Efficient Pb(II) Adsorption by Natural Mugaldzhar Diatomite: Isotherm, Kinetic, and Thermodynamic Analysis
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Characterization of Natural Diatomite
2.3. Batch Adsorption Experiments
2.4. Isotherm, Kinetic, and Thermodynamic Analysis of Adsorption Data
2.4.1. Equilibrium Modelling
2.4.2. Kinetic Modelling
2.4.3. Thermodynamic Modelling
3. Results and Discussion
3.1. The Characteristics of Natural Diatomite
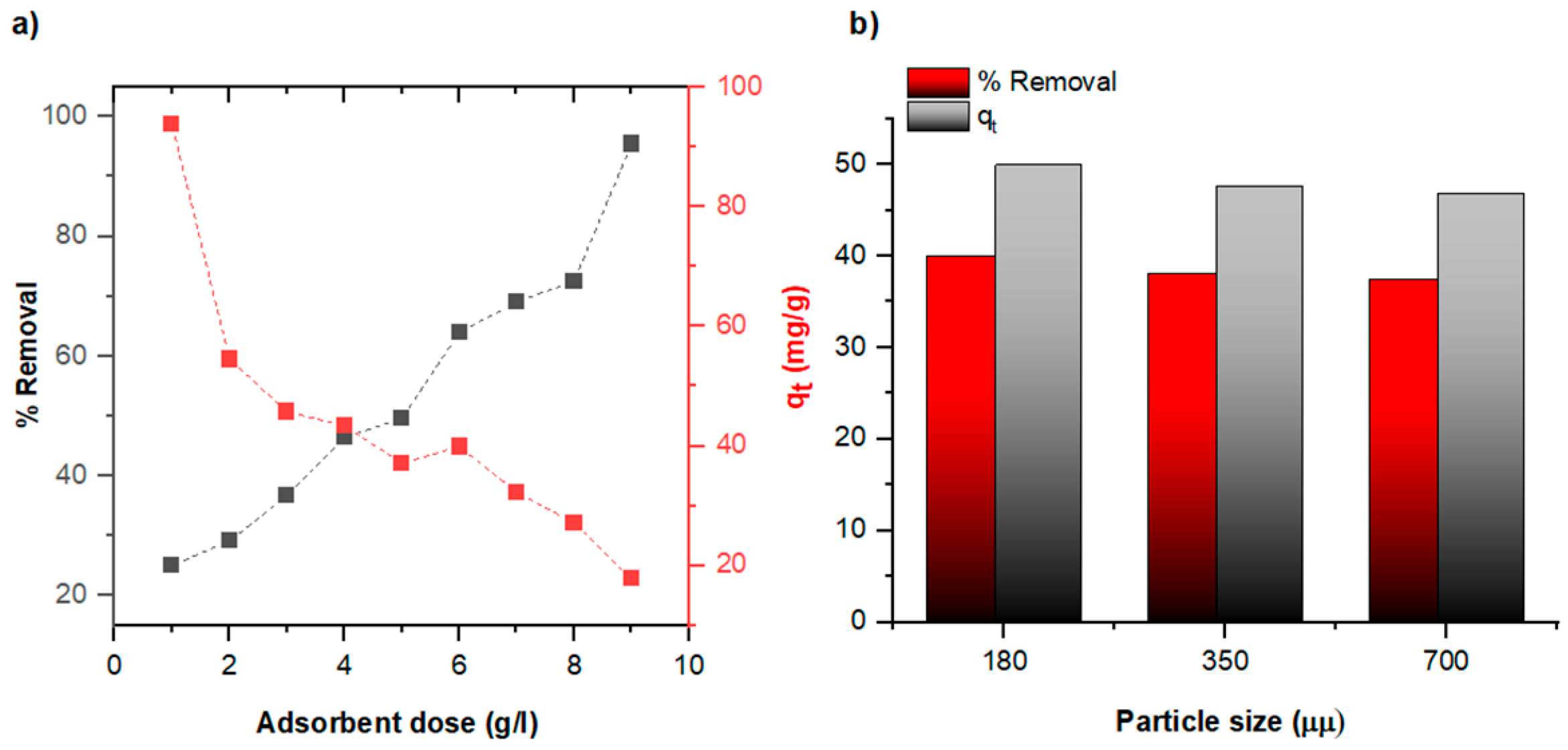
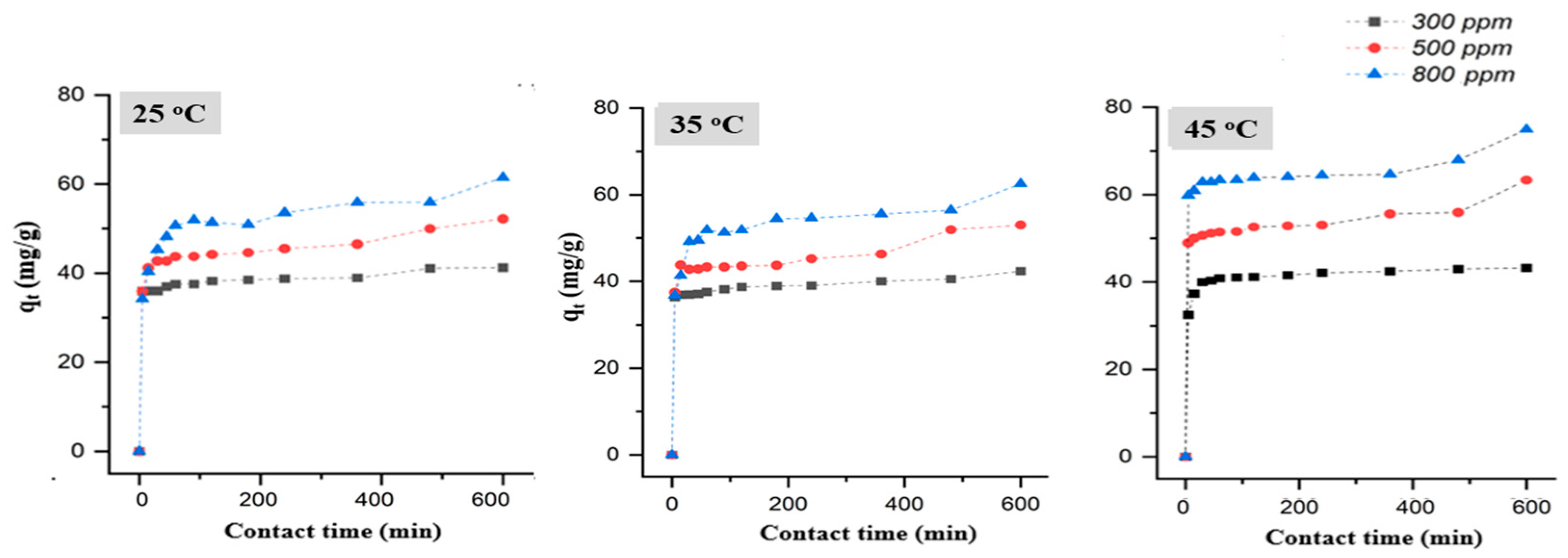
3.2. Pb (II) Removal from Water by Natural Diatomite
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Järup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A. Heavy Metals and Human Health: Mechanistic Insight into Toxicity and Counter Defense System of Antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Özsin, G.; Apaydin Varol, E. Potential of Microwave-Assisted Hydrothermal Modification for Enhanced Fly Ash Adsorption Capacity toward Heavy Metals: A Comprehensive Investigation of Adsorbent Characterization, Isotherms, Kinetics, and Thermodynamics. Sep. Sci. Technol. 2024, 59, 71–86. [Google Scholar] [CrossRef]
- Sanliyuksel Yucel, D. Removal of Heavy Metals from Aqueous Solution Using Fly Ash: Çan Thermal Power Plant, NW Turkey as a Case Study. Karaelmas Sci. Eng. J. 2017, 7, 291–298. [Google Scholar] [CrossRef]
- Al-Enezi, G.; Hamoda, M.F.; Fawzi, N. Ion Exchange Extraction of Heavy Metals from Wastewater Sludges. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2004, 39, 455–464. [Google Scholar] [CrossRef]
- Kilic, M.; Özsin, G.; Apaydin-Varol, E.; Putun, A.E. Biosorption Behaviour of an Arid Land Plant, Euphorbia rigida, towards Heavy Metals: Equilibrium, Kinetic and Thermodynamic Studies. Hittite J. Sci. Eng. 2017, 4, 105–115. [Google Scholar] [CrossRef]
- Ince, M.; Kaplan Ince, O. An Overview of Adsorption Technique for Heavy Metal Removal from Water/Wastewater: A Critical Review. Int. J. Pure Appl. Sci. 2017, 3, 10–19. [Google Scholar] [CrossRef]
- Gadd, G.M. Biosorption: Critical Review of Scientific Rationale, Environmental Importance and Significance for Pollution Treatment. J. Chem. Technol. Biotechnol. 2009, 84, 13–28. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, T.; Das, D.K.; Mittal, A. Phytoremediation of Heavy Metals in Soil—Concepts, Advancements, and Future Directions. J. Soil Sci. Plant Nutr. 2025, 25, 1253–1280. [Google Scholar] [CrossRef]
- Bhagat, N.R.; Giri, A. Nanotechnology for Detection and Removal of Heavy Metals from Contaminated Water. In Functionalized Nanomaterials for Catalytic Application; Wiley: Hoboken, NJ, USA, 2021; pp. 185–226. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sen Gupta, S. Adsorption of a Few Heavy Metals on Natural and Modified Kaolinite and Montmorillonite: A Review. Adv. Colloid Interface Sci. 2008, 140, 114–131. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for Heavy Metals Removal and Their Future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Lata, S.; Singh, P.K.; Samadder, S.R. Regeneration of Adsorbents and Recovery of Heavy Metals: A Review. Int. J. Environ. Sci. Technol. 2015, 12, 1461–1478. [Google Scholar] [CrossRef]
- Raji, Z.; Karim, A.; Karam, A.; Khalloufi, S. Adsorption of Heavy Metals: Mechanisms, Kinetics, and Applications of Various Adsorbents in Wastewater Remediation—A Review. Waste 2023, 1, 775–805. [Google Scholar] [CrossRef]
- Waranusantigul, P.; Pokethitiyook, P.; Kruatrachue, M.; Upatham, E.S. Kinetics of Zn2+, Cd2+ and Pb2+ Adsorption by Cassia fistula Biomass. Environ. Pollut. 2003, 125, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Bulgariu, L.; Bulgariu, D. Sorption of Pb(II) onto a Mixture of Algae Waste Biomass and Clay: Equilibrium and Kinetic Studies. Chem. Eng. J. 2013, 226, 206–213. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M. Applications of Chitin- and Chitosan-Derivatives for the Detoxification of Water and Wastewater—A Short Review. Adv. Colloid Interface Sci. 2009, 152, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Babel, S.; Kurniawan, T.A. Low-Cost Adsorbents for Heavy Metals Uptake from Contaminated Water: A Review. J. Hazard. Mater. 2003, 97, 219–243. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial Wastes as Low-Cost Potential Adsorbents for the Treatment of Wastewater Laden with Heavy Metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of Heavy Metal Ions Using Wheat-Based Biosorbents—A Review of the Recent Literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.-S.; Park, J.M. The Past, Present, and Future Trends of Biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Danh, H.T.; Ly, N.T.T.; Nguyen, V.; Khieu, D.Q.; Du, P.D. Removal of Pb(II) from aqueous solutions with manganese oxide-modified diatomite. Adsorpt. Sci. Technol. 2023, 2023, 7744896. [Google Scholar] [CrossRef]
- Mansour, H.; Nassar, H.F.; Zaghloul, A.; Kabary, H.; Ahmed, S.A. Preparation and evaluation of altered zeolite and diatomite as affordable adsorbents in contaminated water treatment. Appl. Water Sci. 2025, 15, 160. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Temkin, M.I. Adsorption Equilibrium and the Kinetics of Processes on Heterogeneous Surfaces. Acta Physicochim. URSS 1941, 12, 327–356. [Google Scholar]
- Dubinin, M.M.; Radushkevich, L.V. Equation of the Characteristic Curve of Activated Charcoal. Chem. Zentr. 1947, 1, 875. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Redlich, O.; Peterson, D.L. A Useful Adsorption Isotherm. J. Phys. Chem. 1959, 63, 1024. [Google Scholar] [CrossRef]
- Dubinin, M.M. Physical Adsorption of Gases and Vapors in Micropores. Prog. Surf. Membr. Sci. 1975, 9, 1–70. [Google Scholar]
- Toth, J. State Equations of the Solid–Gas Interface Layers. Acta Chim. Acad. Sci. Hung. 1971, 69, 311–317. [Google Scholar]
- Elovich, S.Y.; Larinov, O.G. Theory of Adsorption from Solutions on Solid Adsorbents. Izv. Akad. Nauk SSSR Ser. Khim. 1962, 2, 209–216. [Google Scholar]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Gelöster Stoffe. Kungl. Sven. Vetenskapsakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- McKay, G.; Ho, Y.S. Sorption of Dye from Aqueous Solution by Peat. Chem. Eng. J. 1999, 70, 115–124. [Google Scholar]
- Koble, R.A.; Corrigan, T.E. Adsorption Isotherms for Pure Hydrocarbons. Ind. Eng. Chem. 1952, 44, 383–387. [Google Scholar] [CrossRef]
- Khan, A.R.; Ataullah, R.; Al-Haddad, A. Equilibrium Isotherm Studies for Some Adsorbents: Comparison of Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms. J. Environ. Sci. Health A 1997, 32, 2245–2261. [Google Scholar]
- Vijayaraghavan, K.; Yun, Y.-S. Bacterial Biosorbents and Biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Chen, C.; Wang, X. Adsorption of Ni(II) from Aqueous Solution Using Oxidized Multiwall Carbon Nanotubes. Ind. Eng. Chem. Res. 2006, 45, 9144–9149. [Google Scholar] [CrossRef]
- Kołodyńska, D.; Bąk, J.; Thomas, P. Comparison of sorption and desorption studies of heavy metal ions from biochar and commercial active carbon. Chem. Eng. J. 2016, 307, 353–363. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Insights into the Modeling of Adsorption Isotherm Systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Azizian, S. Kinetic Models of Sorption: A Theoretical Analysis. J. Colloid Interface Sci. 2004, 276, 47–52. [Google Scholar] [CrossRef]
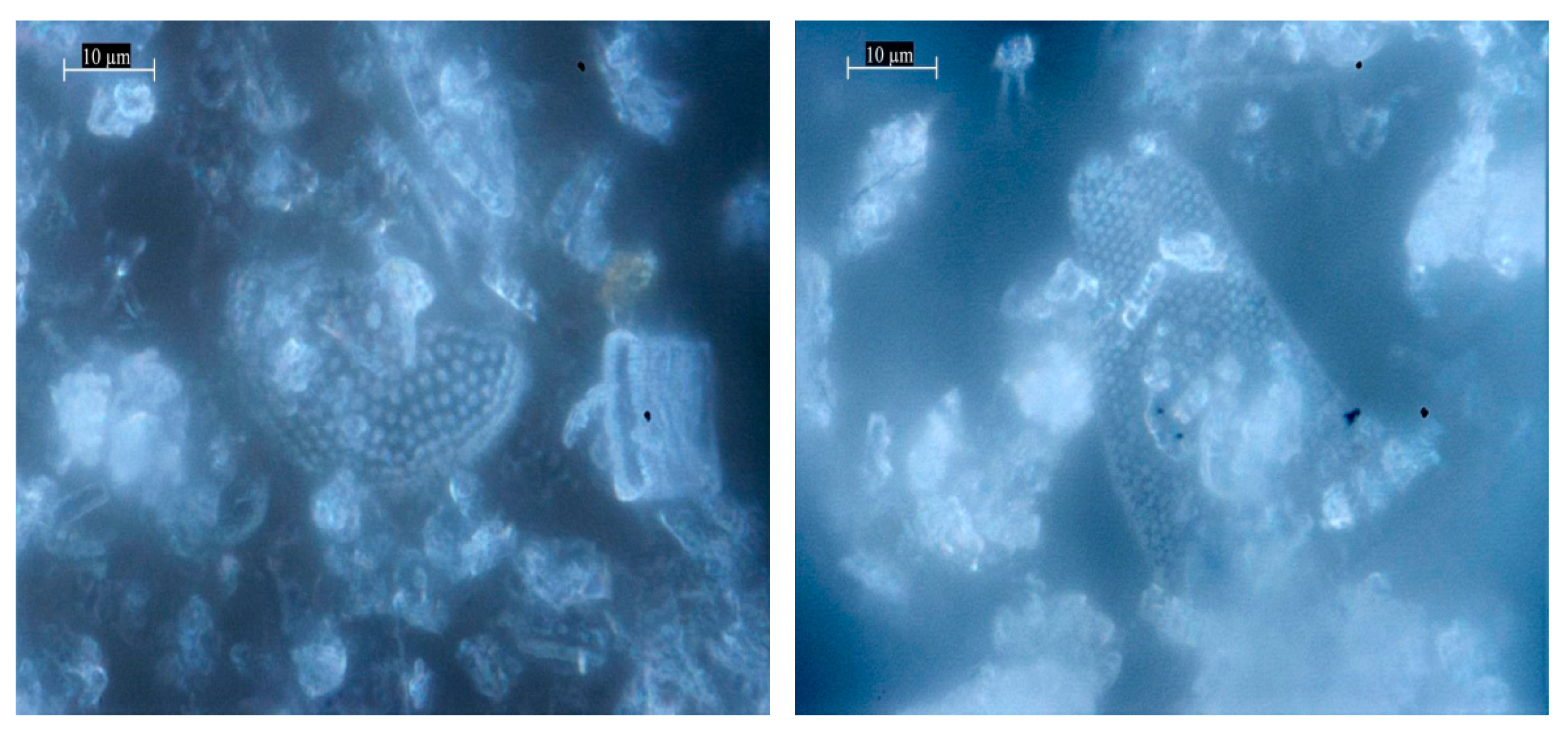
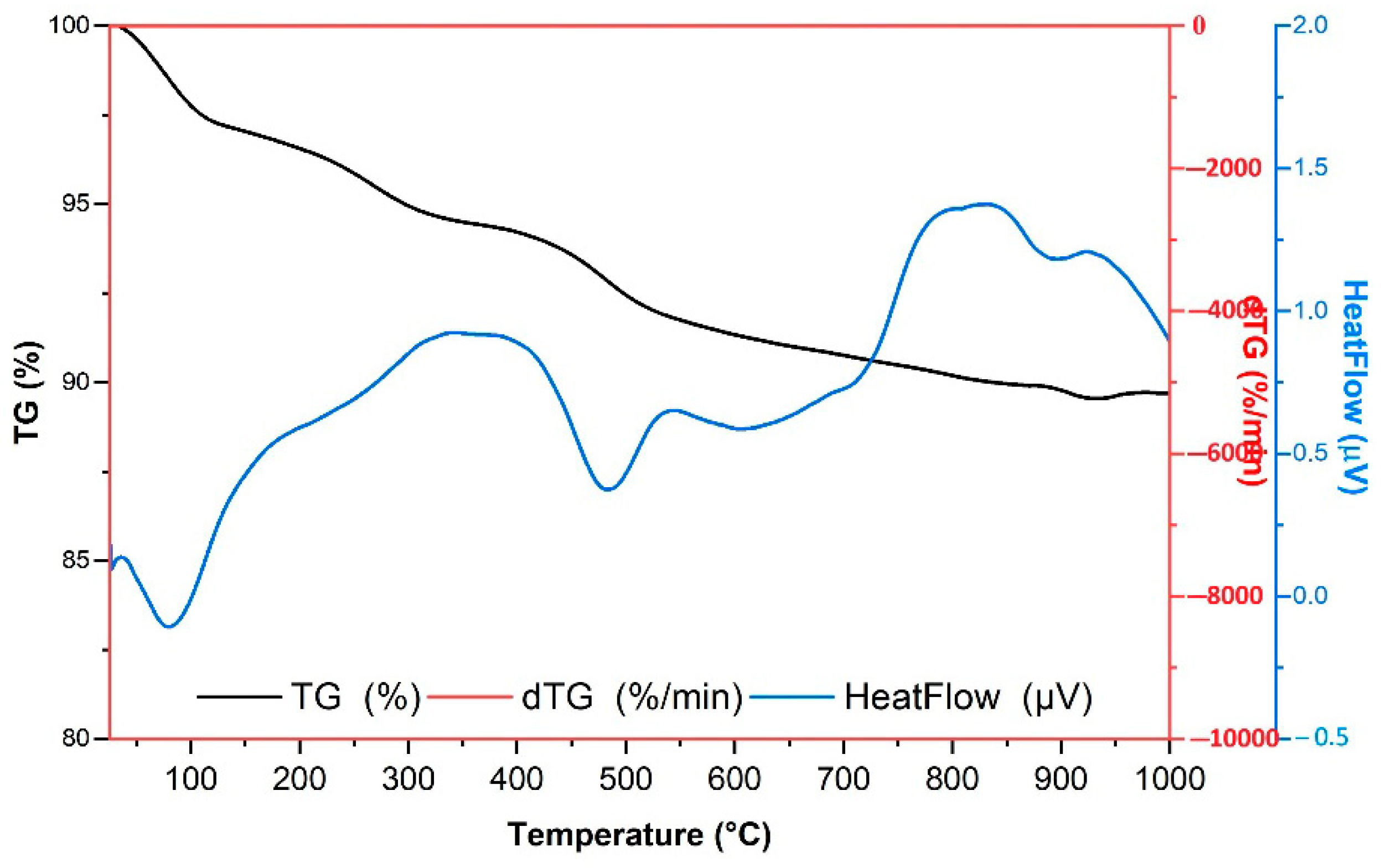


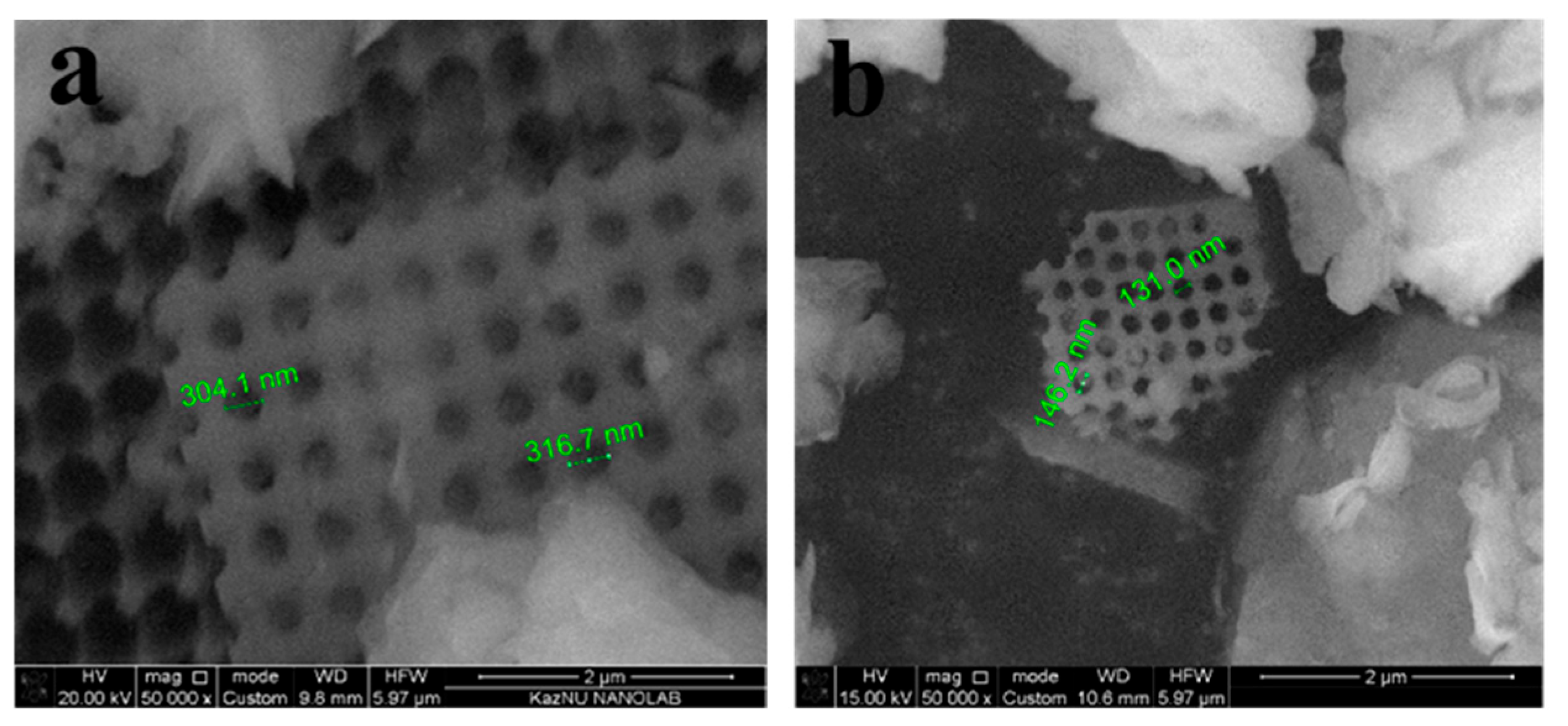
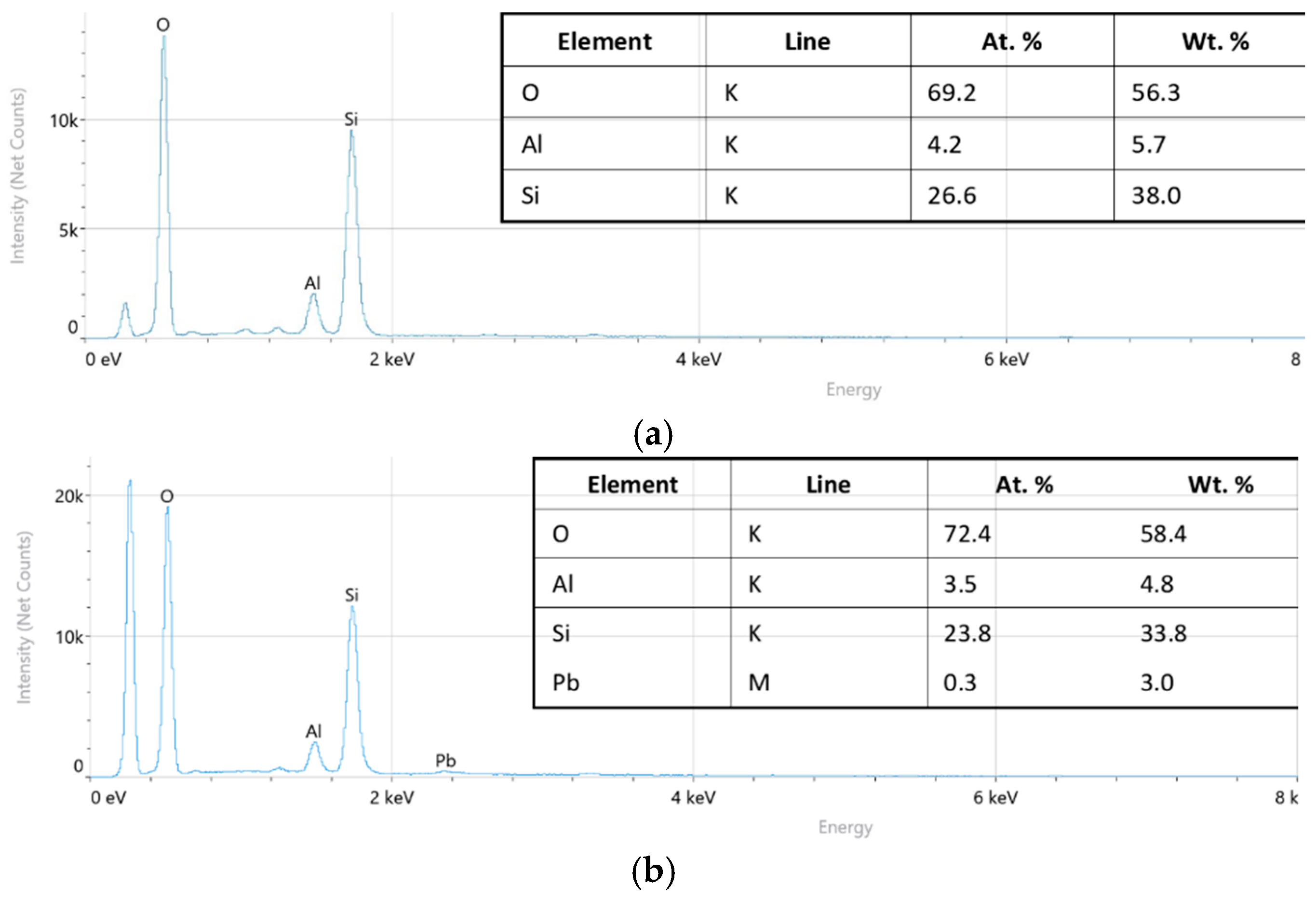
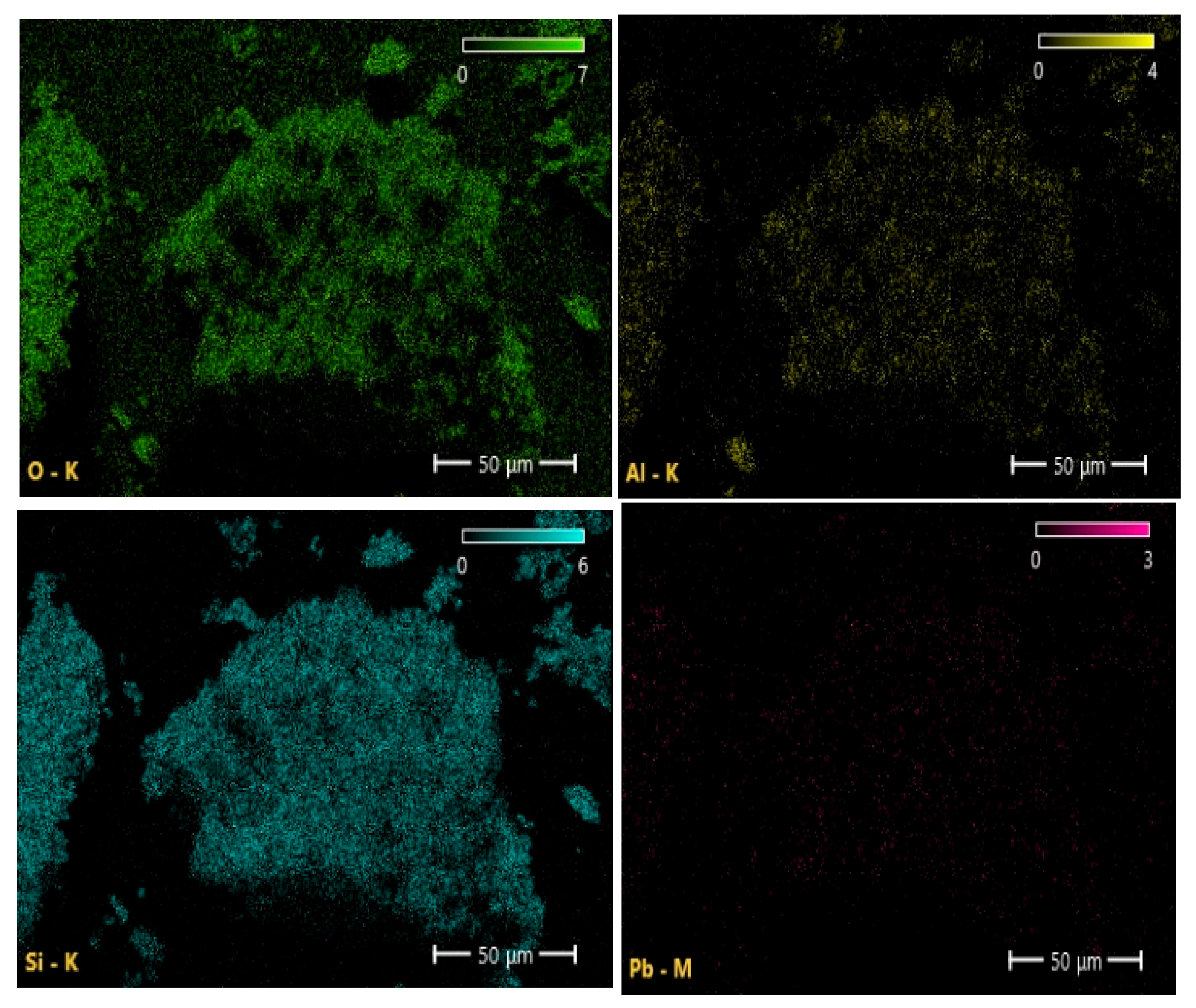
| Samples | Specific Surface Area, m2 g−1 | Average Adsorption Pore Diameter, nm | Average Desorption Pore Diameter, nm |
|---|---|---|---|
| Natural diatomite | 43 | 1.36 | 1.26 |
| Diatomite after adsorption | 38 | 1.20 | 1.19 |
| Temperature | 25 °C | 35 °C | 45 °C |
|---|---|---|---|
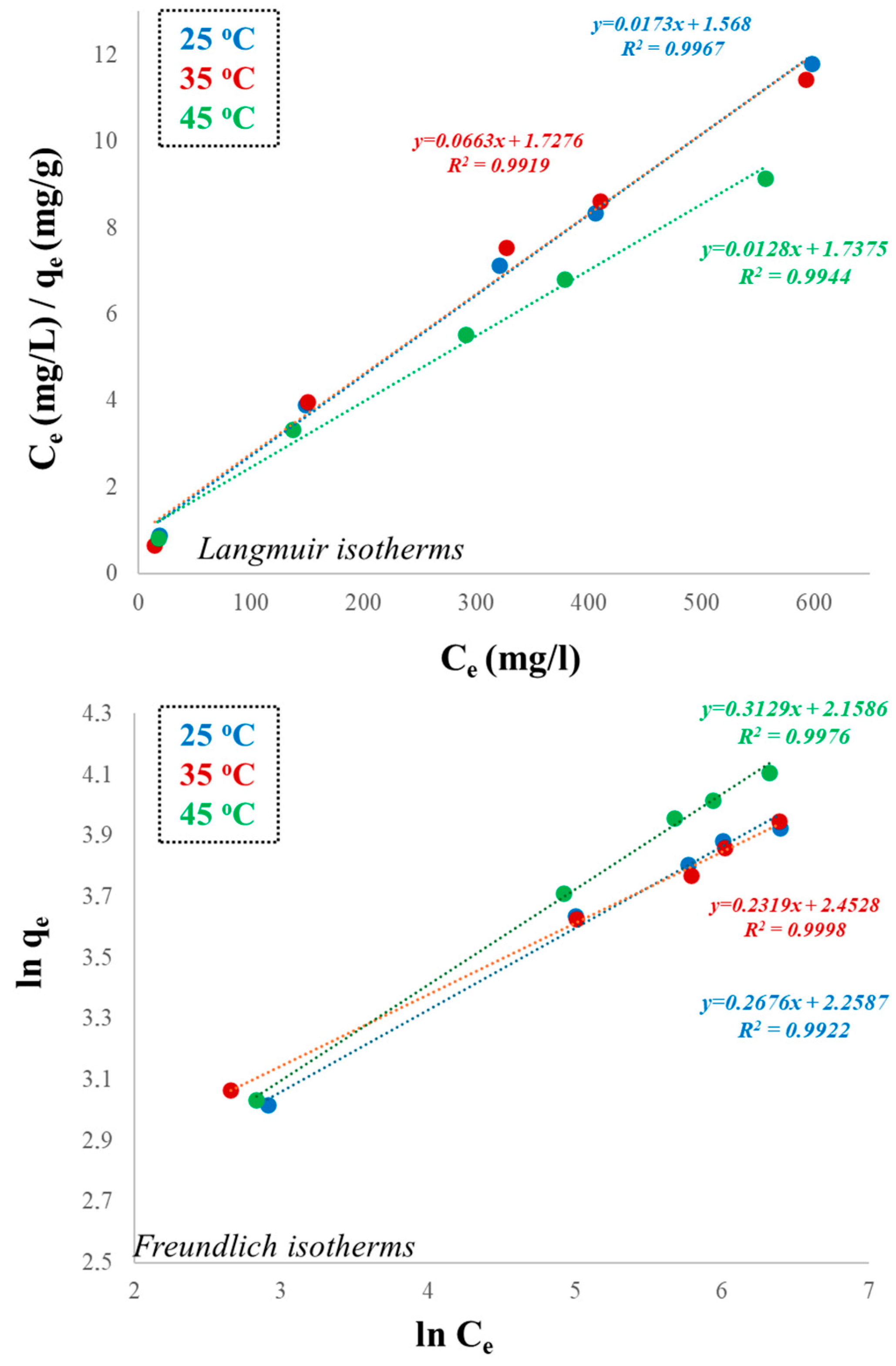
| Langmuir | |||
| qm (mg/g) | 54.05 | 54.06 | 65.78 |
| KL (L/mg) | 0.016 | 0.019 | 0.020 |
| R2 | 0.9956 | 0.9869 | 0.9911 |
| Freundlich | |||
| KF ((mg/g) (L/mg)1/n) | 9.56 | 11.51 | 8.66 |
| N | 2.25 | 2.45 | 2.15 |
| R2 | 0.9922 | 0.9998 | 0.9976 |
| Dubinin-Radushkevich | |||
| qm (mg/g) | 44.80 | 45.50 | 52.15 |
| Β | 1.02 × 10−3 | 1.09 × 10−3 | 1.29 × 10−3 |
| E | 101.1 | 129.0 | 121.2 |
| R2 | 0.8936 | 0.8882 | 0.8991 |
| Temkin | |||
| B | 8.79 | 8.84 | 11.40 |
| KT | 0.3416 | 0.9808 | 0.341 |
| R2 | 0.9873 | 0.9805 | 0.9901 |
| Pseudo-First Order | |
|---|---|
| k1 | 0.0559 |
| qe | 4.48 |
| R2 | 0.8691 |
| Pseudo-Second Order | |
| k2 | 0.0316 |
| qe | 63.7 |
| R2 | 0.9999 |
| Intraparticle Diffusion | |
| kp | 0.6621 |
| C | 58.49 |
| R2 | 0.9271 |
| Elovich | |
| A | 13.051 |
| Β | 0.0372 |
| R2 | 0.9951 |
| T (°C) | ∆G° (kJ/mol) | ∆H° (kJ/mol) | ∆S° (J/mol K) | R2 |
|---|---|---|---|---|
| 20 | –19.17 | 15.9 | 110.4 | 0.9937 |
| 30 | –19.35 | |||
| 40 | –19.39 |
| Adsorbent | Heavy Metal | Adsorption Capacity (mg/g) | Isotherm Fitting | Kinetic Model | Temperature Dependency | Reference |
|---|---|---|---|---|---|---|
| Diatomite (Turkey) | Pb(II) | 26 | Freundlich | Pseudo-2nd order | Endothermic | [38] |
| Diatomite (Iran) | Pb(II) | 25.01 | Langmuir | Pseudo-2nd order | Endothermic | [39] |
| Calcined diatomite (China) | Pb(II) | 47.96 | Langmuir | Pseudo-2nd order | N/A | [40] |
| Perlite | Pb(II) | 13.39 | Langmuir & Freundlich | Pseudo-2nd order | N/A | [41] |
| Natural calcite | Pb(II) | 19.92 | Langmuir | Pseudo-2nd order | Endothermic | [42] |
| Commercial Activated Carbon | Pb(II) | 16.84 | Langmuir & Freundlich | Pseudo-2nd order | Endothermic | [43] |
| Nano-scale zero valent iron (nZVI) | Pb(II) | 50.31 | Langmuir | Pseudo-2nd order | N/A | [44] |
| Magnetic biochar (MBC) | Pb(II) | 27 | Langmuir | Pseudo-2nd order | Endothermic | [45] |
| Diatomite (Kazakhstan) | Pb(II) | 74.9 | Langmuir & Freundlich | Pseudo-2nd order | Endothermic | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurgain, A.; Nazhipkyzy, M.; Özsin, G.; Zhaparova, A.A.; Apaydın-Varol, E. Efficient Pb(II) Adsorption by Natural Mugaldzhar Diatomite: Isotherm, Kinetic, and Thermodynamic Analysis. J. Compos. Sci. 2025, 9, 625. https://doi.org/10.3390/jcs9110625
Nurgain A, Nazhipkyzy M, Özsin G, Zhaparova AA, Apaydın-Varol E. Efficient Pb(II) Adsorption by Natural Mugaldzhar Diatomite: Isotherm, Kinetic, and Thermodynamic Analysis. Journal of Composites Science. 2025; 9(11):625. https://doi.org/10.3390/jcs9110625
Chicago/Turabian StyleNurgain, Araylim, Meruyert Nazhipkyzy, Gamzenur Özsin, Aizhan A. Zhaparova, and Esin Apaydın-Varol. 2025. "Efficient Pb(II) Adsorption by Natural Mugaldzhar Diatomite: Isotherm, Kinetic, and Thermodynamic Analysis" Journal of Composites Science 9, no. 11: 625. https://doi.org/10.3390/jcs9110625
APA StyleNurgain, A., Nazhipkyzy, M., Özsin, G., Zhaparova, A. A., & Apaydın-Varol, E. (2025). Efficient Pb(II) Adsorption by Natural Mugaldzhar Diatomite: Isotherm, Kinetic, and Thermodynamic Analysis. Journal of Composites Science, 9(11), 625. https://doi.org/10.3390/jcs9110625






