Design, Preparation, and Characterization of Polycaprolactone–Chitosan Nanofibers via Electrospinning Techniques for Efficient Methylene Blue Removal from Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
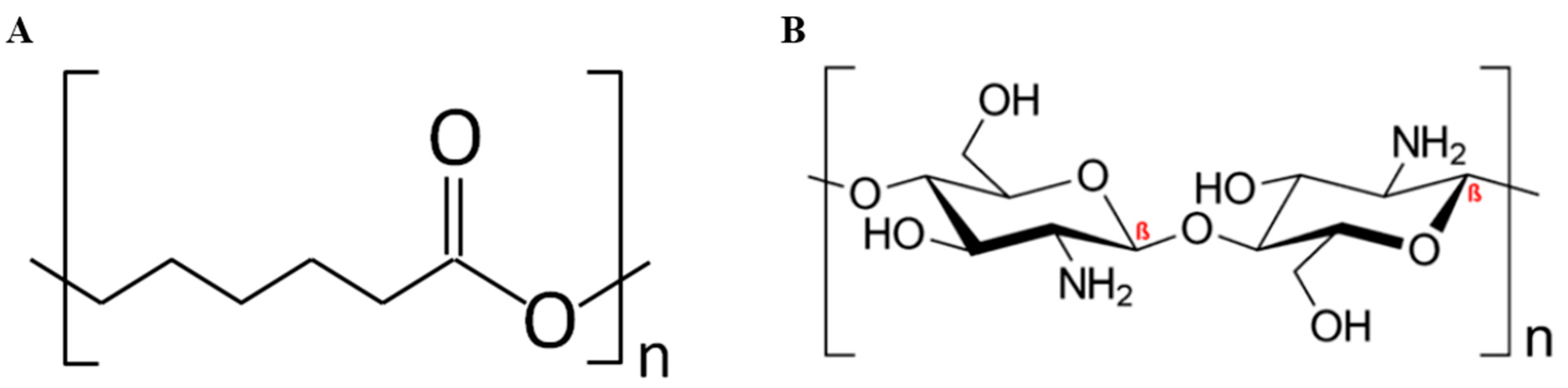
2.2. Preparation of PCL–CH Electrospinning Nanofibers
2.3. Physicochemical Characterization of Nanofibers
2.4. Zeta Potential
2.5. Methylene Blue (MB) Adsorption Study
3. Results and Discussion
3.1. Synthesis and Structural Characterization of PCL–CH Nanofibers
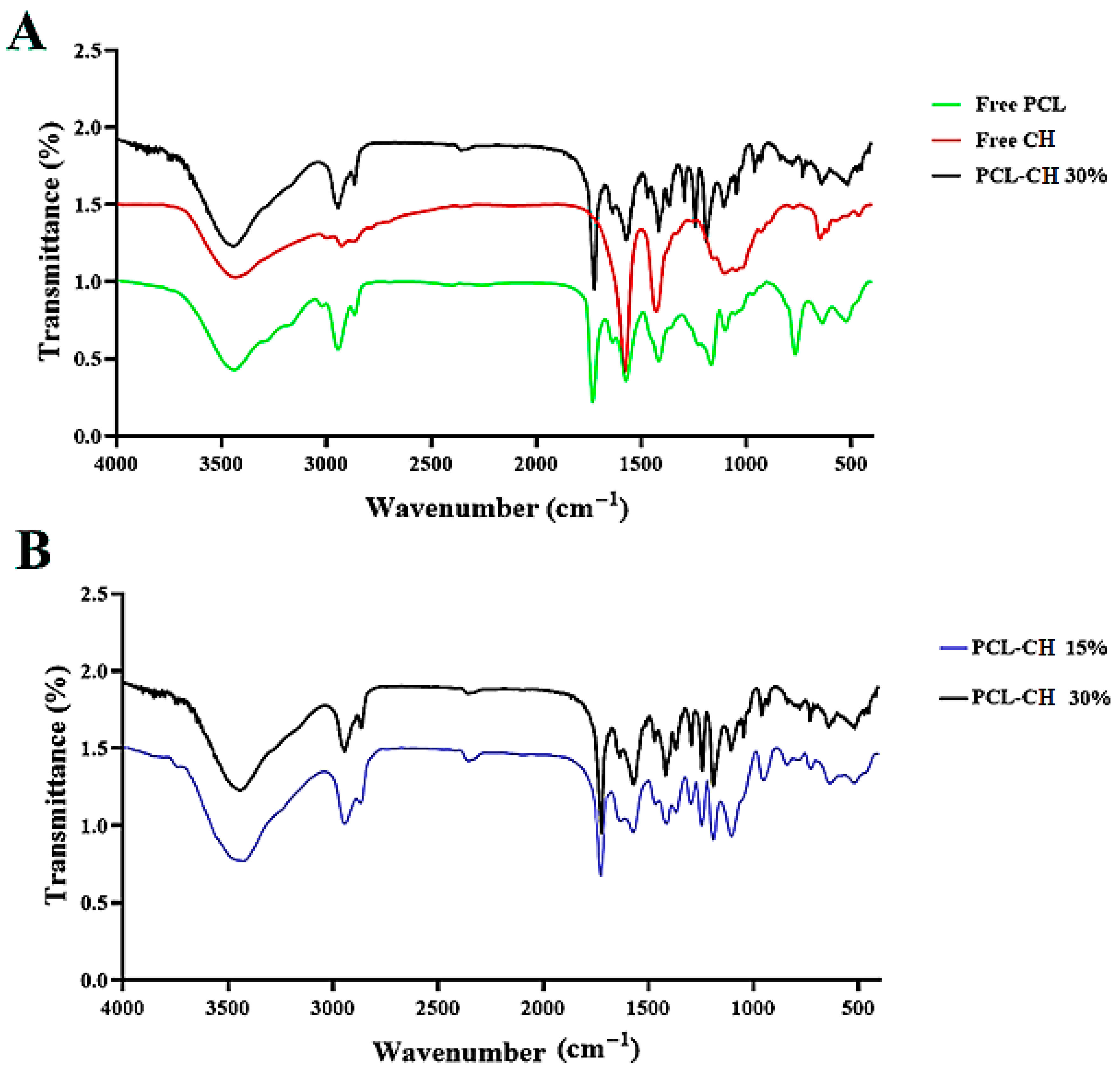
3.1.1. FT–IR Spectroscopy Study
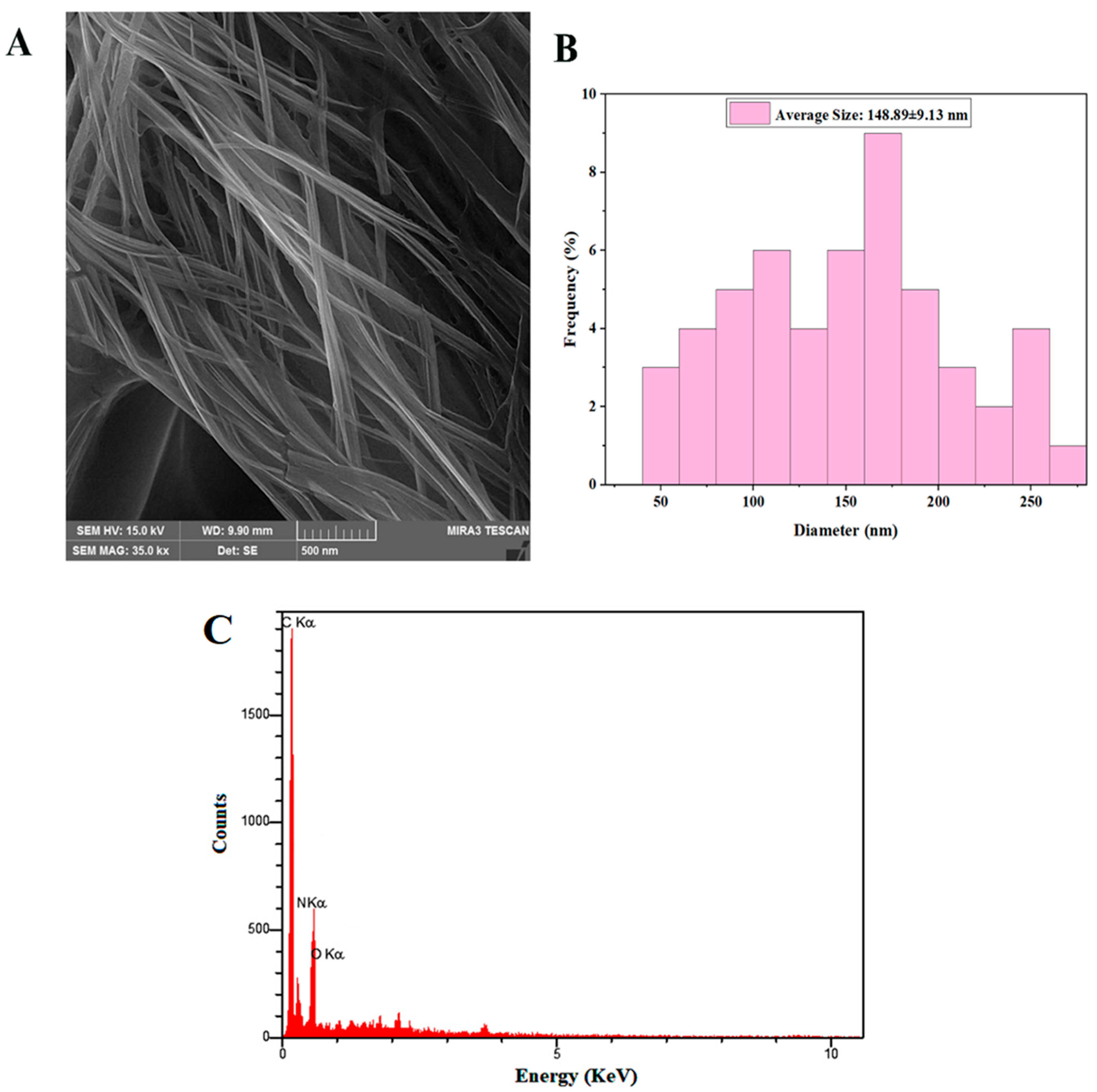
3.1.2. FESEM–EDS Analysis
3.1.3. Zeta Potential Characterization
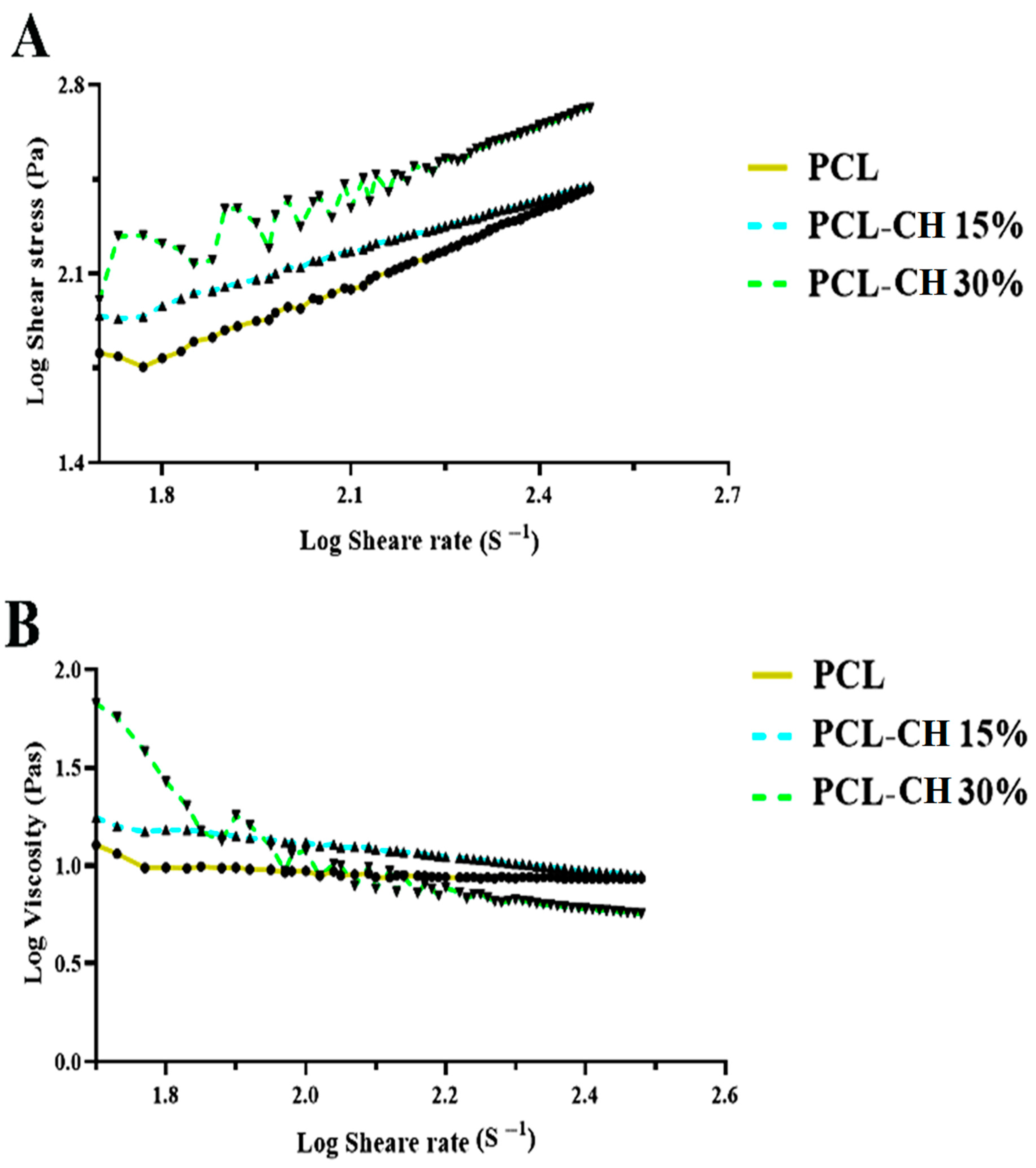
3.1.4. Rheological Behavior Analysis
3.2. MB Dye Adsorption Uptake
3.3. Effects of Original MB Solution pH and Concentration
3.4. Kinetic Analysis
3.5. Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean. Prod. 2020, 250, 119553. [Google Scholar] [CrossRef]
- Shanker, U.; Rani, M.; Jassal, V. Degradation of hazardous organic dyes in water by nanomaterials. Environ. Chem. Lett. 2017, 15, 623–642. [Google Scholar] [CrossRef]
- Fito, J.; Abewaa, M.; Mengistu, A.; Angassa, K.; Ambaye, A.D.; Moyo, W.; Nkambule, T. Adsorption of methylene blue from textile industrial wastewater using activated carbon developed from Rumex abyssinicus plant. Sci. Rep. 2023, 13, 5427. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Peng, Y.J.; Chen, C.M.; Li, Y.F.; Guo, Y.T.; Chen, Y.T.; Chao, K.H.; Yang, J.J. Patent blue versus methylene blue and indigo carmine as a better dye for chromodiscography: In vitro staining efficacy and cytotoxicity study using bovine coccygeal intervertebral discs. Spine J. 2023, 23, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Crossley, K. Methylene Blue-a therapeutic dye for all seasons? J. Chemother. 2002, 14, 431–443. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent Advances in the Treatment of Dye-Containing Wastewater from Textile Industries: Overview and Perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Pragya, B.; Antara, B.; Chandralekha, N.; Salini, S.; Megha, P.; Sandra, K.; Goutam, M.A.; Ramesh, W.U.; Kaviyarasi, R.; Balachandar, V.; et al. Recent advances in green technology and Industrial Revolution 4.0 for a sustainable future. Environ. Sci. Pollut. Res. Int. 2022, 30, 124488–124519. [Google Scholar]
- Haleem, A.; Shafiq, A.; Chen, S.-Q.; Nazar, M. A Comprehensive Review on Adsorption, Photocatalytic and Chemical Degradation of Dyes and Nitro-Compounds over Different Kinds of Porous and Composite Materials. Molecules 2023, 28, 1081. [Google Scholar] [CrossRef]
- Aldalbahi, A.; El-Naggar, M.E.; El-Newehy, M.H.; Rahaman, M.; Hatshan, M.R.; Khattab, T.A. Effects of Technical Textiles and Synthetic Nanofibers on Environmental Pollution. Polymers 2021, 13, 155. [Google Scholar] [CrossRef]
- Al-Kaabi, W.J.; Albukhaty, S.; Al-Fartosy, A.J.M.; Al-Karagoly, H.K.; Al-Musawi, S.; Sulaiman, G.M.; Dewir, Y.H.; Alwahibi, M.S.; Soliman, D.A. Development of Inula graveolens (L.) Plant Extract Electrospun/Polycaprolactone Nanofibers: A Novel Material for Biomedical Application. Appl. Sci. 2021, 11, 828. [Google Scholar] [CrossRef]
- Sogut, E.; Seydim, A.C.; Chiralt, A. Development of chitosan/cycloolefin copolymer and chitosan/polycaprolactone active bilayer films incorporated with grape seed extract and carvacrol. J. Polym. Res. 2021, 28, 319. [Google Scholar] [CrossRef]
- Alyamani, A.A.; Al-Musawi, M.H.; Albukhaty, S.; Sulaiman, G.M.; Ibrahim, K.M.; Ahmed, E.M.; Jabir, M.S.; Al-Karagoly, H.; Aljahmany, A.A.; Mohammed, M.K.A. Electrospun Polycaprolactone/Chitosan Nanofibers Containing Cordia myxa Fruit Extract as Potential Biocompatible Antibacterial Wound Dressings. Molecules 2023, 28, 2501. [Google Scholar] [CrossRef]
- Mandal, P.; Shunmugam, R. Polycaprolactone: A biodegradable polymer with its application in the field of self-assembly study. J. Macromol. Sci. A 2020, 58, 111–129. [Google Scholar] [CrossRef]
- Bakshia, P.S.; Selvakumara, D.; Kadirvelub, K.; Kumara, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Alves, D.C.d.S.; Healy, B.; Yu, T.; Breslin, C.B. Graphene-Based Materials Immobilized within Chitosan: Applications as Adsorbents for the Removal of Aquatic Pollutants. Materials 2021, 14, 3655. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.S.; Haase, H.; Mahltig, B. Chitosan-modified silica sol applications for the treatment of textile fabrics: A view on hydrophilic, antistatic and antimicrobial properties. J. Sol-Gel Sci. Technol. 2019, 91, 461–470. [Google Scholar] [CrossRef]
- Ghahremanzadeh, F.; Alihosseini, F.; Semnani, D. Investigation and comparison of new galactosylation methods on PCL/chitosan scaffolds for enhanced liver tissue engineering. Int. J. Biol. Macromol. 2021, 174, 278–288. [Google Scholar] [CrossRef]
- Lugoloobi, I.; Yuanhao, W.; Marriam, I.; Hu, J.; Tebyetekerwa, M.; Ramakrishna, S. Electrospun biomedical nanofibers and their future as intelligent biomaterials. Curr. Opin. Biomed. Eng. 2022, 24, 100418. [Google Scholar] [CrossRef]
- Albukhaty, S.; Al-Karagoly, H.; Allafchian, A.R.; Jalali, S.A.H.; Al-Kelabi, T.; Muhannad, M. Production and characterization of biocompatible nanofibrous scaffolds made of β-sitosterol loaded polyvinyl alcohol/tragacanth gum composites. Nanotechnology 2021, 33, 085102. [Google Scholar] [CrossRef]
- Chauhan, D.; Dwivedi, J.; Sankararamakrishnan, N. Novel chitosan/PVA/zerovalent iron biopolymeric nanofibers with enhanced arsenic removal applications. Environ. Sci. Pollut. Res. 2014, 21, 9430–9442. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, F.M.; Kaffashi, B.; Shokrollahi, P.; Seyedjafari, E.; Ardeshirylajimi, A. PCL/chitosan/Zn-doped nHA electrospun nanocomposite scaffold promotes adipose derived stem cells adhesion and proliferation. Carbohydr. Polym. 2015, 118, 133–142. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Alwahibi, M.S.; Dewir, Y.H.; Soliman, D.A.; Rizwana, H. Antibacterial Activity of Honey/Chitosan Nanofibers Loaded with Capsaicin and Gold Nanoparticles for Wound Dressing. Molecules 2020, 25, 4770. [Google Scholar] [CrossRef]
- Khan, G.; Yadav, S.K.; Patel, R.R.; Kumar, N.; Bansal, M.; Mishra, B. Tinidazole functionalized homogeneous electrospun chitosan/poly (ε-caprolactone) hybrid nanofiber membrane: Development, optimization and its clinical implications. Int. J. Biol. Macromol. 2017, 103, 1311–1326. [Google Scholar] [CrossRef] [PubMed]
- Pavan, F.A.; Lima, E.C.; Dias, S.L.P.; Mazzocato, A.C. Methylene blue biosorption from aqueous solutions by yellow passion fruit waste. J. Hazard. Mater. 2008, 150, 703–712. [Google Scholar] [CrossRef]
- Kahdim, Q.S.; Abdelmoula, N.; Al-Karagoly, H.; Albukhaty, S.; Al-Saaidi, J. Fabrication of a Polycaprolactone/Chitosan Nanofibrous Scaffold Loaded with Nigella sativa Extract for Biomedical Applications. BioTech 2023, 12, 19. [Google Scholar] [CrossRef]
- Eichhorn, S.; Dufresne, A.; Aranguren, M.; Marcovich, E.; Capadona, J.; Rowan, S.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibres and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Asghari, F.; Rabiei Faradonbeh, D.; Malekshahi, Z.V.; Nekounam, H.; Ghaemi, B.; Yousefpoor, Y.; Ghanbari, H.; Faridi-Majidi, R. Hybrid PCL/Chitosan-PEO Nanofibrous Scaffolds Incorporated with A. Euchroma Extract for Skin Tissue Engineering Application. Carbohydr. Polym. 2022, 278, 118926. [Google Scholar] [CrossRef]
- Miele, D.; Catenacci, L.; Rossi, S.; Sandri, G.; Sorrenti, M.; Terzi, A.; Giannini, C.; Riva, F.; Ferrari, F.; Caramella, C.; et al. Collagen/PCL Nanofibers Electrospun in Green Solvent by DOE Assisted Process. An Insight into Collagen Contribution. Materials 2020, 13, 4698. [Google Scholar] [CrossRef]
- Abasalta, M.; Asefnejad, A.; Khorasani, M.T.; Saadatabadi, A.R. Fabrication of carboxymethyl chitosan/poly(ε-caprolactone)/doxorubicin/nickel ferrite core-shell fibers for controlled release of doxorubicin against breast cancer. Carbohydr. Polym. 2021, 257, 117631. [Google Scholar] [CrossRef]
- Fadaie, M.; Mirzaei, E.; Geramizade, B.; Asvar, Z. Incorporation of nanofibrillated chitosan into electrospun PCL nanofibers makes scaffolds with enhanced mechanical and biological properties. Carbohydr. Polym. 2018, 199, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Aramesh, N.; Bagheri, A.R.; Bilal, M. Chitosan-Based Hybrid Materials for Adsorptive Removal of Dyes and Underlying Interaction Mechanisms. Int. J. Biol. Macromol. 2021, 183, 399–422. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Wang, Y. Recent Developments of Electrospun Nanofibrous Materials as Novel Adsorbents for Water Treatment. Mater. Today Commun. 2021, 27, 102272. [Google Scholar] [CrossRef]
- Kozehkonan, G.S.; Salehi, M.; Farzamfar, S.; Ghanbari, H.; Adabi, M.; Amani, A. Preparation and characterization of PCL polymeric scaffolds coated with chitosan/bioactive glass/gelatin nanoparticles using the tips methodology for bone tissue engineering. Nanomed. J. 2019, 6, 311–320. [Google Scholar]
- Agrawal, S.; Ranjan, R.; Lal, B.; Rahman, A.; Singh, S.P.; Selvaratnam, T.; Nawaz, T. Synthesis and Water Treatment Applications of Nanofibers by Electrospinning. Processes 2021, 9, 1779. [Google Scholar] [CrossRef]
- Tang, X.; Ran, G.; Li, J.; Zhang, Z.; Xiang, C. Extremely efficient and rapidly adsorb methylene blue using porous adsorbent prepared from waste paper: Kinetics and equilibrium studies. J. Hazard. Mater. 2021, 402, 123579. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Shi, Q.; Feng, J.; Yao, J.; Huang, H.; Xie, X. Adsorption Behaviors of Cationic Methylene Blue and Anionic Reactive Blue 19 Dyes onto Nano-Carbon Adsorbent Carbonized from Small Precursors. Nanomaterials 2022, 12, 1814. [Google Scholar] [CrossRef]
- Shahsavar, F.; Babaei, A. Investigating the effect of chitosan functionalized graphene oxide on the performance of biodegradable polycaprolactone. J. Reinf. Plast. Compos. 2023. [Google Scholar] [CrossRef]
- Mosallanezhad, P.; Nazockdast, H.; Ahmadi, Z.; Rostami, A. Fabrication and characterization of polycaprolactone/chitosan nanofibers containing antibacterial agents of curcumin and ZnO nanoparticles for use as wound dressing. Front. Bioeng. Biotechnol. 2022, 10, 1797. [Google Scholar] [CrossRef]
- Modiri-Delshad, T.; Ramazani, A.; Khoobi, M.; Akbari Javar, H.; Akbari, T.; Amin, M. Fabrication of chitosan/polycaprolactone/Myrtus communis L. extract nanofibrous mats with enhanced antibacterial activities. Polym. Polym. Compos. 2023, 31. [Google Scholar] [CrossRef]
- Kayan, G.Ö.; Kayan, A. Polycaprolactone Composites/Blends and Their Applications Especially in Water Treatment. ChemEngineering 2023, 7, 104. [Google Scholar] [CrossRef]
- Martínez, M.E.; Rangel-Méndez, J.R.; Gimeno, M.; Tecante, A.; Lapidus, G.T.; Shirai, K. Removal of Heavy Metal Ions from Wastewater with Poly-ε-Caprolactone-Reinforced Chitosan Composite. Polymers 2022, 14, 5196. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Z.; Chen, B.; Gu, L.; Li, Y.; Yu, S. Design and evaluation of galactosylated chitosan/graphene oxide nanoparticles as a drug delivery system. J. Colloid Interface Sci. 2018, 516, 332–341. [Google Scholar] [CrossRef]
- Heidari, M.; Bahrami, S.H.; Ranjbar-Mohammadi, M.; Milan, P.B. Smart electrospun nanofibers containing PCL/gelatin/graphene oxide for application in nerve tissue engineering. Mater. Sci. Eng. C 2019, 103, 109768. [Google Scholar] [CrossRef]
- Fu, J.; Chen, Z.; Wang, M.; Liu, S.; Zhang, J.; Zhang, J.; Han, R.; Xu, Q. Adsorption of methylene blue by a high-efficiency adsorbent (polydopamine microspheres): Kinetics, isotherm, thermodynamics and mechanism analysis. Chem. Eng. J. 2015, 259, 53–61. [Google Scholar] [CrossRef]
- Zhang, T.J.; Xiao, S.Y.; Fan, K.H.; He, H.; Qin, Z.Y. Preparation and adsorption properties of green cellulose-based composite aerogel with selective adsorption of methylene blue. Polymer 2022, 258, 125320. [Google Scholar] [CrossRef]
- El-Bery, H.M.; Saleh, M.; El-Gendy, R.A.; Saleh, M.R.; Thabet, S.M. High adsorption capacity of phenol and methylene blue using activated carbon derived from lignocellulosic agriculture wastes. Sci. Rep. 2022, 12, 5499. [Google Scholar] [CrossRef]
- Cheng, J.; Zhan, C.; Wu, J.; Cui, Z.; Si, J.; Wang, Q.; Peng, X.; Turng, L.S. Highly Efficient Removal of Methylene Blue Dye from an Aqueous Solution Using Cellulose Acetate Nanofibrous Membranes Modified by Polydopamine. ACS Omega 2020, 5, 5389–5400. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef]
- Taleb, F.; Ammar, M.; Mosbah, M.B.; Salem, R.B.; Moussaoui, Y. Chemical modification of lignin derived from spent coffee grounds for methylene blue adsorption. Sci. Rep. 2020, 10, 11048. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Goswami, M.; Borah, L.; Mahanta, D.; Phukan, P. Equilibrium modeling, kinetic and thermodynamic studies on the adsorption of Cr(VI) using activated carbon derived from matured tea leaves. J. Porous Mater. 2014, 21, 1025–1034. [Google Scholar] [CrossRef]




| Kinetic Analysis | ||||
|---|---|---|---|---|
| Model | Curve Equation | Parameters | ||
| pseudo-first-order | y = −0.1249x + 1.8114 | R2 = 0.9510 | K1 = 0.1249 | qe = 64.774 |
| pseudo-second-order | y = 0.0099x + 0.011 | R2 = 0.9989 | K2 = 0.0089 | qe = 101.010 |
| Adsorption Isotherm | ||||
| Langmuir | y = 0.0544 − 2.399 | R2 = 0.9556 | KL = 0.0226 | qm = 18.382 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, H.M.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M. Design, Preparation, and Characterization of Polycaprolactone–Chitosan Nanofibers via Electrospinning Techniques for Efficient Methylene Blue Removal from Aqueous Solutions. J. Compos. Sci. 2024, 8, 68. https://doi.org/10.3390/jcs8020068
Saleh HM, Albukhaty S, Sulaiman GM, Abomughaid MM. Design, Preparation, and Characterization of Polycaprolactone–Chitosan Nanofibers via Electrospinning Techniques for Efficient Methylene Blue Removal from Aqueous Solutions. Journal of Composites Science. 2024; 8(2):68. https://doi.org/10.3390/jcs8020068
Chicago/Turabian StyleSaleh, Hind M., Salim Albukhaty, Ghassan M. Sulaiman, and Mosleh M. Abomughaid. 2024. "Design, Preparation, and Characterization of Polycaprolactone–Chitosan Nanofibers via Electrospinning Techniques for Efficient Methylene Blue Removal from Aqueous Solutions" Journal of Composites Science 8, no. 2: 68. https://doi.org/10.3390/jcs8020068
APA StyleSaleh, H. M., Albukhaty, S., Sulaiman, G. M., & Abomughaid, M. M. (2024). Design, Preparation, and Characterization of Polycaprolactone–Chitosan Nanofibers via Electrospinning Techniques for Efficient Methylene Blue Removal from Aqueous Solutions. Journal of Composites Science, 8(2), 68. https://doi.org/10.3390/jcs8020068










