Nanostructured K- and Na-Substituted Aluminosilicates for Ni(II) Ions Removal from Liquid Media: Assessment of Sorption Performance and Mechanism
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characterization of Obtained Materials
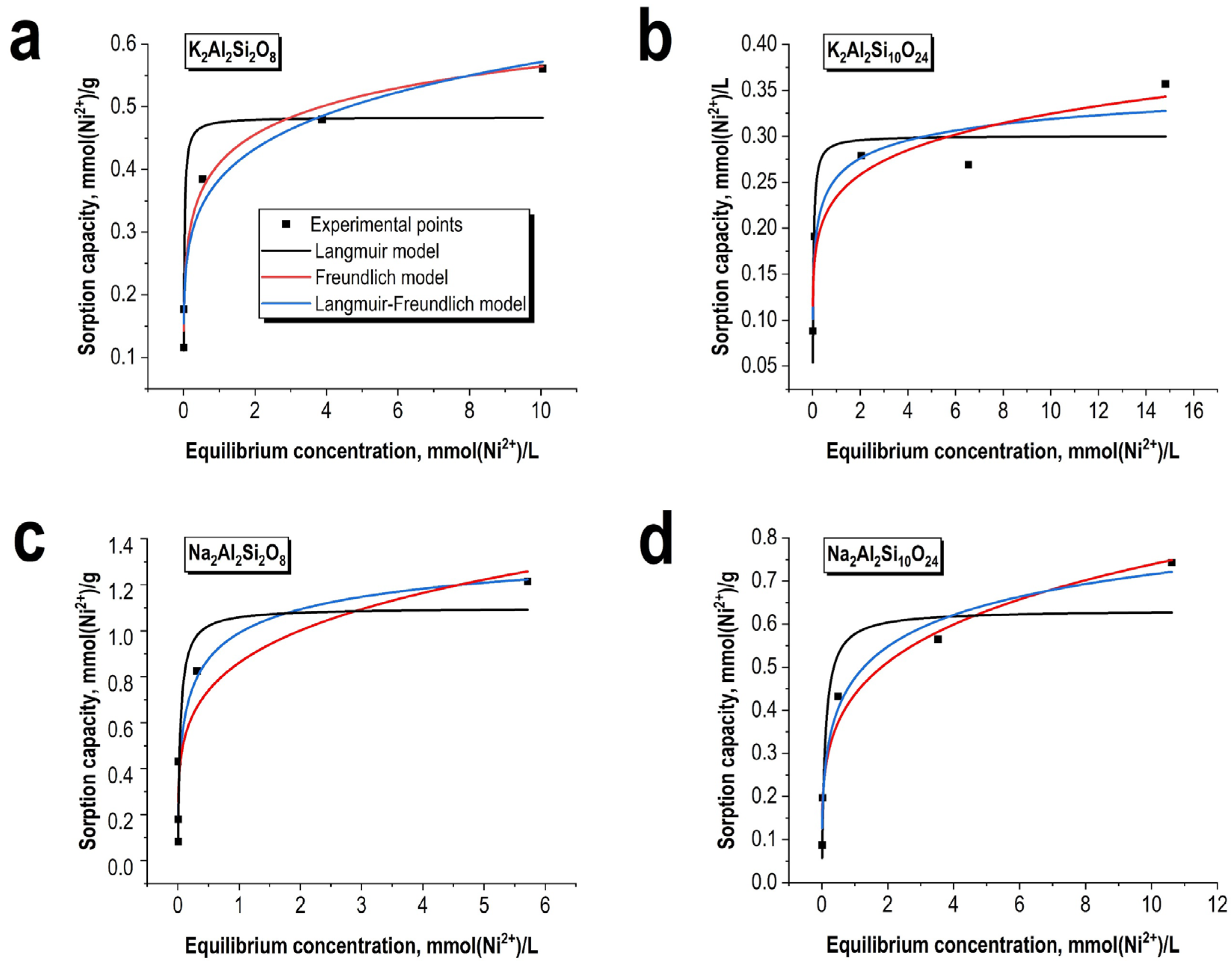
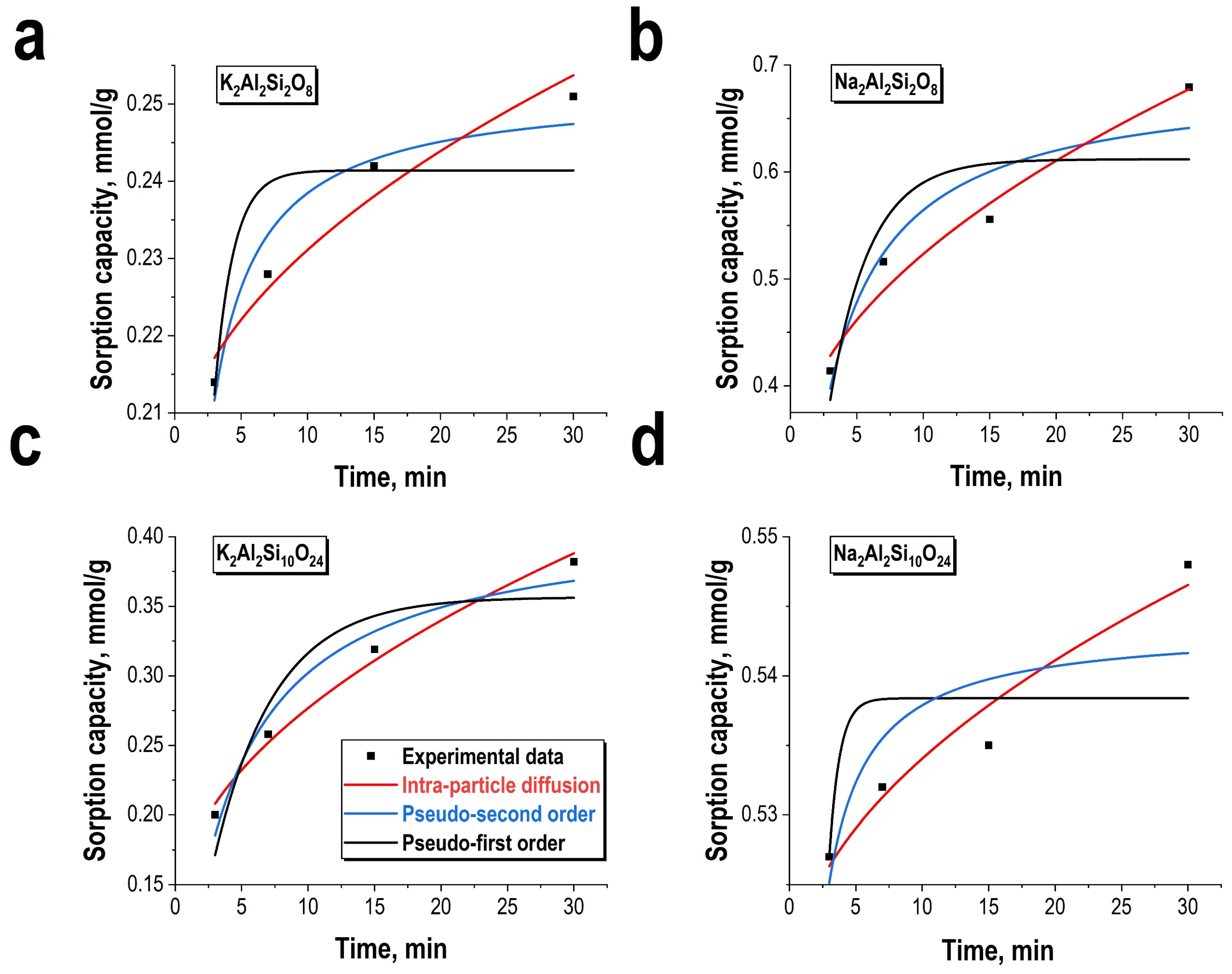
3.2. Sorption Perfomance Towards Ni2+ Ions
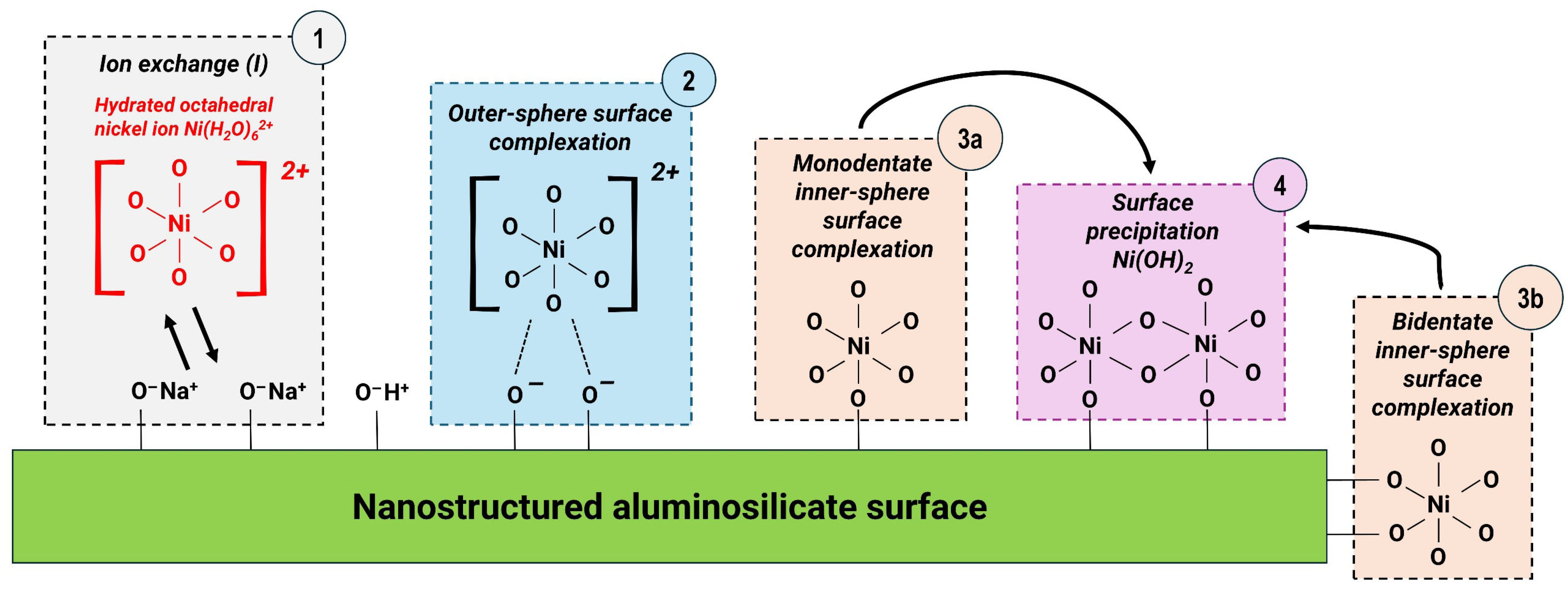
3.3. Mechanism of Adsorption Interaction Between Ni2+ and Aluminosilicate Surface
3.4. Comparative Sorption Capacity Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qu, J.; Liu, J.; Al-Dhabi, N.A.; Leng, Y.; Yi, J.; Jiang, K.; Xing, W.; Yin, D.; Tang, W. Ni-EDTA Decomplexation and Ni Removal from Wastewater by Electrooxidation Coupled with Electrocoagulation: Optimization, Mechanism and Biotoxicity Assessment. Sep. Purif. Technol. 2025, 376, 133980. [Google Scholar] [CrossRef]
- Lee, M.G.; Kam, S.K.; Lee, C.H. Kinetic and Isothermal Adsorption Properties of Strontium and Cesium Ions by Zeolitic Materials Synthesized from Jeju Volcanic Rocks. Environ. Eng. Res. 2021, 26, 200127. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, S. Heavy Metal Contamination in Fish: Sources, Mechanisms and Consequences. Aquat. Sci. 2024, 86, 107. [Google Scholar] [CrossRef]
- Iqbal Khan, Z.; Ghulam Muhammad, F.; Ahmad, K.; Alwahibi, M.S.; Yang, H.-H.; Ishfaq, M.; Anjum, S.; Ali, K.; Iqbal, K.; Radicetti, E.; et al. Nickel Toxicology Testing in Alternative Specimen from Farm Ruminants in a Urban Polluted Environment. J. King Saud. Univ.-Sci. 2024, 36, 103520. [Google Scholar] [CrossRef]
- Rizwan, M.; Usman, K.; Alsafran, M. Ecological Impacts and Potential Hazards of Nickel on Soil Microbes, Plants, and Human Health. Chemosphere 2024, 357, 142028. [Google Scholar] [CrossRef] [PubMed]
- Erulaş, F.A. Sensitive Determination of Nickel at Trace Levels in Surface Water Samples by Slotted Quartz Tube Flame Atomic Absorption Spectrometry after Switchable Solvent Liquid-Phase Microextraction. Environ. Monit. Assess. 2020, 192, 272. [Google Scholar] [CrossRef] [PubMed]
- Koshy, N.; Singh, D.N. Fly Ash Zeolites for Water Treatment Applications. J. Environ. Chem. Eng. 2016, 4, 1460–1472. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.J.; Choi, J.W.; Mtei, K.M.; Hosseini-Bandegharaei, A.; Sillanpää, M. Adsorption and Desorption Processes of Toxic Heavy Metals, Regeneration and Reusability of Spent Adsorbents: Economic and Environmental Sustainability Approach. Adv. Colloid Interface Sci. 2024, 329, 103196. [Google Scholar] [CrossRef]
- Kainth, S.; Sharma, P.; Pandey, O.P. Green Sorbents from Agricultural Wastes: A Review of Sustainable Adsorption Materials. Appl. Surf. Sci. Adv. 2024, 19, 100562. [Google Scholar] [CrossRef]
- Hassan, M.; Naidu, R.; Du, J.; Liu, Y.; Qi, F. Critical Review of Magnetic Biosorbents: Their Preparation, Application, and Regeneration for Wastewater Treatment. Sci. Total Environ. 2020, 702, 134893. [Google Scholar] [CrossRef]
- Godage, N.H.; Gionfriddo, E. Use of Natural Sorbents as Alternative and Green Extractive Materials: A Critical Review. Anal. Chim. Acta 2020, 1125, 187–200. [Google Scholar] [CrossRef]
- Bayuo, J.; Rwiza, M.; Mtei, K. Response Surface Optimization and Modeling in Heavy Metal Removal from Wastewater—A Critical Review. Environ. Monit. Assess. 2022, 194, 351. [Google Scholar] [CrossRef]
- Rostami, M.S.; Khodaei, M.M. Recent Advances in Chitosan-Based Nanocomposites for Adsorption and Removal of Heavy Metal Ions. Int. J. Biol. Macromol. 2024, 270, 132386. [Google Scholar] [CrossRef] [PubMed]
- Villafranca, J.C.; Berton, P.; Ferguson, M.; Clausen, R.; Arancibia-Miranda, N.; Martinis, E.M. Aluminosilicates-Based Nanosorbents for Heavy Metal Removal–A Review. J. Hazard. Mater. 2024, 474, 134552. [Google Scholar] [CrossRef] [PubMed]
- Maleki, A.; Hajizadeh, Z.; Sharifi, V.; Emdadi, Z. A Green, Porous and Eco-Friendly Magnetic Geopolymer Adsorbent for Heavy Metals Removal from Aqueous Solutions. J. Clean. Prod. 2019, 215, 1233–1245. [Google Scholar] [CrossRef]
- Abdel Moamen, O.A.; Murad, G.A.; Hassan, H.S. Experimental and Theoretical Study for the Sorption of Ni2+ and Cd2+ onto Phosphoryl Functionalized Aluminum Silicate Composite. Sep. Purif. Technol. 2024, 344, 127186. [Google Scholar] [CrossRef]
- Tsai, C.-K.; Doong, R.; Hung, H.-Y. Sustainable Valorization of Mesoporous Aluminosilicate Composite from Display Panel Glasses Waste for Adsorption of Heavy Metal Ions. Sci. Total Environ. 2019, 673, 337–346. [Google Scholar] [CrossRef]
- Yarusova, S.B.B.; Shichalin, O.O.O.; Belov, A.A.A.; Azon, S.A.A.; Buravlev, I.Y.; Golub, A.V.V.; Mayorov, V.Y.; Gerasimenko, A.V.V.; Papynov, E.K.K.; Ivanets, A.I.I.; et al. Synthesis of Amorphous KAlSi3O8 for Cesium Radionuclide Immobilization into Solid Matrices Using Spark Plasma Sintering Technique. Ceram. Int. 2022, 48, 3808–3817. [Google Scholar] [CrossRef]
- Gordienko, P.S.; Yarusova, S.B.; Shabalin, I.A.; Slobodyuk, A.B.; Nekhlyudova, E.A.; Shichalin, O.O.; Papynov, E.K.; Kuryavyi, V.G.; Polyakova, N.V.; Parot’kina, Y.A. Synthesis of Calcium Aluminosilicates from Nanostructured Synthetic Na Zeolites and Study of Their Sorption Properties. Russ. J. Inorg. Chem. 2022, 67, 1393–1399. [Google Scholar] [CrossRef]
- Nekhliudova, E.A.; Ivanov, N.P.; Yarusova, S.B.; Papynov, E.K.; Shichalin, O.O.; Mayorov, V.Y.; Fedorets, A.N.; Shkuratov, A.L.; Shlyk, D.K.; Gordienko, P.S. Synthesis and Sorption Properties of Nanostructured Sodium Aluminosilicates Differing in Si/Al Ratio. Inorg. Mater. 2023, 59, 1303–1312. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the Use and Interpretation of Adsorption Isotherm Models: A Review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption Kinetic Models: Physical Meanings, Applications, and Solving Methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Carrio, J.A.G.; Donato, R.K.; Carvalho, A.; Koon, G.K.W.; Donato, K.Z.; Yau, X.H.; Kosiachevskyi, D.; Lim, K.; Ravi, V.; Joy, J.; et al. From 2D Kaolinite to 3D Amorphous Cement. Sci. Rep. 2025, 15, 1669. [Google Scholar] [CrossRef] [PubMed]
- Emminger, Y.H.; Ladner, L.; Ruiz-Agudo, C. Comparative Study of the Early Stages of Crystallization of Calcium Silicate Hydrate (C-S-H) and Calcium Aluminate Silicate Hydrate (C-A-S-H). Cem. Concr. Res. 2025, 193, 107873. [Google Scholar] [CrossRef]
- Yiu, Y.M. Techniques for Structural Investigations (Theory and Experimental). In Applications of Chalcogenides: S, Se, and Te; Springer International Publishing: Cham, Switzerland, 2017; pp. 61–104. [Google Scholar]
- Bates, S.; Zografi, G.; Engers, D.; Morris, K.; Crowley, K.; Newman, A. Analysis of Amorphous and Nanocrystalline Solids from Their X-Ray Diffraction Patterns. Pharm. Res. 2006, 23, 2333–2349. [Google Scholar] [CrossRef]
- Mozgawa, W.; Król, M.; Barczyk, K. FT-IR Studies of Zeolites from Different Structural Groups. Chemik 2011, 65, 671–674. [Google Scholar]
- Ma, Y.K.; Rigolet, S.; Michelin, L.; Paillaud, J.L.; Mintova, S.; Khoerunnisa, F.; Daou, T.J.; Ng, E.P. Facile and Fast Determination of Si/Al Ratio of Zeolites Using FTIR Spectroscopy Technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kumar, D.; Pandey, L.K.; Gaur, J.P. Metal Sorption by Algal Biomass: From Batch to Continuous System. Algal Res. 2016, 18, 95–109. [Google Scholar] [CrossRef]
- Dragan, E.S.; Apopei Loghin, D.F. Enhanced Sorption of Methylene Blue from Aqueous Solutions by Semi-IPN Composite Cryogels with Anionically Modified Potato Starch Entrapped in PAAm Matrix. Chem. Eng. J. 2013, 234, 211–222. [Google Scholar] [CrossRef]
- Urdiales, C.; Gacitua, M.; Villacura, L.; Pizarro, C.; Escudey, M.; Canales, C.; Antilén, M. Variable Surface Charge of Humic Acid-Ferrihydrite Composite: Influence of Electrolytes on Ciprofloxacin Adsorption. J. Hazard. Mater. 2020, 385, 121520. [Google Scholar] [CrossRef] [PubMed]
- Boughriet, A.; Doyemet, G.; Poumaye, N.; Allahdin, O.; Wartel, M. Insight into Adsorption Kinetics of Cs+, Rb+, Co2+, and Sr2+ on a Zeolites-Based Composite: Comprehensive Diffusional Explanation and Modelling. Appl. Sci. 2024, 14, 3511. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, D.; Chen, Y.; He, J.; Li, Q. Mechanistic Study of Highly Effective Phosphate Removal from Aqueous Solutions over a New Lanthanum Carbonate Fabricated Carbon Nanotube Film. J. Environ. Manag. 2024, 359, 120938. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Priya, A.K.; Senthil Kumar, P.; Hoang, T.K.A.; Sekar, K.; Chong, K.Y.; Khoo, K.S.; Ng, H.S.; Show, P.L. A Critical and Recent Developments on Adsorption Technique for Removal of Heavy Metals from Wastewater-A Review. Chemosphere 2022, 303, 135146. [Google Scholar] [CrossRef] [PubMed]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Finish, N.; Ramos, P.; Borojovich, E.J.C.; Zeiri, O.; Amar, Y.; Gottlieb, M. Zeolite Performance in Removal of Multicomponent Heavy Metal Contamination from Wastewater. J. Hazard. Mater. 2023, 457, 131784. [Google Scholar] [CrossRef]
- Mo, X.; Siebecker, M.G.; Gou, W.; Li, W. EXAFS Investigation of Ni(II) Sorption at the Palygorskite-Solution Interface: New Insights into Surface-Induced Precipitation Phenomena. Geochim. Cosmochim. Acta 2021, 314, 85–107. [Google Scholar] [CrossRef]
- Ivanov, N.P.; Shichalin, O.O.; Trigub, A.L.; Rastorguev, V.L.; Nekrasova, N.A.; Provatorova, V.V.; Gnilyak, E.A.; Zaikova, A.R.; Rinchinova, V.B.; Lembikov, A.O.; et al. Optimization of Zn/Al Cationic Ratio in Zn-Al-LDH for Efficient U(VI) Adsorption. J. Water Process Eng. 2025, 69, 106735. [Google Scholar] [CrossRef]
- Akram, M.; Bano, Z.; Bhutto, S.U.A.; Majeed, M.K.; Pan, J.; Li, L.; Xia, M.; Wang, F. Enhanced Nickel (II) Removal from Aqueous Media Using Magnesium-Punica granatum Linn Based Adsorbent: Mechanism and Performance. J. Mol. Liq. 2025, 431, 127808. [Google Scholar] [CrossRef]
- Mozgawa, W.; Fojud, Z.; Handke, M.; Jurga, S. MAS NMR and FTIR Spectra of Framework Aluminosilicates. J. Mol. Struct. 2002, 614, 281–287. [Google Scholar] [CrossRef]
- O’Connor, S.J.; MacKenzie, K.J.D.; Smith, M.E.; Hanna, J.V. Ion Exchange in the Charge-Balancing Sites of Aluminosilicate Inorganic Polymers. J. Mater. Chem. 2010, 20, 10234. [Google Scholar] [CrossRef]
- Wang, Y.; Han, F.; Mu, J. Solidification/Stabilization Mechanism of Pb(II), Cd(II), Mn(II) and Cr(III) in Fly Ash Based Geopolymers. Constr. Build. Mater. 2018, 160, 818–827. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Golikand, A.N.; Ghaemi, M. Synthesis, Characterization, and Electrochemical Properties of Ultrafine β-Ni(OH)2 Nanoparticles. Int. J. Hydrogen Energy 2011, 36, 8674–8679. [Google Scholar] [CrossRef]
- Persson, I. Hydrated Metal Ions in Aqueous Solution: How Regular Are Their Structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy Metal Adsorption with Zeolites: The Role of Hierarchical Pore Architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Sheng, G.; Sheng, J.; Yang, S.; Hu, J.; Wang, X. Behavior and Mechanism of Ni(II) Uptake on MnO2 by a Combination of Macroscopic and EXAFS Investigation. J. Radioanal. Nucl. Chem. 2011, 289, 129–135. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Lu, X.; Meijer, E.J.; Wang, R. An Atomic-Scale Understanding of the Initial Stage of Nucleation of Heavy Metal Cations on Clay Edges. Geochim. Cosmochim. Acta 2019, 248, 161–171. [Google Scholar] [CrossRef]
- Kumar Jha, V.; Kameshima, Y.; Nakajima, A.; Okada, K.; MacKenzie, K.J.D. Effect of Grinding and Heating on Ni2+ Uptake Properties of Waste Paper Sludge. J. Environ. Manag. 2006, 80, 363–371. [Google Scholar] [CrossRef]
- Dinu, M.V.; Dragan, E.S. Evaluation of Cu2+, Co2+ and Ni2+ Ions Removal from Aqueous Solution Using a Novel Chitosan/Clinoptilolite Composite: Kinetics and Isotherms. Chem. Eng. J. 2010, 160, 157–163. [Google Scholar] [CrossRef]
- Panasenko, A.E.; Shichalin, O.O.; Yarusova, S.B.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Azon, S.A.; Fedorets, A.N.; Buravlev, I.Y.; Mayorov, V.Y.; et al. A Novel Approach for Rice Straw Agricultural Waste Utilization: Synthesis of Solid Aluminosilicate Matrices for Cesium Immobilization. Nucl. Eng. Technol. 2022, 54, 3250–3259. [Google Scholar] [CrossRef]
- Isawi, H. Using Zeolite/Polyvinyl Alcohol/Sodium Alginate Nanocomposite Beads for Removal of Some Heavy Metals from Wastewater. Arab. J. Chem. 2020, 13, 5691–5716. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the Selection Mechanism of Heavy Metal (Pb2+, Cu2+, Ni2+, and Cd2+) Adsorption on Clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Chen, C.; Yu, S.; Wang, X. Sorption of Ni2+ on Na-Rectorite Studied by Batch and Spectroscopy Methods. Appl. Geochem. 2008, 23, 2767–2777. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Zhang, G.; Zhao, Y.; Lv, C.; Liu, X.; Chen, L. Preparation of PVDF/Hyperbranched-Nano-Palygorskite Composite Membrane for Efficient Removal of Heavy Metal Ions. Polymers 2019, 11, 156. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Dawodu, F.A. Acid-Modified Montmorillonite for Sorption of Heavy Metals from Automobile Effluent. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 1–12. [Google Scholar] [CrossRef]
- Sharifian, S.; Asasian-Kolur, N.; Najafi, H.; Haddadi, B.; Jordan, C.; Harasek, M. Reusable Granulated Silica Pillared Clay for Wastewater Treatment, Selective for Adsorption of Ni(II). Clean. Eng. Technol. 2023, 14, 100634. [Google Scholar] [CrossRef]
- Pomazkina, O.I.; Filatova, E.G.; Lebedeva, O.V.; Pozhidaev, Y.N. Adsorption of Nickel (II) Ions by Aluminosilicates Modified by Poly-1-Vinyl Imidazole and Poly-4-Vinyl Pyridine. Prot. Met. Phys. Chem. Surfaces 2018, 54, 582–586. [Google Scholar] [CrossRef]
- Inumaru, K. “Sponge Crystal”: A Novel Class of Microporous Single Crystals Formed by Self-Assembly of Polyoxometalate (NH4)3PW12O40 Nanocrystallites. Catal. Surv. Asia 2006, 10, 151–160. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Xu, Z.; Zhang, M. Synthetic Zeolite from Coal Bottom Ash and Its Application in Cadmium and Nickel Removal from Acidic Wastewater. Desalin. Water Treat. 2016, 57, 26089–26100. [Google Scholar] [CrossRef]
- Belova, T.P. Adsorption of Heavy Metal Ions (Cu2+, Ni2+, Co2+ and Fe2+) from Aqueous Solutions by Natural Zeolite. Heliyon 2019, 5, e02320. [Google Scholar] [CrossRef]

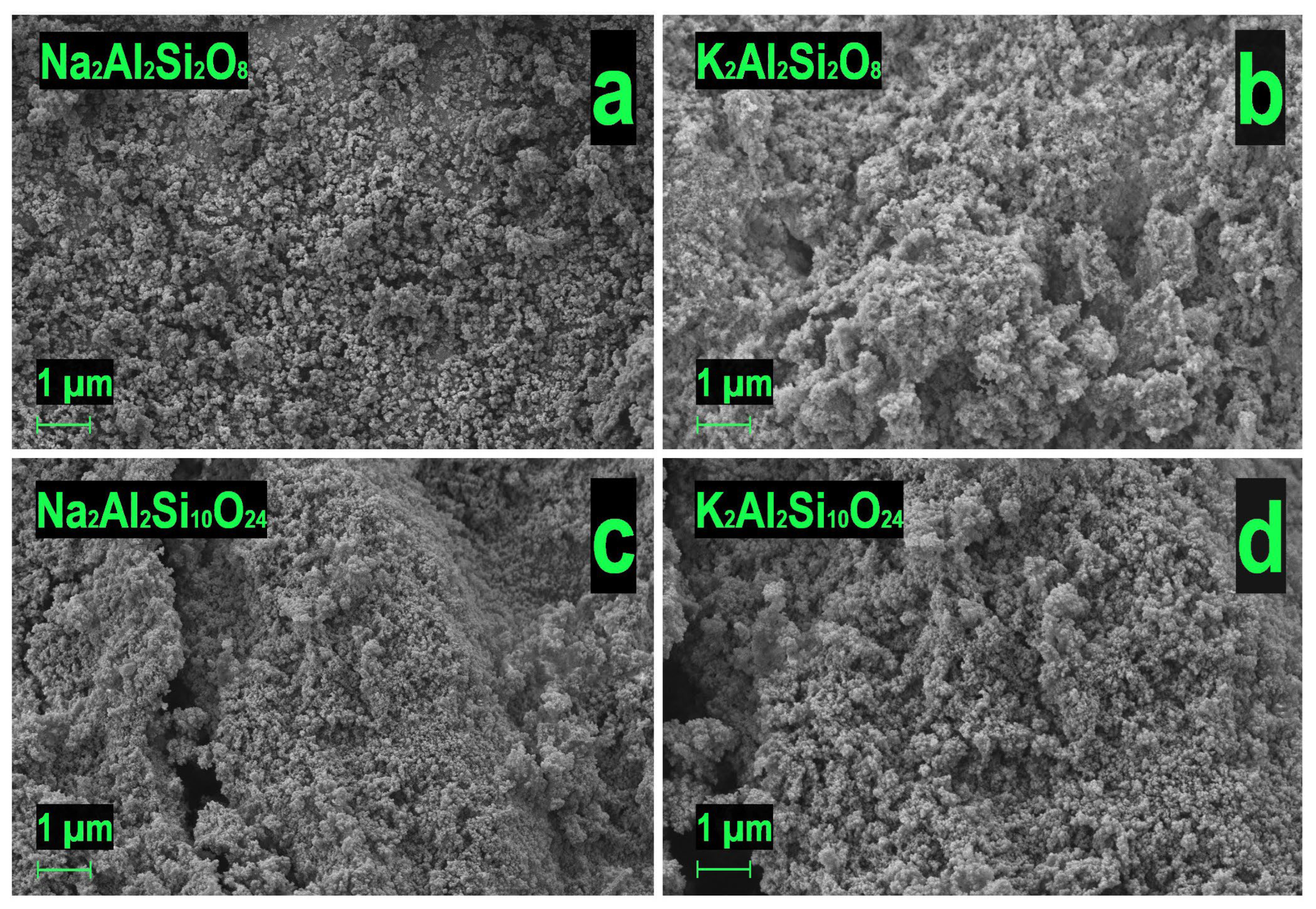
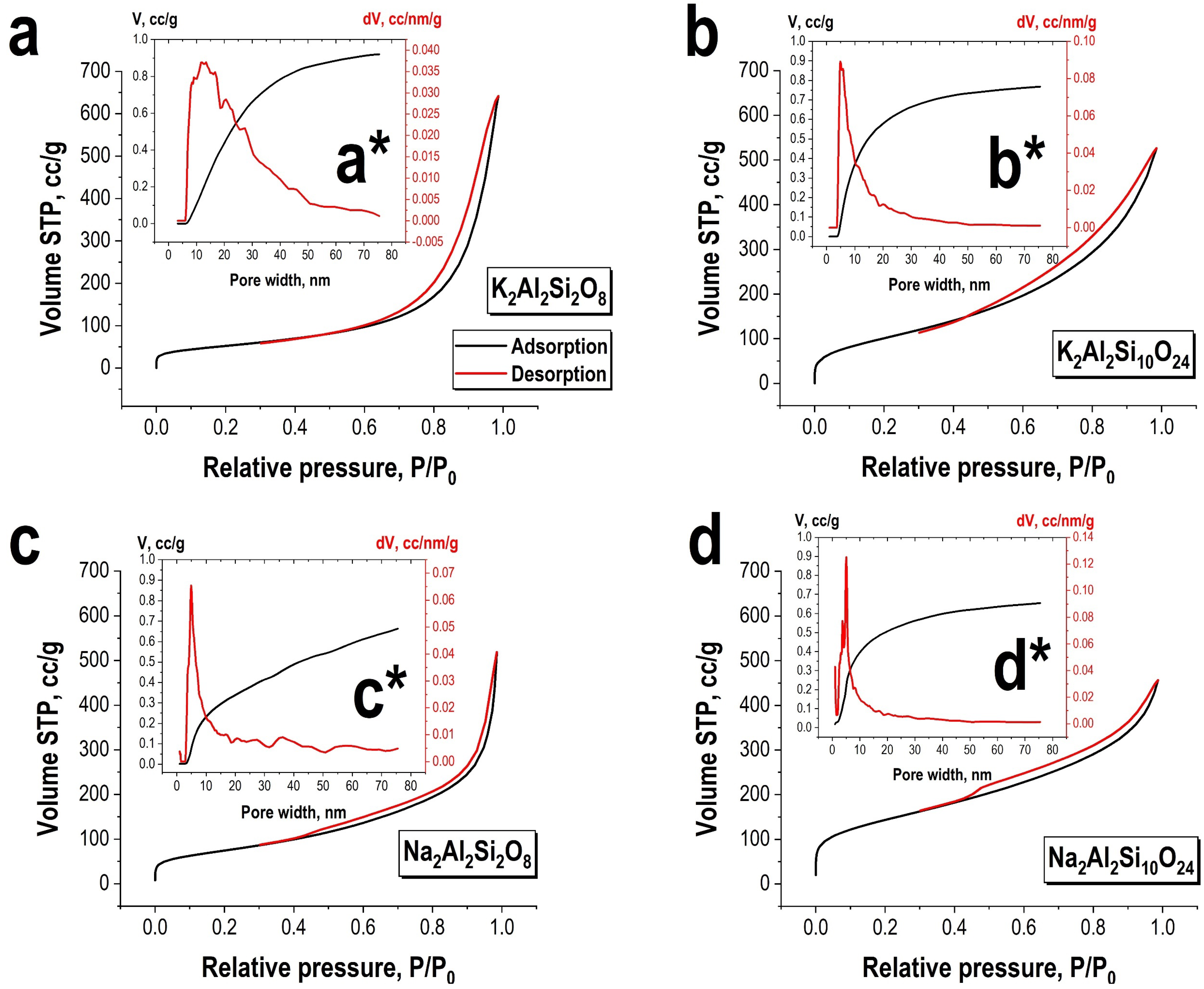
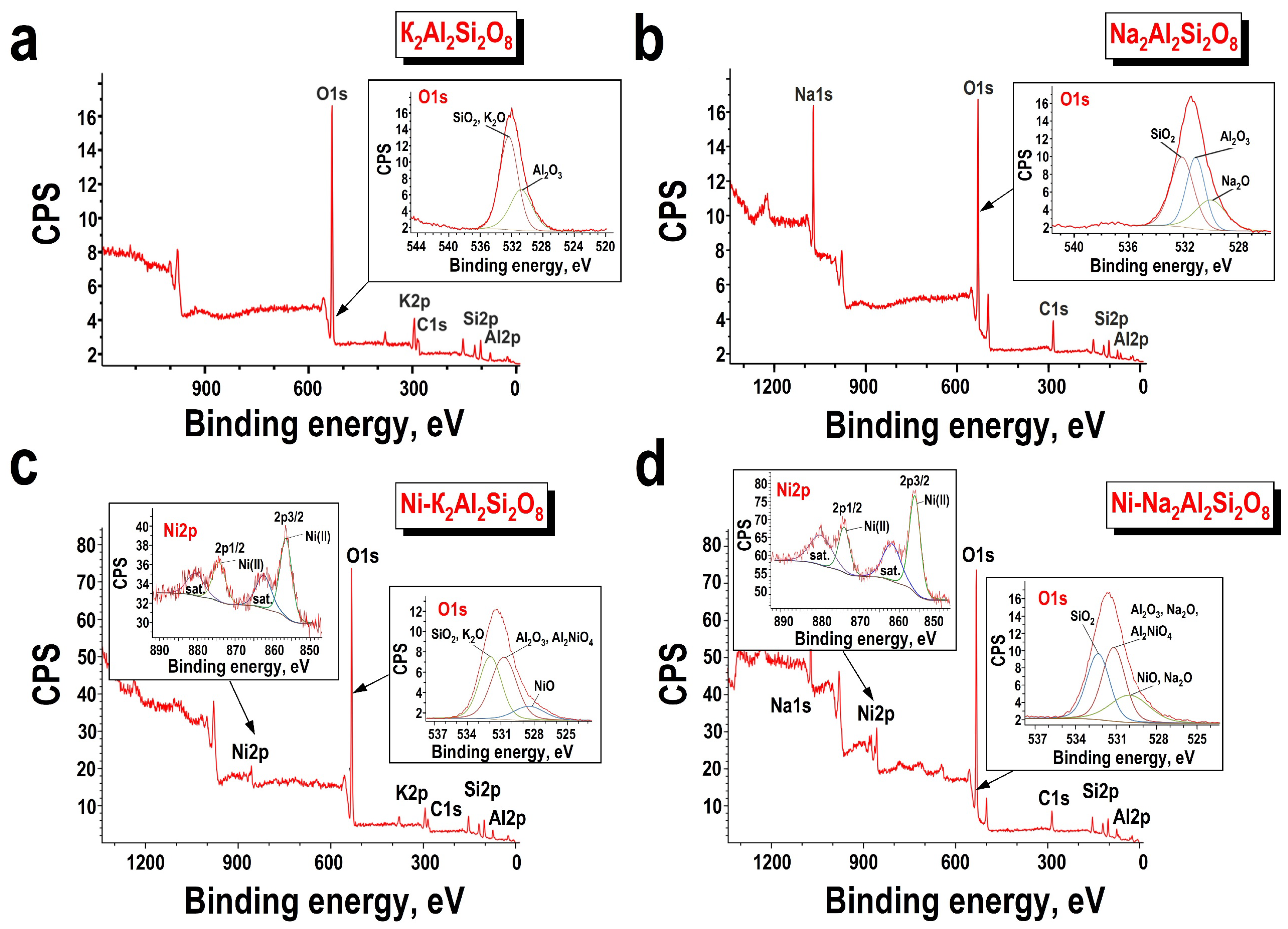
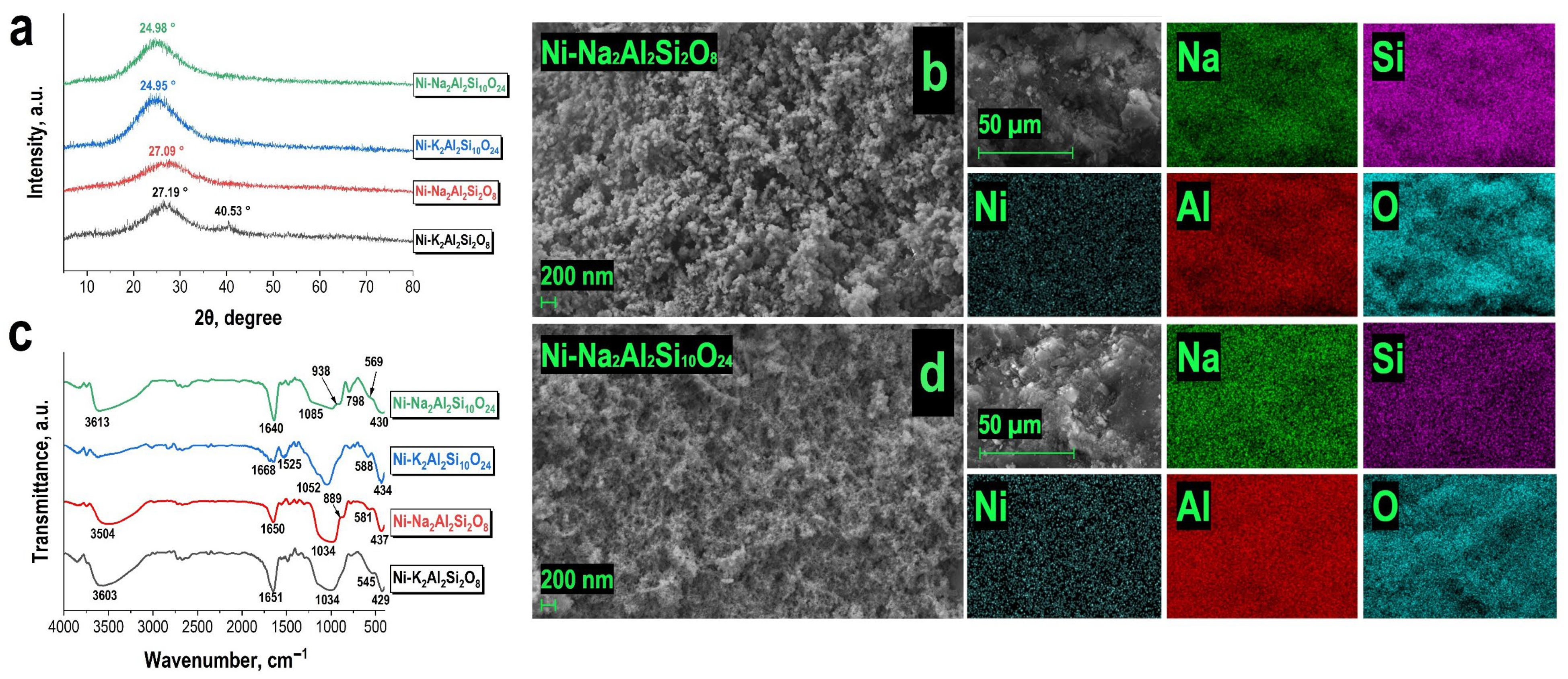
| Predicted Formula (Experimental Formula) | Nominal Si/Al Ratio (Experimental Si/Al Ratio) | Mass Content | Density, g/cm3 | SBET, m2/g |
|---|---|---|---|---|
| K2Al2Si2O8 (K1.1Al2Si2.1On) | 1.0 (1.04) | wt.%(K) = 27.20 wt.%(Al) = 35.10 wt.(Si) = 37.70 | 1.84 | 189.4 |
| K2Al2Si10O24 (K2.1Al2Si10.6On) | 5.0 (5.3) | wt.%(K) = 18.64 wt.%(Al) = 12.53 wt.(Si) = 68.83 | 2.35 | 389.8 |
| Na2Al2Si2O8 (Na1.3Al2Si2.5On) | 1.0 (1.2) | wt.%(Na) = 19.95 wt.%(Al) = 34.96 wt.(Si) = 45.09 | 1.54 | 48.3 |
| Na2Al2Si10O24 (Na1.4Al2Si9.4On) | 5.0 (4.7) | wt.%(Na) = 9.20 wt.%(Al) = 15.42 wt.(Si) = 75.38 | 2.30 | 203.3 |
| Isotherm Model | Parameters | K2Al2Si2O8 | K2Al2Si10O24 | Na2Al2Si2O8 | Na2Al2Si10O24 |
|---|---|---|---|---|---|
| Freundlich | n | 0.17 ± 0.02 | 0.14 ± 0.03 | 0.22 ± 0.06 | 0.23 ± 0.03 |
| Kf | 0.38 ± 0.02 | 0.23 ± 0.02 | 0.86 ± 0.12 | 0.44 ± 0.03 | |
| R2 | 0.98 | 0.92 | 0.87 | 0.97 | |
| Langmuir | Qlmax, mmol/g | 0.48 ± 0.04 | 0.30 ± 0.03 | 1.10 ± 0.19 | 0.63 ± 0.07 |
| Kl, L/mmol | 58 ± 29 | 31 ± 20 | 26 ± 18 | 10.3 ± 8.5 | |
| R2 | 0.91 | 0.85 | 0.82 | 0.89 | |
| Langmuir-Freundlich | Qlmax, mmol/g | 1.00 ± 0.56 | 0.44 ± 0.11 | 1.52 ± 0.90 | 1.13 ± 0.60 |
| Klf, L/mmol | 0.69 ± 0.72 | 1.54 ± 1.18 | 1.89 ± 0.86 | 0.72 ± 0.76 | |
| m | 0.27 ± 0.09 | 0.34 ± 0.12 | 0.46 ± 0.33 | 0.38 ± 0.15 | |
| R2 | 0.99 | 0.99 | 0.90 | 0.98 |
| Kinetics Model | Parameters | K2Al2Si2O8 | K2Al2Si10O24 | Na2Al2Si2O8 | Na2Al2Si10O24 |
|---|---|---|---|---|---|
| Lagergren pseudo-first order | Qmax, mmol/g | 0.241 ± 0.007 | 0.36 ± 0.03 | 0.61 ± 0.05 | 0.54 ± 0.01 |
| K1, 1/min | 0.71 ± 0.14 | 0.22 ± 0.06 | 0.33 ± 0.11 | 1.29 ± 0.28 | |
| R2 | 0.71 | 0.86 | 0.74 | 0.60 | |
| Ho pseudo-second order | Qmax, mmol/g | 0.252 ± 0.004 | 0.41 ± 0.03 | 0.68 ± 0.05 | 0.54 ± 0.01 |
| K, mmol/g·min | 6.9 ± 1.4 | 0.66 ± 0.19 | 0.66 ± 0.27 | 17.48 ± 8.99 | |
| R2 | 0.95 | 0.96 | 0.90 | 0.67 | |
| Morris-Webber intra-particle diffusion | K, mmol (g·min½) | 0.0098 ± 0.0015 | 0.048 ± 0.004 | 0.067 ± 0.008 | 0.0054 ± 0.0008 |
| C, mmol/g | 0.200 ± 0.006 | 0.120 ± 0.010 | 0.310 ± 0.030 | 0.517± 0.003 | |
| R2 | 0.95 | 0.98 | 0.97 | 0.95 |
| Solution Type | Solution Origin | Solution Properties | Solution Composition | Na2Al2Si2O8 Sorption Characteristics | K2Al2Si2O8 Sorption Characteristics |
|---|---|---|---|---|---|
| Seawater contaminated with Ni2+ | Natural Seawater spiked with Ni2+ | pH = 7.17 | C(Ni) = 17.4 mg/L | Q = 7.9 mg(Ni2+)/g R = 45% | Q = 6.1 mg(Ni2+)/g R = 35% |
| Groundwater contaminated with Ni2+ | Natural groundwater spiked with Ni2+ | pH = 6.88 | C(Si) = 4.5 mg/L C(Ca) = 3.4 mg/L C(Na) = 2.1 mg/L C(Mg) = 0.8 mg/L C(K) = 0.3 mg/L C(Ni) = 19.6 mg/L | Q = 18.4 mg(Ni2+)/g R = 94% | Q = 17.7 mg(Ni2+)/g R = 90% |
| Industrial wastewater | Model solution | pH = 3.33 | C(Cu) ≈ 20 mg/L C(Pb) ≈ 20 mg/L C(Mn) ≈ 20 mg/L C(Ni) = 18.9 mg/L | Q = 3.0 mg(Ni2+)/g R = 16% | Q = 1.6 mg(Ni2+)/g R = 8% |
| Characteristic Line | Chemical State * | K2Al2Si2O8 | Ni-K2Al2Si2O8 | K2Al2Si10O24 | Ni-K2Al2Si10O24 |
|---|---|---|---|---|---|
| O 1s | SiO2, KO2 | 532.1 eV | 532.1 eV | 532.4 eV | 532.3 eV |
| (22.44%) | (35.99%) | (38.61%) | |||
| Al2O3, Al2NiO4 | 530.7 eV | 530.9 eV | 530.9 eV | 530.7 eV | |
| (25.65%) | (18.97%) | (18.18%) | |||
| NiO | - | 528.7 eV | - | - | |
| (7.2%) | |||||
| Si 2p | SiO2 | n/d | 102.8 eV | 103.3 eV | 103.1 eV |
| (12.09%) | (18.21%) | (20.42%) | |||
| SiC | 101.2 eV | 101.9 eV | 103.6 eV | ||
| (6.56%) | (8.11%) | (7.69%) | |||
| Al 2p | Al2NiO4 | n/d | 75.2 eV | - | - |
| (6.23%) | |||||
| Al2O3 | 74.4 eV | 74.7 eV | 74.6 eV | ||
| (9.31%) | (4.28%) | (3.57%) | |||
| K 2p 3/2 | K2O | n/d | 293.7 eV | 294.0 eV | 293.8 eV |
| (4.06%) | (1.62%) | (1.16%) | |||
| C 1s | CnHm, C | n/d | 285.0 eV | 285.0 eV | 285.0 eV |
| (2.97%) | (7.56%) | (7.55%) | |||
| SiC | 282.0 eV | 282.0 eV | 282.5 eV | ||
| (2.33%) | (5.26%) | (2.82%) | |||
| Ni 2p 3/2 | NiO, Al2NiO4 | - | 856.6 eV (1.16%) | - | n/d |
| Characteristic Line | Chemical State * | Na2Al2Si2O8 | Ni-Na2Al2Si2O8 | Na2Al2Si10O24 | Ni-Na2Al2Si10O24 |
|---|---|---|---|---|---|
| O 1s | SiO2 | 532.1 eV | 532.3 eV | 532.0 eV | 532.0 eV |
| (18.20%) | (18.40%) | (33.17%) | (32.10%) | ||
| Al2O3, Al2NiO4 | 531.1 eV | 531.2 eV | 531.1 eV | 531.7 eV | |
| (16.75%) | (20.50%) | (3.31%) | (4.38%) | ||
| NiO, Na2O | 530.0 | 530.0 eV | 530.1 eV | 530.1 eV | |
| (8.30%) | (12.90%) | (15.36%) | (11.27%) | ||
| Si 2p | SiO2 | 102.5 eV | 102.6 eV | 102.8 eV | 103.4 eV |
| (11.36%) | (10.41%) | (15.49%) | (12.56%) | ||
| SiC | 101.3 eV | 101.2 eV | 101.4 eV | 102.5 eV | |
| (5.17%) | (5.04%) | (9.26%) | (9.50%) | ||
| Al 2p | Al2NiO4 | - | 74.9 eV | - | - |
| (4.09%) | |||||
| Al2O3 | 74.5 eV | 74.2 eV | 74.4 eV | 74.5 eV | |
| (7.64%) | (4.67%) | (1.37%) | (2.74%) | ||
| SiO2 (Al2O3) | 73.2 eV | - | 73.3 eV | - | |
| (3.47%) | (1.58%) | ||||
| Na 1s | Na2O | 1072.3 eV | 1072.1 eV | 1072.3 eV | 1072.5 eV |
| (3.83%) | (2.17%) | (1.28%) | (0.97%) | ||
| Na (org.) | 1070.7 eV | 1070.4 eV | 1071.0 eV | 1071.1 eV | |
| (0.91%) | (0.52%) | (0.84%) | (0.49%) | ||
| C 1s | NaC2H3O2 | 288.1 eV | 288.9 eV | 289.2 eV | 287.3 eV |
| (3.42%) | (1.38%) | (0.84%) | (2.73%) | ||
| CnHm, C | 285.0 eV | 285.0 eV | 285.0 eV | 285.0 eV | |
| (15.44%) | (9.11%) | (7.58%) | (12.52%) | ||
| SiC | 283.2 eV | 283.3 eV | 283.1 eV | 282.7 eV | |
| (5.51%) | (7.22%) | (8.22%) | (10.65%) | ||
| CO | 287.1 eV | ||||
| (1.70%) | |||||
| Ni 2p 3/2 | NiO, Al2NiO4 | - | 856.2 (3.59%) | - | n/d |
| Sorption Material | Characterization of the Material | Sorption Capacity | Ref. |
|---|---|---|---|
| Zeo/PVA/SA NC | Nanocomposite based on polyvinyl alcohol, sodium alginate and natural zeolite (RaaTec-Zeolite supplements Company, Helsingborg, Sweden) | 47.619 mg/g | [52] |
| Clinoptilolite (Sokyrnytsa, Sokyrnyansky deposit, Ukraine) | Clinoptilolite rock (∼75% clinoptilolite). Associated minerals: quartz, calcite, biotite, muscovite, chlorite, montmorillonite | 15.55 mg/g | [53] |
| Rectorite (Zhongxiang, Hubei Province, China) | Na-rectorite sample Na·Al4[Si,Al]8O20[OH]4·xH2O (SBET = 11.9 m2/g) | 6.3–10.2 mg/g (1.08–1.74·10−4 mol/g) | [54] |
| Calcined paper sludge | Dry-milled and calcined (500–900 °C) paper sludge containing compounds in CaO(MgO)-Al2O3-SiO2 system | 85.1–266.5 mg/g (1.45–4.54 mmol/g) | [49] |
| Palygorskite-based composite membranes | Bicomponent membranes based on polyvinylidene fluoride (PVDF) and hyperbranched polyamidoamine-palygorskite | 124.28 mg/g | [55] |
| Montmorillonite (Ugwuoba, Nigeria) | Acid-treated montmorillonite (0.5–2.5 M H2SO4) | 4.0 mg/g | [56] |
| Silica clay, Mojallali Co., (Isfahan, Iran), modified with biopolymers | Alginate-encapsulated silica clay (SBET = 497 m2/g) | 21.1 mg/g | [57] |
| Bentonite nanoclay-based geopolymer | Bentonite clay-based geopolymer modified with Fe3O4 nanoparticles (geopolymer/Fe3O4 ratio = 13) | 1227.2 mg/g | [15] |
| Polymer-modified natural aluminosilicates (Eastern Transbaikalia, Irkutsk, Russia) | Aluminosilicates modified with poly-1-vinylimidazole (PVIM) and poly-4-vinylpyridine (PVP) | 16.96 mg/g (0.289 mmol/g) 14.73 mg/g (0.251 mmol/g) | [58] |
| Modified natural aluminosilicates (Eastern Transbaikalia Irkutsk, Russia) | Aluminosilicate (75% calcium heulandite Ca[Al2Si7O18]·6H2O and 25% K-feldspar KAlSi3O8) modified with N,N’-bis(3-triethoxysilylpropyl)thiocarbamide | 79.6 mg/g (1.36 mmol/g) | [59] |
| Clinoptilolite-based composite (Cluj County, Romania) | Clinoptilolite-chitosan composite from volcanic tuffs | 247.1 mg/g 4.209 mmol/g | [50] |
| Synthetic zeolite X from coal ash | Zeolite X (SBET = 242.04 m2/g) synthesized by alkaline fusion with NaOH at 600 °C (90 min) followed by hydrothermal treatment at 100 °C (24 h) | 79.2 mg/g (1.35 mmol/g) | [60] |
| Zeolite (Yagodninsky deposit, Nachiki, Kamchatka, Russia) | Zeolitic tuffs containing clinoptilolite (70%), mordenite (10%), and cristobalite, quartz, mica, clay minerals (20%) | 2.34 mg/g | [61] |
| Nanostructured K and Na aluminosilicates | Nanostructured potassium and sodium aluminosilicates with varying Si/Al ratios: M2Al2SixO2(x+4)·nH2O (M = K+, Na+; x = 2, 10) and Na2Al2Si2kO2(2k+2)·nH2O (k = 1–5) | 64.6 mg/g (1.1 mmol/g) | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nekhludova, E.; Ivanov, N.; Yarusova, S.; Shichalin, O.; Parotkina, Y.; Karabtsov, A.; Mayorov, V.; Ivanenko, N.; Barkhudarov, K.; Provatorova, V.; et al. Nanostructured K- and Na-Substituted Aluminosilicates for Ni(II) Ions Removal from Liquid Media: Assessment of Sorption Performance and Mechanism. J. Compos. Sci. 2025, 9, 530. https://doi.org/10.3390/jcs9100530
Nekhludova E, Ivanov N, Yarusova S, Shichalin O, Parotkina Y, Karabtsov A, Mayorov V, Ivanenko N, Barkhudarov K, Provatorova V, et al. Nanostructured K- and Na-Substituted Aluminosilicates for Ni(II) Ions Removal from Liquid Media: Assessment of Sorption Performance and Mechanism. Journal of Composites Science. 2025; 9(10):530. https://doi.org/10.3390/jcs9100530
Chicago/Turabian StyleNekhludova, Ekaterina, Nikita Ivanov, Sofia Yarusova, Oleg Shichalin, Yulia Parotkina, Alexander Karabtsov, Vitaly Mayorov, Natalya Ivanenko, Kirill Barkhudarov, Viktoriya Provatorova, and et al. 2025. "Nanostructured K- and Na-Substituted Aluminosilicates for Ni(II) Ions Removal from Liquid Media: Assessment of Sorption Performance and Mechanism" Journal of Composites Science 9, no. 10: 530. https://doi.org/10.3390/jcs9100530
APA StyleNekhludova, E., Ivanov, N., Yarusova, S., Shichalin, O., Parotkina, Y., Karabtsov, A., Mayorov, V., Ivanenko, N., Barkhudarov, K., Provatorova, V., Rinchinova, V., Afonchenko, V., Savin, S., Nemtinov, V. I., Shurygin, A., Gordienko, P., & Papynov, E. (2025). Nanostructured K- and Na-Substituted Aluminosilicates for Ni(II) Ions Removal from Liquid Media: Assessment of Sorption Performance and Mechanism. Journal of Composites Science, 9(10), 530. https://doi.org/10.3390/jcs9100530







