A Critical Review of Natural and Synthetic Polymer-Based Biological Apatite Composites for Bone Tissue Engineering
Abstract
1. Introduction
2. Biopolymers Used in Tissue Engineering
2.1. Natural Polymers
2.2. Synthetic Biopolymers
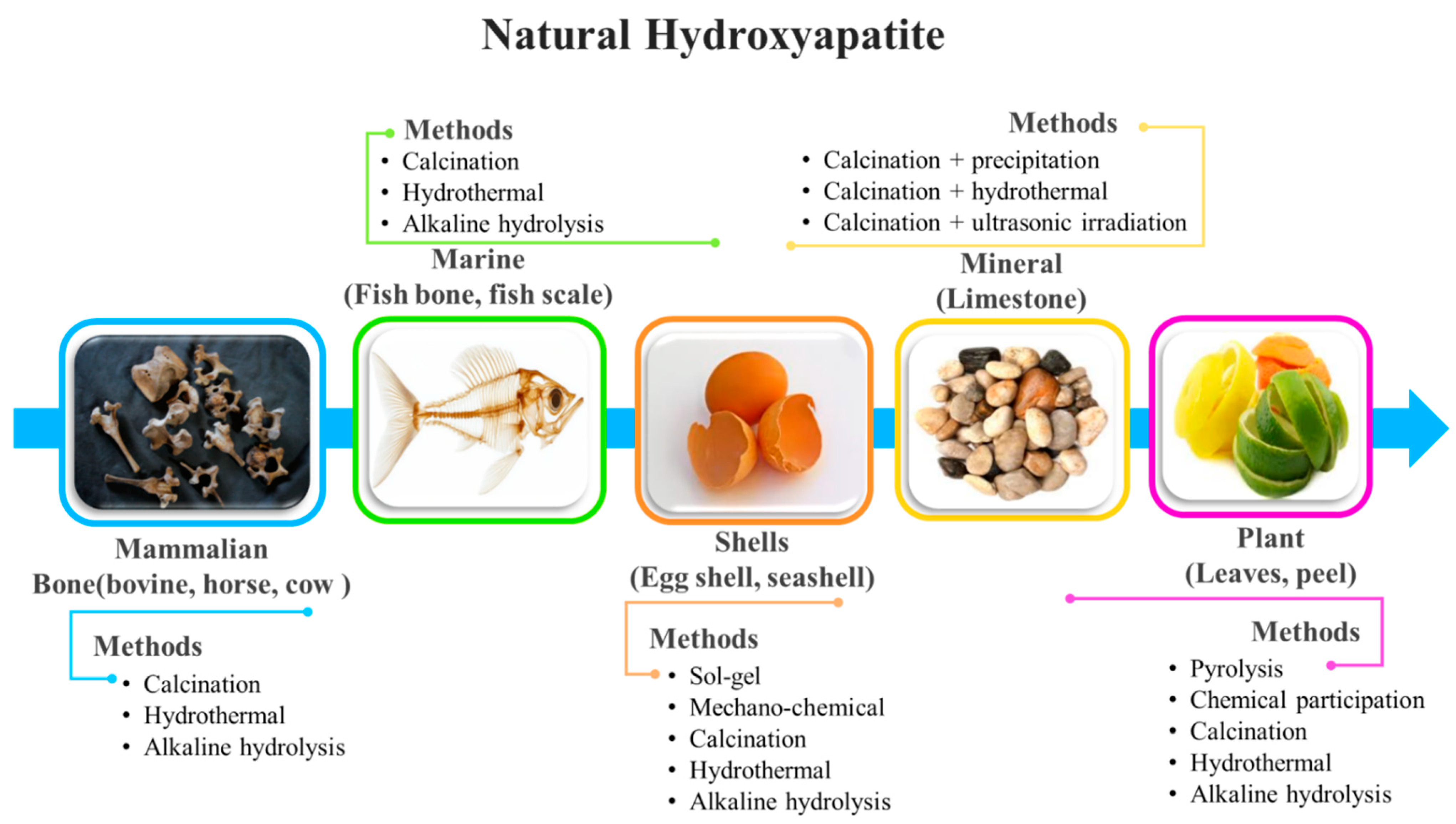
3. Hydroxyapatite as Bioactive Agent in Biocomposites
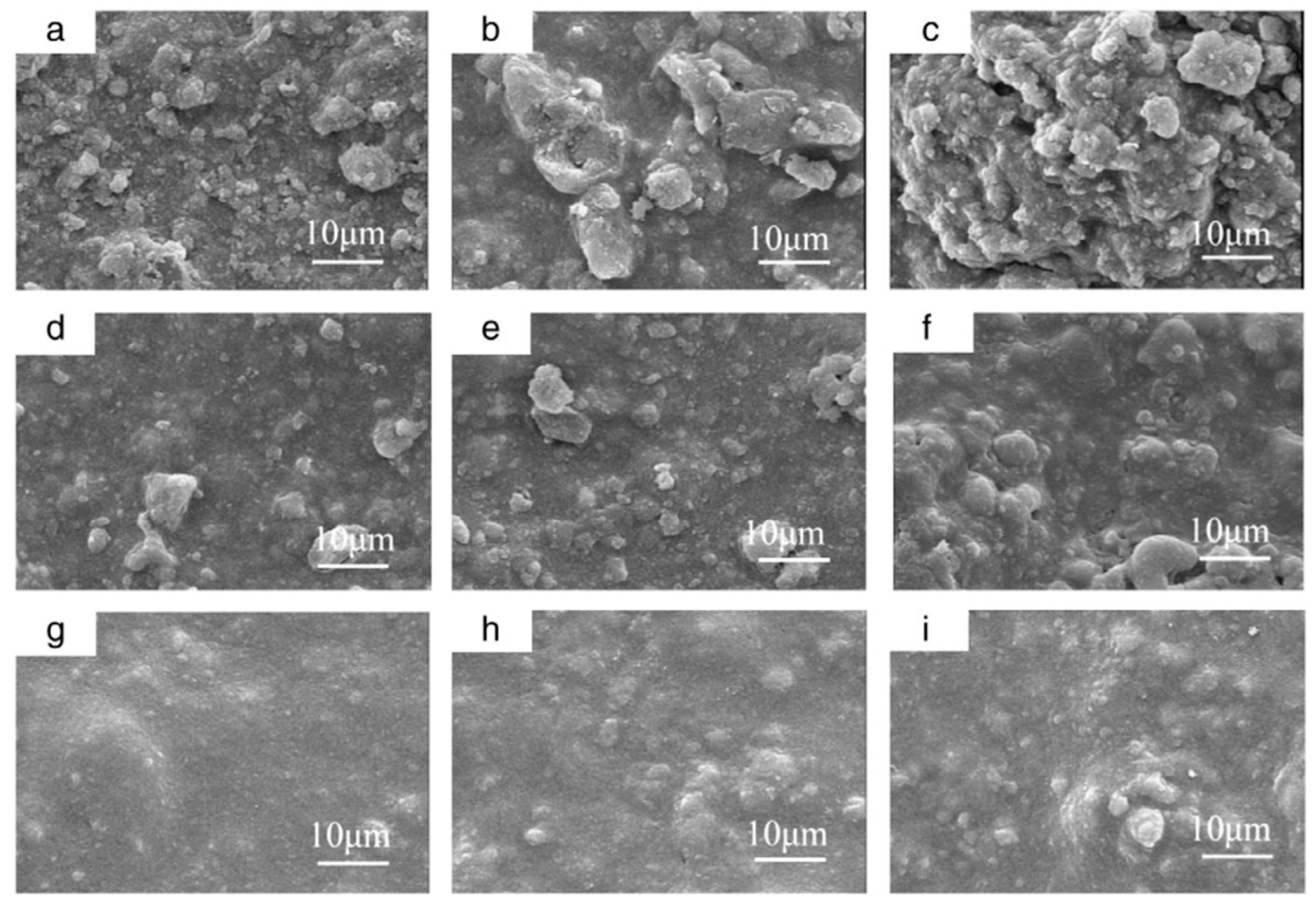
Hydroxyapatite–Polymer Composites for Bone-Tissue Engineering
4. Fabrication of Hydroxyapatite–Polymer Composites
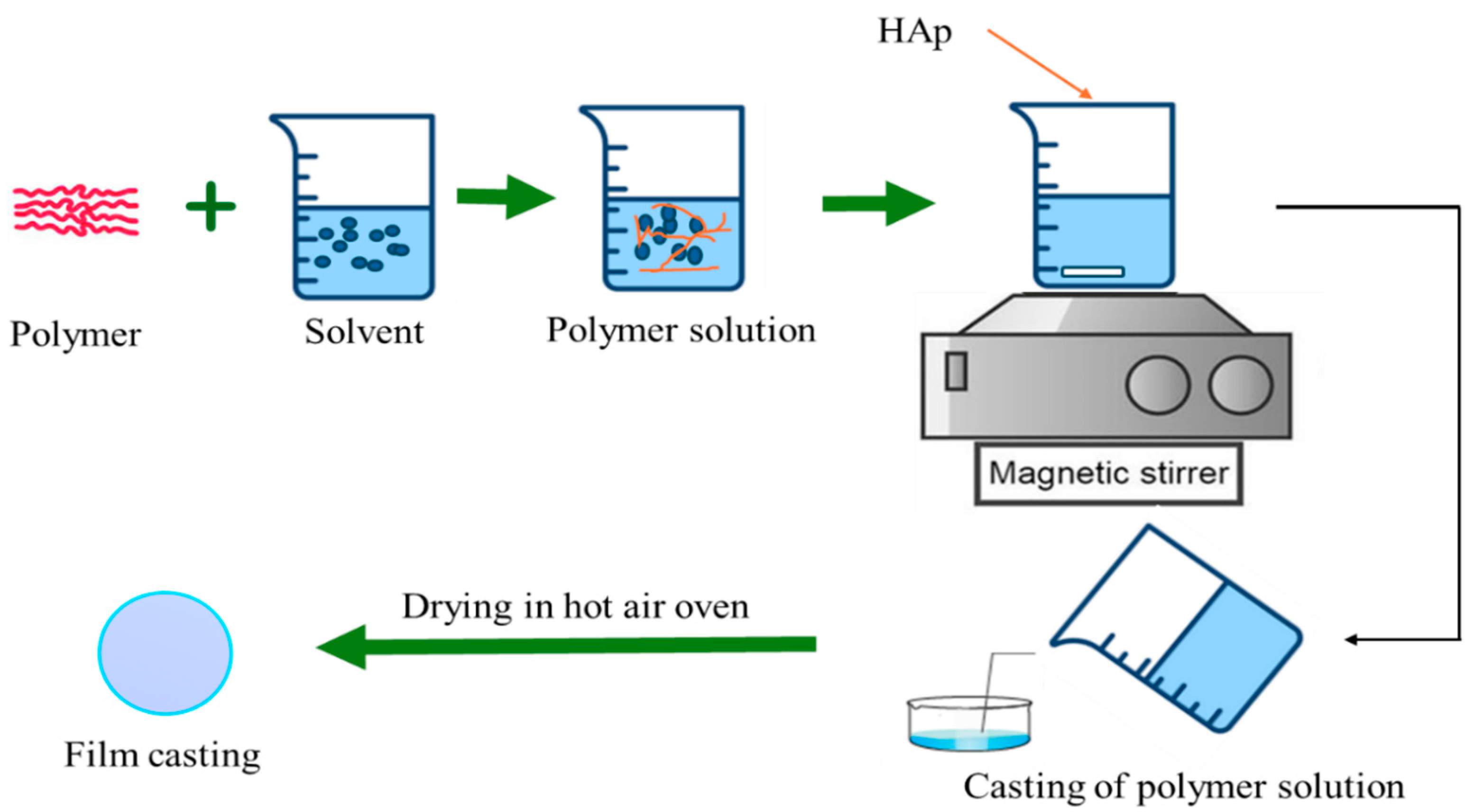
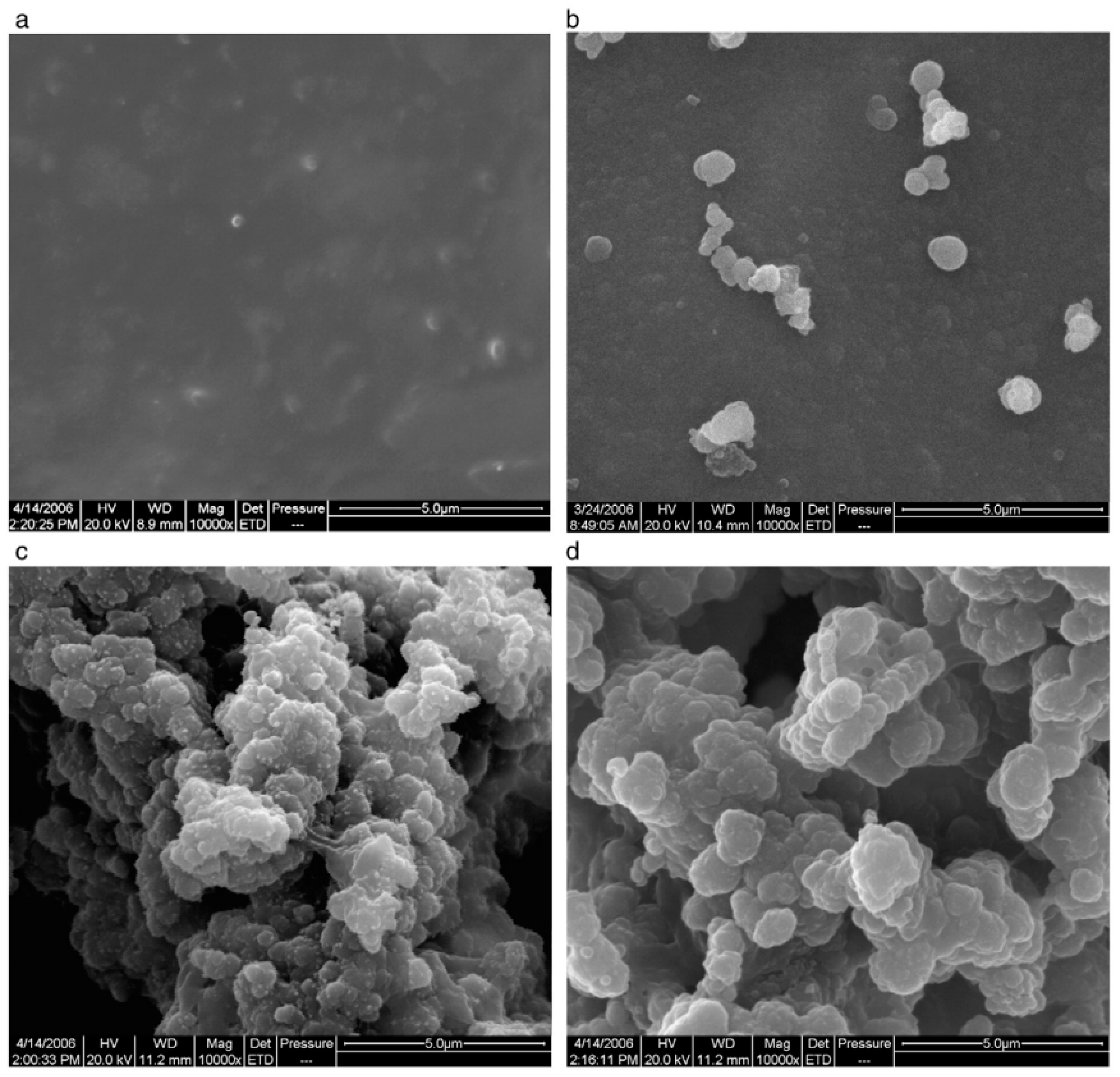
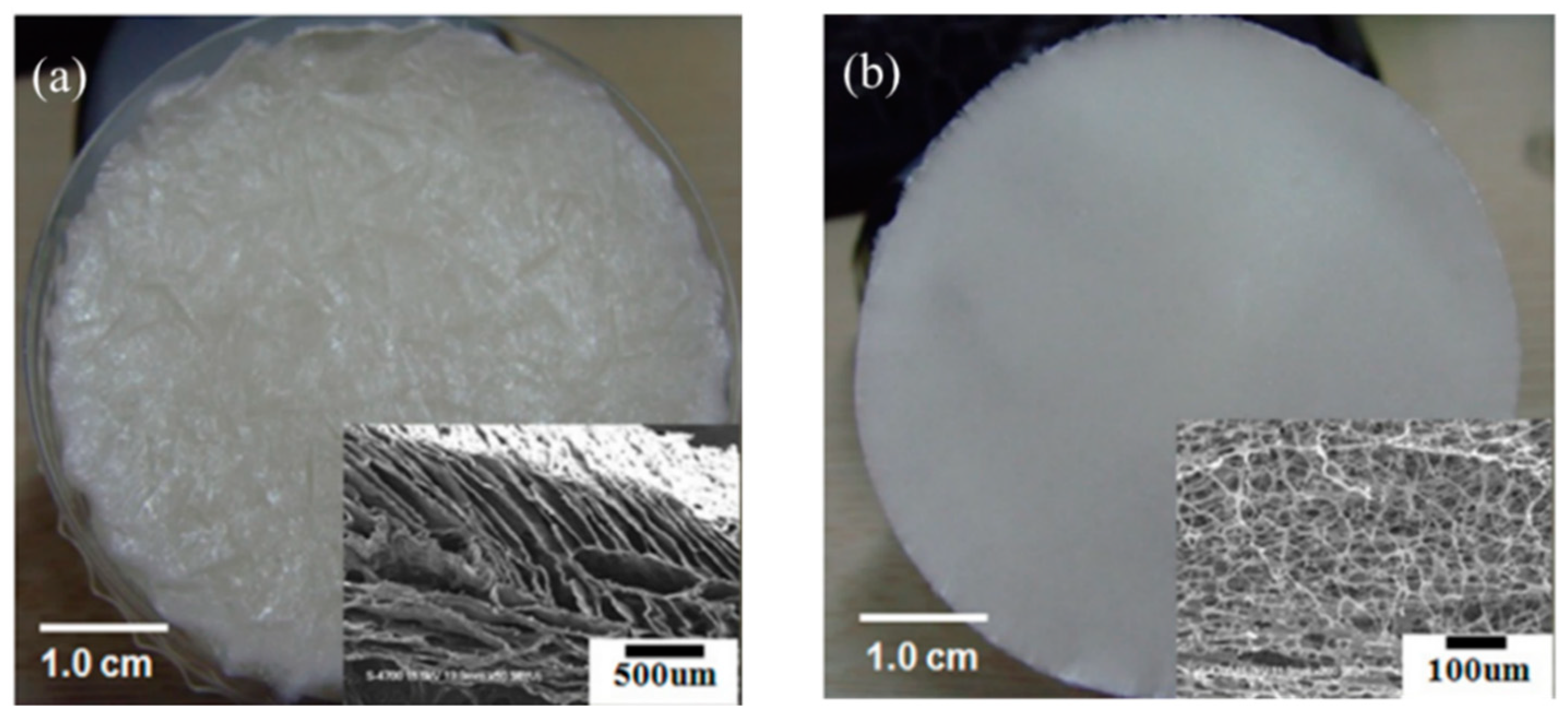
4.1. Solvent/Solution Casting Method
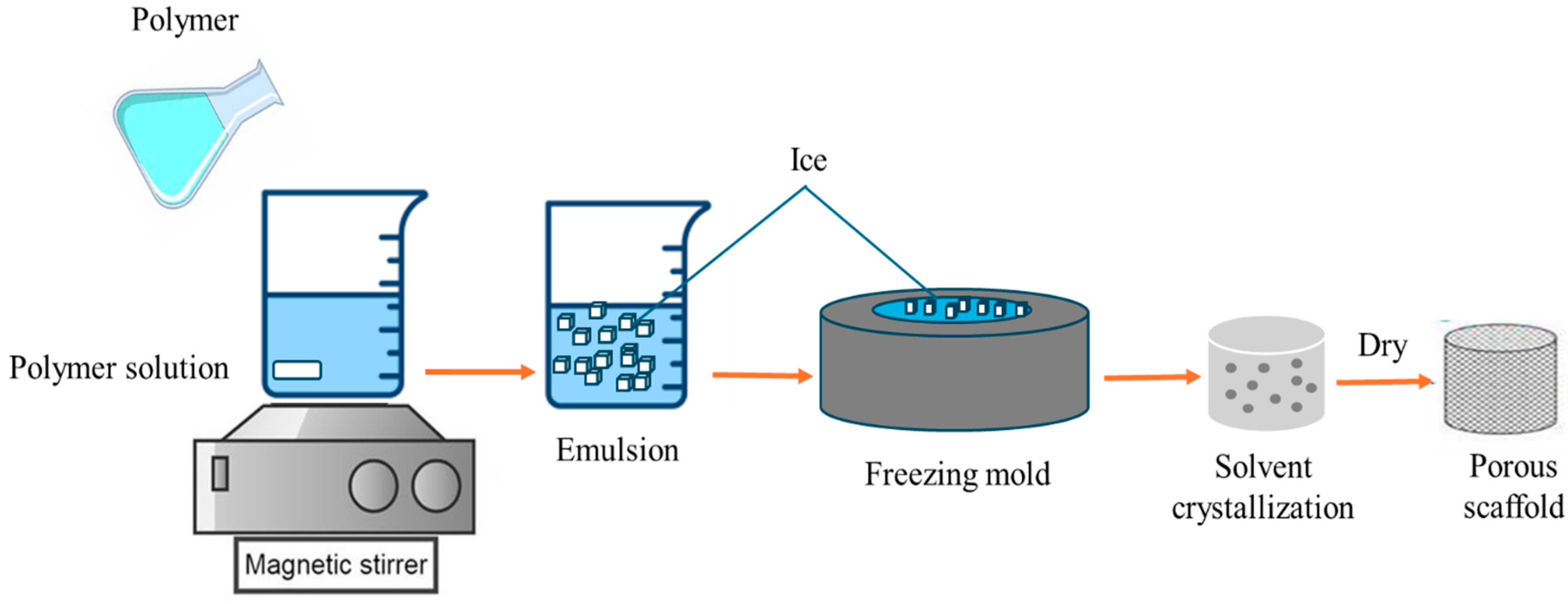
4.2. Freeze-Drying Methods
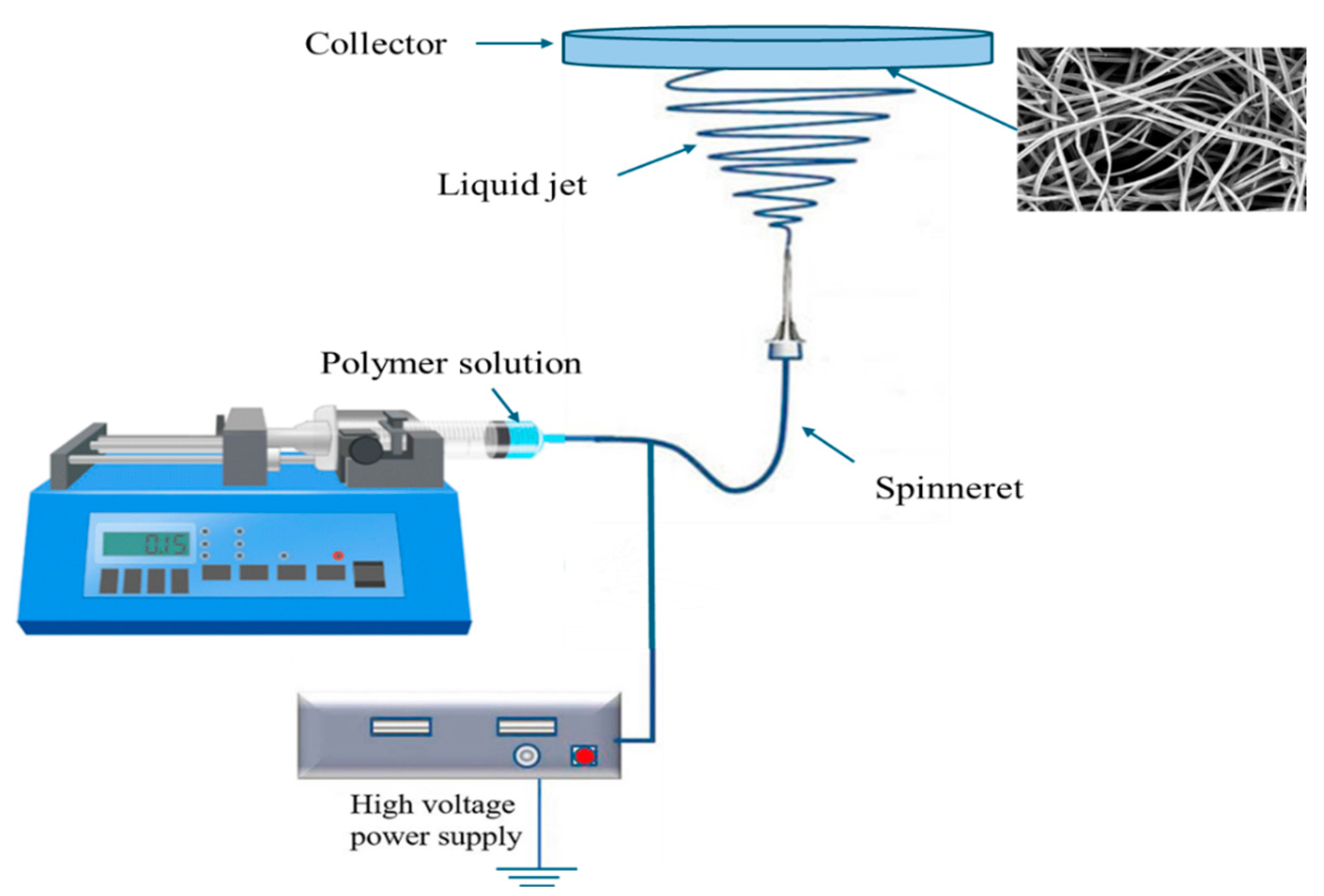
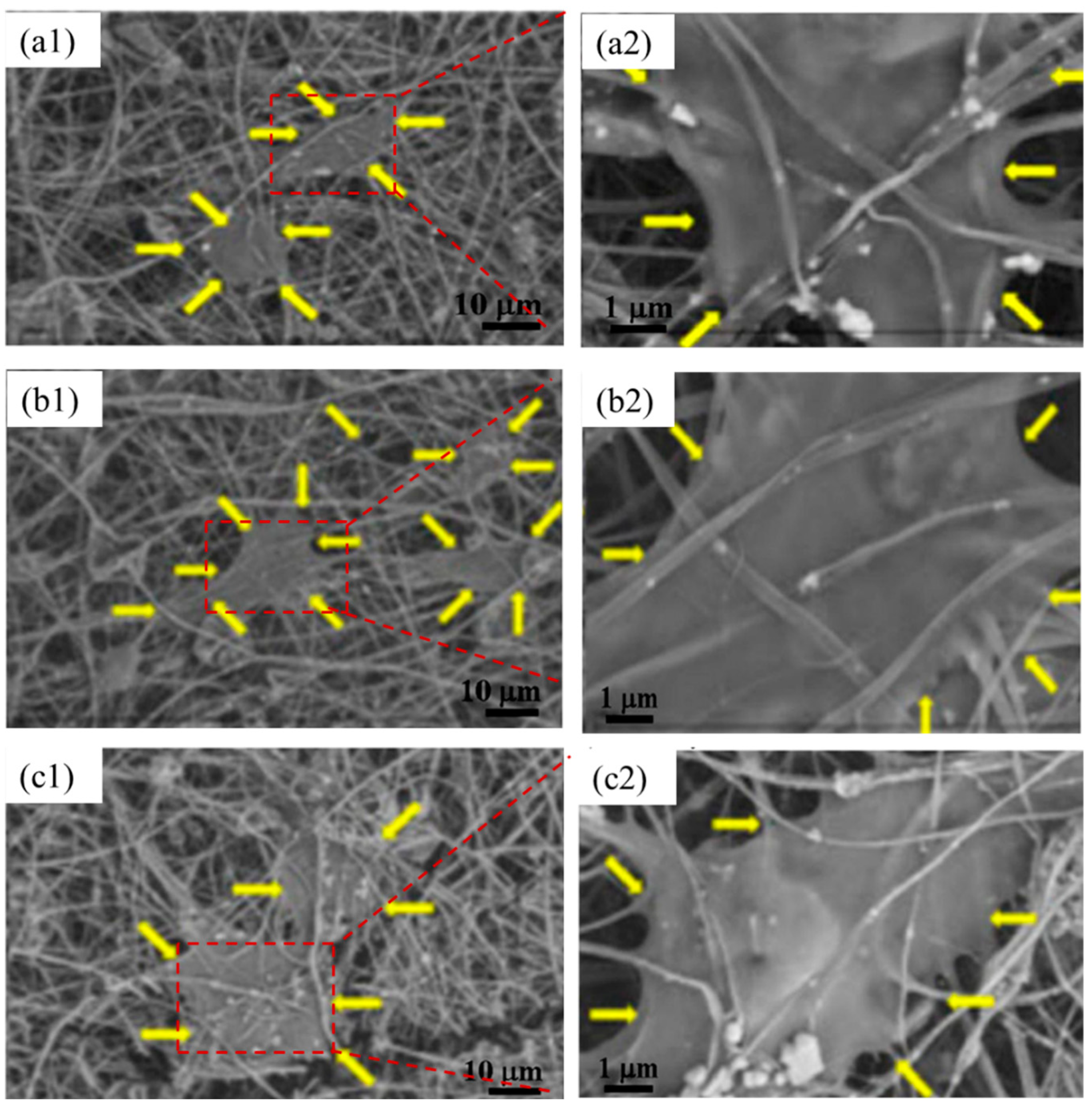
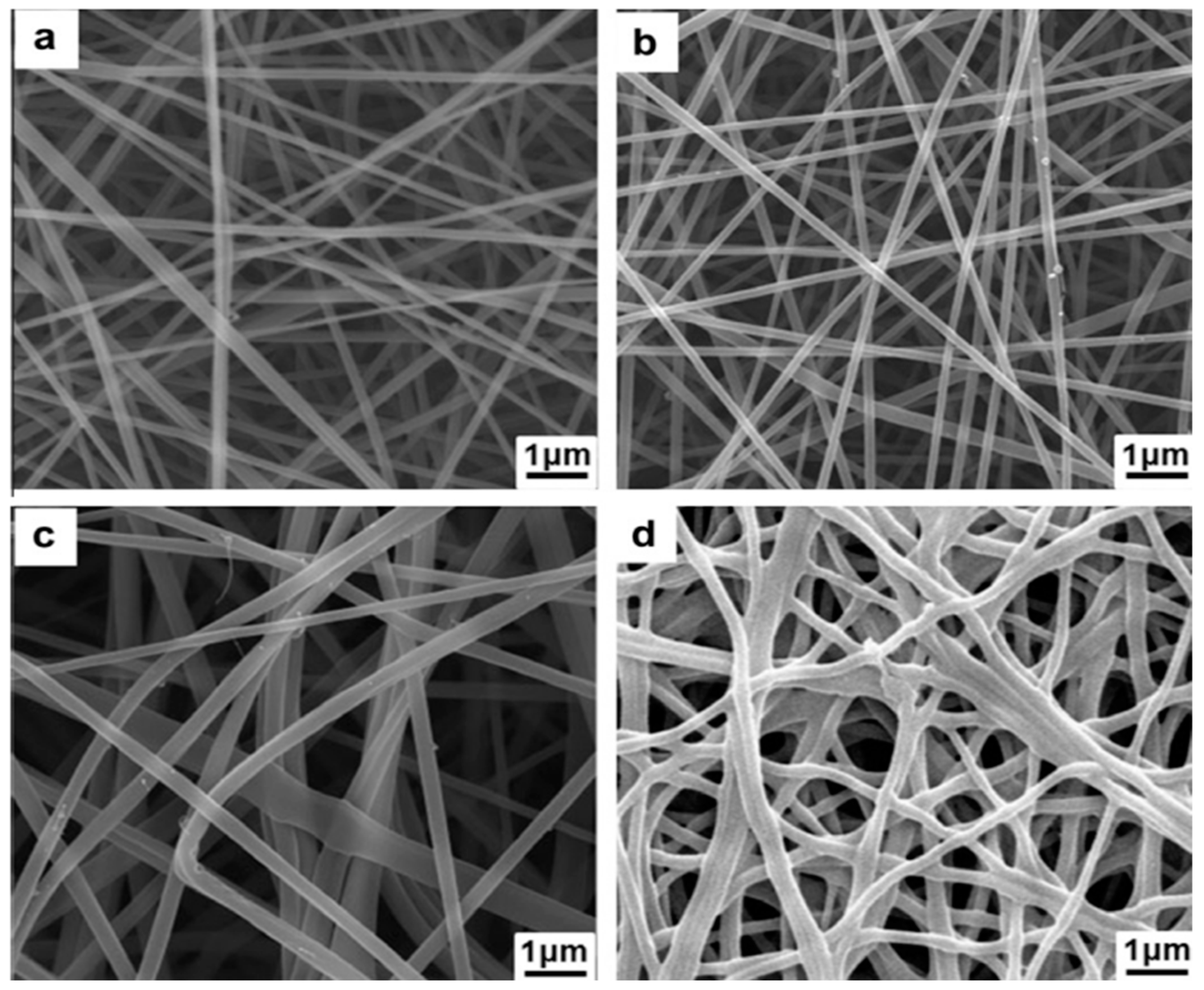
4.3. Electrospinning
4.4. 3D Printing of Microstructure Composites
4.5. Electrodeposition
5. Conclusions and Future Direction
Funding
Data Availability Statement
Conflicts of Interest
References
- Amiryaghoubi, N.; Fathi, M.; Pesyan, N.N.; Samiei, M.; Barar, J.; Omidi, Y. Bioactive polymeric scaffolds for osteogenic repair and bone regenerative medicine. Med. Res. Rev. 2020, 40, 1833–1870. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Kharche, S.; More, N.; Ranglani, D.; Singh, G.; Kapusetti, G. Natural Biopolymers for Bone Tissue Engineering: A Brief Review. Eng. Regen. 2023, 4, 193–204. [Google Scholar] [CrossRef]
- Deng, J.; Song, Q.; Liu, S.; Pei, W.; Wang, P.; Zheng, L.; Huang, C.; Ma, M.; Jiang, Q.; Zhang, K. Advanced applications of cellulose-based composites in fighting bone diseases. Compos. Part B Eng. 2022, 245, 110221. [Google Scholar] [CrossRef]
- Bothe, F.; Lotz, B.; Seebach, E.; Fischer, J.; Hesse, E.; Diederichs, S.; Richter, W. Stimulation of calvarial bone healing with human bone marrow stromal cells versus inhibition with adipose-tissue stromal cells on nanostructured β-TCP-collagen. Acta Biomater. 2018, 76, 135–145. [Google Scholar] [CrossRef]
- Idumah, C.I. Progress in polymer nanocomposites for bone regeneration and engineering. Polym. Polym. Compos. 2021, 29, 509–527. [Google Scholar] [CrossRef]
- Ferraz, M.P. Bone Grafts in Dental Medicine: An Overview of Autografts, Allografts and Synthetic Materials. Materials 2023, 16, 4117. [Google Scholar] [CrossRef]
- Eldeeb, A.E.; Salah, S.; Elkasabgy, N.A. Biomaterials for Tissue Engineering Applications and Current Updates in the Field: A Comprehensive Review. AAPS PharmSciTech 2022, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.M. Recent trends on biomaterials for tissue regeneration applications: Review. J. Mater. Sci. 2023, 58, 527–558. [Google Scholar] [CrossRef]
- Schatkoski, V.M.; do Amaral Montanheiro, T.L.; de Menezes, B.R.C.; Pereira, R.M.; Rodrigues, K.F.; Ribas, R.G.; da Silva, D.M.; Thim, G.P. Current advances concerning the most cited metal ions doped bioceramics and silicate-based bioactive glasses for bone tissue engineering. Ceram. Int. 2021, 47, 2999–3012. [Google Scholar] [CrossRef]
- Zhou, Y.; Chyu, J.; Zumwalt, M. Recent Progress of Fabrication of Cell Scaffold by Electrospinning Technique for Articular Cartilage Tissue Engineering. Int. J. Biomater. 2018, 2018, 1953636. [Google Scholar] [CrossRef]
- Chasan, S.; Hesse, E.; Atallah, P.; Gerstner, M.; Diederichs, S.; Schenker, A.; Grobe, K.; Werner, C.; Richter, W. Sulfation of Glycosaminoglycan Hydrogels Instructs CellFate and Chondral versus Endochondral Lineage Decisionof Skeletal Stem Cells In Vivo, Adv. Funct. Mater. 2022, 32, 2109176. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Chen, X.; Gao, Y.; Wang, W.; Zhuang, X.; Zhang, H.; Dong, X. Bone tissue engineering scaffold materials: Fundamentals, advances, and challenges. Chin. Chem. Lett. 2024, 35, 109197. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A.; Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 3429527. [Google Scholar] [CrossRef]
- Hutmacher, D.W.; Tandon, B.; Dalton, P.D. Scaffold design and fabrication. In Tissue Engineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 355–385. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Kazimierczak, P.; Wessely-Szponder, J.; Palka, K.; Barylyak, A.; Zinchenko, V.; Przekora, A. Hydroxyapatite or Fluorapatite—Which Bioceramic Is Better as a Base for the Production of Bone Scaffold?—A Comprehensive Comparative Study. Int. J. Mol. Sci. 2023, 24, 5576. [Google Scholar] [CrossRef]
- Kokubo, T. Apatite formation on surfaces of ceramics, metals and polymers in body environment. Acta Mater. 1998, 46, 2519–2527. [Google Scholar] [CrossRef]
- Czka, M.; Cholewa-Kowalska, K.; Osyczka, A.M. Bioactivity and osteoinductivity of glasses and glassceramics and their material determinants. Ceram. Int. 2016, 42, 14313–14325. [Google Scholar] [CrossRef]
- ElHawary, H.; Baradaran, A.; Abi-Rafeh, J.; Vorstenbosch, J.; Xu, L.; Efanov, J.I. Bone Healing and Inflammation: Principles of Fracture and Repair. Semin. Plast. Surg. 2021, 35, 198–203. [Google Scholar] [CrossRef]
- Najman, S.; Stojanović, S.; Živković, J.; Najdanović, J.; Radenković, M.; Vasiljević, P.; Ignjatović, N. Applications of Biomaterials in Regenerative Medicine and Tissue Engineering—Concepts and Perspective. Contemp. Mater. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Ferraz, M.P. An Overview on the Big Players in Bone Tissue Engineering: Biomaterials, Scaffolds and Cells. Int. J. Mol. Sci. 2024, 25, 3836. [Google Scholar] [CrossRef] [PubMed]
- Pina, S.; Oliveira, J.M.; Reis, R.L. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv. Mater. 2015, 27, 1143–1169. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef]
- Brahmi, A.; Agsοus, M.; Benkhaoula, B.N.; Ziani, S.; Khireddine, H.; AitAli, S.; Abdullah, M.M.; Chai, B.X.; Belaadi, A. Fluoro-Hydroxyapatite/Chitosan composites as an eco-friendly adsorbent for Direct Red 23 dye removal: Optimization through Response Surface Methodology. J. Mol. Struct. 2024, 1321, 140212. [Google Scholar] [CrossRef]
- Furka, D.; Santana, J.A.D.; Furka, S. Advances in new materials for bone tissue engineering simplifying postoperative rehabilitation: Recent Scientific Breakthroughs. Časopis REHABILITÁCIA 2023, 60, 16–18. [Google Scholar] [CrossRef]
- Mamidi, N.; Ijadi, F.; Norahan, M.H. Leveraging the Recent Advancements in GelMA Scaffolds for Bone Tissue Engineering: An Assessment of Challenges and Opportunities. Biomacromolecules 2024, 25, 2075–2113. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm Regen. 2018, 38, 2. [Google Scholar] [CrossRef]
- Soundarya, S.P.; Sanjay, V.; Menon, A.H.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2018, 110, 74–87. [Google Scholar] [CrossRef]
- Heidari, B.S.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, synthetic and commercially-available biopolymers used to regenerate tendons and ligaments. Bioact. Mater. 2022, 19, 179–197. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymers 2022, 14, 983. [Google Scholar] [CrossRef] [PubMed]
- Awad, Y.M.; Blagodatskaya, E.; Ok, Y.S.; Kuzyakov, Y. Effects of polyacrylamide, biopolymer and biochar on the decomposition of 14C-labelled maize residues and on their stabilization in soil aggregates. Eur. J. Soil Sci. 2013, 64, 488–499. [Google Scholar] [CrossRef]
- Chen, Y.; Hung, S.-T.; Chou, E.; Wu, H.-S. Review of Polyhydroxyalkanoates Materials and other Biopolymers for Medical Applications. Mini-Rev. Org. Chem. 2018, 15, 105–121. [Google Scholar] [CrossRef]
- Pina, S.; Reis, R.L.; Oliveira, J.M. Natural polymeric biomaterials for tissue engineering. In Tissue Engineering Using Ceramics and Polymers; Elsevier: Amsterdam, The Netherlands, 2022; pp. 75–110. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Ficai, A.; Andronescu, E.; Voicu, G.; Ghitulica, C.; Vasile, B.S.; Ficai, D.; Trandafir, V. Self-assembled collagen/hydroxyapatite composite materials. Chem. Eng. J. 2010, 160, 794–800. [Google Scholar] [CrossRef]
- Geçer, A.; Yıldız, N.; Kavak, D.; Çalımlı, A. Comparison of chitosan apatite composites synthesized by different methods. Polym. Compos. 2008, 30, 288–295. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed]
- Ahmady, A.; Abu Samah, N.H. A review: Gelatine as a bioadhesive material for medical and pharmaceutical applications. Int. J. Pharm. 2021, 608, 121037. [Google Scholar] [CrossRef]
- Rahman, M.; Shahid, A.; Hossain, T.; Sheikh, S.; Rahman, S.; Uddin, N.; Rahim, A.; Khan, R.A.; Hossain, I. Sources, extractions, and applications of alginate: A review. Discov. Appl. Sci. 2024, 6, 443. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Harugade, A.; Sherje, A.P.; Pethe, A. Chitosan: A review on properties, biological activities and recent progress in biomedical applications. React. Funct. Polym. 2023, 191, 105634. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, C.; Luo, X.; Wang, X.; Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater. Sci. Eng. C 2019, 99, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef] [PubMed]
- Reizabal, A.; Costa, C.M.; Pérez-Álvarez, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Silk Fibroin as Sustainable Advanced Material: Material Properties and Characteristics, Processing, and Applications. Adv. Funct. Mater. 2023, 33, 2210764. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A Comparative Review of Natural and Synthetic Biopolymer Composite Scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef]
- Moshood, T.D.; Nawanir, G.; Mahmud, F.; Mohamad, F.; Ahmad, M.H.; AbdulGhani, A. Sustainability of biodegradable plastics: New problem or solution to solve the global plastic pollution? Curr. Res. Green Sustain. Chem. 2022, 5, 100273. [Google Scholar] [CrossRef]
- Ibrahim, M.; Ramadan, E.; Elsadek, N.E.; Emam, S.E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O.H.; Sarhan, H.A.; Hussein, A.K.; et al. Polyethylene glycol (PEG): The nature, immunogenicity, and role in the hypersensitivity of PEGylated products. J. Control. Release 2022, 351, 215–230. [Google Scholar] [CrossRef]
- Oun, A.A.; Shin, G.H.; Rhim, J.-W.; Kim, J.T. Recent advances in polyvinyl alcohol-based composite films and their applications in food packaging. Food Packag. Shelf Life 2022, 34, 100991. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- de Oca, H.M.; Ward, I. Structure and mechanical properties of PGA crystals and fibres. Polymer 2006, 47, 7070–7077. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Rahman, S.S.; Mahmud, M.B.; Salehi, A.; Monfared, A.R.; Islam, A.; Filleter, T.; Lee, P.C.; Park, C.B. Mechanically strong and fully transparent PMMA composite with greatly improved toughness and impact strength incorporating PEBA nanofibrils. Chem. Eng. J. 2024, 480, 148311. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Sadasivuni, K.K.; Saha, P.; Adhikari, J.; Deshmukh, K.; Ahamed, M.B.; Cabibihan, J.J. Recent advances in mechanical properties of biopolymer composites: A review. Polym. Compos. 2020, 41, 32–59. [Google Scholar] [CrossRef]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.; Souto, E.B. Polylactic acid (PLA): Properties, synthesis, and biomedical applications—A review of the literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar] [CrossRef]
- Bal, Z.; Korkusuz, F.; Ishiguro, H.; Okada, R.; Kushioka, J.; Chijimatsu, R.; Kodama, J.; Tateiwa, D.; Ukon, Y.; Nakagawa, S.; et al. A novel nano-hydroxyapatite/synthetic polymer/bone morphogenetic protein-2 composite for efficient bone regeneration. Spine J. 2021, 21, 865–873. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]
- Laohavisuti, N.; Boonchom, B.; Boonmee, W.; Chaiseeda, K.; Seesanong, S. Simple recycling of biowaste eggshells to various calcium phosphates for specific industries. Sci. Rep. 2021, 11, 15143. [Google Scholar] [CrossRef]
- Alkaron, W.; Almansoori, A.; Balázsi, K.; Balázsi, C. Hydroxyapatite-Based Natural Biopolymer Composite for Tissue Regeneration. Materials 2024, 17, 4117. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Khil, M.S.; Omran, A.; Sheikh, F.A.; Kim, H.Y.; Barakat, N.A.; Khil, M.S.; Omran, A.; Sheikh, F.A.; Kim, H.Y. Extraction of pure natural hydroxyapatite from the bovine bones bio waste by three different methods. J. Mech. Work. Technol. 2009, 209, 3408–3415. [Google Scholar] [CrossRef]
- Azis, Y.; Adrian, M.; Alfarisi, C.D.; Khairat; Sri, R.M. Synthesis of hydroxyapatite nanoparticles from egg shells by sol-gel method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012040. [Google Scholar] [CrossRef]
- Liandi, A.R.; Rianom, W.H.; Cahyana, A.H.; Fathoni, A.; Wendari, T.P. Transforming seafood waste: Green mussel shell-derived hydroxyapatite as a catalyst for spirooxindole synthesis. Bioresour. Technol. Rep. 2024, 25, 101796. [Google Scholar] [CrossRef]
- Zuliantoni, Z.; Suprapto, W.; Setyarini, P.H.; Gapsari, F. Extraction and characterization of snail shell waste hydroxyapatite. Results Eng. 2022, 14, 100390. [Google Scholar] [CrossRef]
- Alkaron, W.A.; Hamad, S.F.; Sabri, M.M. Studying the Fabrication and Characterization of Polymer Composites Reinforced with Waste Eggshell Powder. Adv. Polym. Technol. 2023, 2023, 7640478. [Google Scholar] [CrossRef]
- Sundaram, N.M.; Rajendran, N. Biodegradation and cytotoxicity of ciprofloxacin-loaded hydroxyapatite-polycaprolactone nanocomposite film for sustainable bone implants. Int. J. Nanomed. 2015, 10, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.A.M.; Portela, T.O.; Correa, G.S.; Oliveira, M.M.; Rangel, J.H.G.; Rodrigues, S.F.; Mercury, J.M.R. Synthesis of hydroxyapatite by hydrothermal and microwave irradiation methods from biogenic calcium source varying pH and synthesis time. Boletín Soc. Española Cerámica Vidr. 2022, 61, 35–41. [Google Scholar] [CrossRef]
- Gergely, G.; Wéber, F.; Lukács, I.; Tóth, A.L.; Horváth, Z.E.; Mihály, J.; Balázsi, C. Preparation and characterization of hydroxyapatite from eggshell. Ceram. Int. 2010, 36, 803–806. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.M.S.; Alipal, J.; Abdullah, H.; Idris, M.; Lee, T. Synthesis of eggshell derived hydroxyapatite via chemical precipitation and calcination method. Mater. Today Proc. 2021, 42, 172–177. [Google Scholar] [CrossRef]
- Lee, S.-W.; Balázsi, C.; Balázsi, K.; Seo, D.-H.; Kim, H.S.; Kim, C.-H.; Kim, S.-G. Comparative Study of hydroxyapatite prepared from seashells and eggshells as a bone graft material. Tissue Eng. Regen. Med. 2014, 11, 113–120. [Google Scholar] [CrossRef]
- Balázsi, K.; Sim, H.-Y.; Choi, J.-Y.; Kim, S.-G.; Chae, C.-H.; Balázsi, C. Biogenic Nanosized Hydroxyapatite for Tissue Engineering Applications. In Proceedings of the International Symposium on Biomedical Engineering and Medical Physics, Riga, Latvia, 10–12 October 2012; Springer: Berlin/Heidelberg, Germany, 2013; pp. 190–193. [Google Scholar] [CrossRef]
- Ofudje, E.A.; Akande, J.A.; Sodiya, E.F.; Ajayi, G.O.; Ademoyegun, A.J.; Al-Sehemi, A.G.; Kavil, Y.N.; Bakheet, A.M. Bioactivity properties of hydroxyapatite/clay nanocomposites. Sci. Rep. 2023, 13, 19896. [Google Scholar] [CrossRef] [PubMed]
- Velu, R.; Calais, T.; Jayakumar, A.; Raspall, F. A Comprehensive Review on Bio-Nanomaterials for Medical Implants and Feasibility Studies on Fabrication of Such Implants by Additive Manufacturing Technique. Materials 2019, 13, 92. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Nazemi, Z.; Salehi, A.O.M.; Seyfoori, A.; John, J.V.; Nourbakhsh, M.S.; Akbari, M. Cellulose-based composite scaffolds for bone tissue engineering and localized drug delivery. Bioact. Mater. 2023, 20, 137–163. [Google Scholar] [CrossRef]
- Song, J.; Li, L.; Fang, L.; Zhang, E.; Zhang, Y.; Zhang, Z.; Vangari, P.; Huang, Y.; Tian, F.; Zhao, Y.; et al. Advanced strategies of scaffolds design for bone regeneration. BMEMat 2023, 1, e12046. [Google Scholar] [CrossRef]
- Radha, G.; Manjubaashini, N.; Balakumar, S. Nano-hydroxyapatite/natural polymer composite scaffolds for bone tissue engineering: A brief review of recent trend. Vitr. Model. 2023, 2, 125–151. [Google Scholar] [CrossRef]
- Jang, J.-W.; Min, K.-E.; Kim, C.; Shin, J.; Lee, J.; Yi, S. Review: Scaffold Characteristics, Fabrication Methods, and Biomaterials for the Bone Tissue Engineering. Int. J. Precis. Eng. Manuf. 2023, 24, 511–529. [Google Scholar] [CrossRef]
- Joseph, B.; Jose, C.; Kavil, S.V.; Kalarikkal, N.; Thomas, S. Solvent-Casting Approach for Design of Polymer Scaffolds and Their Multifunctional Applications. In Functional Biomaterials; Wiley: Hoboken, NJ, USA, 2023; pp. 371–394. [Google Scholar] [CrossRef]
- Li, X.; Nan, K.; Shi, S.; Chen, H. Preparation and characterization of nano-hydroxyapatite/chitosan cross-linking composite membrane intended for tissue engineering. Int. J. Biol. Macromol. 2012, 50, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Türe, H. Characterization of hydroxyapatite-containing alginate–gelatin composite films as a potential wound dressing. Int. J. Biol. Macromol. 2019, 123, 878–888. [Google Scholar] [CrossRef]
- Deng, C.; Weng, J.; Lu, X.; Zhou, S.; Wan, J.; Qu, S.; Feng, B.; Li, X. Preparation and in vitro bioactivity of poly(d,l-lactide) composite containing hydroxyapatite nanocrystals. Mater. Sci. Eng. C 2008, 28, 1304–1310. [Google Scholar] [CrossRef]
- Yang, B.; Li, X.; Shi, S.; Kong, X.; Guo, G.; Huang, M.; Luo, F.; Wei, Y.; Zhao, X.; Qian, Z. Preparation and characterization of a novel chitosan scaffold. Carbohydr. Polym. 2010, 80, 860–865. [Google Scholar] [CrossRef]
- Garg, T. Preparation of Chitosan Scaffolds for Tissue Engineering using Freeze drying Technology. IOSR J. Pharm. 2012, 2, 72–73. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Deschamps, A.A.; Claase, M.B.; Sleijster, W.J.; de Bruijn, J.D.; Grijpma, D.W.; Feijen, J. Design of segmented poly(ether ester) materials and structures for the tissue engineering of bone. J. Control. Release 2002, 78, 175–186. [Google Scholar] [CrossRef]
- Yu, L.-M.; Liu, T.; Ma, Y.-L.; Zhang, F.; Huang, Y.-C.; Fan, Z.-H. Fabrication of Silk-Hyaluronan Composite as a Potential Scaffold for Tissue Repair. Front. Bioeng. Biotechnol. 2020, 8, 578988. [Google Scholar] [CrossRef]
- Priya, G.; Madhan, B.; Narendrakumar, U.; Kumar, R.V.S.; Manjubala, I. In Vitro and In Vivo Evaluation of Carboxymethyl Cellulose Scaffolds for Bone Tissue Engineering Applications. ACS Omega 2021, 6, 1246–1253. [Google Scholar] [CrossRef]
- Wahid, F.; Khan, T.; Hussain, Z.; Ullah, H. Nanocomposite scaffolds for tissue engineering; properties, preparation and applications. In Applications of Nanocomposite Materials in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 701–735. [Google Scholar] [CrossRef]
- Liu, C. Collagen–hydroxyapatite composite scaffolds for tissue engineering. In Hydroxyapatite (Hap) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 211–234. [Google Scholar] [CrossRef]
- Katrilaka, C.; Karipidou, N.; Petrou, N.; Manglaris, C.; Katrilakas, G.; Tzavellas, A.N.; Pitou, M.; Tsiridis, E.E.; Choli-Papadopoulou, T.; Aggeli, A. Freeze-Drying Process for the Fabrication of Collagen-Based Sponges as Medical Devices in Biomedical Engineering. Materials 2023, 16, 4425. [Google Scholar] [CrossRef]
- Ying, R.; Wang, H.; Sun, R.; Chen, K. Preparation and properties of a highly dispersed nano-hydroxyapatite colloid used as a reinforcing filler for chitosan. Mater. Sci. Eng. C 2020, 110, 110689. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz, L.G.; Abt, T.; León, N.; Sánchez-Soto, M. Properties of Freeze-Dried Gelatin/Clay Aerogel Composites Crosslinked with Tannic Acid. ACS Appl. Polym. Mater. 2023, 5, 7774–7785. [Google Scholar] [CrossRef]
- Sun, F.; Lim, B.-K.; Ryu, S.-C.; Lee, D.; Lee, J. Preparation of multi-layered film of hydroxyapatite and chitosan. Mater. Sci. Eng. C 2010, 30, 789–794. [Google Scholar] [CrossRef]
- Ahmadi Bonakdar, M.; Rodrigue, D. Electrospinning: Processes, structures, and materials. Macromolecules 2024, 4, 58–103. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Tao, D.; Higaki, Y.; Ma, W.; Wu, H.; Shinohara, T.; Yano, T.; Takahara, A. Chain orientation in poly(glycolic acid)/halloysite nanotube hybrid electrospun fibers. Polymer 2015, 60, 284–291. [Google Scholar] [CrossRef]
- Xu, J.; Fang, Q.; Liu, Y.; Zhou, Y.; Ye, Z.; Tan, W.-S. In situ ornamenting poly(ε-caprolactone) electrospun fibers with different fiber diameters using chondrocyte-derived extracellular matrix for chondrogenesis of mesenchymal stem cells. Colloids Surf. B Biointerfaces 2021, 197, 111374. [Google Scholar] [CrossRef]
- Shakhssalim, N.; Soleimani, M.; Dehghan, M.M.; Rasouli, J.; Taghizadeh-Jahed, M.; Torbati, P.M.; Naji, M. Bladder smooth muscle cells on electrospun poly(ε-caprolactone)/poly( l -lactic acid) scaffold promote bladder regeneration in a canine model. Mater. Sci. Eng. C 2017, 75, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Sharma, C.; Purohit, S.D.; Singh, H.; Dinda, A.K.; Potdar, P.D.; Chou, C.-F.; Mishra, N.C. Gelatin-polycaprolactone-nanohydroxyapatite electrospun nanocomposite scaffold for bone tissue engineering. Mater. Sci. Eng. C 2021, 119, 111588. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fajardo, A.R.; Gerola, A.P.; Rodrigues, J.H.; Nakamura, C.V.; Muniz, E.C.; Hsieh, Y.-L. First report of electrospun cellulose acetate nanofibers mats with chitin and chitosan nanowhiskers: Fabrication, characterization, and antibacterial activity. Carbohydr. Polym. 2020, 250, 116954. [Google Scholar] [CrossRef]
- Keirouz, A.; Zakharova, M.; Kwon, J.; Robert, C.; Koutsos, V.; Callanan, A.; Chen, X.; Fortunato, G.; Radacsi, N. High-throughput production of silk fibroin-based electrospun fibers as biomaterial for skin tissue engineering applications. Mater. Sci. Eng. C 2020, 112, 110939. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Poly-є-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Nanostructured Biomaterials for Regeneration. Adv. Funct. Mater. 2008, 18, 3568–3582. [Google Scholar] [CrossRef]
- Li, C.; Vepari, C.; Jin, H.-J.; Kim, H.J.; Kaplan, D.L. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3115–3124. [Google Scholar] [CrossRef]
- Luu, Y.; Kim, K.; Hsiao, B.; Chu, B.; Hadjiargyrou, M. Development of a nanostructured DNA delivery scaffold via electrospinning of PLGA and PLA–PEG block copolymers. J. Control. Release 2003, 89, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Gouma, P.; Xue, R.; Goldbeck, C.; Perrotta, P.; Balázsi, C. Nano-hydroxyapatite—Cellulose acetate composites for growing of bone cells. Mater. Sci. Eng. C 2012, 32, 607–612. [Google Scholar] [CrossRef]
- Yang, D.; Jin, Y.; Ma, G.; Chen, X.; Lu, F.; Nie, J. Fabrication and characterization of chitosan/PVA with hydroxyapatite biocomposite nanoscaffolds. J. Appl. Polym. Sci. 2008, 110, 3328–3335. [Google Scholar] [CrossRef]
- Mobarak, M.H.; Islam, A.; Hossain, N.; Al Mahmud, Z.; Rayhan, T.; Nishi, N.J.; Chowdhury, M.A. Recent advances of additive manufacturing in implant fabrication—A review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Bikiaris, N.D.; Koumentakou, I.; Samiotaki, C.; Meimaroglou, D.; Varytimidou, D.; Karatza, A.; Kalantzis, Z.; Roussou, M.; Bikiaris, R.D.; Papageorgiou, G.Z. Recent Advances in the Investigation of Poly(lactic acid) (PLA) Nanocomposites: Incorporation of Various Nanofillers and their Properties and Applications. Polymers 2023, 15, 1196. [Google Scholar] [CrossRef]
- Heo, S.; Kim, S.; Wei, J.; Hyun, Y.; Yun, H.; Kim, D.; Shin, J.W.; Shin, J. Fabrication and characterization of novel nano- and micro-HA/PCL composite scaffolds using a modified rapid prototyping process. J. Biomed. Mater. Res. Part A 2009, 89A, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Nasirpouri, F. Fundamentals and Principles of Electrode-Position. In Electrodeposition of Nanostructured Materials; Springer: Cham, Switzerland, 2017; pp. 75–121. [Google Scholar] [CrossRef]
- Azani, M.-R.; Hassanpour, A. High-performance polymer nanocomposites: Advanced fabrication methods and critical insights. J. Polym. Res. 2024, 31, 168. [Google Scholar] [CrossRef]
- Mahmoodi, S.; Sorkhi, L.; Farrokhi-Rad, M.; Shahrabi, T. Electrophoretic deposition of hydroxyapatite–chitosan nanocomposite coatings in different alcohols. Surf. Coat. Technol. 2013, 216, 106–114. [Google Scholar] [CrossRef]
- Pang, X.; Zhitomirsky, I. Electrodeposition of composite hydroxyapatite–chitosan films. Mater. Chem. Phys. 2005, 94, 245–251. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Shahrabi, T.; Mahmoodi, S.; Khanmohammadi, S. Electrophoretic deposition of hydroxyapatite-chitosan-CNTs nanocomposite coatings. Ceram. Int. 2017, 43, 4663–4669. [Google Scholar] [CrossRef]


| Polymers | Properties | Application | References | |
|---|---|---|---|---|
| Advantages | Limitation | |||
| Gelatin | Excellent biocompatibility, biodegradable, non-toxic, enhancement of cell adhesion and proliferation | Weak mechanical properties, low stability in physiological condition | Scaffolds for hard tissue engineering | [40] |
| Alginate | Biodegradability, biocompatibility, bioresorbable, non-toxicity, mimicking the function of extracellular body tissue | Weak mechanical strength, low cell adhesion, difficult to sterilize | Bone tissue applications | [41] |
| Cellulose | Good water absorption capacity, mechanical performance, structural properties, cell adhesion, biocompatibility | A lower biodegradability in humans | 3D scaffolds, bone replacements | [42] |
| Chitosan | Prevent formation of scar tissue, excellent biocompatibility, biodegradability, and anti-inflammatory | Poor stability, weak mechanical performance | Scaffolds, microspheres | [43] |
| Collagen | Superior biocompatibility, low toxicity, biodegradable, rough surface, low immunogenicity | Weak mechanical properties, poor stability in an aqueous environment. | Scaffolds, drug delivery systems | [44] |
| Hyaluronic Acid | High biocompatibility, biodegradability, good cell adhesion, proliferation, differentiation | Weak mechanical performance, high degradation rate | Scaffolds, hydrogel | [45] |
| Silk Fibroin | Enhanced flexibility, biocompatibility, good mechanical performance | Reduced biodegradation rate | Scaffolds | [46] |
| Polymers | Properties | Application | References | |
|---|---|---|---|---|
| Advantages | Limitation | |||
| Poly (ethylene glycol) (PEG) | Biocompatible, degradable, non-toxic, non-immunogenic blend with various polymers, enzyme-stable, hydrophilic | Limited rheological, mechanical strength, and bioactivity | Scaffolds, BTE, 3D bioprinting, orthopedic implant | [50] |
| Poly (vinyl alcohol) (PVA) | Excellent biocompatibility, biodegradability, good mechanical behavior | Low bioactivity and cell adhesion | Scaffolds, drug delivery systems | [51] |
| Polylactic acid (PLA) | Biodegradability, high mechanical performance, lesser inflammatory response | Low toughness, mechanical support, insufficient biocompatibility | Load bearing applications, orthopedic repair, scaffolds | [52] |
| Poly (glycolic acid) (PGA) | Proliferation, and differentiation, high crystallinity, excellent mechanical performance, good cell attachment | Hydrophobic | Scaffolds, BTE | [53] |
| Poly (-caprolactone) (PCL) | Good biodegradability, and biocompatibility, low elastic modulus, tailorable physical properties, low degradation rate | Poor cell attachment, hydrophobic | Scaffolds, BTE, 3D bioprinting | [54] |
| Poly (methyl methacrylate) (PMMA) | Enhances processability, durability | Undegradable | Scaffolds | [55] |
| Poly (lactic-co-glycolic acid) (PLGA) | Processability, good mechanical performance, high biocompatibility, adjustable degradation rate, low inflammatory response | Possible inflammatory response, low bioactivity | Scaffolds, orthopedic implants, drug delivery systems | [56] |
| Source | Types of CaP | Characteristics | Biomedical Applications | References |
|---|---|---|---|---|
| Animal Bones | Hydroxyapatite | High similarity to human bone, excellent osteoconductive | Bone grafts, dental implants, coatings for prosthetics | [4,63] |
| Eggshells | Calcium carbonate (CaCO3), can be transformed into HAp | Low-cost, abundant, easy to process | Bone repair, drug delivery systems | [64] |
| Corals | Calcium carbonate (CaCO3), transformed into HAp | Porous structure resembling cancellous bone | Bone scaffolds, tissue regeneration | [22] |
| Marine Shells | Calcium carbonate (CaCO3), can be converted to HAp | Biodegradable, porous, naturally occurring structure | Bone regeneration, dental applications | [65] |
| Fish Scales | Hydroxyapatite | Naturally contains calcium phosphate, biocompatible | Bone implants, regenerative medicine | [66] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkaron, W.; Almansoori, A.; Balázsi, C.; Balázsi, K. A Critical Review of Natural and Synthetic Polymer-Based Biological Apatite Composites for Bone Tissue Engineering. J. Compos. Sci. 2024, 8, 523. https://doi.org/10.3390/jcs8120523
Alkaron W, Almansoori A, Balázsi C, Balázsi K. A Critical Review of Natural and Synthetic Polymer-Based Biological Apatite Composites for Bone Tissue Engineering. Journal of Composites Science. 2024; 8(12):523. https://doi.org/10.3390/jcs8120523
Chicago/Turabian StyleAlkaron, Wasan, Alaa Almansoori, Csaba Balázsi, and Katalin Balázsi. 2024. "A Critical Review of Natural and Synthetic Polymer-Based Biological Apatite Composites for Bone Tissue Engineering" Journal of Composites Science 8, no. 12: 523. https://doi.org/10.3390/jcs8120523
APA StyleAlkaron, W., Almansoori, A., Balázsi, C., & Balázsi, K. (2024). A Critical Review of Natural and Synthetic Polymer-Based Biological Apatite Composites for Bone Tissue Engineering. Journal of Composites Science, 8(12), 523. https://doi.org/10.3390/jcs8120523







