The Effect of Calcium Stearate Additives in Concrete on Mass Transfer When Exposed to Aspergillus niger Fungi
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
- -
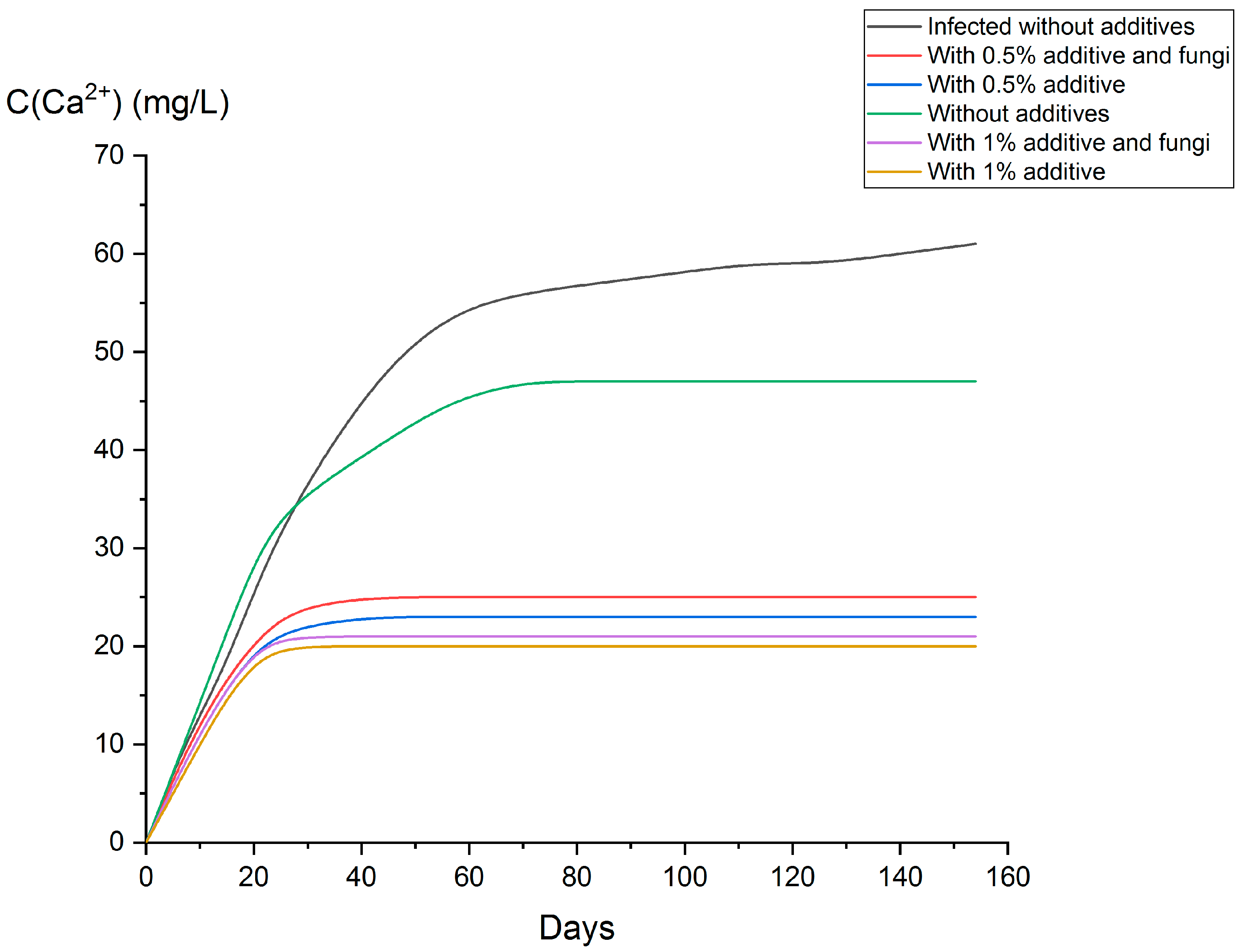
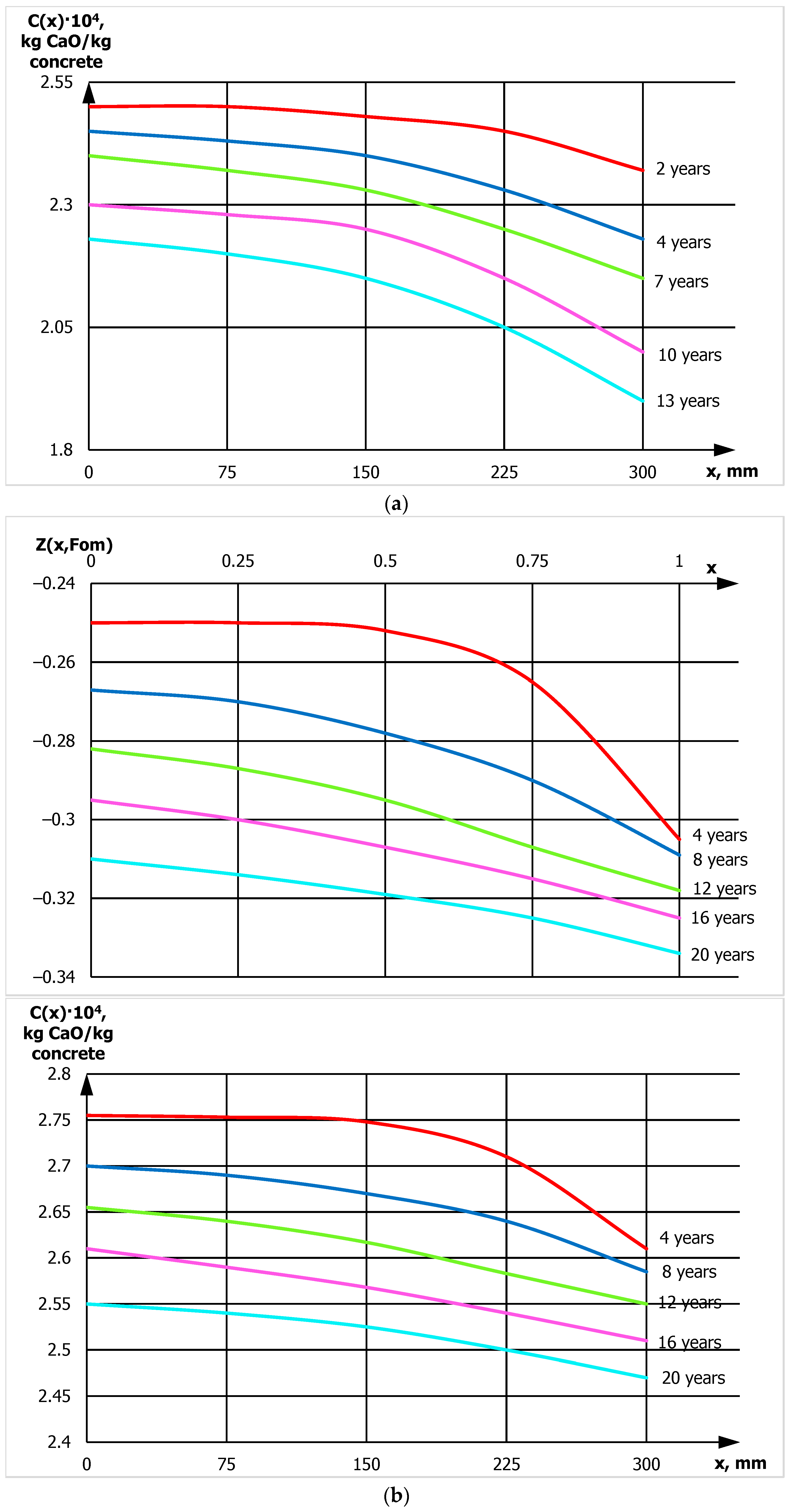
- In concrete of waterproofness classes W6–W10, the degree of decomposition of calcium-containing phases by fungal microorganisms and the removal of calcium from the structure is reduced by approximately 3 times compared to conventional concrete.
- -
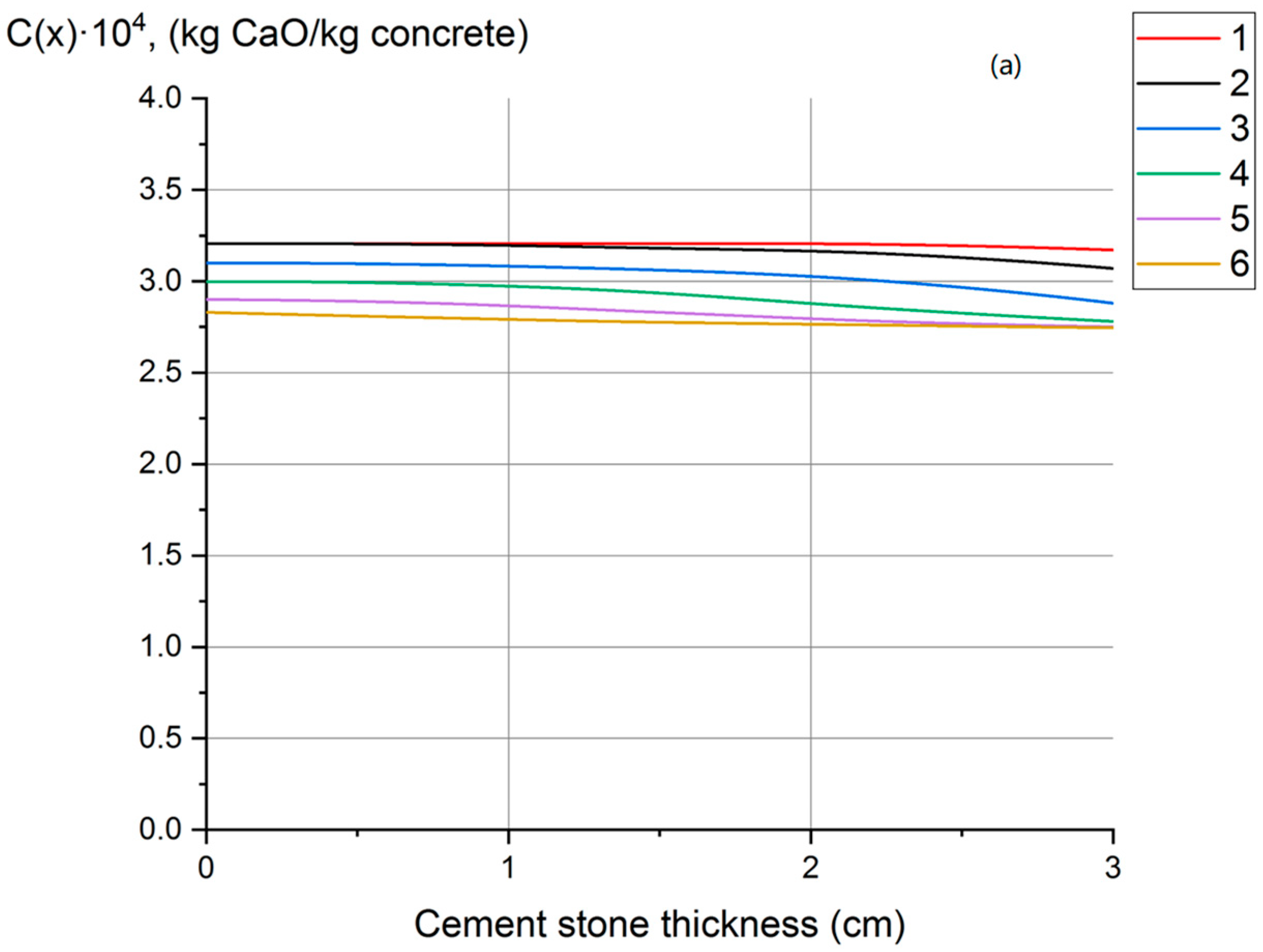
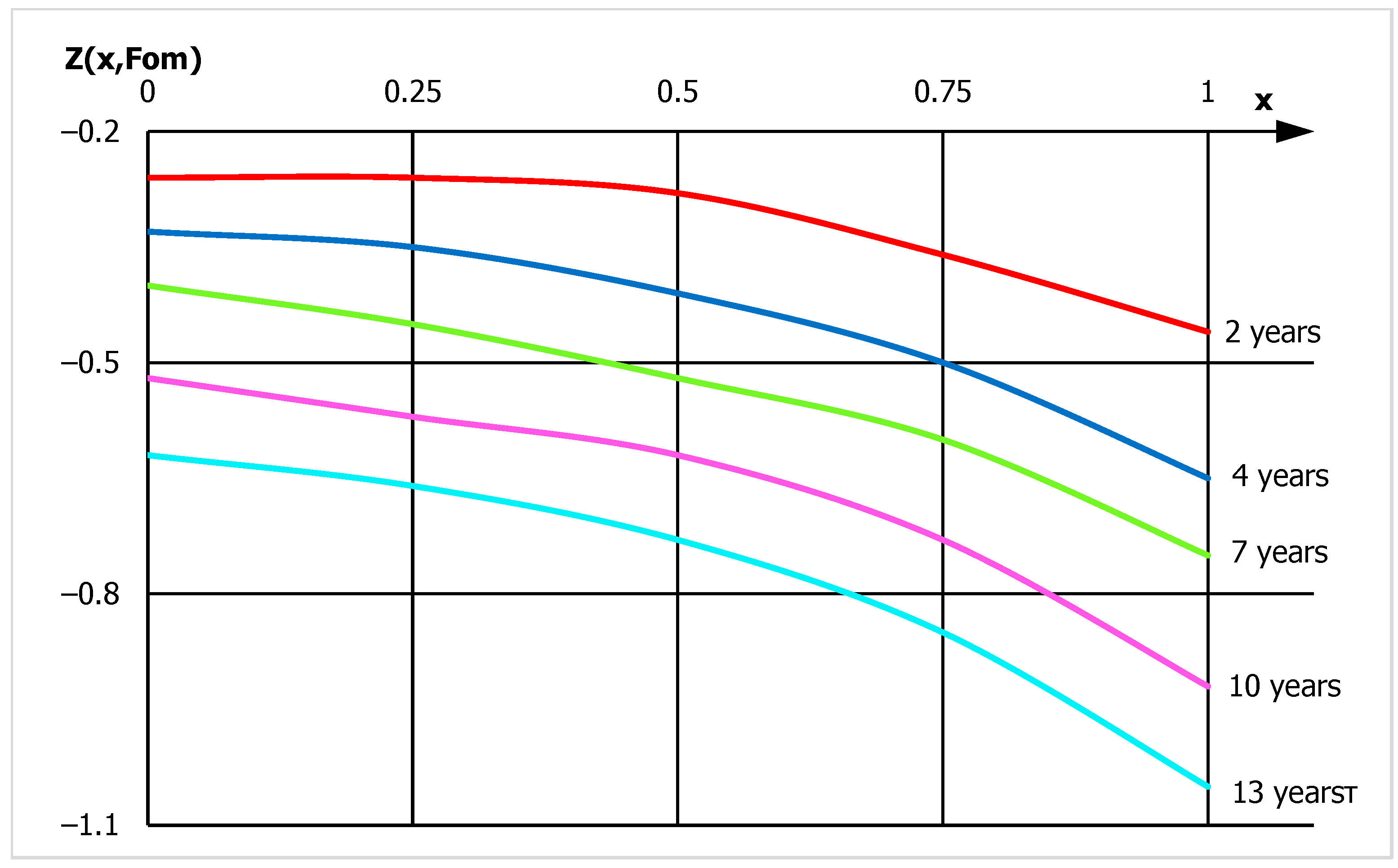
- Profiles of calcium hydroxide concentrations throughout the thickness of concrete have been obtained, indicating that fungal destruction of hydrophobic concrete primarily occurs at the surface. In ordinary cement concrete, the decomposition and leaching of calcium hydroxide occurs throughout the thickness.
- -
- The values of the mass conductivity coefficients for fungal destruction of ordinary concrete during moistening and for concrete with calcium stearate additives differ by two orders of magnitude: 10−9 and 10−11 [m2/s], respectively, indicating a significant slowdown in mass transfer.
- -
- Using a mathematical model of concrete biocorrosion, it is predicted that destructive processes in concrete without additives will lead to dangerous conditions after 15 years of fungal exposure, while for hydrophobic concrete, this will occur 25–30 years.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Arakelyan, G.A.; Karapetyan, A.K.; Badalyan, M.M.; Ghahramanyan, A.A.; Makarov, E.M. Increasing the efficiency of fine-grained lightweight concrete using complex additives. J. Archit. Eng. Res. 2022, 2, 3–8. [Google Scholar] [CrossRef]
- Turgunbaev, U.; Umirova, M. High-strength concrete technology for manufacturing reinforced concrete sleepers from prestressed reinforced concrete. E3S Web Conf. 2021, 264, 02040. [Google Scholar] [CrossRef]
- Almuslehi, O.F.; Fomin, N.I.; Rutkauskas, T.K. Strengthening of Reinforced Concrete Beams in a Damaged Building Using Carbon Fiber Materials. Russ. J. Constr. Sci. Technol. 2022, 8, 30–42. [Google Scholar] [CrossRef]
- Khakimullina, S.; Il’ina, L.; Mukhina, I. Increasing the mechanical strength of fine-grained concrete by introducing finely dispersed mineral additives. AIP Conf. Proc. 2017, 1800, 020009. [Google Scholar]
- Agzamov, F.A.; Lomakina, L.N.; Hababutdinova, N.B.; Davletshin, R.F.; Kriga, A.K.; Tokunov, T.V. Cement stone corrosion processes affected by acidulous components of bedded fluids. Pet. Eng. 2015, 13, 10–28. [Google Scholar]
- Woyciechowski, P.; Adamczewski, G.; Łukowski, P. Chemical corrosion of concrete tank in sewage treatment plant as the cause of failure. MATEC Web Conf. 2019, 284, 07007. [Google Scholar] [CrossRef][Green Version]
- Blikharskyy, Y.; Selejdak, J.; Kopiika, N.; Vashkevych, R. Study of Concrete under Combined Action of Aggressive Environment and Long-Term Loading. Materials 2021, 14, 6612. [Google Scholar] [CrossRef]
- Kozubal, J.; Wyjadłowski, M.; Steshenko, D. Probabilistic analysis of a concrete column in an aggressive soil environment. PLoS ONE 2019, 14, e0212902. [Google Scholar] [CrossRef] [PubMed]
- Manso, S.; Calvo-Torras, M.Á.; De Belie, N.; Segura, I.; Aguado, A. Evaluation of natural colonisation of cementitious materials: Effect of bioreceptivity and environmental conditions. Sci. Total Environ. 2015, 512–513, 444–453. [Google Scholar] [CrossRef]
- Stohl, L.; Manninger, T.; von Werder, J.; Dehn, F.; Gorbushina, A.; Meng, B. Bioreceptivity of concrete: A review. J. Build. Eng. 2023, 76, 107201. [Google Scholar] [CrossRef]
- Guillitte, O. Bioreceptivity: A new concept for building ecology studies. Sci. Total Environ. 1995, 167, 215–220. [Google Scholar] [CrossRef]
- Chunduri, J.P.R. Indoor Fungal Populations Inhabiting Cement Structures-Remedial Measures. IOSR J. Environ. Sci. Toxicol. Food Technol. (IOSR-JESTFT) 2014, 8, 19–24. [Google Scholar] [CrossRef]
- Gupta, S.P.; Kurmi, M.K. Impact of fungi on historical monument with reference to Mahadev temple Bastar of Chhatisgarh. Int. J. Life Sci. Res. Arch. 2023, 4, 1–5. [Google Scholar] [CrossRef]
- De la Rosa-García, S.C.; Ortega-Morales, O.; Gaylarde, C.C.; Beltrán-García, M.; Quintana-Owen, P.; Reyes-Estebanez, M. Influence of fungi in the weathering of limestone of Mayan monuments. Rev. Mex. De Micol. 2011, 33, 43–51. [Google Scholar]
- Farooq, M.; Hassan, M.; Gull, F. Mycobial deterioration of stone monuments of Dharmarajika, Taxila. J. Microbiol. Exp. 2015, 2, 29–33. [Google Scholar] [CrossRef]
- Gupta, S.P.; Chandrol, G.K.; Sharma, D.N. Screening and indexing of dominance of fungal flora on sandstones with special reference to historical monuments. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 99–105. [Google Scholar]
- Bertron, A. Understanding interactions between cementitious materials and microorganisms: A key to sustainable and safe concrete structures in various contexts. Mater. Struct. 2014, 47, 1787–1806. [Google Scholar] [CrossRef]
- Li, X.; Kappler, U.; Jiang, G.; Bond, P.L. The Ecology of Acidophilic Microorganisms in the Corroding Concrete Sewer Environment. Front. Microbiol. 2017, 8, 683. [Google Scholar] [CrossRef]
- Sterflinger, K. Fungi: Their role in deterioration of cultural heritage. Fungal Biol. Rev. 2010, 24, 47–55. [Google Scholar] [CrossRef]
- Bone, J.R.; Stafford, R.; Hall, A.E.; Herbert, R.J.H. The intrinsic primary bioreceptivity of concrete in the coastal environment—A review. Dev. Built Environ. 2022, 10, 100078. [Google Scholar] [CrossRef]
- Łowińska-Kluge, A.; Horbik, D.; ZgoŁa-Grześkowiak, A.; Stanisz, E.; Górski, Z. A comprehensive study on the risk of biocorrosion of building materials. Corr. Eng. Sci. Technol. 2017, 52, 13–21. [Google Scholar] [CrossRef]
- Sazanova, K.V.; Shchiparev, S.M.; Vlasov, D.Y. Formation of organic acids by fungi isolated from the surface of stone monuments. Microbiology 2014, 83, 516–522. [Google Scholar] [CrossRef]
- Long, G.; Xie, Y.; Luo, Z.; Qu, L.; Zhou, J.L.; Li, W. Deterioration mechanism of steam-cured concrete subjected to coupled environmental acid and drying action. J. Infrastruct. Preserv. Resil. 2020, 1, 5. [Google Scholar] [CrossRef]
- Ninan, C.M.; Ajay, A.; Ramaswamy, K.P.; Thomas, A.V.; Bertron, A. A critical review on the effect of organic acids on cement-based materials. IOP Conf. Ser. Earth Environ. Sci. 2020, 491, 012045. [Google Scholar] [CrossRef]
- Yang, S.; Bieliatynskyi, A.; Trachevskyi, V.; Shao, M.; Ta, M. Research of Nano-modified Plain Cement Concrete Mixtures and Cement-Based Concrete. Int. J. Concr. Struct. Mater. 2023, 17, 50. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, Y.; Mu, S.; Cai, J.; Hong, J.; Liu, J.; Zhao, Y. Pore Structure and Permeability of Cementitious Materials Containing a Carboxylic Acid Type Hydrophobic Agent. Front. Mater. 2022, 9, 907638. [Google Scholar] [CrossRef]
- Klisińska-Kopacz, A.; Tišlova, R. Effect of hydrophobization treatment on the hydration of repair Roman cement mortars. Constr. Build. Mater. 2012, 35, 735–740. [Google Scholar] [CrossRef]
- Grabowska, K.B.; Koniorczyk, M. Internal hydrophobization of cementitious materials by using of organosilicon compounds. E3S Web Conf. 2020, 172, 14006. [Google Scholar] [CrossRef]
- Materak, K.; Wieczorek, A.; Chałupka-Śpiewak, K.; Koniorczyk, M.; Kalina, L.; Bílek, V., Jr. Internal hydrophobic treatment for mortar containing supplementary cementitious materials: Thermal analysis of kinetics and products of hydration. J. Therm. Anal. Calorim. 2024, 150, 937–951. [Google Scholar] [CrossRef]
- Tarakanov, O.V.; Erofeeva, I.V.; Belyakova, E.A.; Moskvin, R.N.; Sanyagina, Y.A.; Khristoforova, I.A. Modeling of the processes of early structure formation and hardening of cement materials with organomineral additives. Nanotechnol. Constr. 2024, 16, 510–524. [Google Scholar] [CrossRef]
- Bulanov, P.E.; Ermilova, E.U.; Mavliev, L.F. Structure and mineral composition of soil-cement with complex additive. Mag. Civ. Eng. 2018, 83, 38–48. [Google Scholar]
- Qu, Z.Y.; Wang, F.; Liu, P.; Yu, Q.L.; Brouwers, H.J.H. Super-hydrophobic magnesium oxychloride cement (MOC): From structural control to self-cleaning property evaluation. Mater. Struct. 2020, 53, 30. [Google Scholar] [CrossRef]
- Lanzón, M.; Martínez, E.; Mestre, M.; Madrid, J.A. Use of zinc stearate to produce highly-hydrophobic adobe materials with extended durability to water and acid-rain. Constr. Build. Mat. 2017, 139, 114–122. [Google Scholar] [CrossRef]
- Maravelaki, P.N.; Kapetanaki, K.; Papayianni, I.; Ioannou, I.; Faria, P.; Alvarez, J.; Stefanidou, M.; Nunes, C.; Theodoridou, M.; Ferrara, L.; et al. RILEM TC 277-LHS report: Additives and admixtures for modern lime-based mortars. Mater. Struct. 2023, 56, 106. [Google Scholar] [CrossRef]
- García-Vera, V.E.; Tenza-Abril, A.J.; Lanzón, M.; Saval, J.M. Exposing Sustainable Mortars with Nanosilica, Zinc Stearate, and Ethyl Silicate Coating to Sulfuric Acid Attack. Sustainability 2018, 10, 3769. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; He, X.; Yu, X.; Su, Y.; Huang, J.; Oh, S.-K. Sodium Oleate and Styrene-Acrylate Copolymer Emulsion-Modified Cement Mortar: Functional Combination of Physical Barrier and Hydrophobicity. J. Mater. Civ. Eng. 2023, 35, 04023242. [Google Scholar] [CrossRef]
- Wang, Y.; Zuo, Y.; Tang, Y. Inhibition effect and mechanism of sodium oleate on passivation and pitting corrosion of steel in simulated concrete pore solution. Constr. Build. Mater. 2018, 167, 197–204. [Google Scholar] [CrossRef]
- Izaguirre, A.; Lanas, J.; Álvarez, J.I. Effect of water-repellent admixtures on the behaviour of aerial lime-based mortars. Cem. Concr. Res. 2009, 39, 1095–1104. [Google Scholar] [CrossRef]
- Nemati Chari, M.; Naseroleslami, R.; Shekarchi, M. The impact of calcium stearate on characteristics of concrete. Asian J. Civ. Eng. 2019, 20, 1007–1020. [Google Scholar] [CrossRef]
- Soloviev, V.; Shvetsova, V. Influence of Calcium Stearate Ca(C18H35O2)2 on the Properties of Fine-Grained Concrete. Solid State Phenom. 2022, 334, 259–271. [Google Scholar] [CrossRef]
- Tannous, J.; Salem, T.; Metalssi, O.O.; Fen-Chong, T. Effect of calcium stearate on cellulose acetate-based mortars. J. Clean. Prod. 2024, 460, 142588. [Google Scholar] [CrossRef]
- Park, J.-H.; Yoon, C.-B. Properties and Durability of Cement Mortar Using Calcium Stearate and Natural Pozzolan for Concrete Surface Treatment. Materials 2022, 15, 5762. [Google Scholar] [CrossRef] [PubMed]
- Walloch, C.T.; Ebner, C.L.; Chin, D.; Siadat, B.; Ou, C.-C. Masonry Admixture and Method of Preparing Same. US5460648A, 15 April 1994. [Google Scholar]
- Supplee, W.W. Additive for, Method of Adding Thereof and Resulting Cured Cement-Type Concreations for Improved Heat and Freeze-Thaw Durability. US5922124A, 12 September 1997. [Google Scholar]
- Supplee, W.W. Concrete Admixture with Improved Durability and Efflorescence Control Containing a Highly Resilient Colorant. US6537366B1, 26 December 2000. [Google Scholar]
- Kanduth, B.; Gelinas, J.-B. Concrete Mix Having Anti-Efflorescence Properties and Method of Making Concrete Using the Same. EP2298709A1, 18 September 2009. [Google Scholar]
- Naseroleslami, R.; Chari, M.N. The effects of calcium stearate on mechanical and durability aspects of self-consolidating concretes incorporating silica fume/natural zeolite. Constr. Build. Mater. 2019, 225, 384–400. [Google Scholar] [CrossRef]
- Bazhenov, Y.M.; Lukuttsova, N.P.; Karpikov, E.G. Fine-grained Concrete Modified by Integrated Micro-dispersive Additive. Vestn. MGSU 2013, 2, 94–100. [Google Scholar]
- Maryoto, A.; Sthenly Gan, B.; Intang Setyo Hermanto, N.; Setijadi, R. Effect of Calcium Stearate in the Mechanical and Physical Properties of Concrete with PCC and Fly Ash as Binders. Materials 2020, 13, 1394. [Google Scholar] [CrossRef]
- Li, W.; Wittmann, F.H.; Jiang, R.; Zhao, T.; Wolfseher, R. Integral Water Repellent Concrete Produced by Addition of Metal Soaps. Restor. Build. Monum. 2014, 18, 41–48. [Google Scholar] [CrossRef]
- Maryoto, A.; Gan, B.S.; Aylie, H. Reduction of chloride ion ingress into reinforced concrete using a hydrophobic additive material. J. Teknol. (Sci. Eng.) 2017, 79, 65–72. [Google Scholar] [CrossRef][Green Version]
- Jahandari, S.; Tao, Z.; Alim, A. Md Effects of different integral hydrophobic admixtures on the properties of concrete. In Proceedings of the 30th Biennial National Conference of the Concrete Institute of Australia, Perth, Australia, 5–8 September 2021; Concrete Institute of Australia: Sydney, Australia, 2021; p. 8. [Google Scholar][Green Version]
- Quraishi, M.A.; Kumar, V.; Abhilash, P.P.; Singh, B.N. Calcium Stearate: A Green Corrosion Inhibitor for Steel in Concrete Environment. J. Mater. Environ. Sci. 2011, 2, 365–372. [Google Scholar][Green Version]
- Konovalova, V.S. Investigation of the Effect of Volumetric Hydrophobization on the Kinetics of Mass Transfer Processes Occurring in Cement Concretes during Corrosion. Materials 2023, 16, 3827. [Google Scholar] [CrossRef]
- Maryoto, A.; Gan, B.S.; Hermanto, N.I.S.; Setijadi, R. The Compressive Strength and Resistivity toward Corrosion Attacks by Chloride Ion of Concrete Containing Type I Cement and Calcium Stearate. Int. J. Corros. 2018, 2018, 2042510. [Google Scholar] [CrossRef]
- Konovalova, V.S.; Rumyantseva, V.E.; Strokin, K.B.; Galtsev, A.A.; Novikov, D.G.; Monastyrev, P.V. Degradation of Concrete Cement Stone Under the Influence of Aspergillus niger Fungi. Corros. Mater. Degrad. 2024, 5, 476–489. [Google Scholar] [CrossRef]
- Chromková, I.; Čechmánek, R. Influence of Biocorrosion on Concrete Properties. Key Eng. Mater. 2018, 760, 83–90. [Google Scholar] [CrossRef]
- Hilbig, H.; Gutberlet, T.; Beddoe, R.E. Acid attack on hydrated cement: Effect of organic acids on the degradation process. Mater. Struct. 2024, 57, 83. [Google Scholar] [CrossRef]
- Strokin, K.B.; Galtsev, A.A.; Konovalova, V.S.; Narmaniya, B.E. The effect of calcium stearate on the microbiological corrosion of cement stone concrete. Constr. Mater. 2024, 8, 25–29. [Google Scholar] [CrossRef]
- Lv, Y.; Luo, Y.; Song, C.; Jin, W.; Xiang, T.; Qiao, M.; Dang, J.; Bai, W.; Yang, Z.; Zhao, J. Effect of calcium stearate hydrophobic agent on the performance of mortar and reinforcement corrosion in mortar with cracks. Constr. Build. Mater. 2024, 450, 138684. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Roumyantseva, V.E.; Krasilnikov, I.V.; Konovalova, V.S.; Evsyakov, A.S. Monitoring of the Penetration of Chloride Ions to the Reinforcement Surface Through a Concrete Coating During Liquid Corrosion. IOP Conf. Ser. Mater. Sci. Eng. 2018, 463, 042048. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Rumyantseva, V.E.; Konovalova, V.S.; Evsyakov, A.S. The role of colmatation in liquid corrosion of hydrophobized concrete. IOP Conf. Ser. Mater. Sci. Eng. 2020, 896, 012096. [Google Scholar] [CrossRef]
- Strokin, K.B.; Novikov, D.G.; Konovalova, V.S. Forecasting the durability of reinforced concrete under conditions of microbiological corrosion. E3S Web Conf. 2021, 274, 04003. [Google Scholar] [CrossRef]
- Strokin, K.B.; Novikov, D.G.; Konovalova, V.S. Determination of the degree of microbiological damage to the «Cement Concrete-Steel Reinforcement» system. J. Phys. Conf. Ser. 2022, 2373, 022032. [Google Scholar] [CrossRef]
- Rumyantseva, V.; Konovalova, V.; Narmaniya, B.; Strokin, K.; Gal`tsev, A. Changing the characteristics of cement stone with the calcium stearate additive under the influence of fungal microorganisms. AIP Conf. Proc. 2024, 3243, 020046. [Google Scholar]
- Rumyantseva, V.; Konovalova, V.; Narmaniya, B.; Korinchuk, M. Increasing the Biostability of Concrete by the Introduction Of Additives. E3S Web Conf. 2023, 403, 03005. [Google Scholar] [CrossRef]
- Maryoto, A. Improving microstructures of concrete using Ca(C18H35O2). Procedia Eng. 2015, 125, 631–637. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Aleksandrova, O.V.; Lapidus, A.A.; Kuzmina, T.K.; Topchiy, D.V. An Engineering Method of Analyzing the Dynamics of Mass Transfer during Concrete Corrosion Processes in Offshore Structures. Materials 2023, 16, 3705. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Roumyantseva, V.E.; Krasilnikov, I.V.; Konovalova, V.S. Physical and mathematical modelling of the mass transfer process in heterogeneous systems under corrosion destruction of reinforced concrete structures. IOP Conf. Ser. Mater. Sci. Eng. 2018, 456, 012039. [Google Scholar] [CrossRef]
- Danilov, I.Z.; Prokhorenko, P.P.; Zhuravleva, V.P. Effect of ultrasound on the process of mass transfer in cement mortar and concrete. J. Eng. Phys. 1976, 31, 1162–1168. [Google Scholar] [CrossRef]
- Atkinson, A.; Claisse, P.A.; Harris, A.W.; Nickerson, A.K. Mass Transfer in Water-Saturated Concretes. MRS Online Proc. Libr. 1989, 176, 741. [Google Scholar] [CrossRef]
- Ibragimov, A.M.; Aksakovskaya, L.N.; Gerasimova, S.V. Mathematical Model for Heat and Mass Transfer of Concrete Heat Treatment Using Solar Power. Light Eng. 2023, 31, 12–21. [Google Scholar] [CrossRef]
- Nakamura, H.; Srisoros, W.; Yashiro, R.; Kunieda, M. Time-Dependent Structural Analysis Considering Mass Transfer to Evaluate Deterioration Process of RC Structures. J. Adv. Concr. Technol. 2006, 4, 147–158. [Google Scholar] [CrossRef]
- Tang, L.; Utgenannt, P.; Boubitsas, D. Durability and Service Life Prediction of Reinforced Concrete Structures. J. Chin. Ceram. Soc. 2015, 43, 1408–1419. [Google Scholar]
- Chen, X.; Ming, Y.; Fu, F.; Chen, P. Numerical and Empirical Models for Service Life Assessment of RC Structures in Marine Environment. Int. J. Concr. Struct. Mater. 2022, 16, 11. [Google Scholar] [CrossRef]
- Silvestro, L.; Andrade, J.J.O.; Dal Molin, D.C.C. Evaluation of service-life prediction model for reinforced concrete structures in chloride-laden environments. J. Build. Rehabil. 2019, 4, 20. [Google Scholar] [CrossRef]
- Martin-Perez, B.; Lounis, Z. Numerical modelling of service life of reinforced concrete structures. In Proceedings of the 2nd International RILEM Workshop on Life Prediction and Aging Management of Concrete Structures, Paris, France, 5–6 May 2003; Naus, D.J., Ed.; RILEM Publications: Bagneux, France, 2003; pp. 71–79. [Google Scholar]
- Oliveira, M.; Azenha, M.; Lourenço, P. A multi-physics modelling based on coupled diffusion equations to simulate the carbonation process. Rev. IBRACON Estrut. Mater. 2020, 13, e13506. [Google Scholar] [CrossRef]
- Titi, A.; Biondini, F. On the accuracy of diffusion models for life-cycle assessment of concrete structures. Struct. Infrastruct. Eng. 2016, 12, 1202–1215. [Google Scholar] [CrossRef]
- Kuzina, V.V.; Samchenko, S.V.; Kozlova, I.V.; Koshev, A.N. Mathematical modeling of physical and chemical processes in porous media in solving the problems of nanocomposite materials and water-filling. Nanotechnol. Constr. 2023, 15, 298–309. [Google Scholar] [CrossRef]
- Acuña-Aguero, O.H.; Corral-Higuera, R.; Barrios-Durstewitz, C.P.; Gómez-Soberón, J.M.; Arredondo-Rea, S.P.; Alamaral-Sánchez, J.L.; Rosas-Casarez, C.A.; Chinchillas-Chinchillas, M.J. Modeling and simulation of chloride diffusion in concrete with recycled aggregates. In Proceedings of the International Conference on Advances in Civil and Structural Engineering—CSE 2014, Kuala Lumpur, Malaysia, 2–3 August 2014; Institute of Research Engineers and Doctors (the IRED): Kuala Lumpur, Malaysia, 2014; pp. 15–19. [Google Scholar]
- Xu, J.; Mo, R.; Wang, P.; Zhou, J.; Dong, X.; She, W. Coupled Transport of Sulfate and Chloride Ions with Adsorption Effect: A Numerical Analysis. Front. Mater. 2020, 7, 536517. [Google Scholar] [CrossRef]
- Hidayat, M.N.; Kusuma, J.; Aris, N. A Two-Dimensional Mathematical Model of Carbon Dioxide (CO2) Transport in Concrete Carbonation Proses. J. Mat. Komputasi Dan Stat. 2021, 17, 405–417. [Google Scholar] [CrossRef]
- Leonovich, S.N.; Shalyi, E.E.; Kim, L.V. Reinforced Concrete Under the Action of Carbonization and Chloride Aggression: A Probabilistic Model for Service Life Prediction. Sci. Technol. 2019, 18, 284–291. [Google Scholar] [CrossRef]
- Mahima, S.; Moorthi, P.V.P.; Bahurudeen, A.; Gopinath, A. Influence of chloride threshold value in service life prediction of reinforced concrete structures. Sādhanā 2018, 43, 115. [Google Scholar] [CrossRef]
- Andrade, C. Rebar corrosion modelling and deterioration limit state. Rev. Alconpat 2020, 10, 165–179. [Google Scholar]
- Isgor, O.B.; Razaqpur, A.G. Modelling steel corrosion in concrete structures. Mater. Struct. 2006, 39, 291–302. [Google Scholar] [CrossRef]
- Zhakipbekov, S.K.; Aruova, L.B.; Toleubayeva, S.T.; Ahmetganov, T.B.; Utkelbaeva, A.O. The features of the hydration and structure formation process of modified low-clinker binders. Mag. Civ. Eng. 2021, 103, 10302. [Google Scholar]
- Zhou, J.; Yu, B.; Kong, Y.; Yang, W.; Cheng, B.; Wu, J. Effect of calcium hydroxide on the microstructure and performance of super sulfated cement. Ceram. Silikáty 2022, 66, 85–94. [Google Scholar] [CrossRef]
- Rao, M.; Wei, J.; Gao, Z.; Zhou, W.; Li, Q.; Liu, S. Study on Strength and Microstructure of Cement-Based Materials Containing Combination Mineral Admixtures. Adv. Mater. Sci. Eng. 2016, 2016, 7243670. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Ding, J.; Chen, F.-Z.; Liu, M.-Z.; Liu, J.; Hou, Z. Performance Evaluation and Microstructure Study of Pervious Concrete Prepared from Various Solid Waste Admixtures. Int. J. Concr. Struct. Mater. 2025, 19, 7. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Rumyantseva, V.E.; Krasilnikov, I.V.; Konovalova, V.S.; Karavaev, I.V. Determination of safe service life of structures made of concrete containing hydrophobic additives. Izv. Vyss. Uchebnykh Zaved. Seriya Teknol. Tekst. Promyshlennosti 2017, 6, 268–276. [Google Scholar]
- Park, J.H.; Hasegawa, T.; Senbu, O.; Park, D.-C. Study on Neutralization Progress Model of Concrete with Coating Finishing Materials in Outdoor Exposure Conditions Based on the Diffusion Reaction of Calcium Hydroxide. Int. J. Concr. Struct. Mater. 2012, 6, 155–163. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Yuan, Q.; Deng, D. Modelling calcium leaching kinetics of cement asphalt paste. Mater. Struct. 2018, 51, 133. [Google Scholar] [CrossRef]
- Marchand, J.; Bentz, D.P.; Samson, E.; Maltais, Y. Influence of calcium hydroxide dissolution on the transport properties of hydrated cement systems. In Materials Science of Concrete: Calcium Hydroxide in Concrete; Skalny, J.P., Gebauer, J., Odler, I., Eds.; The American Ceramic Society: Westerville, OH, USA, 2001; pp. 113–129. [Google Scholar]
- Fedosov, S.V.; Roumyantseva, V.E.; Konovalova, V.S.; Loginova, S.A. Mathematical modeling of diffusion processes of mass transfer of «free calcium hydroxide» during corrosion of cement concretes. Int. J. Comput. Civ. Struct. Eng. 2018, 14, 161–168. [Google Scholar] [CrossRef][Green Version]
- Fedosov, S.V.; Rumyantseva, V.E.; Krasilnikov, I.V.; Krasilnikova, I.A. Mathematical modeling of mass transfer in the «cement concrete–liquid medium» system, limited by the internal diffusion of the transferred component at liquid corrosion of the first type. Stroit. Mater. [Constr. Mater.] 2021, 7, 4–9. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Rumyantseva, V.E.; Krasilnikov, I.V.; Konovalova, V.S.; Evsyakov, A.S. Mathematical modeling of the colmatation of concrete pores during corrosion. Mag. Civ. Eng. 2018, 83, 198–207. [Google Scholar]
- Fedosov, S.V.; Rumyantseva, V.E.; Kasyanenko, N.S.; Manokhina, Y.V.; Vitalova, N.M. Mathematical modeling of cement concrete corrosion destruction proceeding via the second kind mechanism at small values of fourier number. News High. Educ. Inst. Constr. [Izv. Vyss. Uchebnyh zavedenij. Stroit.] 2014, 5, 21–25. [Google Scholar]
- Fedosov, S.V.; Rumyantseva, V.E.; Konovalova, V.S.; Loginova, S.A. Mathematical Model of Mass Transfer Processes in Biological Corrosion of Cement Concretes. IOP Conf. Ser. Mater. Sci. Eng. 2020, 869, 052059. [Google Scholar] [CrossRef]
- Fedosov, S.V.; Narmaniya, B.E. Modelling the influence of mass transfer parameters on the kinetics of corrosion interaction of concrete and biological media. Vestn. MGSU 2024, 19, 596–605. [Google Scholar] [CrossRef]
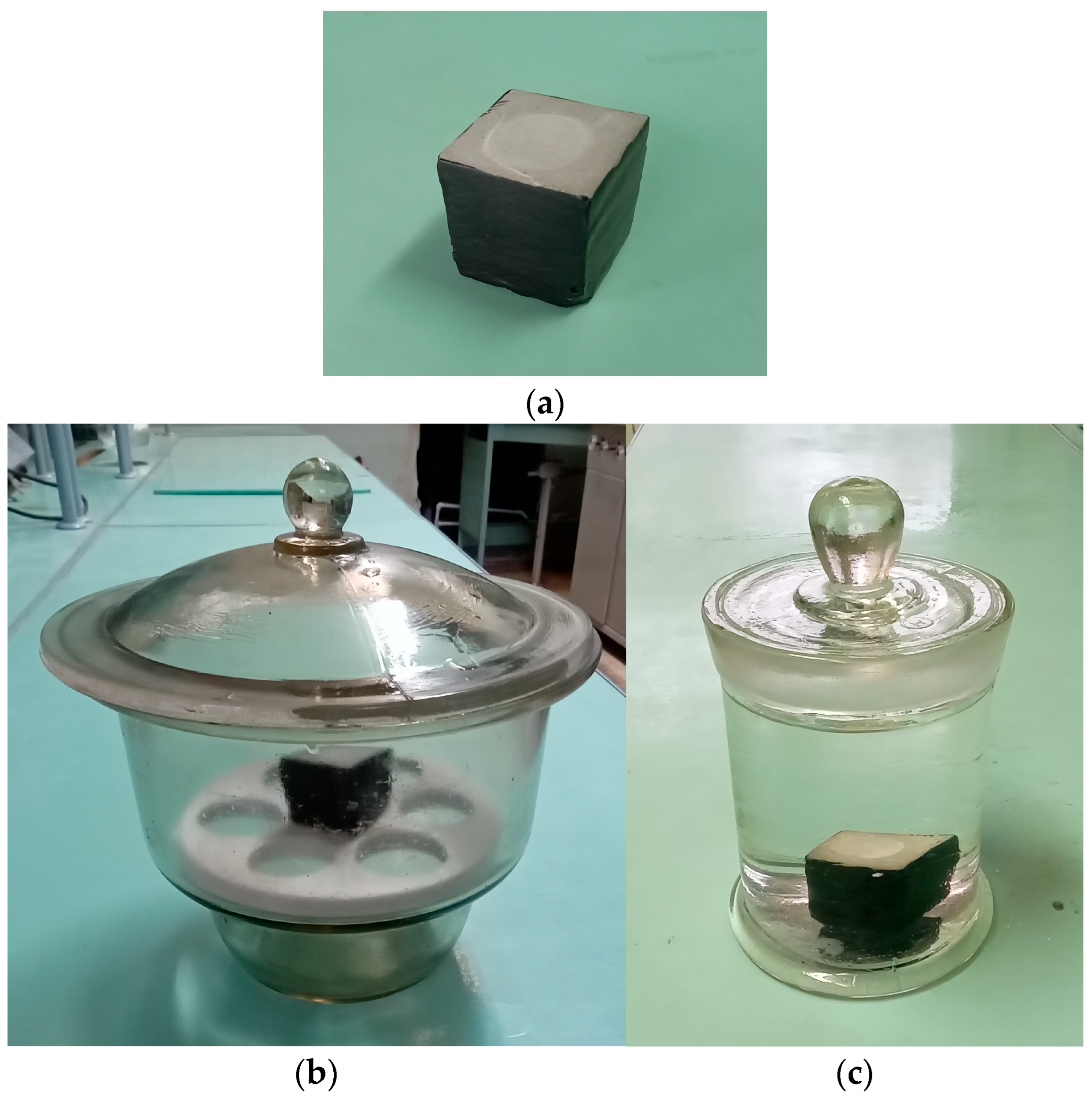
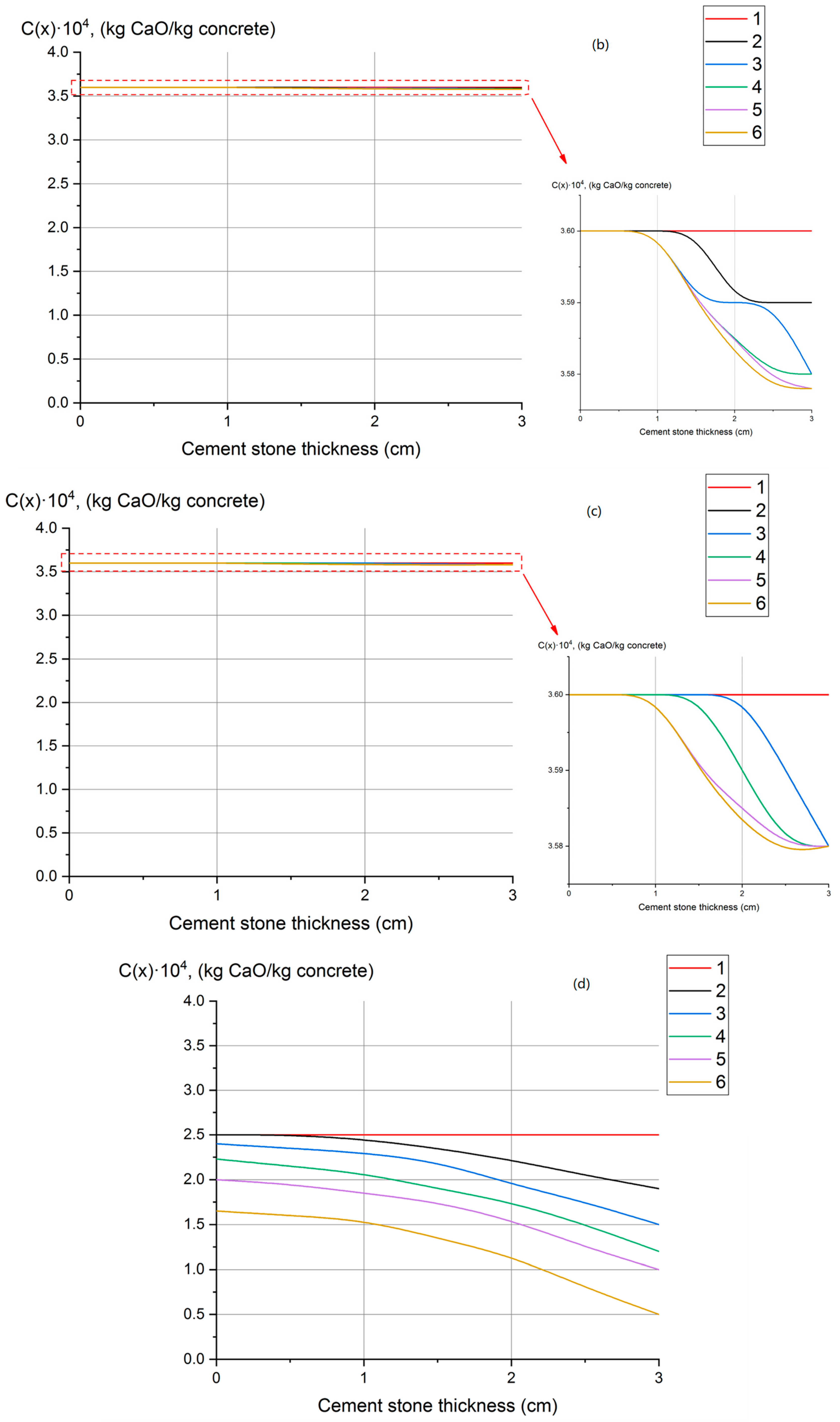
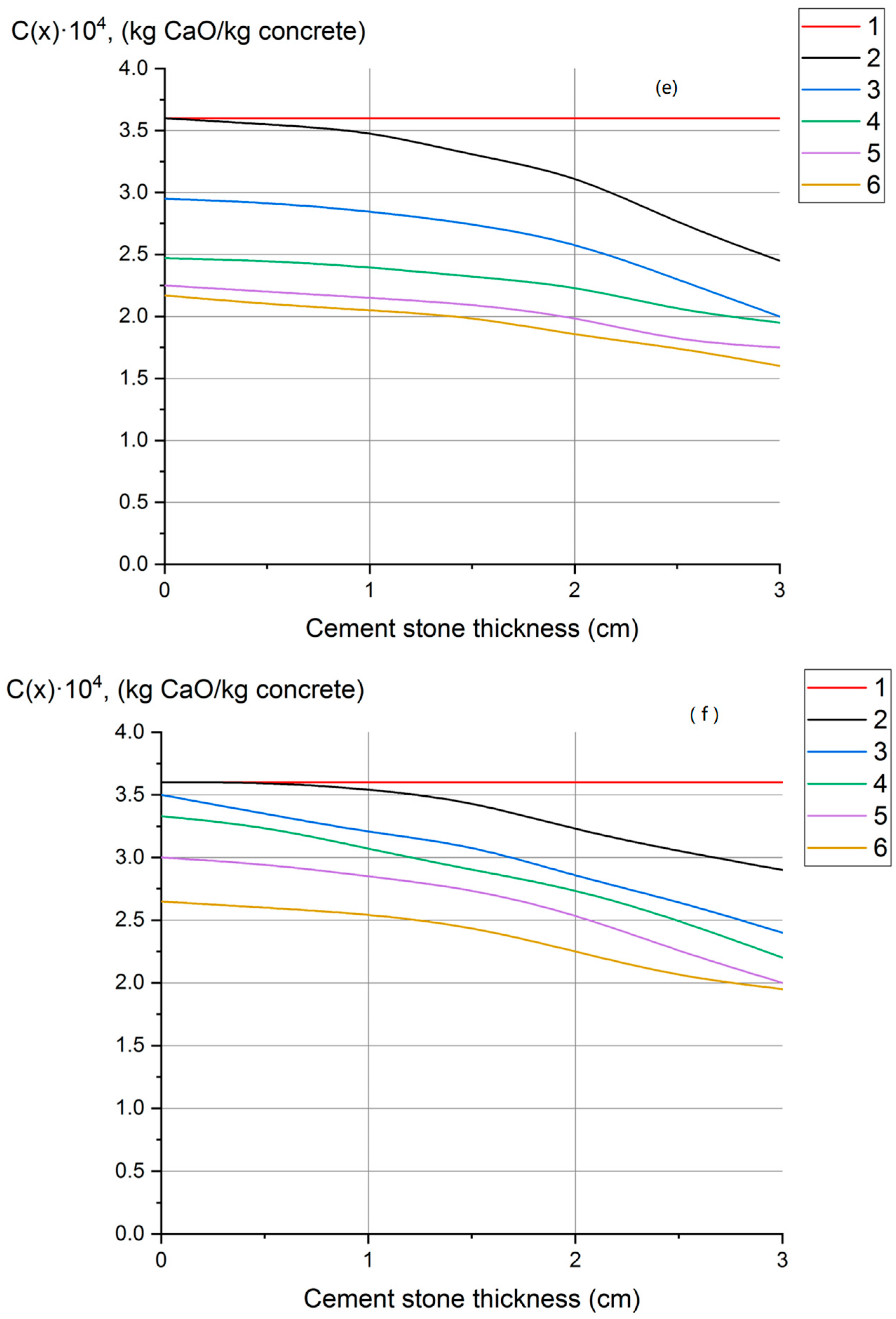
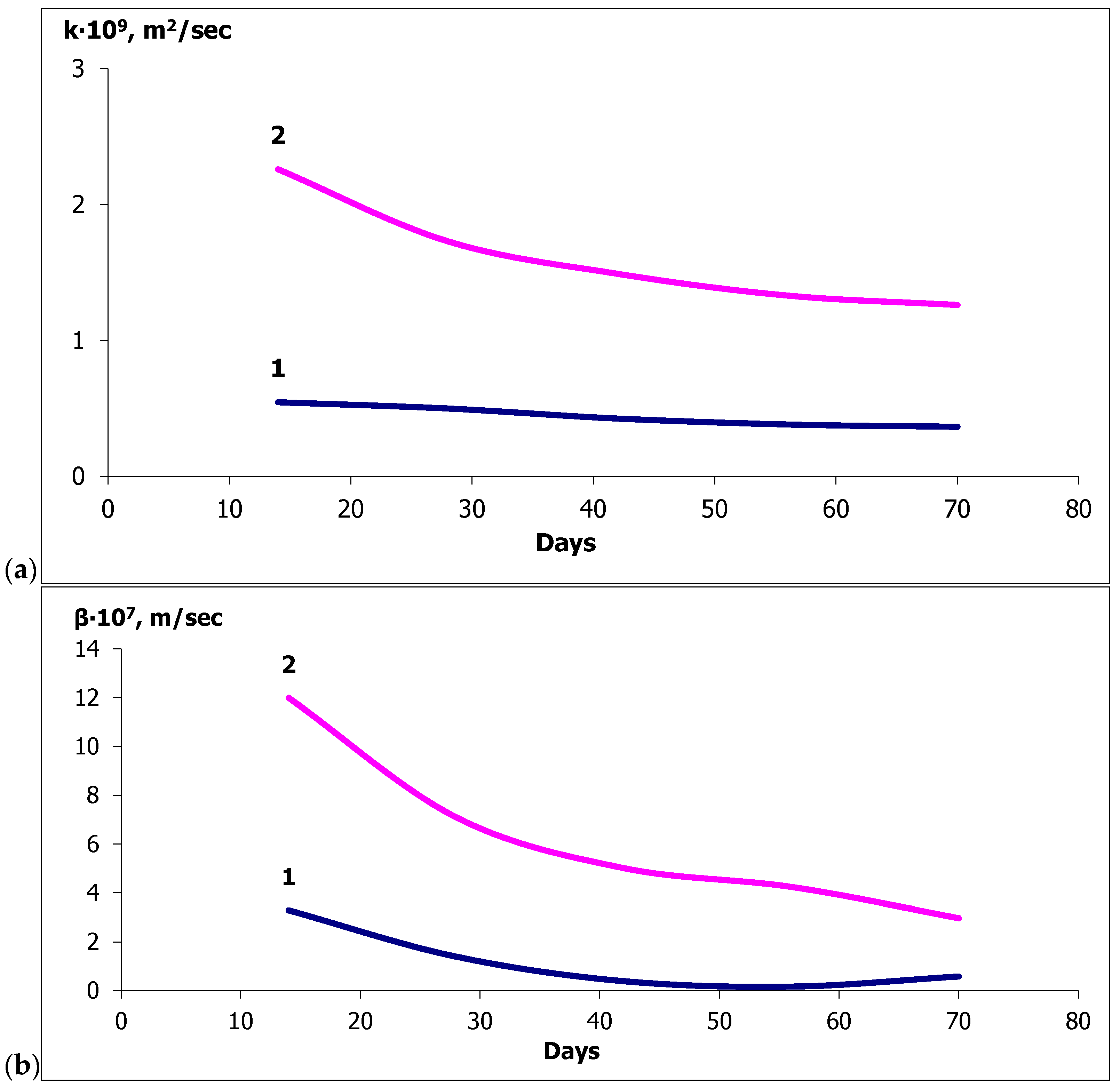
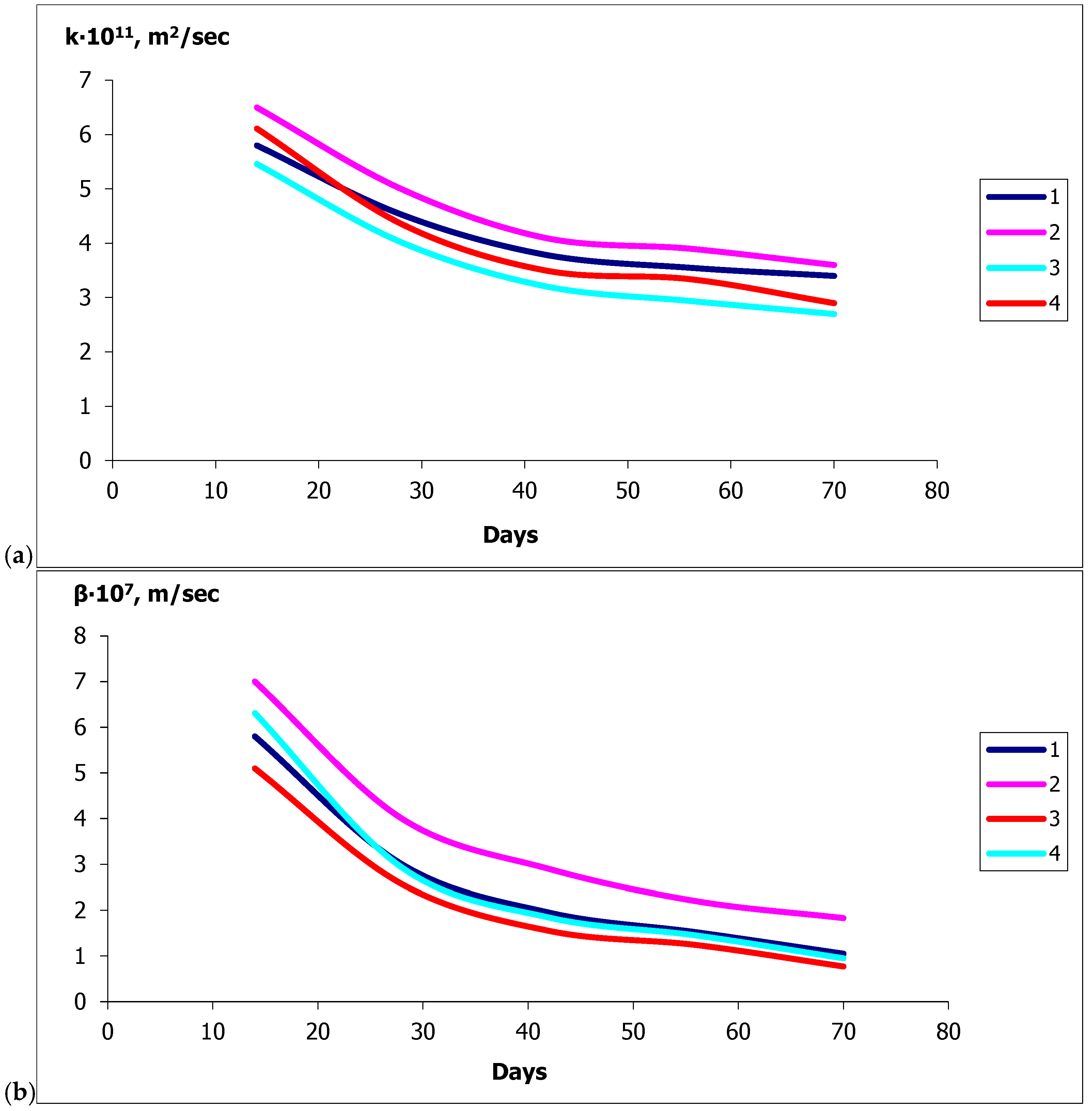

| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | R2O a |
|---|---|---|---|---|---|---|
| 21.02 | 5.42 | 4.19 | 65.91 | 0.40 | 2.40 | 0.66 |
| C3S | C2S | C3A | C4AF |
|---|---|---|---|
| 64.31 | 11.75 | 7.24 | 12.76 |
| Type of Sample | Cement | Water | Calcium Stearate |
|---|---|---|---|
| Without additives | 769.23 | 230.77 | - |
| 0.5 wt.% additive | 766.28 | 229.89 | 3.83 |
| 1 wt.% additive | 763.36 | 229 | 7.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konovalova, V.S.; Strokin, K.B.; Galtsev, A.A.; Novikov, D.G. The Effect of Calcium Stearate Additives in Concrete on Mass Transfer When Exposed to Aspergillus niger Fungi. J. Compos. Sci. 2025, 9, 569. https://doi.org/10.3390/jcs9100569
Konovalova VS, Strokin KB, Galtsev AA, Novikov DG. The Effect of Calcium Stearate Additives in Concrete on Mass Transfer When Exposed to Aspergillus niger Fungi. Journal of Composites Science. 2025; 9(10):569. https://doi.org/10.3390/jcs9100569
Chicago/Turabian StyleKonovalova, Viktoriya S., Konstantin B. Strokin, Aleksey A. Galtsev, and Denis G. Novikov. 2025. "The Effect of Calcium Stearate Additives in Concrete on Mass Transfer When Exposed to Aspergillus niger Fungi" Journal of Composites Science 9, no. 10: 569. https://doi.org/10.3390/jcs9100569
APA StyleKonovalova, V. S., Strokin, K. B., Galtsev, A. A., & Novikov, D. G. (2025). The Effect of Calcium Stearate Additives in Concrete on Mass Transfer When Exposed to Aspergillus niger Fungi. Journal of Composites Science, 9(10), 569. https://doi.org/10.3390/jcs9100569






