A PVA–Brookite Composite: The Effect of Plasma Pre-Treatment on the Thermal, Mechanical, and Photochromic Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Physical Measurements
3. Results
3.1. FTIR Measurements
3.2. Thermal Analysis
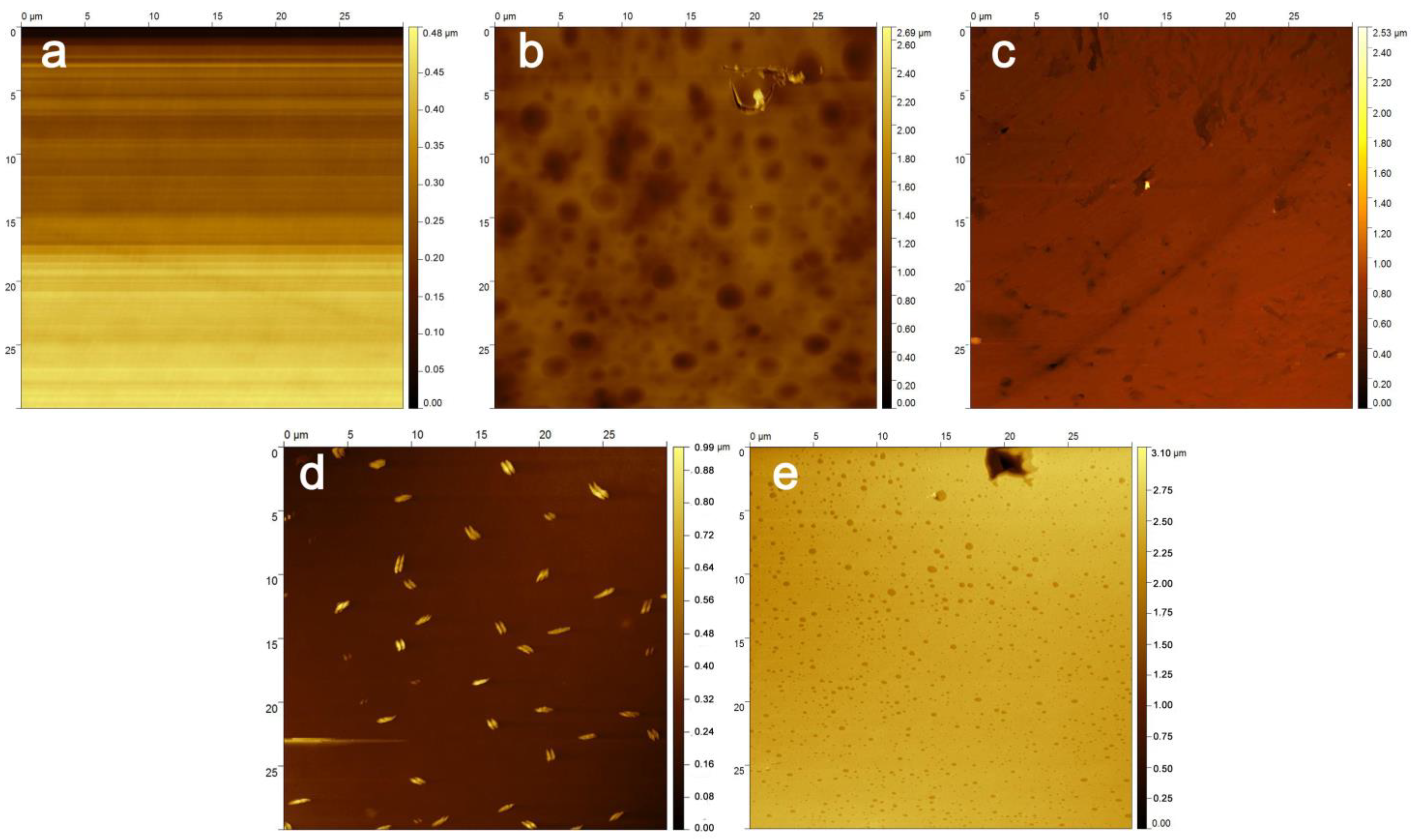
3.3. Surface Morphology
3.4. Mechanical Properties
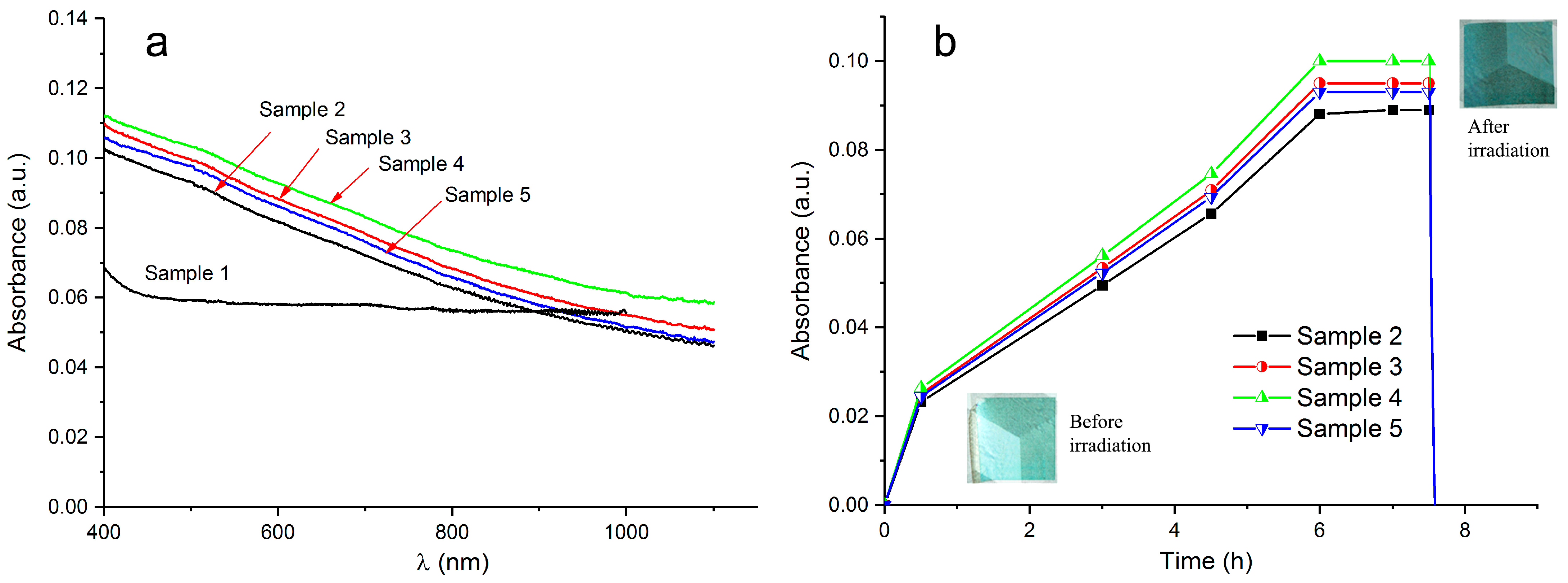
3.5. Photochromic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hsissou, R.; Seghiri, R.; Benzekri, Z.; Hilali, M.; Rafik, M.; Elharfi, A. Polymer composite materials: A comprehensive review. Compos. Struct. 2021, 262, 113640. [Google Scholar] [CrossRef]
- Ramaraj, B.; Nayak, S.K.; Yoon, K.R. Poly(vinyl alcohol) and layered double hydroxide composites: Thermal and mechanical properties. J. Appl. Polym. Sci. 2010, 116, 1671–1677. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kraev, A.; Agafonov, A.; Titov, V. Composites based on PVA and Al–Zn structures with excellent mechanical properties. Polym. Compos. 2022, 43, 4029–4037. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Titov, V.A.; Evdokimova, A.V.; Shibaeva, V.D.; Kraev, A.S.; Sirotkin, N.A.; Khlyustova, A.V. Composites based on biodegradable polymers and layered structures. Polym. Sci. B 2023, 65, 692–699. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Z.; Li, M.; Yin, Z.; Butt, H.A. The synthesis, mechanisms, and additives for bio-compatible polyvinyl alcohol hydrogels: A review on current advances, trends, and future outlook. J. Vinyl Addit. Technol. 2023, 29, 939–959. [Google Scholar] [CrossRef]
- Chang, J.H. Comparative analysis of properties of PVA composites with various nanofillers: Pristine clay, organoclay, and functionalized graphene. Nanomaterials 2019, 9, 323. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Robben, L.; Alkaima, A.; Bahnemann, D. Brookite versus anatase TiO2 photocatalysts: Phase transformations and photocatalytic activities. Photochem. Photobiol. Sci. 2013, 12, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Di Paola, A.; Bellardita, M.; Palmisano, L. Brookite, the least known TiO2 photocatalyst. Catalysts 2013, 3, 36–73. [Google Scholar] [CrossRef]
- Biancardo, M.; Argazzi, R.; Bignozzi, C.A. Solid-state photochromic device based on nanocrystalline TiO2 functionalized with electron donor–acceptor species. Inorg. Chem. 2005, 44, 9619–9621. [Google Scholar] [CrossRef]
- Belhomme, L.; Duttine, M.; Labrugère, C.; Coicaud, E.; Rougier, A.; Penin, N.; Dandre, A.; Ravaine, S.; Gaudon, M. Investigation of the photochromism of WO3, TiO2, and composite WO3–TiO2 nanoparticles. Inorg. Chem. 2024, 63, 10079–10091. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, M.; Sadrnia, H.; Khojastehpour, M.; Hosseini, F.; Thibault, J. Effect of TiO2 nanoparticles on barrier and mechanical properties of PVA films. J. Membr. Sci. Res. 2021, 7, 67–73. [Google Scholar] [CrossRef]
- Alfahed, R.K.F.; Mohammad, K.K.; Majeed, M.S.; Badran, H.A.; Ali, K.M.; Kadem, B.Y. Preparation, morphological, and mechanical characterization of titanium dioxide (TiO2)/polyvinyl alcohol (PVA) composite for gamma-rays radiation shielding. J. Phys. Conf. Ser. 2019, 1279, 012019. [Google Scholar] [CrossRef]
- Vishwas, M.; Rao, K.N.; Priya, D.N.; Raichur, A.M.; Chakradhar, R.; Venkateswarlu, K. Effect of TiO2 nano-particles on optical, electrical and mechanical properties of poly(vinyl alcohol) films. Procedia Mater. Sci. 2014, 5, 847–854. [Google Scholar] [CrossRef][Green Version]
- Khalid, F.; Roy, A.S.; Parveen, A.; Castro-Muñoz, R. Fabrication of the cross-linked PVA/TiO2/C nanocomposite membrane for alkaline direct methanol fuel cells. Mater. Sci. Eng. B 2024, 299, 116929. [Google Scholar] [CrossRef]
- Beenarani, B.B.; Sugumaran, C.P. The electrochemical performance of simple, flexible and highly thermally stable PVA-TiO2 nanocomposite in an all-solid-state supercapacitor. IEEE Trans. Nanotechnol. 2021, 20, 215–223. [Google Scholar] [CrossRef]
- Bozdoğan, A.; Aksakal, B.; Yargi, O. Film formation and mechanical properties of an opaque titanium dioxide and transparent polyvinyl alcohol composite films. Polym. Compos. 2020, 41, 939–950. [Google Scholar] [CrossRef]
- Aparicio, G.M.; Vargas, R.A.; Bueno, P.R. Protonic conductivity and thermal properties of cross-linked PVA/TiO2 nanocomposite polymer membranes. J. Non-Cryst. Solids 2019, 522, 119520. [Google Scholar] [CrossRef]
- Ahmad, J.; Deshmukh, K.; Habib, M.; Hägg, M.B. Influence of TiO2 nanoparticles on the morphological, thermal and solution properties of PVA/TiO2 nanocomposite membranes. Arab. J. Sci. Eng. 2014, 39, 6805–6814. [Google Scholar] [CrossRef]
- Radoicic, M.; Saponjic, Z.; Marinovic-Cincovic, M.; Ahrenkiel, S.; Bibic, N.; Nedeljkovic, J. The influence of shaped TiO2 nanofillers on thermal properties of polyvinyl alcohol. J. Serbian Chem. Soc. 2012, 77, 699–714. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kusova, T.; Kraev, A.; Titov, V.; Agafonov, A. Doped TiO2: The effect of doping elements on photocatalytic activity. Mater. Adv. 2020, 1, 1193–1201. [Google Scholar] [CrossRef]
- Bi, D.; Li, Y.; Yao, Y.; Tao, T.; Liang, B.; Lu, S. Preparation and characterizations of flexible photothermal Ti2O3-PVA nanocomposites. J. Alloy Compd. 2020, 825, 153998. [Google Scholar] [CrossRef]
- Shang, M.; He, Y.; Yu, J.; Yan, J.; Xie, H.; Li, J. Preparation, Characterization and Photothermal Study of PVA/Ti2O3 Composite Films. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2024, 39, 658–663. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, Q.; Liu, Q.; Qiu, J.; Li, J.; Zheng, W.; Cao, J.; Wang, L.; Wang, W.; Yuan, S.; et al. Full-spectrum-responsive Ti4O7-PVA nanocomposite hydrogel with ultrahigh evaporation rate for efficient solar steam generation. Desalination 2024, 577, 117400. [Google Scholar] [CrossRef]
- Arnal, P.; Corriu, R.J.P.; Leclercq, D.; Mutin, P.H.; Vioux, A. Preparation of anatase, brookite and rutile at low temperature by non-hydrolytic sol–gel methods. J. Mater. Chem. 1996, 6, 1925–1932. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kraev, A.; Kusova, T.; Titov, V.; Agafonov, A. Mo-doped TiO2 using plasma in contact with liquids: Advantages and limitations. J. Chem. Technol. Biotechnol. 2021, 96, 1125–1131. [Google Scholar] [CrossRef]
- Khlyustova, A.; Evdokimova, A.; Sirotkin, N.; Shibaeva, V. Green Method of Doping Photochromic TiO2. Appl. Sci. 2024, 14, 8877. [Google Scholar] [CrossRef]
- Lv, K.; Li, Y.; Sirotkin, N.; Agafonov, A.; Evdokimova, A.; Shibaeva, V.; Khlyustova, A. An underwater diaphragm discharge: A new tool for ZnFe LDH obtaining. Appl. Clay Sci. 2024, 258, 107482. [Google Scholar] [CrossRef]
- Agafonov, A.; Alekseeva, O.; Vokhidova, N.; Evdokimova, A.; Kraev, A.; Shibaeva, V.; Khlyustova, A. Effect of production method on the properties of PVA/Ag–Cu composites. Polym. Bull. 2024, 81, 6457–6472. [Google Scholar] [CrossRef]
- Sirotkin, N.; Khlyustova, A. The electrophysical characteristics of underwater impulse discharge plasma in the processes of creating multifunctional composites. Plasma Chem. Plasma Proc. 2023, 43, 561–575. [Google Scholar] [CrossRef]
- Balaganapathi, T.; KaniAmuthan, B.; Vinoth, S.; Thilakan, P. Synthesis, characterization and dye adsorption studies of porous brookite and mixed brookite with rutile TiO2 using PEG assisted sol-gel synthesis process. Mater. Res. Bull. 2017, 91, 114–121. [Google Scholar] [CrossRef]
- Arbelaiz, A.; Fernández, B.; Valea, A.; Mondragon, I. Mechanical properties of short flax fibre bundle/poly (ε-caprolactone) composites: Influence of matrix modification and fibre content. Carbohydr. Polym. 2006, 64, 224–232. [Google Scholar] [CrossRef]
- Finch, C.A. Polyvinyl Alcohol; Properties and Applications; John Wiley & Sons: Hoboken, NJ, USA, 1973. [Google Scholar]
- Abdel-Hady, E.E.; Mohamed, H.F.; Abdel-Hamed, M.O.; Gomaa, M.M. Physical and electrochemical properties of PVA/TiO2 nanocomposite membrane. Adv. Polym. Technol. 2018, 37, 3842–3853. [Google Scholar] [CrossRef]
- ASTM D882-10; ASTM Subcommittee D20. 10 on Mechanical Properties. Standard Test Method for Tensile Properties of Thin Plastic Sheeting. American Society for Testing and Materials: West Conshohocken, PA, USA, 1995.
- Agalya, K.; Vijayakumar, S.; Vidhya, E.; Prathipkumar, S.; Mythil, R.; Devanesan, S.; AlSalhi, M.; Kim, W. Fabrication of PVA/TiO2 Composites Via Green Synthesis and Assessment of their Photodegradation and Anti-Germ Capabilities. Waste Biomass Valorization 2024, 15, 6441–6451. [Google Scholar] [CrossRef]
- El-Desoky, M.M.; Morad, I.; Wasfy, M.H.; Mansour, A.F. Synthesis, structural and electrical properties of PVA/TiO2 nanocomposite films with different TiO2 phases prepared by sol–gel technique. J. Mater. Sci. Mater. Electr. 2020, 31, 17574–17584. [Google Scholar] [CrossRef]
- Feldstein, M.M.; Shandryuk, G.A.; Kuptsov, S.A.; Platé, N.A. Coherence of thermal transitions in poly (N-vinyl pyrrolidone)–poly (ethylene glycol) compatible blends 1. Interrelations among the temperatures of melting, maximum cold crystallization rate and glass transition. Polymer 2000, 41, 5327–5338. [Google Scholar] [CrossRef]
- Mohammed, G.; El Sayed, A.M.; Morsi, W.M. Spectroscopic, thermal, and electrical properties of MgO/polyvinyl pyrrolidone/polyvinyl alcohol nanocomposites. J. Phys. Chem. Solids 2018, 115, 238–247. [Google Scholar] [CrossRef]
- Restrepo, I.; Medina, C.; Meruane, V.; Akbari-Fakhrabadi, A.; Flores, P.; Rodríguez-Llamazares, S. The effect of molecular weight and hydrolysis degree of poly (vinyl alcohol)(PVA) on the thermal and mechanical properties of poly (lactic acid)/PVA blends. Polímeros 2018, 28, 169–177. [Google Scholar] [CrossRef]
- Nam, E.; Wong, E.H.; Tan, S.; Fu, Q.; Blencowe, A.; Qiao, G.G. Antifogging Surface Facilitated by Nanoscale Coatings with Controllable Hydrophobicity and Cross-Linking Density. Macromol. Mater. Eng. 2017, 302, 1600199. [Google Scholar] [CrossRef]
- Song, Y.J.; Wang, M.; Zhang, X.Y.; Wu, J.Y.; Zhang, T. Investigation on the role of the molecular weight of polyvinyl pyrrolidone in the shape control of high-yield silver nanospheres and nanowires. Nanoscale Res. Lett. 2014, 9, 17. [Google Scholar] [CrossRef]
- Díaz-Cruz, C.; Nuñez, G.A.; Espinoza-Gómez, H.; Flores-López, L.Z. Effect of molecular weight of PEG or PVA as reducing-stabilizing agent in the green synthesis of silver-nanoparticles. Eur. Polym. J. 2016, 83, 265–277. [Google Scholar] [CrossRef]
- Logutenko, O.A.; Titkov, A.I.; Vorob’yov, A.M.; Balaev, D.A.; Shaikhutdinov, K.A.; Semenov, S.V.; Lyakhov, N.Z. Effect of molecular weight of sodium polyacrylates on the size and morphology of nickel nanoparticles synthesized by the modified polyol method and their magnetic properties. Eur. Polym. J. 2018, 99, 102–110. [Google Scholar] [CrossRef]
- Mansour, L.M.; Taj, B.M.; Mokhtari, M. Synthesis and swelling characterization of cross-linked PVP/PVA hydrogels. Iran. Polym. J. 2005, 14, 1022–1030. [Google Scholar]
- Yurovskikh, S.V.; Chvalun, S.N.; Lyoo, W.S. Structure and properties of poly (vinyl alcohol) of different stereoregularity. Polym. Sci. A 2001, 43, 278–284. [Google Scholar]
- Nunes, R.W.; Martin, J.R.; Johnson, J.F. Influence of molecular weight and molecular weight distribution on mechanical properties of polymers. Polym. Eng. Sci. 1982, 22, 205–228. [Google Scholar] [CrossRef]
- Ohtani, B.; Adzuma, S.; Nishimoto, S.I.; Kagiya, T. Ultraviolet and visible light-induced photochromic action of poly (vinyl alcohol) film containing colloidal and suspended semiconductor materials. J. Polym. Sci. C Polym. Lett. 1987, 25, 383–387. [Google Scholar] [CrossRef]
- Russo, M.; Rigby, S.E.; Caseri, W.; Stingelin, N. Pronounced photochromism of titanium oxide hydrates (hydrous TiO2). J. Mater. Chem. 2010, 20, 1348–1356. [Google Scholar] [CrossRef]
- Nussbaumer, R.J.; Caseri, W.R.; Smith, P. Reversible photochromic properties of TiO2-polymer nanocomposites. J. Nanosci. Nanotechnol. 2006, 6, 459–463. [Google Scholar] [CrossRef] [PubMed]
- George, S.C.; Thomas, S. Transport phenomena through polymeric systems. Prog. Polym. Sci. 2001, 26, 985–1017. [Google Scholar] [CrossRef]
- Tretinnikov, O.N.; Sushko, N.I. Preparation and photochromic properties of nanocomposites based on chemically cross-linked polyvinyl alcohol and phosphotungstic acid. Russ. J. Phys. Chem. A 2011, 85, 2177–2182. [Google Scholar] [CrossRef]




| Sample | Sol | Plasma Treatment of Sol (Material of Electrode)/Dopant Content, % | Film Thickness, mm |
|---|---|---|---|
| 1 | - | -/0 | 0.10 |
| 2 | TiO2 | - | 0.09 |
| 3 | TiO2 | +(Mo)/3.8 | 0.05 |
| 4 | TiO2 | +(Nb)/3.0 | 0.06 |
| 5 | TiO2 | +(W)/1.3 | 0.07 |
| Peak Designation | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|---|
| O-H stretching | 3265 | 3270 | 3267 | 3270 | 3266 |
| C-H stretching | 2938 | 2915 | 2916 | 2915 | 2918 |
| C=O stretching | 1704 | 1729 | 1729 | 1728 | 1729 |
| δ(O-H) | 1652 | 1639 | 1644 | 1643 | 1647 |
| CH2 bending | 1423 | 1417 | 1416 | 1422 | 1417 |
| C-O stretching | 1092 | 1092 | 1092 | 1091 | 1091 |
| C-C | 917 | 917 | 918 | 917 | 915 |
| C-H out-of-plane | 844 | 845 | 845 | 845 | 844 |
| Me-O-Me | – | 652 | 650 | 654 | 652 |
| Sample | Tg, °C | Tc, °C | Tm, °C | Tm − Tc, °C | Tc/Tm | ΔHm, J g−1 | x, % |
|---|---|---|---|---|---|---|---|
| 1 | 34.67 | 186.64 | 226.99 | 40.35 | 0.82 | 45.05 | 32.51 |
| 2 | 31.11 | 188.24 | 242.2 | 53.96 | 0.78 | 18.46 | 13.18 |
| 3 | 43.75 | 189.61 | 241.90 | 52.29 | 0.78 | 67.06 | 47.89 |
| 4 | 44.88 | 194.33 | 246.60 | 52.27 | 0.79 | 97.66 | 69.75 |
| 5 | 44.09 | 192.49 | 245.90 | 53.41 | 0.78 | 88.64 | 63.32 |
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|
| 3.80 | 17.94 | 5.90 | 16.59 | 46.77 |
| Sample | Stress, MPa | Young’s Modulus, MPa | Elongation at Break, % |
|---|---|---|---|
| 1 | 3.17 | 2.78 | 11.2 |
| 2 | 0.11 | 0.19 | 3.21 |
| 3 | 0.42 | 2.00 | 10.9 |
| 4 | 0.25 | 1.21 | 7.71 |
| 5 | 0.29 | 0.98 | 11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evdokimova, A.; Shibaeva, V.; Sirotkin, N.; Kraev, A.; Manakhov, A.; Khlyustova, A. A PVA–Brookite Composite: The Effect of Plasma Pre-Treatment on the Thermal, Mechanical, and Photochromic Properties. J. Compos. Sci. 2025, 9, 7. https://doi.org/10.3390/jcs9010007
Evdokimova A, Shibaeva V, Sirotkin N, Kraev A, Manakhov A, Khlyustova A. A PVA–Brookite Composite: The Effect of Plasma Pre-Treatment on the Thermal, Mechanical, and Photochromic Properties. Journal of Composites Science. 2025; 9(1):7. https://doi.org/10.3390/jcs9010007
Chicago/Turabian StyleEvdokimova, Anastasia, Valeriya Shibaeva, Nikolay Sirotkin, Anton Kraev, Anton Manakhov, and Anna Khlyustova. 2025. "A PVA–Brookite Composite: The Effect of Plasma Pre-Treatment on the Thermal, Mechanical, and Photochromic Properties" Journal of Composites Science 9, no. 1: 7. https://doi.org/10.3390/jcs9010007
APA StyleEvdokimova, A., Shibaeva, V., Sirotkin, N., Kraev, A., Manakhov, A., & Khlyustova, A. (2025). A PVA–Brookite Composite: The Effect of Plasma Pre-Treatment on the Thermal, Mechanical, and Photochromic Properties. Journal of Composites Science, 9(1), 7. https://doi.org/10.3390/jcs9010007







