ [95] |
| WPI | CNC (crown leaves, H2SO4 hydrolysis) | 3–7% (optimum 3.5% via RSM). Glycerol (4–8%, 4 wt.% optimum). Solution casting. | @ 3.5% (RSM-optimized). TS: 7.16 MPa; EB: 39.10%; WVP: 2.21× 10−11 → 2.17 × 10−11 g·m−1·s−1·Pa−1 (−2%); SOL: 30.21 → 27.15% (−10%); CI: 74.82 → 81.14% (+8%); TP: ↓ [97] |
| WPI/PVA (70/30) | CNC (crown leaves, H2SO4 hydrolysis) | 3.5% (Fixed). Glycerol (4 wt.%). Solution casting. | @ 3.5%; no baseline reported for 70/30 blend. TS: 7.061 MPa; EB: 68.831%; WVP: 1.765 × 10−11 g·m−1·s−1·Pa−1; SOL: 24.507%; Tonset: 258 °C; Tmax: 330 °C; TP: ↑ vs. WPI; Degradation: 35% weight loss @ 24d. [102] |
| Gelatin | CNC (leaves, H2SO4 hydrolysis) | 1–5% (optimum 5). Glycerol (10% w/v) + banana leaf extract (BL). Solution casting. | @ 5% + BL. TS: 4.71 → 82.23 MPa (+1646%); EB: 2 → 16% (+700%); Thermal stability: ↑; OP: ↓; SW ↓; WVP ↓; Antimic: E. coli, S. aureus, C. perfringens [14] |
| 1–5% (optimum 5). Glycerol + lotus extract (LE). Tissue paper spray coating. | @ 5% + LE, baseline: uncoated tissue paper. Th: 0.125 → 0.591 mm (+373%); Grammage: 23.48 → 32.36 g/m2 (+38%); d: 0.765 → 0.498 g/cm3 (−35%); WVP: 24.56 × 10−10 → 2.09 × 10−10 g·m−1·s−1·Pa−1 (−91%); WCA: 74.6 → 70.2°; TS: ↑; EB: ↓; Antimic: S. aureus, E. coli, C. glabrata [109] | ||
| Carrageenan (+ starch granules) | CNF (leaves, acid hydrolysis, oxalic acid in autoclave) | 0.2–0.3% (optimum 0.2). Glycerol (0.6 wt.% + sesame oil + aloe vera gel 3%). Solution casting. | @ 0.2% (Carr:Starch 1.5:1); no baseline reported. TS: 5.64 MPa; ME: 4.62 mm; WVP: 6.4 × 10−10 g·m−1·s−1·Pa−1; OP: 2.0%; SOL: 42%; MA: 29%; Th: 150 μm; d: 1.34 g/cm3; WCA: 71°; Thermal stability: stable to 200 °C [98] |
| Gellan gum | CNC (peel, H2SO4 hydrolysis) | 2–10% (optimum 4, relative to gellan gum). Glycerol 15 wt.% (relative to gellan gum). Solution casting. | @4%. TS: 2.24 → 3.32 MPa (+48.2%); Tonset: 161 → 165 °C (+2.5%); Tmax: 241 → 243 °C (+0.8%); EB: ↓; CI: ↑; TP @ 800 nm ↓; [77] |
| 5% (1.2 mL) (+ essential oil 4%). Glycerol 0.01% + Tween20 5 vol.% + Anethum graveolens oil. | @5 (1.2 mL) + 4% oil, no baseline reported. TS: 10.36 MPa; EB: 78.87%; SOL: 60.11%; WVP: 5.16 × 10−12 g·m−1·s−1·Pa−1; Antimic: A. niger. [86] | ||
| PLA | CNC (leaves, H2SO4 hydrolysis, cinnamate functionalized) | 0.5–5% (optimum 3). CNC cinnamate grafting via esterification. Solution casting. | @ 3%. TS: ↑ (+70%); E: ↑ (+37%); EB: ↓; WVP: ↓ (−54%); OP: ↓ (−55%); Tg: 58.3 → 55.2 °C (−5.3%); Tc: 116.7 → 108.1 °C (−7.4%); Tm: 149.4 → 140.8/150.5 °C; UVB: Effective absorption (280–320 nm); TP @visible: ↔ [85] |
| CNF (leaves, TEMPO oxidation) | 1–3% (optimum 3). MMA grafting. Melt blending + compression molding. | @ 3%. TS: 49.8 → 61.1 MPa (+22.7%); IS: 17.1 → 26.7 J/m (+56.1%); Tmax: 380.7 → 377.4 °C (−0.9%); Tc: 108.5 → 114.1 °C (+5.2%); Tm: 147.2/157.5 → 147.4/153.1 °C [20] | |
| PHBV | CNC (crown leaves, H2SO4 hydrolysis) | 1–5% (optimum 3, relative to PHBV). Solution casting. | @ 3%: CI: 37.0 → 41.0% (+10.8%); Tonset: 262 → 264 °C (+0.8%); Tmax: 286 → 287 °C (+0.3%); Tm: 156.0 → 153.5 °C (−1.6%); WVP: 1.39 × 10−11 → 4.14 × 10−11 g·m−1·s−1·Pa−1 (+198%); Tc: ↓; Crystallization rate: ↑; Lifetime @180 °C: no degradation 1000 min [13] |
| CMC | CNC (leaves, H2SO4 hydrolysis) | 15–45% (optimum 30, relative to CMC). Glycerol 30 wt.% (relative to CMC). Solution casting. | @ 30%. TS: 1.60 → 5.07 MPa (+217%); WVTR: 2.77 → 2.10 g·h−1·m−2 (−24%); Tonset: 179 → 184 °C (+3%); Tmax: 260 → 261 °C (+0.4%); EB: 200 → 220% (+10%); TP: ↓ [99] |
| Rubber (NR) | CNC (leaves, H2SO4 hydrolysis) | 2.5–10 phr (~2.4–9.1%, optimum (~2.4%). SV or EB (200 kGy). Casting + vulcanization (SV)/irradiation + casting (EB). | @ 2.5 phr (~2.4%) (S-vul). TS: 17.4 → 19.4 MPa (+11%); EB: 604 → 634% (+5%); E@100%: 1.08 → 1.83 MPa (+69%); SW: 4.42 → 4.86 (+10%) (toluene, 7 d). @ 2.5 phr (~2.4%) (EB). TS: 13.5 → 15.8 MPa (+17%); EB: 783 → 806% (+3%); E@100%: 0.63 → 0.87 MPa (+38%); SW: 6.26 → 6.18 (−1%) (toluene, 1 d) [84] |
| PP | CNC (peel, HCl hydrolysis) | 3% (fixed). MAPP 5 wt.%. Melt blending + injection molding. | @ 3% + MAPP. TS: 33.60 → 38.75 MPa (+15.3%); E: 1238 → 1665 MPa (+34.5%); EB: 420 → 61% (−85%); IS: 21.96 → 25.7 J/m (+17%); E′−25 °C: 3975 → 4998 MPa (+26%); WCA: 92 → 80° (−13%); Tg ↑ [61] |
| PS | CNF (leaves, TEMPO oxidation) | 0.5–3% (optimum 1.0 SF; 3.0 GF). Styrene suspension polymerization (SF) or Sol–gel + phenyltriethoxysilane (GF). Melt blending + compression molding. | @ 1.0% (SF). TS: 23.1 → 31.1 MPa (+34.6%); HDT: 90.3 → 92.8 °C (+2.66%). @ 3.0% (GF). TS: 23.1 → 28.9 MPa (+25.1%); HDT: 90.3 → 93.8 °C (+3.9%) [105] |
| PMMA | CNF (leaves, TEMPO oxidation) | 0.5–3% (optimum 1%). Suspension polymerization with MMA. Melt blending + compression molding. | @ 1%. IS: 14.8 → 18.2 J/m (+22.9%); TS: 43.5 → 44.2 MPa (+1.6%); E: 2350 → 3020 MPa (+28.5%); EB: 12.95 → 9.52% (−26.5%); Tmax: 371.4 → 373.9 °C (+0.7%); TP @ visible: ↔ [104] |
| PU | CNF (leaves, oxalic acid hydrolysis, and steam explosion) | 2–10% (optimum 5%). Film-stacking + compression molding. | @ 5%. TS: 17.5 → 52.6 MPa (+201%); E: 37.5 → 992.4 MPa (+2546%); Fatigue: 608 million cycles (~15 years); EB: ↑ [54] |
| Cellulose matrix (pineapple peel) | CNF (peels, Pretreatment with Fe2+ assisted cold plasma + nanofibrillation) | 5–20% (application 15%, relative to cellulose). Carnauba wax 20 wt.% (relative to cellulose). Coating + aqueous coagulation bath + annealing. | @ 15%. Tmax: 332.7 → 337.1 °C (+1.3%); WCA: >100° (↔); OTR: ↓; TS:↑; E:↑; EB:↑ [75] |
6. Conceptual Framework and Potential for Bioeconomy Integration
7. Limitations and Challenges
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paz-Arteaga, S.L.; Cadena-Chamorro, E.; Goméz-García, R.; Serna-Cock, L.; Aguilar, C.N.; Torres-León, C. Unraveling the Valorization Potential of Pineapple Waste to Obtain Value-Added Products towards a Sustainable Circular Bioeconomy. Sustainability 2024, 16, 7236. [Google Scholar] [CrossRef]
- Bukhari, I.; Haq, F.; Kiran, M.; Aziz, T.; Mehmood, S.; Haroon, M. Lignocellulosic biomass as a renewable resource: Driving second-generation biofuel innovation from agricultural waste. Biomass Bioenergy 2025, 201, 108133. [Google Scholar] [CrossRef]
- Ubando, A.T.; Felix, C.B.; Chen, W.-H. Biorefineries in circular bioeconomy: A comprehensive review. Bioresour. Technol. 2020, 299, 122585. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Singh, T.A.; Singh, N.J.; Shadangi, K.P.; Srivastava, R.K.; Singh, A.K.; Chandel, A.K.; Pareek, N.; Vivekanand, V. Sustainable utilization of pineapple wastes for production of bioenergy, biochemicals and value-added products: A review. Bioresour. Technol. 2022, 351, 127085. [Google Scholar] [CrossRef]
- Aguilar, A.; Twardowski, T.; Wohlgemuth, R. Bioeconomy for sustainable development. Biotechnol. J. 2019, 14, 1800638. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Ureña, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Xu, C.; Nasrollahzadeh, M.; Selva, M.; Issaabadi, Z.; Luque, R. Waste-to-wealth: Biowaste valorization into valuable bio (nano) materials. Chem. Soc. Rev. 2019, 48, 4791–4822. [Google Scholar] [CrossRef]
- Sarangi, P.K.; Srivastava, R.K.; Sahoo, U.K.; Singh, A.K.; Parikh, J.; Bansod, S.; Parsai, G.; Luqman, M.; Shadangi, K.P.; Diwan, D.; et al. Biotechnological innovations in nanocellulose production from waste biomass with a focus on pineapple waste. Chemosphere 2024, 349, 140833. [Google Scholar] [CrossRef] [PubMed]
- FAO. Major Tropical Fruits Market Review: Preliminary Results 2023; Food and Agriculture Organization: Québec City, QC, Canada, 2024. [Google Scholar]
- Araya-Chavarría, K.; Rojas, R.; Ramírez-Amador, K.; Sulbarán-Rangel, B.; Rojas, O.; Esquivel-Alfaro, M. Cellulose nanofibers as functional biomaterial from pineapple stubbles via TEMPO oxidation and mechanical process. Waste Biomass Valorization 2022, 13, 1749–1758. [Google Scholar] [CrossRef]
- Rini; Suryanto, H.; Hari, P.D.; Syukri, D.; Jaswandi; Kurniawan, F.; Makky, M. Application of Nanocellulose Biofilter from Pineapple Peel Waste for Water Microbes Removal. J. Environ. Public Health 2023, 2023, 5823207. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- de Carvalho Benini, K.C.C.; Ornaghi Jr, H.L.; de Medeiros, N.M.; Fernandes Pereira, P.H.; Hilário Cioffi, M.O. Thermal characterization and lifetime prediction of the PHBV/nanocellulose biocomposites using different kinetic approaches. Cellulose 2020, 27, 7503–7522. [Google Scholar] [CrossRef]
- Sasikala, M.; Umapathy, M. Preparation and characterization of pineapple leaf cellulose nanocrystal reinforced gelatin bio-nanocomposite with antibacterial banana leaf extract for application in food packaging. New J. Chem. 2018, 42, 19979–19986. [Google Scholar] [CrossRef]
- Palacios, H.; Urena-Saborio, H.; Zurita, F.; Guerrero de León, A.A.; Sundaram, G.; Sulbarán-Rangel, B. Nanocellulose and Polycaprolactone Nanospun Composite Membranes and Their Potential for the Removal of Pollutants from Water. Molecules 2020, 25, 683. [Google Scholar] [CrossRef]
- Choudhary, N.; Singh, S.; Bhardwaj, S.; Gupta, S.; Nandi, U.; Chandra, R.; Maji, P.K. Recent advancements in nanocellulose-based supercapacitors for energy storage devices: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100416. [Google Scholar] [CrossRef]
- Kumari, S.; Chauhan, R.P.S.; Mishra, A.; Kumar, P. A review on nanocellulose and its potential biomedical applications. Trends Biomater. Artif. Organs 2021, 35, 303–315. [Google Scholar] [CrossRef]
- Cherian, B.M.; Leão, A.L.; De Souza, S.F.; Thomas, S.; Pothan, L.A.; Kottaisamy, M. Isolation of nanocellulose from pineapple leaf fibres by steam explosion. Carbohydr. Polym. 2010, 81, 720–725. [Google Scholar] [CrossRef]
- Dos Santos, R.M.; Neto, W.P.F.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Chou, M.-Y.; Chang, W.-C.; Lian, H.-Y.; Chen, C.-M. Completely biodegradable composites reinforced by the cellulose nanofibers of pineapple leaves modified by eco-friendly methods. J. Polym. Res. 2017, 24, 209. [Google Scholar] [CrossRef]
- Wahyuningsih, K.; Iriani, E.S.; Fahma, F. Utilization of cellulose from pineapple leaf fibers as nanofiller in polyvinyl alcohol-based film. Indones. J. Chem. 2016, 16, 181–189. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, H.; Ma, L.; Zhou, H.; Yu, Y.; Guo, T.; Zhang, Y.; Huang, H. Green pH/magnetic sensitive hydrogels based on pineapple peel cellulose and polyvinyl alcohol: Synthesis, characterization and naringin prolonged release. Carbohydr. Polym. 2019, 209, 51–61. [Google Scholar] [CrossRef]
- Asim, M.; Abdan, K.; Jawaid, M.; Nasir, M.; Dashtizadeh, Z.; Ishak, M.; Hoque, M.E. A review on pineapple leaves fibre and its composites. Int. J. Polym. Sci. 2015, 2015, 950567. [Google Scholar] [CrossRef]
- Liao, S.; Chen, J.; Wang, X. An Update on Pineapple Leaf Fibers. J. Nat. Fibers 2025, 22, 2509129. [Google Scholar] [CrossRef]
- Sethupathi, M.; Khumalo, M.V.; Skosana, S.J.; Muniyasamy, S. Recent developments of pineapple leaf fiber (PALF) utilization in the polymer composites—A review. Separations 2024, 11, 245. [Google Scholar] [CrossRef]
- Norizan, M.N.; Shazleen, S.S.; Alias, A.H.; Sabaruddin, F.A.; Asyraf, M.R.M.; Zainudin, E.S.; Abdullah, N.; Samsudin, M.S.; Kamarudin, S.H.; Norrrahim, M.N.F. Nanocellulose-based nanocomposites for sustainable applications: A review. Nanomaterials 2022, 12, 3483. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zhang, C.; Liu, K.; Duan, G. Source of nanocellulose and its application in nanocomposite packaging material: A review. Nanomaterials 2022, 12, 3158. [Google Scholar] [CrossRef]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Jose, S.A.; Cowan, N.; Davidson, M.; Godina, G.; Smith, I.; Xin, J.; Menezes, P.L. A comprehensive review on cellulose Nanofibers, nanomaterials, and composites: Manufacturing, properties, and applications. Nanomaterials 2025, 15, 356. [Google Scholar] [CrossRef]
- Meena, L.; Sengar, A.S.; Neog, R.; Sunil, C. Pineapple processing waste (PPW): Bioactive compounds, their extraction, and utilisation: A review. J. Food Sci. Technol. 2022, 59, 4152–4164. [Google Scholar] [CrossRef] [PubMed]
- Monteiro Vieira, I.M.P.S.; Passos Santos, B.L.; Marques Santos, C.V.; Santos Ruzene, D.; Pereira Silva, D. Valorization of pineapple waste: A review on how the fruit’s potential can reduce residue generation. BioEnergy Res. 2022, 15, 924–934. [Google Scholar] [CrossRef]
- Cheng, Y.; Bartholomew, D.; Qin, Y. Biology of the pineapple plant. In Genetics and Genomics of Pineapple; Springer: Berlin/Heidelberg, Germany, 2018; pp. 27–40. [Google Scholar]
- Vargas Céspedes, A.; Morales, M.; Watler, W.; Vignola, R. Prácticas efectivas para la reducción de impactos por eventos climáticos / no climáticos: Cultivo de piña en Costa Rica (technical inform F01-8166). Ministerio de Agricultura y Ganadería de Costa Rica. 2018. Available online: http://www.mag.go.cr/bibliotecavirtual/F01-8166.pdf (accessed on 25 October 2025).
- Pandit, P.; Pandey, R.; Singha, K.; Shrivastava, S.; Gupta, V.; Jose, S. Pineapple leaf fibre: Cultivation and production. In Pineapple Leaf Fibers: Processing, Properties and Applications; Jawaid, M., Asim, M., Tahir, P.M., Nasir, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–20. [Google Scholar]
- d’Eeckenbrugge, G.C.; Leal, F. Morphology, anatomy and taxonomy. In The pineapple: Botany, Production and Uses; CAB International: Wallingford, UK, 2018; pp. 11–31. [Google Scholar]
- Ming, R. Genetics and Genomics of Pineapple; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Singha, K.; Pandit, P.; Shrivastava, S. Anatomical structure of pineapple leaf fiber. In Pineapple Leaf Fibers: Processing, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 21–39. [Google Scholar]
- Lobo, M.G.; Yahia, E. Biology and postharvest physiology of pineapple. In Handbook of Pineapple Technology: Production, Postharvest Science, Processing and Nutrition; Wiley: Hoboken, NJ, USA, 2017; pp. 39–61. [Google Scholar]
- Rigg-Aguilar, P.; Moya, R.; Oporto-Velásquez, G.S.; Vega-Baudrit, J.; Starbird, R.; Puente-Urbina, A.; Méndez, D.; Potosme, L.D.; Esquivel, M. Micro-and Nanofibrillated Cellulose (MNFC) from Pineapple (Ananas comosus) Stems and Their Application on Polyvinyl Acetate (PVAc) and Urea-Formaldehyde (UF) Wood Adhesives. J. Nanomater. 2020, 2020, 1393160. [Google Scholar] [CrossRef]
- Zhou, W.; Ye, C.; Geng, L.; Chen, G.; Wang, X.; Chen, W.; Sa, R.; Zhang, J.; Zhang, X. Purification and characterization of bromelain from pineapple (Ananas comosus L.) peel waste. J. Food Sci. 2021, 86, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Liao, J.; Dai, H.; Liu, Y.; Huang, H. Comparison of cellulose nanocrystals from pineapple residues and its preliminary application for Pickering emulsions. Nanotechnology 2021, 32, 495708. [Google Scholar] [CrossRef] [PubMed]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of nanocellulose from pineapple leaf fibers via high-shear homogenization and ultrasonication. Fibers 2018, 6, 28. [Google Scholar] [CrossRef]
- Abraham, E.; Deepa, B.; Pothan, L.A.; Jacob, M.; Thomas, S.; Cvelbar, U.; Anandjiwala, R. Extraction of nanocellulose fibrils from lignocellulosic fibres: A novel approach. Carbohydr. Polym. 2011, 86, 1468–1475. [Google Scholar] [CrossRef]
- Fitriani, F.; Aprilia, S.; Arahman, N.; Bilad, M.R.; Amin, A.; Huda, N.; Roslan, J. Isolation and characterization of nanocrystalline cellulose isolated from pineapple crown leaf fiber agricultural wastes using acid hydrolysis. Polymers 2021, 13, 4188. [Google Scholar] [CrossRef]
- Faria, L.U.S.; Pacheco, B.J.S.; Oliveira, G.C.; Silva, J.L. Production of cellulose nanocrystals from pineapple crown fibers through alkaline pretreatment and acid hydrolysis under different conditions. J. Mater. Res. Technol. 2020, 9, 12346–12353. [Google Scholar] [CrossRef]
- Madureira, A.R.; Atatoprak, T.; Cabuk, D.; Sousa, F.; Pullar, R.C.; Pintado, M. Extraction and characterisation of cellulose nanocrystals from pineapple peel. Int. J. Food Stud. 2018, 7, 24–33. [Google Scholar] [CrossRef]
- Neenu, K.; Dominic, C.M.; Begum, P.S.; Parameswaranpillai, J.; Kanoth, B.P.; David, D.A.; Sajadi, S.M.; Dhanyasree, P.; Ajithkumar, T.; Badawi, M. Effect of oxalic acid and sulphuric acid hydrolysis on the preparation and properties of pineapple pomace derived cellulose nanofibers and nanopapers. Int. J. Biol. Macromol. 2022, 209, 1745–1759. [Google Scholar] [CrossRef]
- Chu, P.H.; Jenol, M.A.; Phang, L.Y.; Ibrahim, M.F.; Prasongsuk, S.; Bankeeree, W.; Punnapayak, H.; Lotrakul, P.; Abd-Aziz, S. Starch extracted from pineapple (Ananas comosus) plant stem as a source for amino acids production. Chem. Biol. Technol. Agric. 2021, 8, 29. [Google Scholar] [CrossRef]
- Mansor, A.M.; Lima, J.S.; Anib, F.N.; Hashima, H.; Hoa, W.S. Characteristics of cellulose, hemicellulose and lignin of MD2 pineapple biomass. Chem. Eng. 2019, 72, 79–84. [Google Scholar] [CrossRef]
- Iwamoto, S.; Abe, K.; Yano, H. The effect of hemicelluloses on wood pulp nanofibrillation and nanofiber network characteristics. Biomacromolecules 2008, 9, 1022–1026. [Google Scholar] [CrossRef]
- Krasznai, D.J.; Champagne Hartley, R.; Roy, H.M.; Champagne, P.; Cunningham, M.F. Compositional analysis of lignocellulosic biomass: Conventional methodologies and future outlook. Crit. Rev. Biotechnol. 2018, 38, 199–217. [Google Scholar] [CrossRef]
- Fareez, I.M.; Haque, N.; Juin Ooi, D.; Jasni, A.H.; Aziz, F.A. Physicochemical properties of nanocellulose extracted from pineapple leaf fibres and its composites. In Pineapple Leaf Fibers: Processing, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 167–183. [Google Scholar]
- Prado, K.S.; Jacinto, A.A.; Spinacé, M.A. Cellulose nanostructures extracted from pineapple fibres. In Pineapple Leaf Fibers: Processing, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2020; pp. 185–234. [Google Scholar]
- Cherian, B.M.; Leão, A.L.; de Souza, S.F.; Costa, L.M.M.; de Olyveira, G.M.; Kottaisamy, M.; Nagarajan, E.; Thomas, S. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr. Polym. 2011, 86, 1790–1798. [Google Scholar] [CrossRef]
- Esquivel-Alfaro, M.; Sulbarán-Rangel, B.; Rojas-Carrillo, O.; Chen, J.; Rodríguez-Quesada, L.; Sáenz-Arce, G.; Rojas, O.J. Processing of Pineapple Leaf Fibers for the Production of Oxidized Micro-/Nanofibrillated Cellulose. Polymers 2025, 17, 2671. [Google Scholar] [CrossRef]
- Tian, Y.; Huang, R.; Chen, Y.; Wang, T.; Wu, J.; Wang, S. The preparation and characterization of pineapple peel cellulose nanofibers and its application in oil-water emulsions. Carbohydr. Polym. 2025, 353, 123245. [Google Scholar] [CrossRef]
- Fernandes Pereira, P.H.; Ornaghi Júnior, H.L.; Venâncio Coutinho, L.D.; Duchemin, B.; Hilário Cioffi, M.O. Obtaining cellulose nanocrystals from pineapple crown fibers by free-chlorite hydrolysis with sulfuric acid: Physical, chemical and structural characterization. Cellulose 2020, 27, 5745–5756. [Google Scholar] [CrossRef]
- Moreno, G.; Ramirez, K.; Esquivel, M.; Jimenez, G. Isolation and characterization of nanocellulose obtained from industrial crop waste resources by using mild acid hydrolysis. J. Renew. Mater. 2018, 6, 362–369. [Google Scholar] [CrossRef]
- Camacho, M.; Ureña, Y.R.C.; Lopretti, M.; Carballo, L.B.; Moreno, G.; Alfaro, B.; Baudrit, J.R.V. Synthesis and characterization of nanocrystalline cellulose derived from pineapple peel residues. J. Renew. Mater. 2017, 5, 271–279. [Google Scholar] [CrossRef]
- Phosanam, A.; Moreira, J.; Adhikari, B.; Adhikari, A.; Losso, J.N. Stabilization of ginger essential oil Pickering emulsions by pineapple cellulose nanocrystals. Curr. Res. Food Sci. 2023, 7, 100575. [Google Scholar] [CrossRef]
- Agarwal, J.; Mohanty, S.; Nayak, S.K. Valorization of pineapple peel waste and sisal fiber: Study of cellulose nanocrystals on polypropylene nanocomposites. J. Appl. Polym. Sci. 2020, 137, 49291. [Google Scholar] [CrossRef]
- Nasrudin, R.F.; Samat, N.; Mokhtar Nur, A.; Yacobb, N. Production of cellulose nanocrystals from oil palm empty fruit bunch and pineapple leaf fibre using double oxidation approach. J. Teknol. (Sci. Eng.) 2022, 84, 73–81. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.; Verma, V.; Kumar, S.; Bhatia, M. Isolation, characterization and evaluation of pineapple crown waste nanofiber gel entrapping ampicillin in topical bacterial infections. Iran. Polym. J. 2024, 33, 687–698. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Hu, S.; Yu, J.; Huang, Y.; Dai, H. Preparation of Agrowaste-Based Nanocellulose by NaOH-Assisted Ball Milling Technique: Influence of Component Intervention. Gels 2025, 11, 631. [Google Scholar] [CrossRef]
- Romruen, O.; Kaewprachu, P.; Karbowiak, T.; Rawdkuen, S. Isolation and characterization cellulose nanosphere from different agricultural by-products. Polymers 2022, 14, 2534. [Google Scholar] [CrossRef]
- Nguyen, N.T.T.; Phan-Huynh, M.-A.; Le Anh, K.; Van Hong Thien, D.; Hara, K.; Van-Pham, D.-T. Pineapple leaf-derived TEMPO-oxidized cellulose nanospheres and graphene oxide composite: A green solution for ciprofloxacin adsorption. Cellulose 2025, 32, 3317–3334. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.H.; Wei, X.Y.; Li, J.H.; Wang, F. Preparation of pineapple leaf cellulose membrane. Adv. Mater. Res. 2014, 1046, 13–17. [Google Scholar] [CrossRef]
- Parsai, G.; Parikh, P.A.; Parikh, J.K. Novel DES-ultrasonication assisted process for nanocellulose synthesis using Box Behnken design. Ind. Crops Prod. 2024, 217, 118856. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Sreekala, M.; Kunaver, M.; Huskić, M.; Thomas, S. Morphology, transport characteristics and viscoelastic polymer chain confinement in nanocomposites based on thermoplastic potato starch and cellulose nanofibers from pineapple leaf. Carbohydr. Polym. 2017, 169, 176–188. [Google Scholar] [CrossRef]
- Lv, T.; Luo, Y.; Chen, Y.; Dai, D.; Feng, X.; Chen, H.; Yu, Y.; Ma, L.; Zhang, Y.; Dai, H. Tuning the properties of pineapple peel cellulose nanofibrils by TEMPO-mediated oxidation and ball milling. Cellulose 2022, 29, 9609–9625. [Google Scholar] [CrossRef]
- Qian, Y.; Kuang, Y.; Zhang, Y.; Wei, Y.; Liu, Y.; Wang, C.; Chen, G. High-value utilization of pineapple leaf fibers towards high-performance electromagnetic shielding materials. Mater. Today Nano 2023, 24, 100393. [Google Scholar] [CrossRef]
- Raharjo, Y.; Darmokoesoemo, H.; Fetty, A.J.T.; Aziz, R.A.; Salsabila, F.; Ishma, E.F. Cellulose nanocrystals based on pineapple leaf fibers in hemoperfusion applications for creatinine removal: Batch method adsorption study. J. Kim. Ris. 2024, 9, 163–181. [Google Scholar] [CrossRef]
- Gao, T.M.; Huang, M.F.; Li, P.W.; Han, Z.P.; Xie, R.H.; Chen, H.L. Preparation and characterization nano-cellulose and its surface modification by Silane Coupling Agent. Appl. Mech. Mater. 2012, 217, 260–263. [Google Scholar] [CrossRef]
- Zhu, H.; Cheng, J.-H.; Han, Z. Construction of a sustainable and hydrophobic high-performance all-green pineapple peel cellulose nanocomposite film for food packaging. Int. J. Biol. Macromol. 2024, 256, 128396. [Google Scholar] [CrossRef]
- Dai, H.; Huang, Y.; Huang, H. Enhanced performances of polyvinyl alcohol films by introducing tannic acid and pineapple peel-derived cellulose nanocrystals. Cellulose 2018, 25, 4623–4637. [Google Scholar] [CrossRef]
- Dai, H.; Ou, S.; Huang, Y.; Huang, H. Utilization of pineapple peel for production of nanocellulose and film application. Cellulose 2018, 25, 1743–1756. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Gopi, S.; Geethamma, V.; Kalarikkal, N.; Thomas, S. Cellulose nanofiber vs nanocrystals from pineapple leaf fiber: A comparative studies on reinforcing efficiency on starch nanocomposites. Macromol. Symp. 2018, 380, 1800102. [Google Scholar] [CrossRef]
- Balakrishnan, P.; Gopi, S.; MS, S.; Thomas, S. UV resistant transparent bionanocomposite films based on potato starch/cellulose for sustainable packaging. Starch-Stärke 2018, 70, 1700139. [Google Scholar] [CrossRef]
- Ravindran, L.; Sreekala, M.; Thomas, S. Novel processing parameters for the extraction of cellulose nanofibres (CNF) from environmentally benign pineapple leaf fibres (PALF): Structure-property relationships. Int. J. Biol. Macromol. 2019, 131, 858–870. [Google Scholar] [CrossRef] [PubMed]
- Asri, L.A.; Rahmatika, A.; Fahreza, M.Z.; Insanu, M.; Purwasasmita, B.S. Preparation and release behavior of carboxylated cellulose nanocrystals-alginate nanocomposite loaded with rutin. Mater. Res. Express 2018, 5, 095303. [Google Scholar] [CrossRef]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Hafizulhaq, F.; Asrofi, M. Properties of cellulose nanofiber/bengkoang starch bionanocomposites: Effect of fiber loading. LWT 2019, 116, 108554. [Google Scholar] [CrossRef]
- Deewan, R.; Tanboonchuy, V.; Khamdahsag, P.; Yan, D.Y.-S. Utilization of agricultural waste: Mango peels and pineapple crown leaves as precursors for nanomaterial production for arsenate remediation. Environ. Sci. Pollut. Res. 2025, 32, 14508–14526. [Google Scholar] [CrossRef]
- Chawalitsakunchai, W.; Dittanet, P.; Loykulnant, S.; Sae-oui, P.; Tanpichai, S.; Seubsai, A.; Prapainainar, P. Properties of natural rubber reinforced with nano cellulose from pineapple leaf agricultural waste. Mater. Today Commun. 2021, 28, 102594. [Google Scholar] [CrossRef]
- Pornbencha, K.; Sringam, S.; Piyanirund, S.; Seubsai, A.; Prapainainar, P.; Niumnuy, C.; Roddecha, S.; Dittanet, P. Functionalization of cellulose nanocrystals extracted from pineapple leaves as a UV-absorbing agent in poly (lactic acid). RSC Adv. 2023, 13, 15311–15321. [Google Scholar] [CrossRef]
- Sripahco, T.; Khruengsai, S.; Pripdeevech, P. Biodegradable antifungal films from nanocellulose-gellan gum incorporated with Anethum graveolens essential oil for bread packaging. Int. J. Biol. Macromol. 2023, 243, 125244. [Google Scholar] [CrossRef]
- Sainorudin, M.H.; Abdullah, N.A.; Asmal Rani, M.S.; Mohammad, M.; Mahizan, M.; Shadan, N.; Abd Kadir, N.H.; Yaakob, Z.; El-Denglawey, A.; Alam, M. Structural characterization of microcrystalline and nanocrystalline cellulose from Ananas comosus L. leaves: Cytocompatibility and molecular docking studies. Nanotechnol. Rev. 2021, 10, 793–806. [Google Scholar] [CrossRef]
- Sainorudin, M.H.; Abdullah, N.A.; Rani, M.S.; Mohammad, M.; Abd Kadir, N.H.; Razali, H.; Asim, N.; Yaakob, Z. Investigation of the structural, thermal and morphological properties of nanocellulose synthesised from pineapple leaves and sugarcane bagasse. Curr. Nanosci. 2022, 18, 68–77. [Google Scholar] [CrossRef]
- Gnanasekaran, S.; Nordin, N.I.A.A.; Jamari, S.S.; Shariffuddin, J.H. Isolation and characterisation of nanofibrillated cellulose from N36 Ananas comosus leaves via ball milling with green solvent. Ind. Crops Prod. 2022, 178, 114660. [Google Scholar] [CrossRef]
- Nguyen, C.T.X.; Bui, K.H.; Vo, N.; Le, T.M.; Pham, C.D.; Do, N.H.N.; Mai, P.T.; Le, A.K.; Le, P.T.K. Fabrication of chitosan-based film by incorporating with nanocellulose produced from agricultural waste. Chem. Eng. Trans. 2022, 97, 85–90. [Google Scholar] [CrossRef]
- Nguyen, C.X.T.; Bui, K.H.; Truong, B.Y.; Dang, M.D.T.; Nguyen, G.K.; Nguyen, P.T.X.; Do, N.H.N.; Le, P.K. Chemo-Mechanical Production of Cellulose Nanocrystal from Pineapple Leaf Waste as Eco-Friendly Reinforcement for Biocomposite Films. Available online: https://ssrn.com/abstract=4054258 (accessed on 25 October 2025). [CrossRef]
- Prado, K.S.; Spinacé, M.A. Isolation and characterization of cellulose nanocrystals from pineapple crown waste and their potential uses. Int. J. Biol. Macromol. 2019, 122, 410–416. [Google Scholar] [CrossRef]
- Barros, S.d.S.; Junior, W.A.G.P.; Pereira, B.; Mendoza, S.L.Y.; de Freitas, F.A.; Saron, C.; Manzato, L. Extraction of cellulose nanofibrils and nanocrystals from a new cultivar of pineapple (Ananas comosus ‘Vitória’) from the Amazon region. Biofuels Bioprod. Biorefining 2025, 19, 1595–1610. [Google Scholar] [CrossRef]
- Araya, J.; Esquivel, M.; Jimenez, G.; Navia, D.; Poveda, L. Antimicrobial activity and physicochemical characterization of thermoplastic films based on bitter cassava starch, nanocellulose and rosemary essential oil. J. Plast. Film. Sheeting 2022, 38, 46–71. [Google Scholar] [CrossRef]
- Almendárez-Camarillo, A.; Flores-Hernandez, C.; Balcázar-Enríquez, V.; Aguirre-García, M.; Martínez-Hernandez, A.L.; Velasco-Santos, C. Nanocellulose extraction of pineapple leaves for chitosan-starch nanocomposites. J. Nat. Fibers 2022, 19, 3624–3637. [Google Scholar] [CrossRef]
- Fitriani, F.; Aprilia, S.; Arahman, N.; Bilad, M.R.; Suhaimi, H.; Huda, N. Properties of biocomposite film based on whey protein isolate filled with nanocrystalline cellulose from pineapple crown leaf. Polymers 2021, 13, 4278. [Google Scholar] [CrossRef] [PubMed]
- Fitriani, F.; Aprilia, S.; Bilad, M.R.; Arahman, N.; Usman, A.; Huda, N.; Kobun, R. Optimization of biocomposite film based on whey protein isolate and nanocrystalline cellulose from pineapple crown leaf using response surface methodology. Polymers 2022, 14, 3006. [Google Scholar] [CrossRef] [PubMed]
- Dmitrenko, M.; Kuzminova, A.; Cherian, R.M.; Joshy, K.; Pasquini, D.; John, M.J.; Hato, M.J.; Thomas, S.; Penkova, A. Edible carrageenan films reinforced with starch and nanocellulose: Development and characterization. Sustainability 2023, 15, 15817. [Google Scholar] [CrossRef]
- Siribanluehan, N.; Wattanachai, P. Preparation and Characterization of Durian Husk-Based Biocomposite Films Reinforced With Nanocellulose From Corn Husk and Pineapple Leaf. Biopolymers 2024, 115, e23619. [Google Scholar] [CrossRef]
- Mahardika, M.; Masruchin, N.; Amelia, D.; Ilyas, R.A.; Septevani, A.A.; Syafri, E.; Hastuti, N.; Karina, M.; Khan, M.A.; Jeon, B.-H. Nanocellulose reinforced polyvinyl alcohol-based bio-nanocomposite films: Improved mechanical, UV-light barrier, and thermal properties. RSC Adv. 2024, 14, 23232–23239. [Google Scholar] [CrossRef]
- Sumaiyah, S.; Hasibuan, P.A.Z.; Syahputra, H.; Lubis, M.F. The nanocrystalline cellulose from Ananas comosus leaf wastes: An overview to extraction, purification, and applications as curcumin drug delivery system. J. Appl. Pharm. Sci. 2024, 14, 165–173. [Google Scholar] [CrossRef]
- Fitriani, F.; Aprilia, S.; Mahardika, M.; Masruchin, N.; Rinaldi, W. Development of hybrid biodegradable polymer based on polyvinyl alcohol and whey protein isolate reinforced with cellulose nanocrystal from pineapple crown leaf. Case Stud. Chem. Environ. Eng. 2025, 11, 101061. [Google Scholar] [CrossRef]
- da Costa, F.A.T.; Dufresne, A.; Parra, D.F. Impact of High-Dose Gamma Irradiation on PLA/PBAT Blends Reinforced with Cellulose Nanoparticles from Pineapple Leaves. ACS Omega 2025, 33, 38182–38202. [Google Scholar] [CrossRef]
- Shih, Y.; Chou, M.; Lian, H.; Hsu, L.; Chen-Wei, S. Highly transparent and impact-resistant PMMA nanocomposites reinforced by cellulose nanofibers of pineapple leaves modified by eco-friendly methods. Express Polym. Lett. 2018, 12, 844–854. [Google Scholar] [CrossRef]
- Shih, Y.-F.; Kotharangannagari, V.K.; Tsou, T.-C. Development of eco-friendly modified cellulose nanofiber reinforced polystyrene nanocomposites: Thermal, mechanical, and optical properties. J. Polym. Res. 2020, 27, 181. [Google Scholar] [CrossRef]
- Grumo, J.; Lubguban, A.; Capangpangan, R.; Yabuki, A.; Alguno, A. Corrosion protection properties of pineapple (Ananas comosus) leaves nanocellulose reinforced in epoxy nanocomposite coatings on mild steel. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Chandrashekar, A.; Vargheese, S.; Vijayan, J.G.; Gopi, J.A.; Prabhu, T.N. Highly efficient removal of Rhodamine B dye using nanocomposites made from cotton seed oil-based polyurethane and silylated nanocellulose. J. Polym. Environ. 2022, 30, 4999–5011. [Google Scholar] [CrossRef]
- Aththanayaka, S.; Thiripuranathar, G.; Ekanayake, S. Sustainable approach for fabrication of pineapple agro-waste mediated cellulose nanocrystals embedded with Ag/Ag2O/ZnO nanocomposites for efficient removal of waterborne pathogens in wastewater. Int. J. Biol. Macromol. 2025, 310, 143272. [Google Scholar] [CrossRef]
- Sasikala, M.; Magesan, P.; Dhanalekshmi, K.; Umapathy, M. Eco-friendly bio-nanocomposites: Incorporation of nano-cellulose from pineapple leaf waste into tissue paper. Cellulose 2024, 31, 9369–9383. [Google Scholar] [CrossRef]
- Claro, P.I.; Marques, A.C.; Cunha, I.; Martins, R.F.o.P.; Pereira, L.M.; Marconcini, J.M.; Mattoso, L.H.; Fortunato, E. Tuning the electrical properties of cellulose nanocrystals through laser-induced graphitization for UV photodetectors. ACS Appl. Nano Mater. 2021, 4, 8262–8272. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Feng, X.; Ma, L.; Zhang, Y.; Dai, H. Lignocellulose nanocrystals from pineapple peel: Preparation, characterization and application as efficient Pickering emulsion stabilizers. Food Res. Int. 2021, 150, 110738. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Zhang, S.; Feng, X.; Cui, B.; Ma, L.; Zhang, Y. Enhanced interface properties and stability of lignocellulose nanocrystals stabilized pickering emulsions: The leading role of tannic acid. J. Agric. Food Chem. 2021, 69, 14650–14661. [Google Scholar] [CrossRef]
- Tang, L.; Huang, H. Evaluation of pineapple peel cellulose nanocrystals/EGCG complexes for improving the stability of curcumin emulsion. Cellulose 2022, 29, 6123–6141. [Google Scholar] [CrossRef]
- Nurani, D.; Masruchin, N.; Widyaningrum, B.A.; Kusumah, S.S.; Ningrum, R.S.; Darmokoesoemo, H.; Kusuma, H.S. Pickering emulsion stabilized by cellulose nanofibril from pineapple leaves for biofoam manufacture. Nano-Struct. Nano-Objects 2024, 39, 101223. [Google Scholar] [CrossRef]
- Karimi, S.; Dufresne, A.; Md. Tahir, P.; Karimi, A.; Abdulkhani, A. Biodegradable starch-based composites: Effect of micro and nanoreinforcements on composite properties. J. Mater. Sci. 2014, 49, 4513–4521. [Google Scholar] [CrossRef]
- Feng, P.; Luo, Y.; Ke, C.; Qiu, H.; Wang, W.; Zhu, Y.; Hou, R.; Xu, L.; Wu, S. Chitosan-based functional materials for skin wound repair: Mechanisms and applications. Front. Bioeng. Biotechnol. 2021, 9, 650598. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An overview of its properties and applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Panda, P.K.; Sadeghi, K.; Seo, J. Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging applications: A review. Food Packag. Shelf Life 2022, 33, 100904. [Google Scholar] [CrossRef]
- Chen, C.; Yang, H.; Yang, X.; Ma, Q. Tannic acid: A crosslinker leading to versatile functional polymeric networks: A review. RSC Adv. 2022, 12, 7689–7711. [Google Scholar] [CrossRef]
- Etxabide, A.; Arregi, M.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Whey protein films for sustainable food packaging: Effect of incorporated ascorbic acid and environmental assessment. Polymers 2023, 15, 387. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.; Souto, E.B. Polylactic acid (PLA): Properties, synthesis, and biomedical applications–A review of the literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Rivera-Briso, A.L.; Serrano-Aroca, Á. Poly (3-Hydroxybutyrate-co-3-Hydroxyvalerate): Enhancement strategies for advanced applications. Polymers 2018, 10, 732. [Google Scholar] [CrossRef]
- Thivya, P.; Gururaj, P.; Reddy, N.B.P.; Rajam, R. Recent advances in protein-polysaccharide based biocomposites and their potential applications in food packaging: A review. Int. J. Biol. Macromol. 2024, 268, 131757. [Google Scholar] [CrossRef]
- Zhong, K.; Yang, B.; Liu, C.; Xiong, Y.; Wen, Q.; Li, W.; Ren, J. Progress in the application of polysaccharide/nanomaterial composites in food safety. Food Chem. 2025, 495, 146317. [Google Scholar] [CrossRef] [PubMed]
- Metha, C.; Pawar, S.; Suvarna, V. Recent advancements in alginate-based films for active food packaging applications. Sustain. Food Technol. 2024, 2, 1246–1265. [Google Scholar] [CrossRef]
- Kokkuvayil Ramadas, B.; Rhim, J.-W.; Roy, S. Recent progress of carrageenan-based composite films in active and intelligent food packaging applications. Polymers 2024, 16, 1001. [Google Scholar] [CrossRef]
- Wu, S.; Ma, S.; Zhang, Q.; Yang, C. A comprehensive review of polyurethane: Properties, applications and future perspectives. Polymer 2025, 327, 128361. [Google Scholar] [CrossRef]
- Omar, M.F.; Ali, F.; Jami, M.S.; Azmi, A.S.; Ahmad, F.; Marzuki, M.Z.; Muniyandi, S.K.; Zainudin, Z.; Kim, M.P. A Comprehensive Review of Natural Rubber Composites: Properties, Compounding Aspects, and Renewable Practices with Natural Fibre Reinforcement. J. Renew. Mater. 2025, 13, 497–538. [Google Scholar] [CrossRef]
- Jin, F.-L.; Li, X.; Park, S.-J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Chakrabarty, A.; Teramoto, Y. Recent advances in nanocellulose composites with polymers: A guide for choosing partners and how to incorporate them. Polymers 2018, 10, 517. [Google Scholar] [CrossRef]
- Cardona Alzate, C.A.; Ortiz-Sanchez, M.; Solarte-Toro, J.C. Design strategy of food residues biorefineries based on multifeedstocks analysis for increasing sustainability of value chains. Biochem. Eng. J. 2023, 194, 108857. [Google Scholar] [CrossRef]
- Chaudhary, S.; Singh, B. Pineapple by-products utilization: Progress towards the circular economy. Food Humanit. 2024, 2, 100243. [Google Scholar] [CrossRef]
- Pandey, A.K.; Thakur, S.; Mehra, R.; Kaler, R.S.S.; Paul, M.; Kumar, A. Transforming Agri-food waste: Innovative pathways toward a zero-waste circular economy. Food Chem. X 2025, 28, 102604. [Google Scholar] [CrossRef]
- Banerjee, S.; Patti, A.F.; Ranganathan, V.; Arora, A. Hemicellulose based biorefinery from pineapple peel waste: Xylan extraction and its conversion into xylooligosaccharides. Food Bioprod. Process. 2019, 117, 38–50. [Google Scholar] [CrossRef]
- Sumiati, T.; Suryadi, H. Comparison of the deep euteutic solvent (DES) solvent for extracting lignin from the lignocellulosic material of pineapple leaves. Pharmacogn. J. 2021, 13, 1702–1709. [Google Scholar] [CrossRef]
- Mateo, S.; Fabbrizi, G.; Moya, A.J. Lignin from plant-based agro-industrial biowastes: From extraction to sustainable applications. Polymers 2025, 17, 952. [Google Scholar] [CrossRef] [PubMed]
- Santos Pompêu, G.C.; Pasquini, D.; Ruggiero, R. Characterization of distinct types of isolated lignins from industrial waste and their potential use calorific. Obs. Econ. Latinoam. 2024, 22, 291. [Google Scholar] [CrossRef]
- Bruce, B.B.; Boateng, I.D. Pineapple by-products: A critical review of their bioactive compounds as eco-friendly pesticides in pest management. Food Chem. X 2025, 28, 102567. [Google Scholar] [CrossRef] [PubMed]
- Nakthong, N.; Wongsagonsup, R.; Amornsakchai, T. Characteristics and potential utilizations of starch from pineapple stem waste. Ind. Crops Prod. 2017, 105, 74–82. [Google Scholar] [CrossRef]
- Roskan, N.F.M.; Zainol, N.; Samad, K.A. Chlorophyll extraction from pineapple plantation waste through mechanical extraction. Biocatal. Agric. Biotechnol. 2022, 39, 102264. [Google Scholar] [CrossRef]
- Mandal, D.D.; Singh, G.; Majumdar, S.; Chanda, P. Challenges in developing strategies for the valorization of lignin—A major pollutant of the paper mill industry. Environ. Sci. Pollut. Res. 2023, 30, 11119–11140. [Google Scholar] [CrossRef]
- Bröring, S.; Laibach, N.; Wustmans, M. Innovation types in the bioeconomy. J. Clean. Prod. 2020, 266, 121939. [Google Scholar] [CrossRef]
- Sodhi, A.S.; Bhatia, S.; Batra, N. Advances in Green Technologies and Global Circular Bioeconomy Framework for a Sustainable Society. Circ. Econ. Sustain. 2025, 5, 2991–3016. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esquivel-Alfaro, M.; Rojas-Carrillo, O.; Sulbarán-Rangel, B.; Rodríguez-Barquero, L.; Palacios-Hinestroza, H.; Rojas, O.J. Pineapple-Derived Nanocellulose for Nanocomposites: Extraction, Processing, and Properties. J. Compos. Sci. 2025, 9, 652. https://doi.org/10.3390/jcs9120652
Esquivel-Alfaro M, Rojas-Carrillo O, Sulbarán-Rangel B, Rodríguez-Barquero L, Palacios-Hinestroza H, Rojas OJ. Pineapple-Derived Nanocellulose for Nanocomposites: Extraction, Processing, and Properties. Journal of Composites Science. 2025; 9(12):652. https://doi.org/10.3390/jcs9120652
Chicago/Turabian StyleEsquivel-Alfaro, Marianelly, Oscar Rojas-Carrillo, Belkis Sulbarán-Rangel, Lilliana Rodríguez-Barquero, Hasbleidy Palacios-Hinestroza, and Orlando J. Rojas. 2025. "Pineapple-Derived Nanocellulose for Nanocomposites: Extraction, Processing, and Properties" Journal of Composites Science 9, no. 12: 652. https://doi.org/10.3390/jcs9120652
APA StyleEsquivel-Alfaro, M., Rojas-Carrillo, O., Sulbarán-Rangel, B., Rodríguez-Barquero, L., Palacios-Hinestroza, H., & Rojas, O. J. (2025). Pineapple-Derived Nanocellulose for Nanocomposites: Extraction, Processing, and Properties. Journal of Composites Science, 9(12), 652. https://doi.org/10.3390/jcs9120652










