A Comparative Environmental and Economic Analysis of Carbon Fiber-Reinforced Polymer Recycling Processes Using Life Cycle Assessment and Life Cycle Costing
Abstract
1. Introduction
2. Life Cycle Assessment and Life Cycle Costing Methodology
- Goal and Scope Definition: This stage establishes the study’s objectives. Key methodological decisions are also determined here, such as defining the functional unit, setting system boundaries, identifying impact categories, and selecting Life Cycle Impact Assessment (LCIA) models.
- Life Cycle Inventory (LCI): This phase collects data and calculates system inputs and outputs. Data gathering includes foreground operations (such as manufacturing and packing) and background processes (such as the production of bought power and materials).
- Life Cycle Impact Assessment: This phase connects LCI data with environmental impact categories and indicators. LCIA methods categorize emissions into impact categories and quantify them into equivalent units, allowing for a more accurate evaluation of ecological impacts.
- Life Cycle Interpretation: The final phase aligns the outcomes of LCI and LCIA with the purpose and scope. This stage comprises checking for completeness, sensitivity, and consistency.
2.1. Goal and Scope Definition
2.2. Life Cycle Inventory
2.2.1. Production of Carbon Fiber-Reinforced Polymers
2.2.2. End-of-Life Processes—Recycling Processes
2.3. Life Cycle Impact Assessment
3. Results
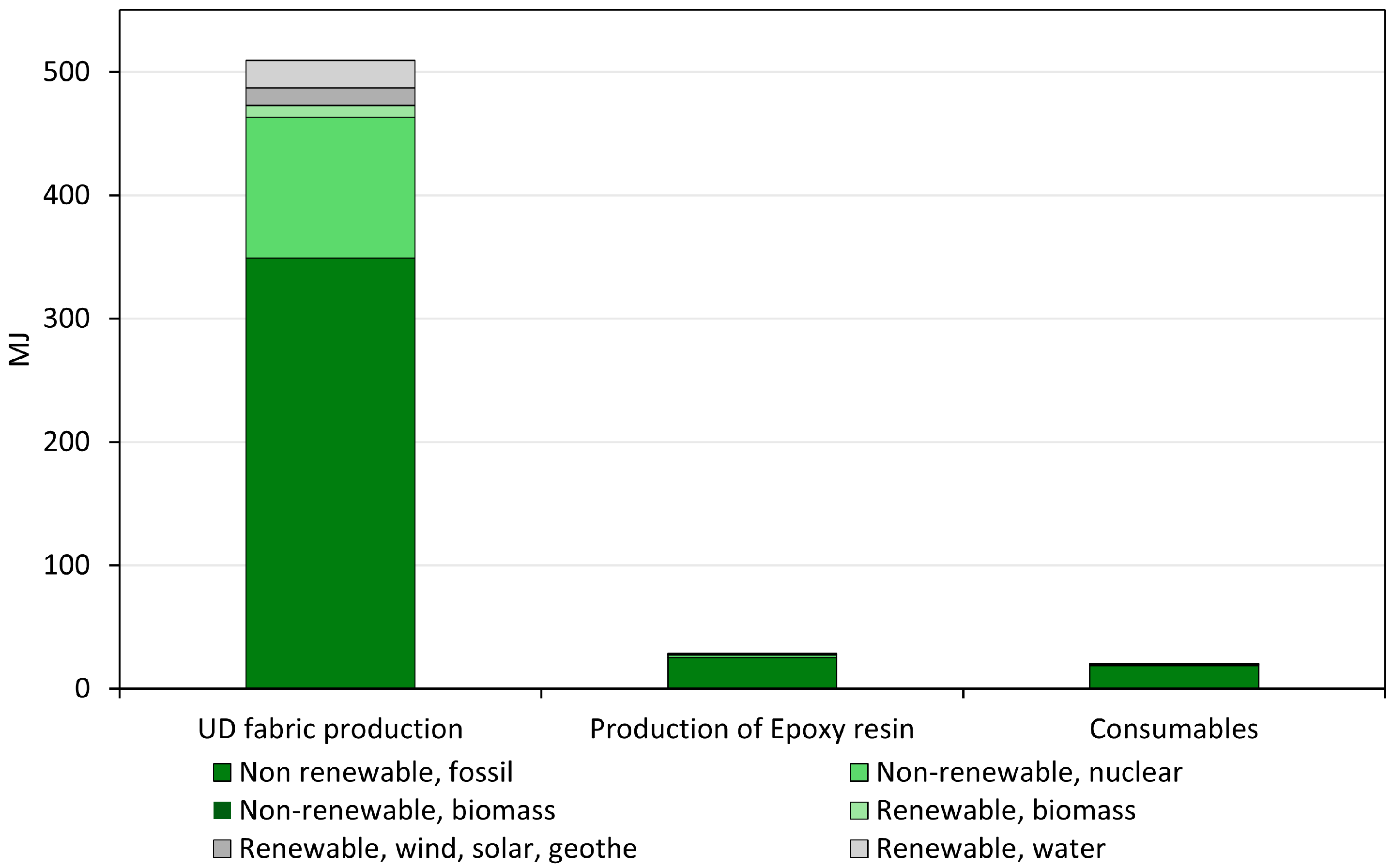
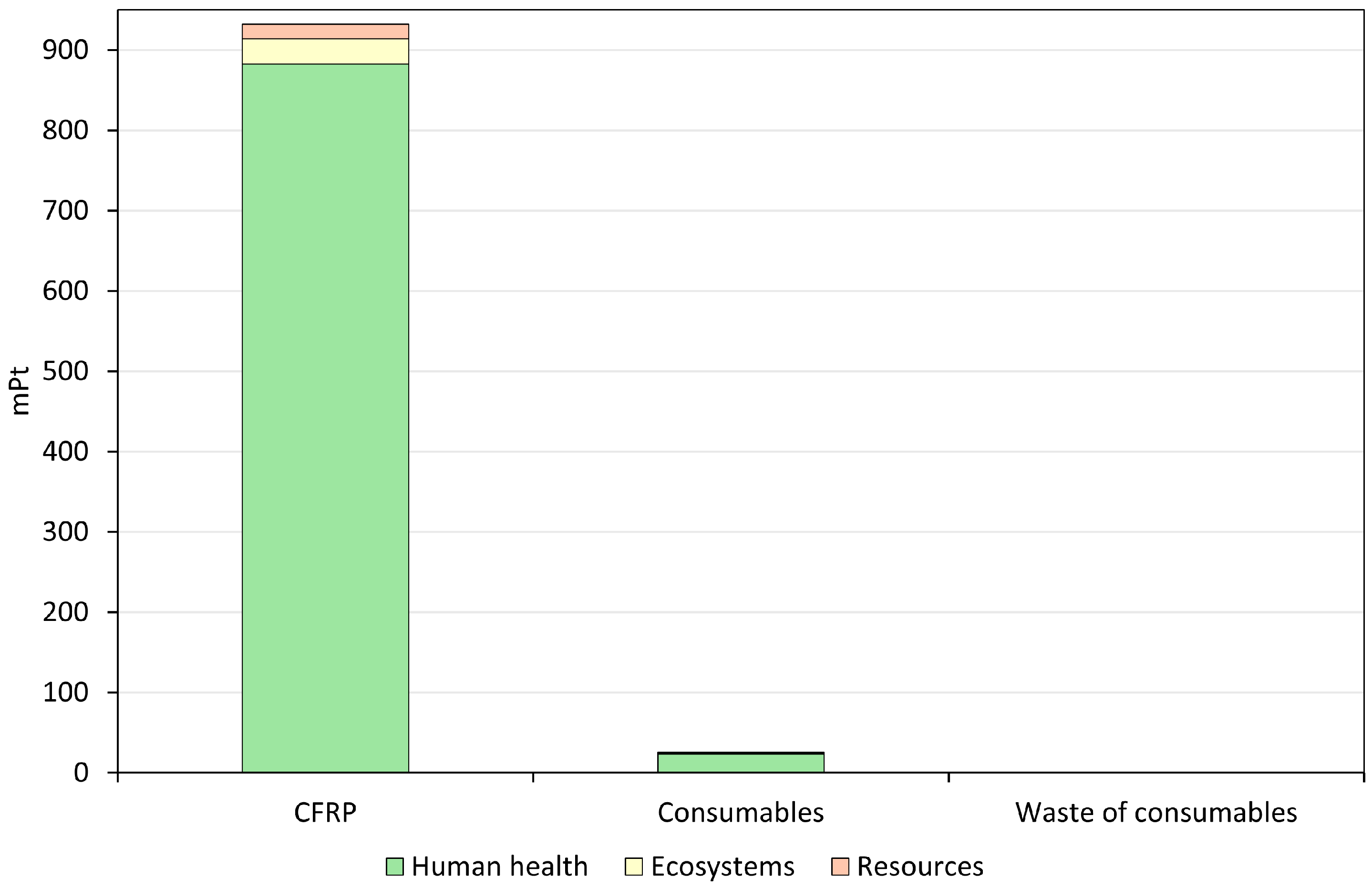
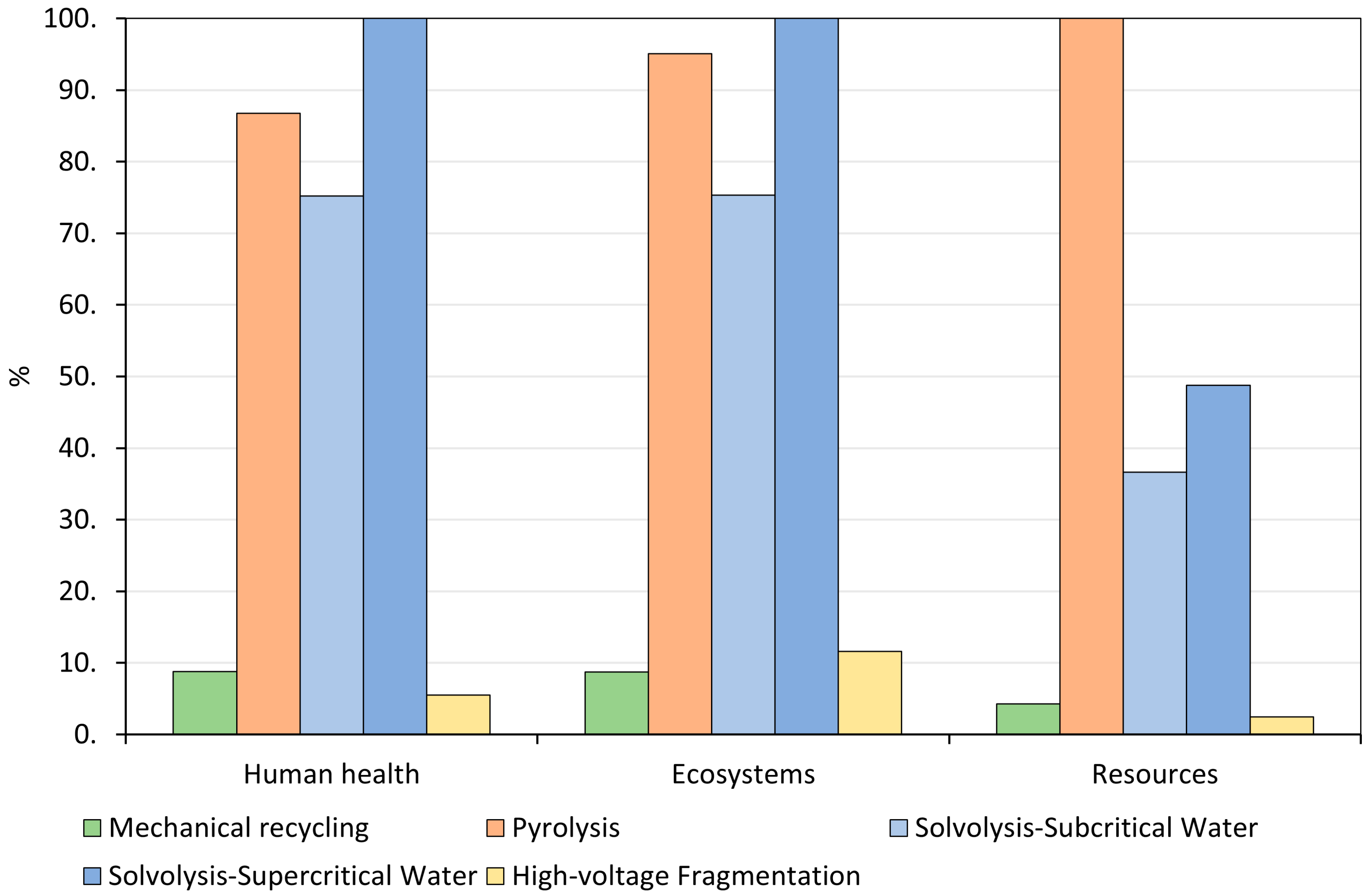
3.1. Interpretation of Life Cycle Assessment Results
- 23.3 kg CO2eq from UD fabric production, reflecting the high impact of the carbon fiber production stage,
- 1.31 kg CO2eq from epoxy resin production, and
- 0.624 kg CO2eq from consumables such as vacuum bags.
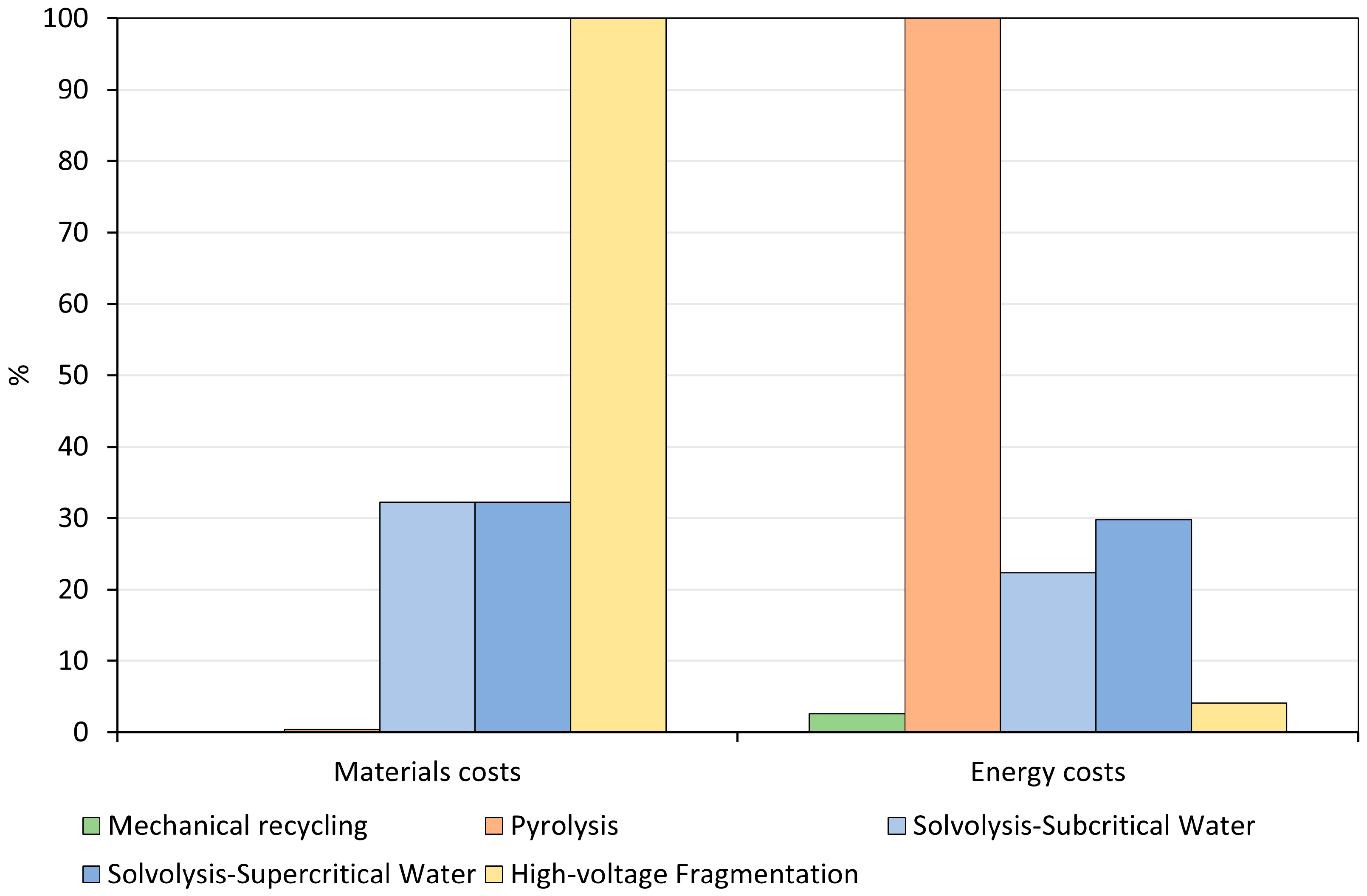
3.2. Life Cycle Costing Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CFRPs | Carbon Fiber-Reinforced Polymers |
| EoL | End-of-Life |
| LCA | Life Cycle Assessment |
| eLCC | Environmental Life Cycle Costing |
| LCC | Life Cycle Costing |
| GWP | Global Warming Potential |
| PED | Primary Energy Demand |
| LCIA | Life Cycle Impact Assessment |
| LCI | Life Cycle Inventory |
| eLCA | Environmental Life Cycle Assessment |
| vCFs | Virgin Carbon Fibers |
| AN | Acrylonitrile |
| PAN | Polyacrylonitrile |
| DMF | Dimethylformamide |
| PDMS | Polydimethylsiloxane |
| UD | Unidirectional |
| LCM | Liquid Composite Molding |
| LDPE | Low-Density Polyethylene |
| PES | Polyethersulfone |
| LRI | Liquid Resin Infusion |
| VARI | Vacuum-Assisted Resin Injection |
| HVF | High-Voltage Fragmentation |
| CED | Cumulative Energy Demand |
| Pt | Points |
| rCFs | Recycled Carbon Fibers |
| TRL | Technology Readiness Level |
References
- Mouritz, A.P.; Gibson, A.G. Fire Properties of Polymer Composite Materials; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2007; ISBN 978-1-4020-5356-6. [Google Scholar]
- Pantelakis, S.; Tserpes, K. (Eds.) Revolutionizing Aircraft Materials and Processes; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-35345-2. [Google Scholar]
- Borjan, D.; Knez, Ž.; Knez, M. Recycling of Carbon Fiber-Reinforced Composites—Difficulties and Future Perspectives. Materials 2021, 14, 4191. [Google Scholar] [CrossRef] [PubMed]
- Pickering, S.J. Recycling Technologies for Thermoset Composite Materials—Current Status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Jacob, A. Composites Can Be Recycled. Reinf. Plast. 2011, 55, 45–46. [Google Scholar] [CrossRef]
- Ribeiro, M.C.S.; Meira-Castro, A.C.; Silva, F.G.; Santos, J.; Meixedo, J.P.; Fiúza, A.; Dinis, M.L.; Alvim, M.R. Re-Use Assessment of Thermoset Composite Wastes as Aggregate and Filler Replacement for Concrete-Polymer Composite Materials: A Case Study Regarding GFRP Pultrusion Wastes. Resour. Conserv. Recycl. 2015, 104, 417–426. [Google Scholar] [CrossRef]
- Karuppannan Gopalraj, S.; Kärki, T. A Review on the Recycling of Waste Carbon Fibre/Glass Fibre-Reinforced Composites: Fibre Recovery, Properties and Life-Cycle Analysis. SN Appl. Sci. 2020, 2, 433. [Google Scholar] [CrossRef]
- Aldosari, S.M.; AlOtaibi, B.M.; Alblalaihid, K.S.; Aldoihi, S.A.; AlOgab, K.A.; Alsaleh, S.S.; Alshamary, D.O.; Alanazi, T.H.; Aldrees, S.D.; Alshammari, B.A. Mechanical Recycling of Carbon Fiber-Reinforced Polymer in a Circular Economy. Polymers 2024, 16, 1363. [Google Scholar] [CrossRef] [PubMed]
- Marsh, G. Reclaiming Value from Post-Use Carbon Composite. Reinf. Plast. 2008, 52, 36–39. [Google Scholar] [CrossRef]
- Butenegro, J.A.; Bahrami, M.; Abenojar, J.; Martínez, M.Á. Recent Progress in Carbon Fiber Reinforced Polymers Recycling: A Review of Recycling Methods and Reuse of Carbon Fibers. Materials 2021, 14, 6401. [Google Scholar] [CrossRef]
- Oliveux, G.; Dandy, L.O.; Leeke, G.A. Current Status of Recycling of Fibre Reinforced Polymers: Review of Technologies, Reuse and Resulting Properties. Prog. Mater. Sci. 2015, 72, 61–99. [Google Scholar] [CrossRef]
- Vogiantzi, C.; Tserpes, K. A Preliminary Investigation on a Water- and Acetone-Based Solvolysis Recycling Process for CFRPs. Materials 2024, 17, 1102. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Varughese, S. A Novel Sonochemical Approach for Enhanced Recovery of Carbon Fiber from CFRP Waste Using Mild Acid–Peroxide Mixture. ACS Sustain. Chem. Eng. 2016, 4, 2080–2087. [Google Scholar] [CrossRef]
- Cho, C.M.; Wang, X.; Tsumura, S.K.; Thitsartarn, W.; Tay, S.W. Reusing Bisphenol—A Type of Epoxy Polymer Recyclates from the Solvolysis of CFRP. J. Compos. Sci. 2024, 8, 2. [Google Scholar] [CrossRef]
- Diani, M.; Torvi, S.; Colledani, M. Application of High Voltage Fragmentation to Treat End-of-Life Wind Blades; Materials Research Forum LLC.: Millersville, PA, USA, 2023; pp. 266–274. [Google Scholar]
- Li, Z.; Yang, Y.; Teng, Z.; Zhang, C.; Yang, W.; Cao, Y.; Li, S. Eco-Efficient Recycling of Carbon Fibers from CFRP Waste: A Two-Step Strategy by Nanosecond Pulsed Laser. Polym. Compos. 2024, 1–17. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Feng, R.; Xu, Y.; Zhu, J.-H. Recycling and Reutilization of Waste Carbon Fiber Reinforced Plastics: Current Status and Prospects. Polymers 2023, 15, 3508. [Google Scholar] [CrossRef] [PubMed]
- Ballout, W.; Sallem-Idrissi, N.; Sclavons, M.; Doneux, C.; Bailly, C.; Pardoen, T.; Van Velthem, P. High Performance Recycled CFRP Composites Based on Reused Carbon Fabrics through Sustainable Mild Solvolysis Route. Sci. Rep. 2022, 12, 5928. [Google Scholar] [CrossRef] [PubMed]
- Meng, F. Environmental and Cost Analysis of Carbon Fibre Composites Recycling. Ph.D. Thesis, University of Nottingham, Nottingham, UK, 2017. [Google Scholar]
- Maheshwari, S.; Deswal, D.S. Role of Waste Management at Landfills in Sustainable Waste Management. Int. J. Emerg. Technol. 2017, 8, 324–328. [Google Scholar]
- Helbig, C.; Gemechu, E.D.; Pillain, B.; Young, S.B.; Thorenz, A.; Tuma, A.; Sonnemann, G. Extending the Geopolitical Supply Risk Indicator: Application of Life Cycle Sustainability Assessment to the Petrochemical Supply Chain of Polyacrylonitrile-Based Carbon Fibers. J. Clean. Prod. 2016, 137, 1170–1178. [Google Scholar] [CrossRef]
- Stergiou, V.; Konstantopoulos, G.; Charitidis, C.A. Carbon Fiber Reinforced Plastics in Space: Life Cycle Assessment Towards Improved Sustainability of Space Vehicles. J. Compos. Sci. 2022, 6, 144. [Google Scholar] [CrossRef]
- Chatzipanagiotou, K.-R.; Antypa, D.; Petrakli, F.; Karatza, A.; Pikoń, K.; Bogacka, M.; Poranek, N.; Werle, S.; Amanatides, E.; Mataras, D.; et al. Life Cycle Assessment of Composites Additive Manufacturing Using Recycled Materials. Sustainability 2023, 15, 12843. [Google Scholar] [CrossRef]
- Vinci, G.; Gobbi, L.; Porcaro, D.; Pinzi, S.; Carmona-Cabello, M.; Ruggeri, M. Environmental Evaluation of Chemical Plastic Waste Recycling: A Life Cycle Assessment Approach. Resources 2024, 13, 176. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, M.; Zhu, P.; Gao, K.; Xuan, Y.; Lu, Q.; Ding, Y.; Zhang, B.; Yue, M. Comparative Life Cycle Assessment and Life Cycle Cost Analysis of Bonded Nd-Fe-B Magnets: Virgin Production versus Recycling. Sustainability 2024, 16, 8599. [Google Scholar] [CrossRef]
- Howarth, J.; Mareddy, S.S.R.; Mativenga, P.T. Energy Intensity and Environmental Analysis of Mechanical Recycling of Carbon Fibre Composite. J. Clean. Prod. 2014, 81, 46–50. [Google Scholar] [CrossRef]
- Li, X.; Bai, R.; McKechnie, J. Environmental and Financial Performance of Mechanical Recycling of Carbon Fibre Reinforced Polymers and Comparison with Conventional Disposal Routes. J. Clean. Prod. 2016, 127, 451–460. [Google Scholar] [CrossRef]
- Dong, J.; Tang, Y.; Nzihou, A.; Chi, Y.; Weiss-Hortala, E.; Ni, M. Life Cycle Assessment of Pyrolysis, Gasification and Incineration Waste-to-Energy Technologies: Theoretical Analysis and Case Study of Commercial Plants. Sci. Total Environ. 2018, 626, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Kawajiri, K.; Kobayashi, M. Cradle-to-Gate Life Cycle Assessment of Recycling Processes for Carbon Fibers: A Case Study of Ex-Ante Life Cycle Assessment for Commercially Feasible Pyrolysis and Solvolysis Approaches. J. Clean. Prod. 2022, 378, 134581. [Google Scholar] [CrossRef]
- Yousef, S.; Eimontas, J.; Stasiulaitiene, I.; Zakarauskas, K.; Striūgas, N. Recovery of Energy and Carbon Fibre from Wind Turbine Blades Waste (Carbon Fibre/Unsaturated Polyester Resin) Using Pyrolysis Process and Its Life-Cycle Assessment. Environ. Res. 2024, 245, 118016. [Google Scholar] [CrossRef]
- La Rosa, A.D.; Greco, S.; Tosto, C.; Cicala, G. LCA and LCC of a Chemical Recycling Process of Waste CF-Thermoset Composites for the Production of Novel CF-Thermoplastic Composites. Open Loop and Closed Loop Scenarios. J. Clean. Prod. 2021, 304, 127158. [Google Scholar] [CrossRef]
- Leißner, T.; Hamann, D.; Wuschke, L.; Jäckel, H.-G.; Peuker, U.A. High Voltage Fragmentation of Composites from Secondary Raw Materials—Potential and Limitations. Waste Manag. 2018, 74, 123–134. [Google Scholar] [CrossRef]
- Shuaib, N.A.; Mativenga, P.T. Carbon Footprint Analysis of Fibre Reinforced Composite Recycling Processes. Procedia Manuf. 2017, 7, 183–190. [Google Scholar] [CrossRef]
- Vo Dong, P.A.; Azzaro-Pantel, C.; Cadene, A.-L. Economic and Environmental Assessment of Recovery and Disposal Pathways for CFRP Waste Management. Resour. Conserv. Recycl. 2018, 133, 63–75. [Google Scholar] [CrossRef]
- Meng, F.; Olivetti, E.A.; Zhao, Y.; Chang, J.C.; Pickering, S.J.; McKechnie, J. Comparing Life Cycle Energy and Global Warming Potential of Carbon Fiber Composite Recycling Technologies and Waste Management Options. ACS Sustain. Chem. Eng. 2018, 6, 9854–9865. [Google Scholar] [CrossRef]
- Khalil, Y.F. Comparative Environmental and Human Health Evaluations of Thermolysis and Solvolysis Recycling Technologies of Carbon Fiber Reinforced Polymer Waste. Waste Manag. 2018, 76, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Pillain, B.; Loubet, P.; Pestalozzi, F.; Woidasky, J.; Erriguible, A.; Aymonier, C.; Sonnemann, G. Positioning Supercritical Solvolysis among Innovative Recycling and Current Waste Management Scenarios for Carbon Fiber Reinforced Plastics Thanks to Comparative Life Cycle Assessment. J. Supercrit. Fluids 2019, 154, 104607. [Google Scholar] [CrossRef]
- ISO 14040:2006; Environmental Management: Life Cycle Assessment. Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management, Life Cycle Assessment. Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- European Platform on LCA EPLCA. Available online: https://eplca.jrc.ec.europa.eu/lifecycleassessment.html (accessed on 23 July 2024).
- Frank, E.; Hermanutz, F.; Buchmeiser, M.R. Carbon Fibers: Precursors, Manufacturing, and Properties. Macromol. Mater. Eng. 2012, 297, 493–501. [Google Scholar] [CrossRef]
- Kaur, J.; Millington, K.; Smith, S. Producing High-Quality Precursor Polymer and Fibers to Achieve Theoretical Strength in Carbon Fibers: A Review. J. Appl. Polym. Sci. 2016, 133, 43963. [Google Scholar] [CrossRef]
- Duflou, J.R.; De Moor, J.; Verpoest, I.; Dewulf, W. Environmental Impact Analysis of Composite Use in Car Manufacturing. CIRP Ann. 2009, 58, 9–12. [Google Scholar] [CrossRef]
- Meng, F.; McKechnie, J.; Turner, T.A.; Pickering, S.J. Energy and Environmental Assessment and Reuse of Fluidised Bed Recycled Carbon Fibres. Compos. Part A Appl. Sci. Manuf. 2017, 100, 206–214. [Google Scholar] [CrossRef]
- Park, S.-J. Carbon Fibers; Springer Series in Materials Science; Springer: Singapore, 2018; Volume 210, ISBN 978-981-13-0537-5. [Google Scholar]
- Gill, A.S.; Visotsky, D.; Mears, L.; Summers, J.D. Cost Estimation Model for PAN Based Carbon Fiber Manufacturing Process. In Proceedings of the ASME 2016 11th International Manufacturing Science and Engineering Conference, Blacksburg, VA, USA, 27 June 2016; p. V001T02A044. [Google Scholar]
- Stiller, H. Material Intensity of Advanced Composite Materials: Results of Asudy for the Verbundwerkstofflabor Bremen e.V.; Wuppertal Papers; Wuppertal Institute for Climate, Environment and Energy: Wuppertal, Germany, 1999. [Google Scholar]
- Terekhov, I.V.; Chistyakov, E.M. Binders Used for the Manufacturing of Composite Materials by Liquid Composite Molding. Polymers 2021, 14, 87. [Google Scholar] [CrossRef] [PubMed]
- Karachalios, E.; Muñoz, K.; Jimenez, M.; Prentzias, V.; Goossens, S.; Geernaert, T.; Plagianakos, T.S. LRI-Fabricated Composite Demonstrators for an Aircraft Fuselage on the Basis of a Building Block Design Approach. Compos. Part C Open Access 2021, 6, 100178. [Google Scholar] [CrossRef]
- Suzuki, T.; Takahashi, J. Prediction of Energy Intensity of Carbon Fiber Reinforced Plastics for Mass-Produced Passenger Cars. In Proceedings of the 9th Japan International SAMPE Symposium, Tokyo, Japan, 29 November 2005. [Google Scholar]
- Pimenta, S.; Pinho, S.T. Recycling Carbon Fibre Reinforced Polymers for Structural Applications: Technology Review and Market Outlook. Waste Manag. 2011, 31, 378–392. [Google Scholar] [CrossRef]
- Palmer, J.; Ghita, O.R.; Savage, L.; Evans, K.E. Successful Closed-Loop Recycling of Thermoset Composites. Compos. Part A Appl. Sci. Manuf. 2009, 40, 490–498. [Google Scholar] [CrossRef]
- Palmer, J.; Savage, L.; Ghita, O.R.; Evans, K.E. Sheet Moulding Compound (SMC) from Carbon Fibre Recyclate. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1232–1237. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Characterisation of Products from the Recycling of Glass Fibre Reinforced Polyester Waste by Pyrolysis. Fuel 2003, 82, 2223–2230. [Google Scholar] [CrossRef]
- Meyer, L.O.; Schulte, K.; Grove-Nielsen, E. CFRP-Recycling Following a Pyrolysis Route: Process Optimization and Potentials. J. Compos. Mater. 2009, 43, 1121–1132. [Google Scholar] [CrossRef]
- Witik, R.A.; Gaille, F.; Teuscher, R.; Ringwald, H.; Michaud, V.; Månson, J.-A.E. Economic and Environmental Assessment of Alternative Production Methods for Composite Aircraft Components. J. Clean. Prod. 2012, 29, 91–102. [Google Scholar] [CrossRef]
- Lefeuvre, A.; Yerro, X.; Jean-Marie, A.; Vo Dong, P.A.; Azzaro-Pantel, C. Modelling Pyrolysis Process for CFRP Recycling in a Closed-Loop Supply Chain Approach. In Computer Aided Chemical Engineering; Elsevier: Barcelona, Spain, 2017; Volume 40, pp. 2029–2034. ISBN 978-0-444-63965-3. [Google Scholar]
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical Recycling of Glass Fiber Reinforced Epoxy Resin Cured with Amine Using Nitric Acid. Polymer 2005, 46, 1905–1912. [Google Scholar] [CrossRef]
- Vogiantzi, C.; Tserpes, K. On the Definition, Assessment, and Enhancement of Circular Economy across Various Industrial Sectors: A Literature Review and Recent Findings. Sustainability 2023, 15, 16532. [Google Scholar] [CrossRef]
- Water—Specific Heat, vs. Temperature. Available online: https://www.engineeringtoolbox.com/specific-heat-capacity-water-d_660.html (accessed on 25 November 2024).
- Roux, M.; Eguémann, N.; Dransfeld, C.; Thiébaud, F.; Perreux, D. Thermoplastic Carbon Fibre-Reinforced Polymer Recycling with Electrodynamical Fragmentation: From Cradle to Cradle. J. Thermoplast. Compos. Mater. 2017, 30, 381–403. [Google Scholar] [CrossRef]
- Bluhm, H.; Bohme, R.; Frey, W.; Giese, H.; Hoppe, P.; Kessler, G.; Muller, G.; Neubert, N.; Rusch, D.; Schultheiss, C.; et al. Industrial Applications of High Voltage Pulsed Power Techniques: Developments at Forschungszentrum Karlsruhe (FZK). In Proceedings of the Digest of Technical Papers. 11th IEEE International Pulsed Power Conference (Cat. No.97CH36127), Baltimore, MD, USA, 29 June–2 July 1997; IEEE: Baltimore, MA, USA, 1997; Volume 1, pp. 1–12. [Google Scholar]
- Electricity Price Statistics. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Electricity_price_statistics (accessed on 27 November 2024).
- Rybicka, J.; Tiwari, A.; Leeke, G.A. Technology Readiness Level Assessment of Composites Recycling Technologies. J. Clean. Prod. 2016, 112, 1001–1012. [Google Scholar] [CrossRef]
- Utekar, S.; K, S.V.; More, N.; Rao, A. Comprehensive Study of Recycling of Thermosetting Polymer Composites—Driving Force, Challenges and Methods. Compos. Part B Eng. 2021, 207, 108596. [Google Scholar] [CrossRef]
- Dai, Y.; Liang, W.; Ye, D.; Xie, S.; Sang, Y.; Li, D. Modification Effects of Nanosilica on Asphalt Binders: A Review. Nanotechnol. Rev. 2023, 12, 20230138. [Google Scholar] [CrossRef]
- Chen, W.; Lin, B.; Li, D.; Zhang, J.; Cui, S. Progressive Collapse Performance of Shear Strengthened RC Frames by Nano CFRP. Nanotechnol. Rev. 2022, 11, 811–823. [Google Scholar] [CrossRef]
- Shehab, E.; Meiirbekov, A.; Amantayeva, A.; Suleimen, A.; Tokbolat, S.; Sarfraz, S.; Ali, M.H. A Fuzzy Logic-Based Cost Modelling System for Recycling Carbon Fibre Reinforced Composites. Polymers 2021, 13, 4370. [Google Scholar] [CrossRef] [PubMed]

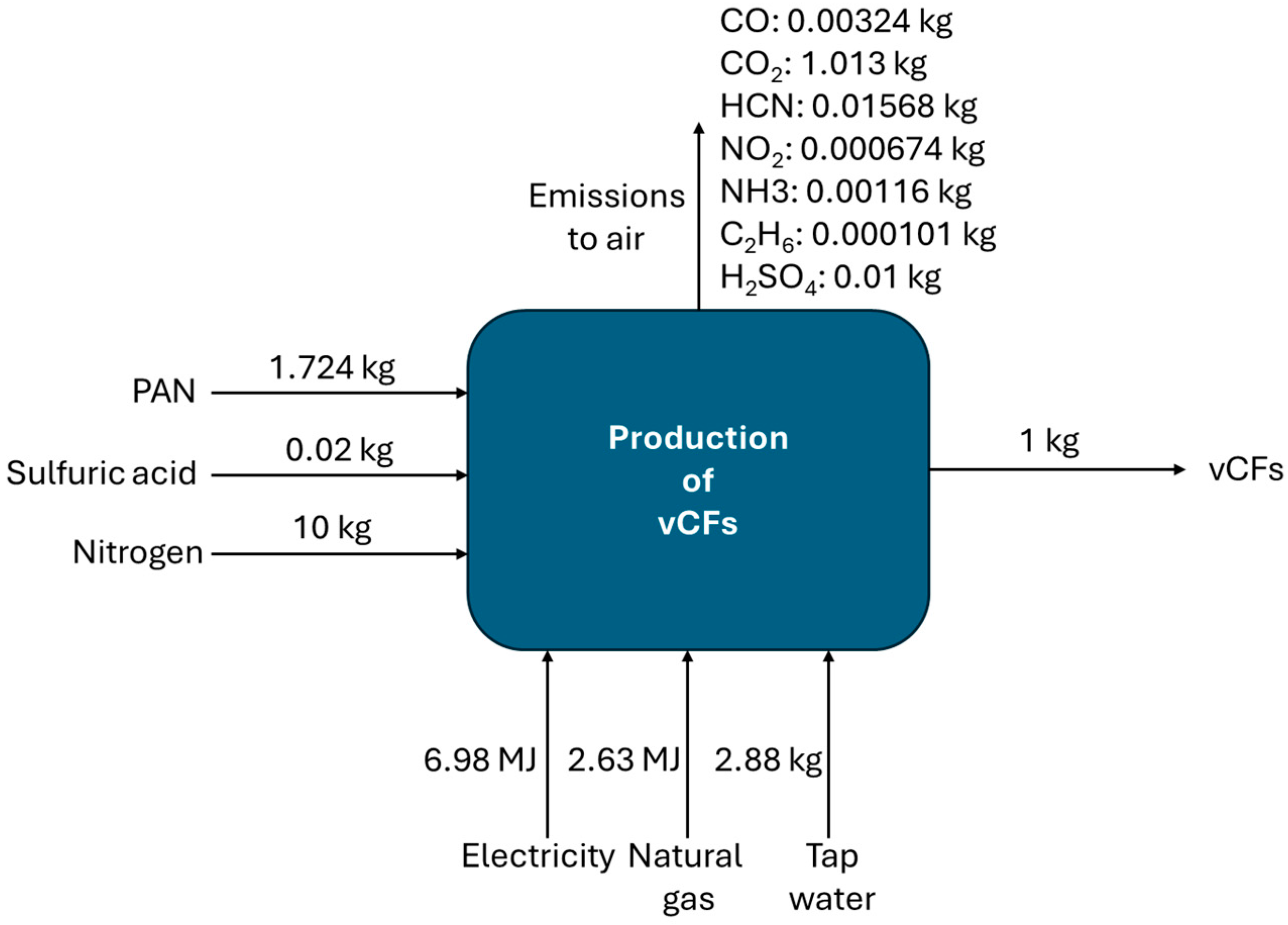





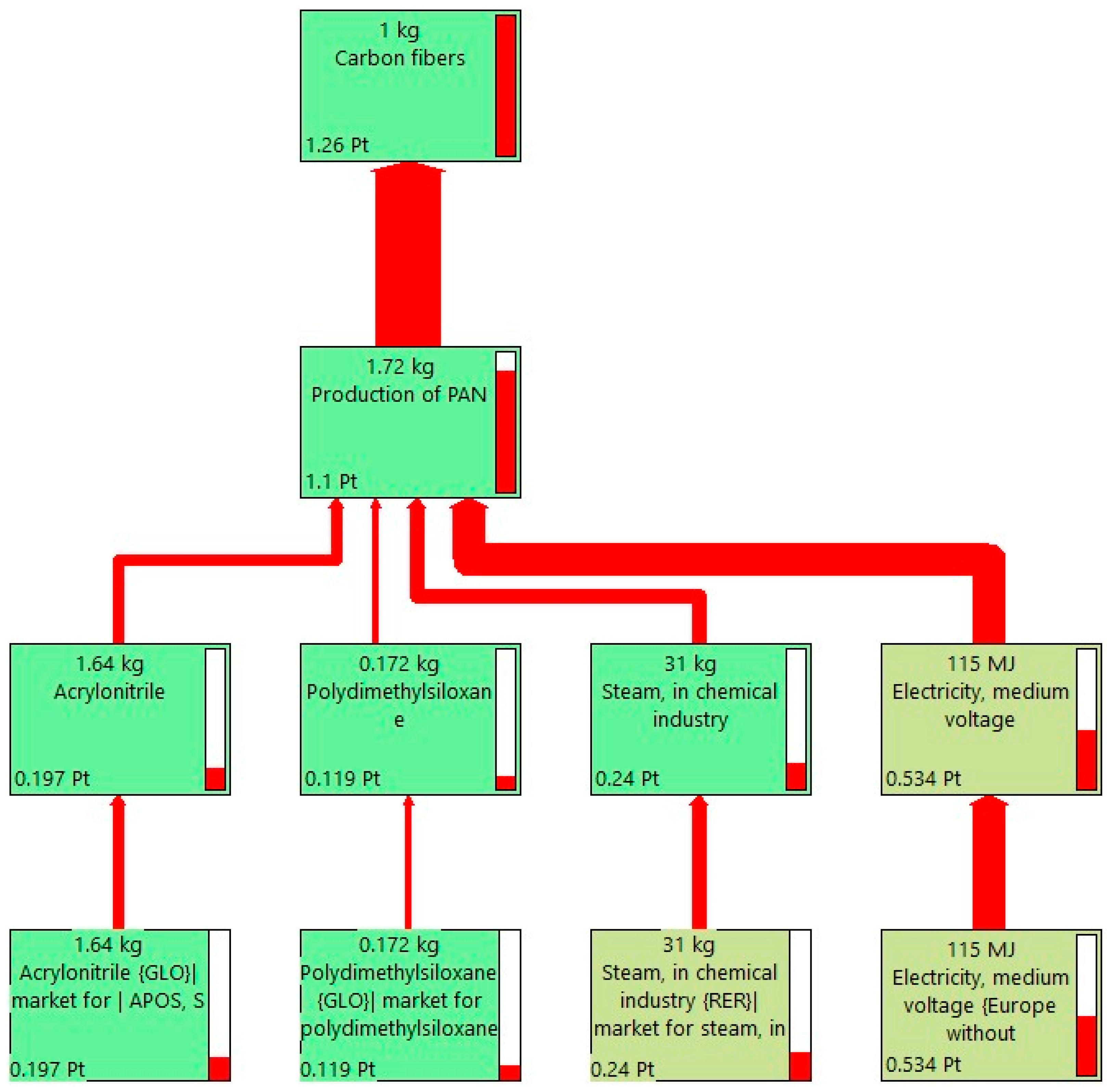
| Input | Quantity |
|---|---|
| Acrylonitrile | 0.95 kg |
| Methyl acrylate | 0.05 kg |
| Dimethylformamide solvent | 0.0061 kg |
| Polydimethylsiloxane | 0.1 kg |
| Electric energy | 66.87 MJ |
| Steam | 18 kg |
| Product | Material |
|---|---|
| Vacuum bag | Nylon 6-6 |
| Peel ply | Polyethylene terephthalate, granulate |
| Infusion mesh | Polypropylene, granulate |
| Tacky tape | Synthetic rubber |
| Aspiration tubes | Nylon 6-6 |
| Vacuum hose | Polypropylene, granulate |
| Valves, spiral tubing, etc. | Polypropylene, granulate |
| Recycling Process | CED (MJ/kg CFRP Waste) | GWP (kg CO2eq/kg CFRP Waste) |
|---|---|---|
| Mechanical recycling | 5.82 | 0.218 |
| Pyrolysis | 66.3 | 2.84 |
| Solvolysis-subcritical water | 49.8 | 1.87 |
| Solvolysis-supercritical water | 66.3 | 2.49 |
| HVF | 4.97 | 0.0796 |
| Input | Impact Category | Factor | Unit |
|---|---|---|---|
| UD production | Material costs | 246 | EUR/kg |
| Epoxy resin | Material costs | 100 | EUR/kg |
| Consumables | Material costs | 16.5 | EUR/kg |
| Electricity | Energy costs | 0.052 | EUR/MJ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogiantzi, C.; Tserpes, K. A Comparative Environmental and Economic Analysis of Carbon Fiber-Reinforced Polymer Recycling Processes Using Life Cycle Assessment and Life Cycle Costing. J. Compos. Sci. 2025, 9, 39. https://doi.org/10.3390/jcs9010039
Vogiantzi C, Tserpes K. A Comparative Environmental and Economic Analysis of Carbon Fiber-Reinforced Polymer Recycling Processes Using Life Cycle Assessment and Life Cycle Costing. Journal of Composites Science. 2025; 9(1):39. https://doi.org/10.3390/jcs9010039
Chicago/Turabian StyleVogiantzi, Christina, and Konstantinos Tserpes. 2025. "A Comparative Environmental and Economic Analysis of Carbon Fiber-Reinforced Polymer Recycling Processes Using Life Cycle Assessment and Life Cycle Costing" Journal of Composites Science 9, no. 1: 39. https://doi.org/10.3390/jcs9010039
APA StyleVogiantzi, C., & Tserpes, K. (2025). A Comparative Environmental and Economic Analysis of Carbon Fiber-Reinforced Polymer Recycling Processes Using Life Cycle Assessment and Life Cycle Costing. Journal of Composites Science, 9(1), 39. https://doi.org/10.3390/jcs9010039







