Biomechanical Comparison of Titanium and CFR-PEEK Intramedullary Nails Using Finite Element Analysis
Abstract
1. Introduction
2. Materials and Methods
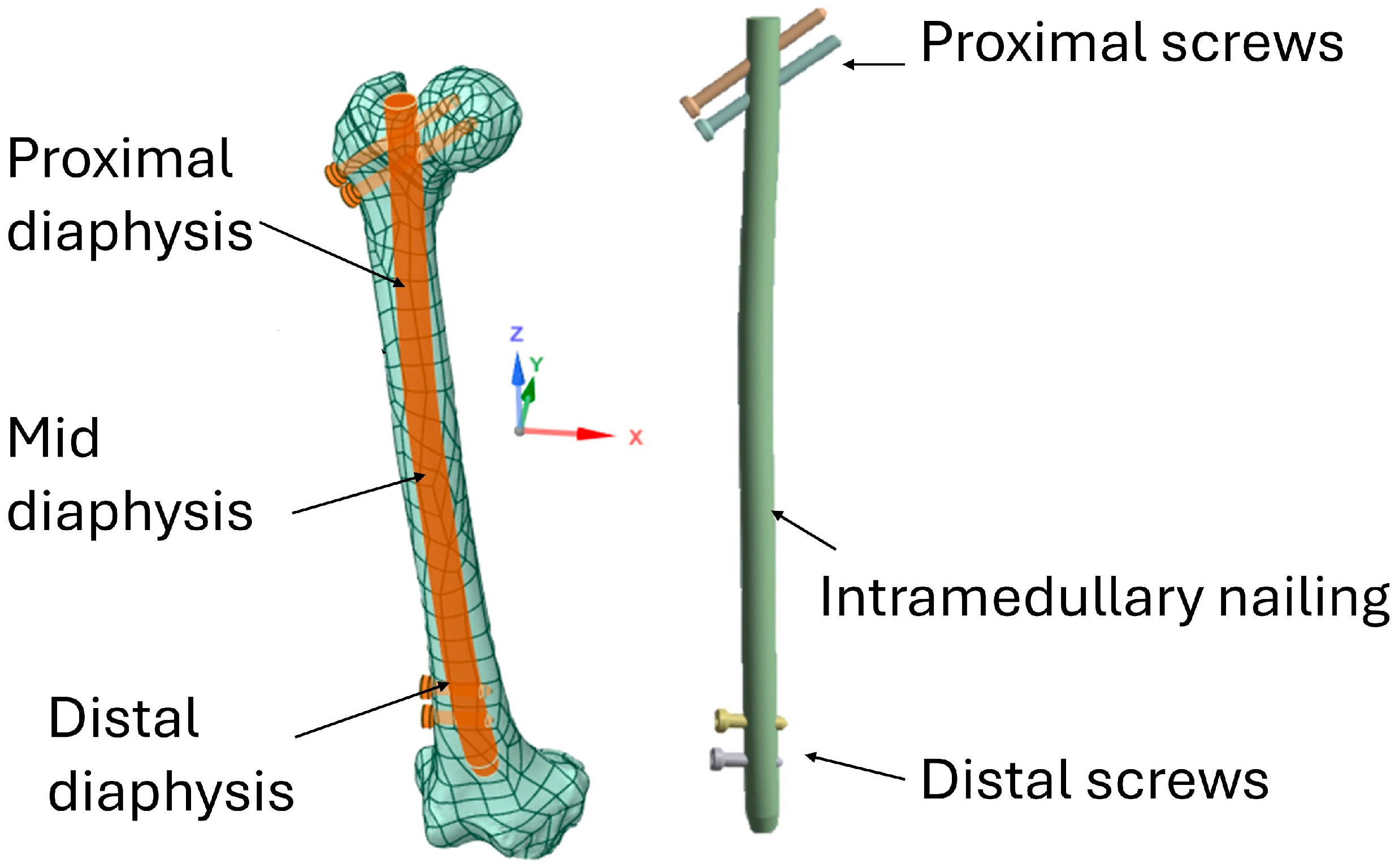
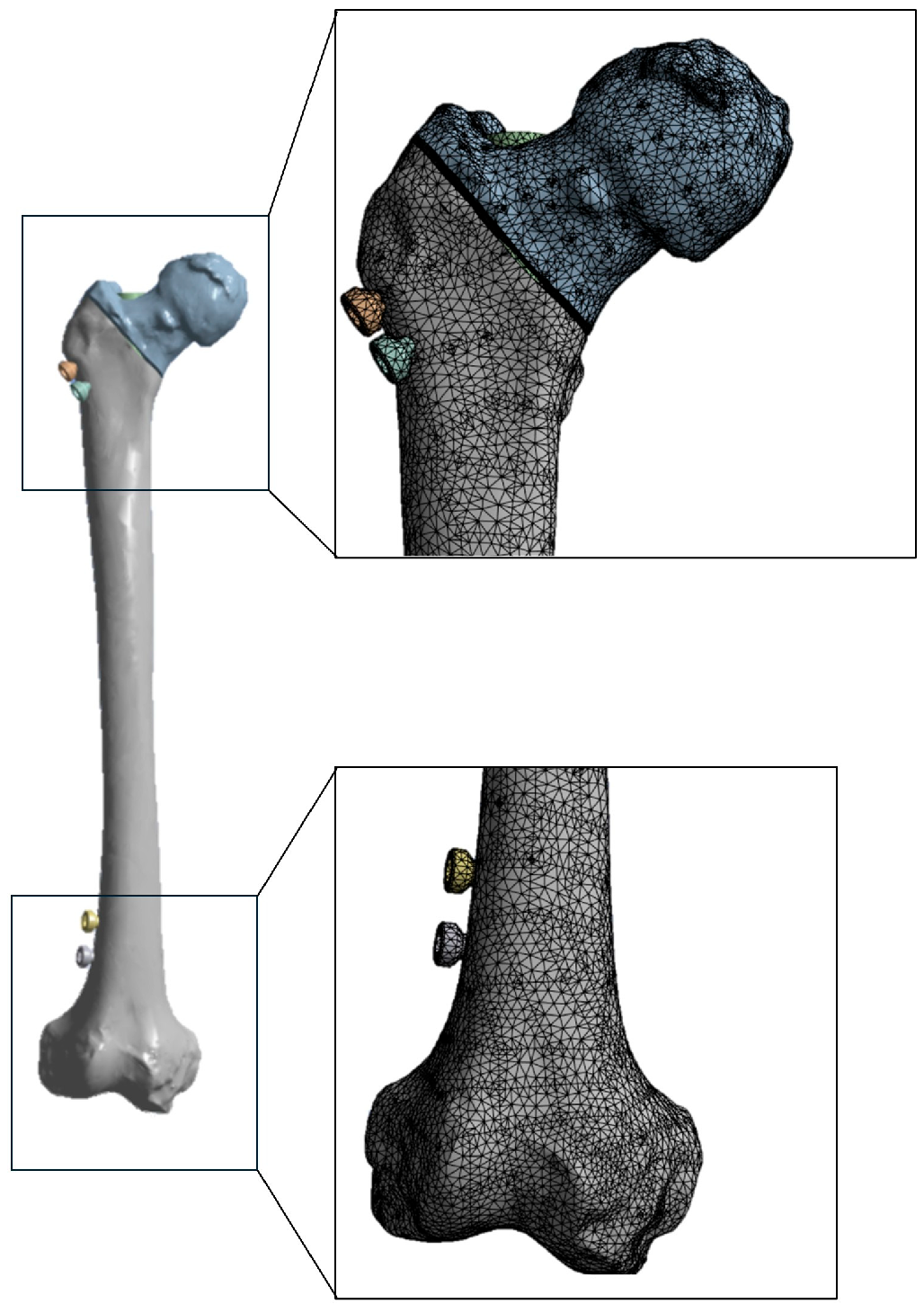
2.1. Modeling
2.2. Materials Properties
2.3. FEA Modeling
2.4. Evaluation of the Osteogenic Response of Callus Based on Stress
3. Results
3.1. Distribution of Von Mises Stresses in the Femur
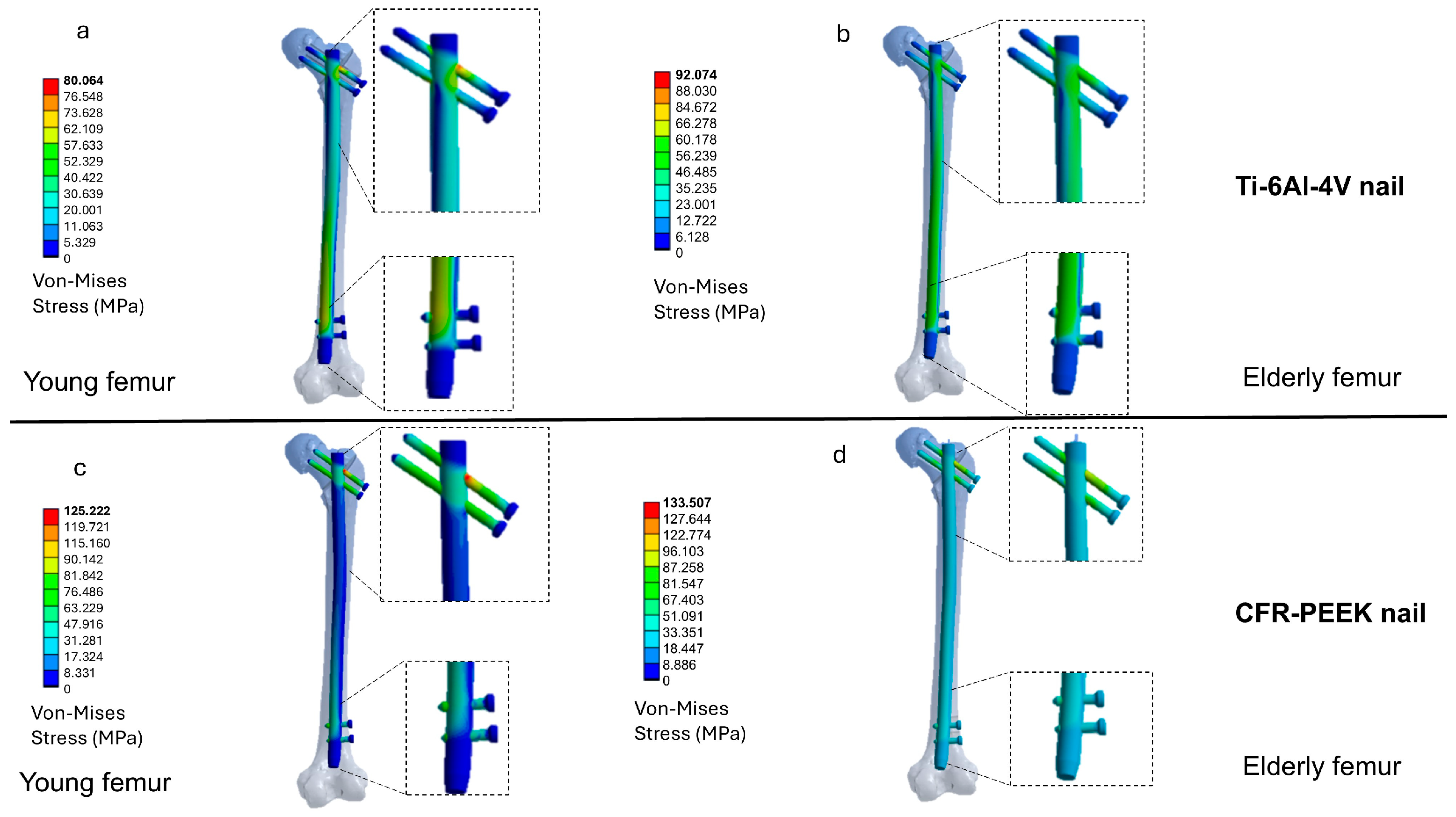
3.2. Distribution of Von Mises Stresses in the Intramedullary Nail
3.3. Evaluation of the Osteogenic Stimulus Index
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lindskog, D.M.; Baumgaertner, M.R. Unstable intertrochanteric hip fractures in the elderly. JAAOS—J. Am. Acad. Orthop. Surg. 2004, 12, 179–190. [Google Scholar] [CrossRef]
- Parker, M.J.; Gillespie, W.J.; Gillespie, L.D. Effectiveness of hip protectors for preventing hip fractures in elderly people: Systematic review. BMJ 2006, 332, 571–574. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Bifolco, G.; Caffarelli, C.; Mazziotti, G.; Migliaccio, S.; Napoli, N.; Ruggiero, C.; Cipriani, C. Nutrition, vitamin D, and calcium in elderly patients before and after a hip fracture and their impact on the musculoskeletal system: A narrative review. Nutrients 2024, 16, 1773. [Google Scholar] [CrossRef]
- Shen, Y.; Tan, B.; Zhang, J.; Zhang, N.; Wang, Z. Epidemiology and disease burden of fractures in Asia, 1990–2021: An analysis for the Global Burden of Disease Study 2021. J. Orthop. Transl. 2025, 52, 281–290. [Google Scholar] [CrossRef]
- Gonzalez-Martin, D.; Hernández-Castillejo, L.E.; Herrera-Perez, M.; Pais-Brito, J.L.; Gonzalez-Casamayor, S.; Garrido-Miguel, M. Osteosynthesis versus revision arthroplasty in Vancouver B2 periprosthetic hip fractures: A systematic review and meta-analysis. Eur. J. Trauma Emerg. Surg. 2023, 49, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.H. Brief History of Evolution of Femoral Nail. In The Art of Intramedullary Nailing for Femoral Fracture; Springer Nature: Singapore, 2022; pp. 1–2. [Google Scholar]
- Kanakamedala, A.C.; Schoof, L.H.; Schultz, B.J.; Kyriakides, P.W.; Ganta, A.; Konda, S.R. A review and critical analysis of the history of intramedullary nailing. Curr. Orthop. Pract. 2024, 35, 135–144. [Google Scholar] [CrossRef]
- Roy, S.; Sekhar, S.; Pal, J. Comparative Study of Outcome of Treatment of Fracture Shaft of Femur by Open Intramedullary Kuntscher’s Nail and Closed Intramedullary Interlocking Nail. J. Indian Med. Assoc. 2022, 120, 44–48. [Google Scholar]
- Bekos, A.; Sioutis, S.; Kostroglou, A.; Saranteas, T.; Mavrogenis, A.F. The history of intramedullary nailing. Int. Orthop. 2021, 45, 1355–1361. [Google Scholar] [CrossRef]
- Sigterman, T.; Verbruggen, J. Proximal humeral fractures in the elderly. Treatment with an intramedullary nail. Eur. J. Trauma Emerg. Surg. 2025, 51, 266. [Google Scholar] [CrossRef]
- Barber, C.C.; Burnham, M.; Ojameruaye, O.; McKee, M.D. A systematic review of the use of titanium versus stainless steel implants for fracture fixation. OTA Int. 2021, 4, e138. [Google Scholar] [CrossRef]
- Dávid, Á.L.; Mucsina, F.; Antal, E.; Lamberti, A.G.; Lőrincz, A.; Józsa, G. Comparison of titanium versus resorbable intramedullary nailing in pediatric forearm fractures. Children 2024, 11, 942. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; Lee, P.Y. Mechanical behaviors of titanium, nickel–titanium, and stainless elastic intramedullary nail in fixation of tibial diaphyseal fractures. Injury 2023, 54, 111097. [Google Scholar] [CrossRef]
- Yaqoob, K.; Amjad, I.; Munir Awan, M.A.; Liaqat, U.; Zahoor, M.; Kashif, M. Novel method for the production of titanium foams to reduce stress shielding in implants. ACS Omega 2023, 8, 1876–1884. [Google Scholar] [CrossRef] [PubMed]
- Al Zoubi, N.F.; Tarlochan, F.; Mehboob, H.; Jarrar, F. Design of titanium alloy femoral stem cellular structure for stress shielding and stem stability: Computational analysis. Appl. Sci. 2022, 12, 1548. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, Z.; Wang, Y.; Zhu, G.; Tan, K.; Liu, M.; Li, L. Biomechanical effects of mechanical stress on cells involved in fracture healing. Orthop. Surg. 2024, 16, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Zhang, J.; Jia, J.; Li, X. Influence of knee flexion on early femoral fracture healing: A combined analysis of musculoskeletal dynamics and finite elements. Comput. Methods Programs Biomed. 2023, 241, 107757. [Google Scholar] [CrossRef]
- Ma, Q.; Miri, Z.; Haugen, H.J.; Moghanian, A.; Loca, D. Significance of mechanical loading in bone fracture healing, bone regeneration, and vascularization. J. Tissue Eng. 2023, 14, 20417314231172573. [Google Scholar] [CrossRef]
- Pan, X.; Gencturk, B.; Alnaggar, M.; Sohail, M.G.; Kahraman, R.; Al Nuaimi, N.; Rodrigues, D.F.; Yildirim, Y. Numerical simulation of the fracture and compression response of self-healing concrete containing engineered aggregates. Cem. Concr. Compos. 2023, 136, 104858. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Z.; Qi, X.; Hu, Y.; Li, B.; Zhang, L. Computational modeling of Bone Fracture Healing under different initial conditions and mechanical load. IEEE Trans. Biomed. Eng. 2024, 71, 2105–2118. [Google Scholar] [CrossRef]
- Schmidt, I.; Albert, J.; Ritthaler, M.; Papastavrou, A.; Steinmann, P. Bone fracture healing within a continuum bone remodelling framework. Comput. Methods Biomech. Biomed. Eng. 2022, 25, 1040–1050. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, G.; Yang, H.; Jin, X. Computational models of bone fracture healing and applications: A review. Biomed. Eng./Biomed. Tech. 2024, 69, 219–239. [Google Scholar] [CrossRef]
- Kang, J.H.; Sakthiabirami, K.; Jang, K.J.; Jang, J.G.; Oh, G.J.; Park, C.; Fisher, J.G.; Park, S.W. Mechanical and biological evaluation of lattice structured hydroxyapatite scaffolds produced via stereolithography additive manufacturing. Mater. Des. 2022, 214, 110372. [Google Scholar] [CrossRef]
- Song, C.; Liu, L.; Deng, Z.; Lei, H.; Yuan, F.; Yang, Y.; Li, Y.; Yu, J. Research progress on the design and performance of porous titanium alloy bone implants. J. Mater. Res. Technol. 2023, 23, 2626–2641. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, Q.; Zhou, H.; Jing, Z.; Liu, X.; Zheng, Y.; Cai, H.; Wei, F.; Jiang, L.; Yu, M.; et al. Three-dimensional-printed individualized porous implants: A new “implant-bone” interface fusion concept for large bone defect treatment. Bioact. Mater. 2021, 6, 3659–3670. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Montesani, L.; Comuzzi, L.; Cipollina, A.; Deporter, D.A.; Di Pietro, N.; Trentadue, B. The Influence of Insertion Torque on Stress Distribution in Peri-Implant Bones Around Ultra-Short Implants: An FEA Study. J. Funct. Biomater. 2025, 16, 260. [Google Scholar] [CrossRef]
- Sokac, M.; Milosevic, A.; Santosi, Z.; Vukelic, D.; Budak, I. Design and Verification of a New Fixture for Machining of Porous Blocks for Medical CAD/CAM Systems. Appl. Sci. 2025, 15, 794. [Google Scholar] [CrossRef]
- Feng, J.; Li, A.; Safaei, B.; Qin, Z.; Chu, F. Revolutionary coatings: Unlocking the full potential of energy dissipation and mechanical properties in nickel foam. Chem. Eng. J. 2025, 505, 159461. [Google Scholar] [CrossRef]
- Ceddia, M.; Morizio, A.; Solarino, G.; Trentadue, B. Reduction of Ceramic Wear by Concave Dimples on the Bearing Surface in CoC Hip Implants: A Finite Element Analysis. Ceramics 2025, 8, 51. [Google Scholar] [CrossRef]
- Moharil, S.; Reche, A.; Durge, K.; Moharil, S.S. Polyetheretherketone (PEEK) as a biomaterial: An overview. Cureus 2023, 15, e44307. [Google Scholar] [CrossRef]
- de Ruiter, L.; Rankin, K.; Browne, M.; Briscoe, A.; Janssen, D.; Verdonschot, N. Decreased stress shielding with a PEEK femoral total knee prosthesis measured in validated computational models. J. Biomech. 2021, 118, 110270. [Google Scholar] [CrossRef]
- Dallal, S.; Eslami, B.; Tiari, S. Recent Advances in PEEK for Biomedical Applications: A Comprehensive Review of Material Properties, Processing, and Additive Manufacturing. Polymers 2025, 17, 1968. [Google Scholar] [CrossRef] [PubMed]
- Ziran, B.H.; O’Pry, E.K.; Harris, R.M. Carbon Fiber-Reinforced PEEK Versus Titanium Tibial Intramedullary Nailing: A Preliminary Analysis and Results. J. Orthop. Trauma 2020, 34, 8429–8433. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Hu, Y.C.; Xu, Z.M.; Zhao, Y.W.; Wu, J.M. An intramedullary nail with multifunctional interlocking for all types of fracture in both femurs. Orthop. Surg. 2009, 1, 121–126. [Google Scholar] [CrossRef]
- Giannoudis, V.P.; Rodham, P.; Antypas, A.; Mofori, N.; Chloros, G.; Giannoudis, P.V. Patient perspective on the use of carbon fibre plates for extremity fracture fixation. Eur. J. Orthop. Surg. Traumatol. 2023, 33, 2573–2577. [Google Scholar] [CrossRef]
- Lewis, G.S.; Mischler, D.; Wee, H.; Reid, J.S.; Varga, P. Finite element analysis of fracture fixation. Curr. Osteoporos. Rep. 2021, 19, 403–416. [Google Scholar] [CrossRef]
- Verma, A.; Jain, A.; Sethy, S.S.; Verma, V.; Goyal, N.; Vathulya, M.; Kandwal, P. Finite element analysis and its application in Orthopaedics: A narrative review. J. Clin. Orthop. Trauma 2024, 58, 102803. [Google Scholar] [CrossRef]
- Wang, K.Y.; Farid, A.R.; Comtesse, S.; von Keudell, A.G. Segmentation and finite element analysis in orthopaedic trauma. 3D Print. Med. 2025, 11, 39. [Google Scholar] [CrossRef]
- Kim, C.J.; Lee, J.S.; Goh, T.S.; Shin, W.C.; Lee, C. Finite element analysis of fixation stability according to reduction position for internal fixation of intertrochanteric fractures. Sci. Rep. 2024, 14, 19214. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Lv, Y.; Zhu, Z.; Lu, Y.; Zhou, H.; Zhang, Y.; Liao, Y.; Wang, B. Biomechanical characteristic differences of two new types of intramedullary nail devices in the treatment of comminuted intertrochanteric fractures of femur: A comparative study based on finite element analysis. J. Orthop. Surg. Res. 2024, 19, 583. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Giannini, G.; De Giosa, G.; Tucci, M.; Trentadue, B. A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite. J. Compos. Sci. 2024, 8, 254. [Google Scholar] [CrossRef]
- Ceddia, M.; Romasco, T.; Comuzzi, L.; Specchiulli, A.; Piattelli, A.; Lamberti, L.; Di Pietro, N.; Trentadue, B. Finite-element analysis study comparing titanium and polyetheretherketone caps in a conometric connection between implant and prosthesis. Adv. Eng. Mater. 2024, 26, 2400198. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Pulcrano, A.; Benedetto, A.; Trentadue, B. Finite Element Analysis of a 3D-Printed Acetabular Prosthesis for an Acetabular Defect According to the Paprosky Classification. Materials 2025, 18, 1295. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.F.; Li, Y.; Lin, J.; Cai, D.; Cai, S.; Yan, L.; Yao, X. Cortical thickness in the intertrochanteric region may be relevant to hip fracture type. BMC Musculoskelet. Disord. 2017, 18, 305. [Google Scholar] [CrossRef]
- Yamamoto, N.; Yamakawa, Y.; Inokuchi, T.; Iwamoto, Y.; Inoue, T.; Noda, T.; Kawasaki, K.; Ozaki, T. Hip fractures following intramedullary nailing fixation for femoral fractures. Injury 2022, 53, 1190–1195. [Google Scholar] [CrossRef]
- Bonfiglio, N.; Smimmo, A.; Carosini, A.; Perna, A.; Ruberto, P.; Minutillo, F.; De Santis, V.; Malerba, G. Subtrochanteric fractures in elderly people: Functional and radiographic outcomes after intramedullary locked nail fixation with or without cerclage. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 127–137. [Google Scholar]
- Samiezadeh, S.; Avval, P.T.; Fawaz, Z.; Bougherara, H. An Effective Approach for Optimization of a Composite Intramedullary Nail for Treating Femoral Shaft Fractures. J. Biomech. Eng. 2015, 137, 121001. [Google Scholar] [CrossRef]
- Cen, H.; Gong, H.; Liu, H.; Jia, S.; Wu, X.; Fan, Y. A Comparative Study on the Multiscale Mechanical Responses of Human Femoral Neck Between the Young and the Elderly Using Finite Element Method. Front. Bioeng. Biotechnol. 2022, 10, 893337. [Google Scholar] [CrossRef]
- Bazyar, P.; Baumgart, A.; Altenbach, H.; Usbeck, A. An overview of selected material properties in finite element modeling of the human femur. Biomechanics 2023, 3, 124–135. [Google Scholar] [CrossRef]
- Mathai, B.; Dhara, S.; Gupta, S. Orthotropic bone remodelling around uncemented femoral implant: A comparison with isotropic formulation. Biomech. Model. Mechanobiol. 2021, 20, 1115–1134. [Google Scholar] [CrossRef]
- Chytła, P.; Wojnarowska, W. Finite Element Analysis of Mechanical Behavior in Human Femoral Bone: A Comparative Study of Homogeneous Isotropic and Heterogeneous Isotropic Material Model. Authorea Prepr. 2025; ahead of print. [Google Scholar]
- Levy, M.; Yosibash, Z. Heterogeneous fracture toughness of human cortical bone tissue. Int. J. Fract. 2025, 249, 17. [Google Scholar] [CrossRef]
- Katz, Y.; Dahan, G.; Sosna, J.; Shelef, I.; Cherniavsky, E.; Yosibash, Z. Scanner influence on the mechanical response of QCT-based finite element analysis of long bones. J. Biomech. 2019, 86, 149–159. [Google Scholar] [CrossRef]
- Schileo, E.; Dall’Ara, E.; Taddei, F.; Malandrino, A.; Schotkamp, T.; Baleani, M.; Viceconti, M. An accurate estimation of bone density improves the accuracy of subject-specific finite element models. J. Biomech. 2008, 41, 2483–2491. [Google Scholar] [CrossRef]
- Knowles, N.K.; Whittier, D.E.; Besler, B.A.; Boyd, S.K. Proximal tibia bone stiffness and strength in HR-pQCT-and QCT-based finite element models. Ann. Biomed. Eng. 2021, 49, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Yang, Z.; Qu, D.; Hu, B.; Li, L. Orthotropic Constitutive Modeling and Tsai-Wu Failure Criterion for Carbon Fiber-Reinforced PEEK Composites. Polymers 2025, 17, 1076. [Google Scholar] [CrossRef]
- Tseng, J.-C.; Huang, W.-C.; Chang, W.; Jeromin, A.; Keller, T.F.; Shen, J.; Chuang, A.C.; Wang, C.-C.; Lin, B.-H.; Amalia, L.; et al. Deformations of Ti-6Al-4V additive-manufacturing-induced isotropic and anisotropic columnar structures: Insitu measurements and underlying mechanisms. Addit. Manuf. 2020, 35, 101322. [Google Scholar] [CrossRef]
- Shash, Y.H.; El-Wakad, M.T.; El-Dosoky, M.A.A.; Dohiem, M.M. Evaluation of stresses on mandible bone and prosthetic parts in fixed prosthesis by utilizing CFR-PEEK, PEKK and PEEK frameworks. Sci. Rep. 2023, 13, 11542. [Google Scholar] [CrossRef]
- Pala, E.; Ozdemir, I.; Grund, T.; Lampke, T. The Influence of Design on Stress Concentration Reduction in Dental Implant Systems Using the Finite Element Method. Crystals 2023, 14, 20. [Google Scholar] [CrossRef]
- Toksoy, S.; Demirtaş, İ.; Güngörürler, M.; Öztuna, V. Finite element analysis of intramedullary nailing and nail-plate combinations for treating unstable proximal tibial metaphyseal fractures. Turk. J. Trauma Emerg. Surg./Ulus. Travma Acil Cerrahi Derg. 2024, 30, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Keller, K.; Engelhardt, M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2014, 3, 346–350. [Google Scholar] [CrossRef]
- Lunn, D.E.; Redmond, A.C.; Chapman, G.J.; Lund, M.E.; Ferguson, S.J.; De Pieri, E. Hip contact force pathways in total hip replacement differ between patients and activities of daily living. J. Biomech. 2024, 176, 112309. [Google Scholar] [CrossRef]
- Amiri, P.; Bull, A.M. Prediction of in vivo hip contact forces during common activities of daily living using a segment-based musculoskeletal model. Front. Bioeng. Biotechnol. 2022, 10, 995279. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, G.; Deuretzbacher, G.; Heller, M.; Graichen, F.; Rohlmann, A.; Strauss, J.; Duda, G.N. Hip contact forces and gait patterns from routine activities. J. Biomech. 2001, 34, 859–871. [Google Scholar] [CrossRef]
- Wong, M.; Carter, D.R. Mechanical stress and morphogenetic endochondral ossification of the sternum. J. Bone Jt. Surg. Am. 1988, 70, 992–1000. [Google Scholar] [CrossRef]
- Gardner, T.N.; Mishra, S.; Marks, L. The role of osteogenic index, octahedral shear stress and dilatational stress in the ossification of a fracture callus. Med. Eng. Phys. 2004, 26, 493–501. [Google Scholar] [CrossRef]
- O’Connor, M.I.; Switzer, J.A. AAOS clinical practice guideline summary: Management of hip fractures in older adults. JAAOS–J. Am. Acad. Orthop. Surg. 2022, 30, e1291–e1296. [Google Scholar] [CrossRef]
- Hu, L.; Xiong, Y.; Mi, B.; Panayi, A.C.; Zhou, W.; Liu, Y.; Liu, J.; Xue, H.; Yan, C.; Abududilibaier, A.; et al. Comparison of intramedullary nailing and plate fixation in distal tibial fractures with metaphyseal damage: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2019, 14, 30. [Google Scholar] [CrossRef]
- Schemitsch, E.H.; Nowak, L.L.; Schulz, A.P.; Brink, O.; Poolman, R.W.; Mehta, S.; Stengel, D.; Zhang, C.Q.; Martinez, S.; Kinner, B.; et al. Intramedullary nailing vs sliding hip screw in trochanteric fracture management: The INSITE randomized clinical trial. JAMA Netw. Open 2023, 6, e2317164. [Google Scholar] [CrossRef]
- Kenedi, P.P.; de Oliveira e Silva Neto, J.R. Evaluating the bone load share of a femur-nail set—An analytical stiffness model. Res. Biomed. Eng. 2024, 40, 213–223. [Google Scholar] [CrossRef]
- Chen, P.; Fan, Z.; Xu, N.; Wang, H. A biomechanical investigation of a novel intramedullary nail used to salvage failed internal fixations in intertrochanteric fractures. J. Orthop. Surg. Res. 2023, 18, 632. [Google Scholar] [CrossRef] [PubMed]
- Vles, G.F.; Brodermann, M.H.; Roussot, M.A.; Youngman, J. Carbon-fiber-reinforced PEEK intramedullary nails defining the niche. Case Rep. Orthop. 2019, 2019, 1538158. [Google Scholar] [CrossRef] [PubMed]
- Takashima, K.; Nakahara, I.; Uemura, K.; Hamada, H.; Ando, W.; Takao, M.; Sugano, N. Clinical outcomes of proximal femoral fractures treated with a novel carbon fiber-reinforced polyetheretherketone intramedullary nail. Injury 2020, 51, 678–682. [Google Scholar] [CrossRef]
- Sacchetti, F.; Andreani, L.; Palazzuolo, M.; Cherix, S.; Bonicoli, E.; Neri, E.; Capanna, R. Carbon/PEEK nails: A case–control study of 22 cases. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 643–651. [Google Scholar] [CrossRef]
- Samiezadeh, S.; Avval, P.T.; Fawaz, Z.; Bougherara, H. Biomechanical assessment of composite versus metallic intramedullary nailing system in femoral shaft fractures: A finite element study. Clin. Biomech. 2014, 29, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Samiezadeh, S.; Schemitsch, E.H.; Zdero, R.; Bougherara, H. Biomechanical response under stress-controlled tension-tension fatigue of a novel carbon fiber/epoxy intramedullary nail for femur fractures. Med. Eng. Phys. 2020, 80, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, E.L.; Rath, E.; Shlaifer, A.; Chechik, O.; Maman, E.; Salai, M. Carbon fiber reinforced PEEK Optima—A composite material biomechanical properties and wear/debris characteristics of CF-PEEK composites for orthopedic trauma implants. J. Mech. Behav. Biomed. Mater. 2013, 17, 221–228. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, Y.; Dong, L.; Jia, P.; Lu, F. Finite Element Analysis of Absorbable Sheath to Prevent Stress Shielding of Tibial Interlocking Intramedullary Nail. IOP Conf. Ser. Mater. Sci. Eng. 2017, 224, 012052. [Google Scholar] [CrossRef]
- Chen, L.; Gao, L.; Cui, H.; Guo, X.; Han, J.; Liu, J.; Yao, Y. Finite element comparison of titanium and polyetheretherketone materials for mandibular defect reconstruction. Am. J. Transl. Res. 2024, 16, 6097–6105. [Google Scholar] [CrossRef]
- Potter, B.K. From Bench to Bedside: Radiolucent Implants—Better Visualization or Camouflaged Gimmick? Clin. Orthop. Relat. Res. 2022, 480, 461–463. [Google Scholar] [CrossRef]





| Materials | Young Modulus [GPa] | Shear Modulus [GPa] | Poisson’s Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cortical bone | 0.37 | 0.3 | 0.3 | ||||||
| Trabecular bone | |||||||||
| Materials | Young Modulus [GPa] | Shear Modulus [GPa] | Poisson’s Ratio | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CFR-PEEK | 18 | 18 | 3.6 | 4.8 | 4.8 | 3.0 | 0.30 | 0.30 | 0.30 |
| Ti-6Al-4V | 110 | 110 | 110 | 41.2 | 41.2 | 41.2 | 0.34 | 0.34 | 0.34 |
| Force | Young (N) | Elderly (N) |
|---|---|---|
| JRF (Joint Reaction Force) | 1170 | 1100 |
| Fabd (Abductors) | 300 | 180 |
| Flp (Iliopsoas) | 188 | 112.8 |
| Fvl (Vastus lateralis) | 292 | 175.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceddia, M.; Pesare, E.; Solarino, G.; Lamberti, L.; Trentadue, B. Biomechanical Comparison of Titanium and CFR-PEEK Intramedullary Nails Using Finite Element Analysis. J. Compos. Sci. 2025, 9, 576. https://doi.org/10.3390/jcs9110576
Ceddia M, Pesare E, Solarino G, Lamberti L, Trentadue B. Biomechanical Comparison of Titanium and CFR-PEEK Intramedullary Nails Using Finite Element Analysis. Journal of Composites Science. 2025; 9(11):576. https://doi.org/10.3390/jcs9110576
Chicago/Turabian StyleCeddia, Mario, Elisa Pesare, Giuseppe Solarino, Luciano Lamberti, and Bartolomeo Trentadue. 2025. "Biomechanical Comparison of Titanium and CFR-PEEK Intramedullary Nails Using Finite Element Analysis" Journal of Composites Science 9, no. 11: 576. https://doi.org/10.3390/jcs9110576
APA StyleCeddia, M., Pesare, E., Solarino, G., Lamberti, L., & Trentadue, B. (2025). Biomechanical Comparison of Titanium and CFR-PEEK Intramedullary Nails Using Finite Element Analysis. Journal of Composites Science, 9(11), 576. https://doi.org/10.3390/jcs9110576






