Nitrogen-Doped Borophene Quantum Dots: A Novel Sensing Material for the Detection of Hazardous Environmental Gases
Abstract
1. Introduction
2. Calculation Methods
3. Results and Discussion
3.1. Structural and Electronic Properties of and BQDs
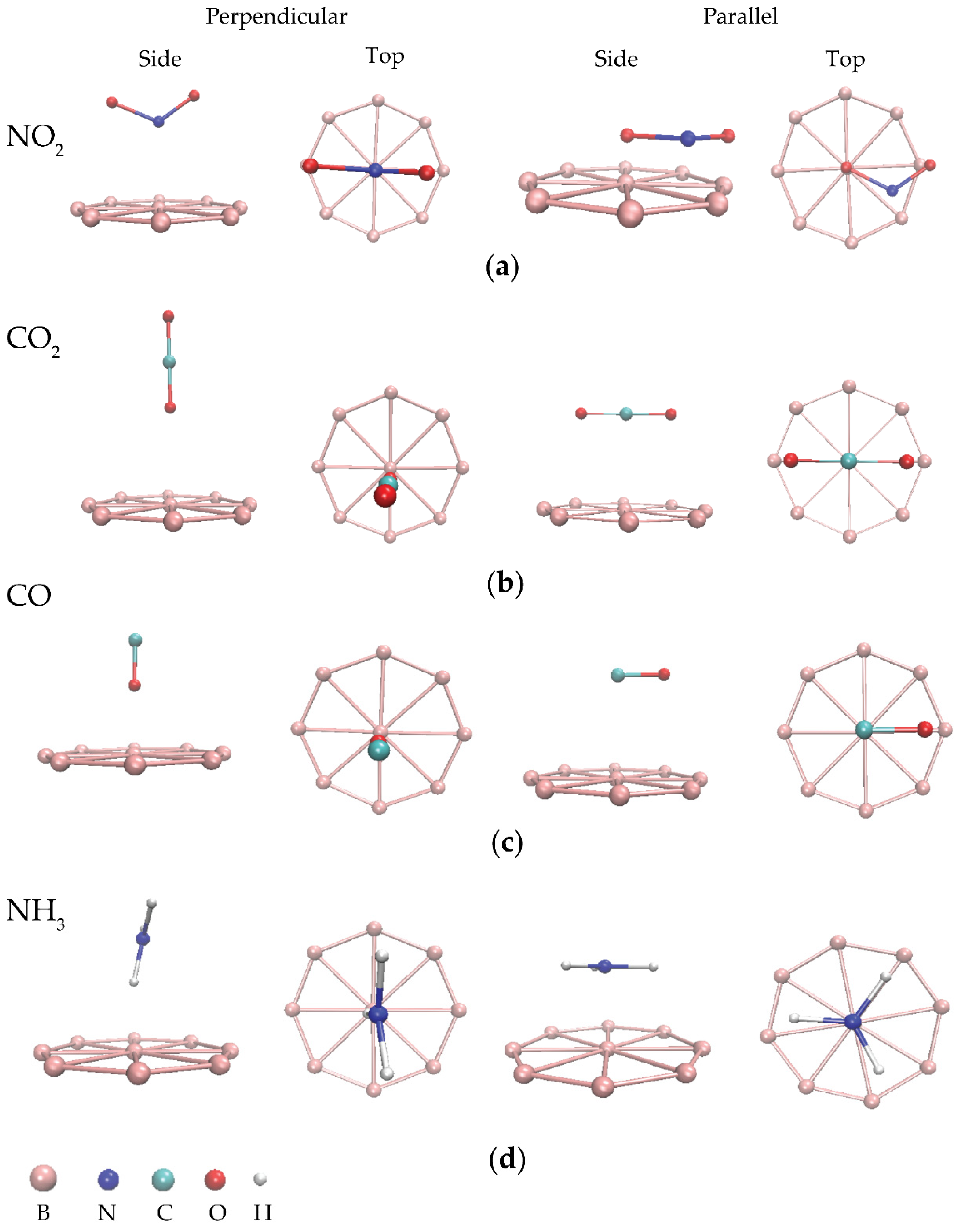
3.2. Adsorption of NO2, CO2, CO, and NH3 Gas Molecules on BQDs
3.3. Adsorption of NO2, CO2, CO, and NH3 Gas Molecules on BQDs
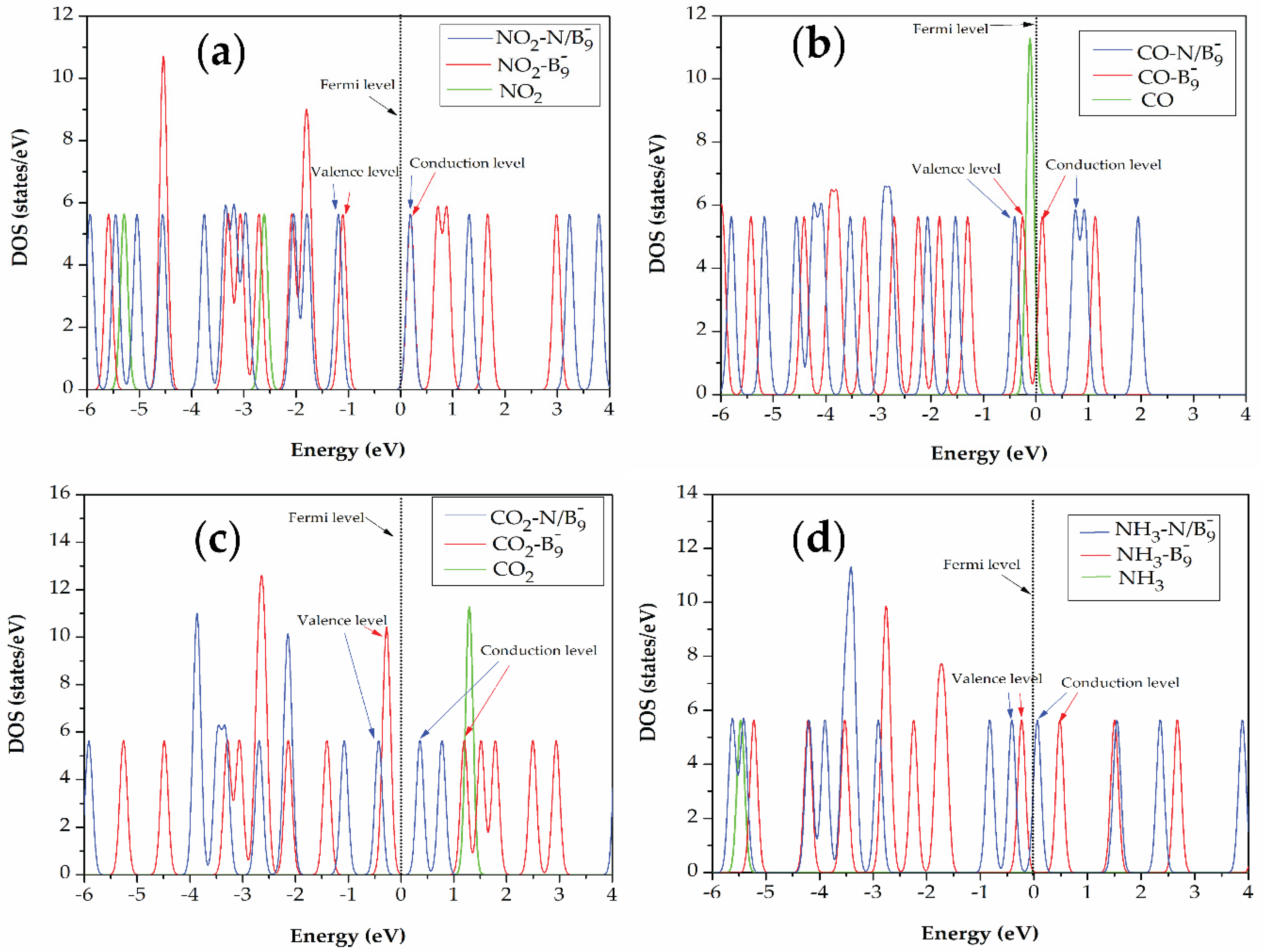
3.4. Total Densities of State (DOS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seesaard, T.; Kamjornkittikoon, K.; Wongchoosuk, C. A comprehensive review on advancements in sensors for air pollution applications. Sci. Total Environ. 2024, 951, 175696. [Google Scholar] [CrossRef] [PubMed]
- Chaloeipote, G.; Wongchoosuk, C. Flexible humidity sensor based on PEDOT:PSS/Mxene nanocomposite. Flex. Print. Electron. 2024, 9, 015015. [Google Scholar] [CrossRef]
- Seekaew, Y.; Kamlue, S.; Wongchoosuk, C. Room-temperature ammonia gas sensor based on Ti3C2Tx MXene/Graphene Oxide/CuO/ZnO Nanocomposite. ACS Appl. Nano Mater. 2023, 6, 9008–9020. [Google Scholar] [CrossRef]
- Aufray, B.; Kara, A.; Vizzini, S.; Oughaddou, H.; Léandri, C.; Ealet, B.; Le Lay, G. Graphene-like silicon nanoribbons on Ag(110): A possible formation of silicene. Appl. Phys. Lett. 2010, 96, 183102. [Google Scholar] [CrossRef]
- Liu, H.; Neal, A.T.; Zhu, Z.; Luo, Z.; Xu, X.; Tománek, D.; Ye, P.D. Phosphorene: An unexplored 2D semiconductor with a high hole mobility. ACS Nano 2014, 8, 4033–4041. [Google Scholar] [CrossRef]
- Pumera, M.; Sofer, Z.; Ambrosi, A. Layered transition metal dichalcogenides for electrochemical energy generation and storage. J. Mater. Chem. A 2014, 2, 8981–8987. [Google Scholar] [CrossRef]
- Chen, X.; Hu, J.; Chen, P.; Yin, M.; Meng, F.; Zhang, Y. UV-light-assisted NO2 gas sensor based on WS2/PbS heterostructures with full recoverability and reliable anti-humidity ability. Sens. Actuators B Chem. 2021, 339, 129902. [Google Scholar] [CrossRef]
- Pak, Y.; Lim, N.; Kumaresan, Y.; Lee, R.; Kim, K.; Kim, T.H.; Kim, S.M.; Kim, J.T.; Lee, H.; Ham, M.H.; et al. Palladium nanoribbon array for fast hydrogen gas sensing with ultrahigh sensitivity. Adv. Mater. 2015, 27, 6945–6952. [Google Scholar] [CrossRef]
- Shukla, V.; Wärma, J.; Jena, N.K.; Grigoriev, A.; Ahuja, R. Toward the realization of 2D borophene based gas sensor. J. Phys. Chem. C 2017, 121, 26869–26876. [Google Scholar] [CrossRef]
- Yuan, W.; Shi, G. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078. [Google Scholar] [CrossRef]
- Wang, T.; Huang, D.; Yang, Z.; Xu, S.; He, G.; Li, X.; Hu, N.; Yin, G.; He, D.; Zhang, L. A review on graphene-based gas/vapor sensors with unique properties and potential applications. Nano-Micro Lett. 2016, 8, 95–119. [Google Scholar] [CrossRef] [PubMed]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Mannix, A.J.; Zhou, X.-F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorps. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Zhang, J.; Zhong, Q.; Li, W.; Li, S.; Li, H.; Cheng, P.; Meng, S.; Chen, L.; Wu, K. Experimental realization of two-dimensional boron sheets. Nat. Chem. 2016, 8, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Kaneti, Y.V.; Benu, D.P.; Xu, X.; Yuliarto, B.; Yamauchi, Y.; Golberg, D. Borophene: Two-dimensional boron monolayer: Synthesis, properties, and potential applications. Chem. Rev. 2022, 122, 1000–1051. [Google Scholar] [CrossRef]
- Hou, C.; Tai, G.; Liu, Y.; Liu, X. Borophene gas sensor. Nano Res. 2022, 15, 2537–2544. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Penev, E.S.; Yakobson, B.I. Two-dimensional boron: Structures, properties and applications. Chem. Soc. Rev. 2017, 46, 6746–6763. [Google Scholar] [CrossRef]
- Sergeeva, A.P.; Popov, I.A.; Piazza, Z.A.; Li, W.L.; Romanescu, C.; Wang, L.S.; Boldyrev, A.I. Understanding boron through size-selected clusters: Structure, chemical bonding, and fluxionality. Acc. Chem. Res. 2014, 47, 1349–1358. [Google Scholar] [CrossRef]
- Hou, C.; Tai, G.A.; Wu, Z.H.; Hao, J.Q. Borophene: Current status, challenges and opportunities. ChemPlusChem 2020, 85, 2186–2196. [Google Scholar] [CrossRef]
- Sabokdast, S.; Horri, A.; Azar, Y.T.; Momeni, M.; Tavakoli, M.B. Detection of nucleobases on borophene nanosheet: A DFT investigation. Bioelectrochemistry 2021, 138, 107721. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Lü, T.-Y.; Wang, H.-Q.; Feng, Y.P.; Zheng, J.-C. Review of borophene and its potential applications. Front. Phys. 2019, 14, 33403. [Google Scholar] [CrossRef]
- Yang, X.; Ding, Y.; Ni, J. Ab initio prediction of stable boron sheets and boron nanotubes: Structure, stability, and electronic properties. Phys. Rev. B 2008, 77, 041402. [Google Scholar] [CrossRef]
- Liu, Y.; Penev, E.S.; Yakobson, B.I. Probing the synthesis of two-dimensional boron by first-principles computations. Angew. Chem. 2013, 52, 3156–3159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yang, Y.; Gao, G.; Yakobson, B.I. Two-dimensional boron monolayers mediated by metal substrates. Angew. Chem. 2015, 127, 13214–13218. [Google Scholar] [CrossRef]
- Penev, E.S.; Bhowmick, S.; Sadrzadeh, A.; Yakobson, B.I. Polymorphism of two-dimensional boron. Nano Lett. 2012, 12, 2441–2445. [Google Scholar] [CrossRef]
- Piazza, Z.A.; Hu, H.S.; Li, W.L.; Zhao, Y.F.; Li, J.; Wang, L.S. Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nat. Commun. 2014, 5, 3113. [Google Scholar] [CrossRef]
- Romanescu, C.; Galeev, T.R.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. Transition-metal-centered monocyclic boron wheel clusters (M©Bn): A new class of aromatic borometallic compounds. Acc. Chem. Res. 2013, 46, 350–358. [Google Scholar] [CrossRef]
- Zhai, H.J.; Alexandrova, A.N.; Birch, K.A.; Boldyrev, A.I.; Wang, L.S. Hepta- and octacoordinated boron in molecular wheels of eight- and nine-atom boron clusters: Observation and confirmation. Angew. Chem. Int. Ed. 2003, 42, 6004–6008. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, M.; Sharma, D.K.; Auluck, S. Enhancing gas adsorption properties of borophene by embedding transition metals. Comput. Condens. Matter 2020, 22, e00436. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, X.; Song, E.; Hou, X.; Yang, X.; Mi, J.; Huang, J.; Stampfl, C. Transition metal-doped α-borophene as potential oxygen and hydrogen evolution electrocatalyst: A density functional theory study. Catal. Commun. 2020, 144, 106090. [Google Scholar] [CrossRef]
- Xu, X.; Hou, X.; Lu, J.; Zhang, P.; Xiao, B.; Mi, J. Metal-doped two-dimensional borophene nanosheets for the carbon dioxide electrochemical reduction reaction. J. Phys. Chem. C 2020, 124, 24156–24163. [Google Scholar] [CrossRef]
- Li, W.L.; Romanescu, C.; Galeev, T.R.; Piazza, Z.A.; Boldyrev, A.I.; Wang, L.S. Transition-metal-centered nine membered boron rings: M©B9 and M©B9− (M = Rh, Ir). J. Am. Chem. Soc. 2012, 134, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Altalhi, T.; Yang, J.-H.; Yakobson, B.I. Semiconducting ά-boron sheet with high mobility and low all-boron contact resistance: A first-principles study. Nanoscale 2021, 13, 8474–8480. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Yang, J.-H.; Xiang, H.; Gong, X.-G. Fully boron sheet-based field effect transistors from first-principles: Inverse design of semiconducting boron sheets. J. Phys. Chem. Lett. 2021, 12, 576–584. [Google Scholar] [CrossRef]
- Jiang, H.R.; Lu, Z.; Wu, M.C.; Ciucci, F.; Zhao, T.S. Borophene: A promising anode material offering high specific capacity and high rate capability for lithium-ion batteries. Nano Energy 2016, 23, 97–104. [Google Scholar] [CrossRef]
- Jena, N.K.; Araujo, R.B.; Shukla, V.; Ahuja, R. Borophane as a bencmate of graphene: A potential 2D material for anode of Li and Na-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 16148–16158. [Google Scholar] [CrossRef]
- Huang, C.S.; Murat, A.; Babar, V.; Montes, E.; Schwingenschlögl, U. Adsorption of the gas molecules NH3, NO, NO2, and CO on borophene. J. Phys. Chem. C 2018, 122, 14665–14670. [Google Scholar] [CrossRef]
- Ta, L.T.; Hamada, I.; Morikawa, Y.; Dinh, V.A. Adsorption of toxic gases on borophene: Surface deformation links to chemisorptions. RSC Adv. 2021, 11, 18279. [Google Scholar] [CrossRef]
- Zhao, A.; Han, Y.; Che, Y.; Liu, Q.; Wang, X.; Li, Q.; Sun, J.; Lei, Z.; He, X.; Liu, Z.H. High-quality borophene quantum dot realization and their application in a photovoltaic device. J. Mater. Chem. A 2021, 9, 24036–24043. [Google Scholar] [CrossRef]
- Gogoi, D.; Hazarika, C.; Neog, G.; Mridha, P.; Bora, H.K.; Das, M.R.; Szunerits, S.; Boukherroub, R. Borophene quantum dots as novel peroxidase-mimicking nanozyme: A dual-mode assay for the detection of oxytetracycline and tetracycline antibiotics. ACS Appl. Mater. Interfaces 2024, 16, 14645–14660. [Google Scholar] [CrossRef]
- Joshi, D.J.; Malek, N.I.; Jung Park, T.; Kailasa, S.K. Ultrasonication-assisted synthesis of fluorescent borophene quantum dots for sensing of dehydroepiandrosterone biomarker. J. Mol. Liq. 2023, 385, 122294. [Google Scholar] [CrossRef]
- Yashwanth, H.J.; Hareesh, K.; Rondiya, S.R.; Choudhary, R.J.; Dhole, S.D. The borophene quantum dots scaffolded TiO2 nanocomposite as an efficient photo electrocatalyst for water splitting application. Appl. Surf. Sci. 2024, 646, 158910. [Google Scholar] [CrossRef]
- Ranjan, P.; Lee, J.M.; Kumar, P.; Vinu, A. Borophene: New sensation in flatland. Adv. Mater. 2020, 1, 2000531. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Sadique, M.A.; Kaushik, A.; Ranjan, P.; Khan, R.; Srivastava, K.A. Borophene as an emerging 2D flatland for biomedical applications: Current challenges and future prospects. J. Mater. Chem. B 2022, 10, 1146–1175. [Google Scholar] [CrossRef]
- Liu, X.; Hou, C.; Liu, Y.; Chen, S.; Wu, Z.; Liang, X.; Tai, G. Borophene and BC2N quantum dot heterostructures: Ultrasensitive humidity sensing and multifunctional applications. J. Mater. Chem. A 2023, 11, 24789–24799. [Google Scholar] [CrossRef]
- Wang, H.; An, D.; Wang, M.; Sun, L.; Li, Y.; Li, H.; Li, N.; Hu, S.; He, Y.B. Crystalline borophene quantum dots and their derivative boron nanospheres. Mater. Adv. 2021, 2, 3269–3273. [Google Scholar] [CrossRef]
- Liang, R.; Swanson, J.M.J.; Voth, G.A. Benchmark study of the SCC-DFTB approach for a biomolecular proton channel. J. Chem. Theory Comput. 2014, 10, 451–462. [Google Scholar] [CrossRef]
- Porezag, D.; Frauenheim, T.; Kohler, T.; Seifert, G.; Kaschner, R. Construction of tight-binding-like potentials on the basis of density-functional theory: Application to carbon. Phys. Rev. B 1995, 51, 12947. [Google Scholar] [CrossRef]
- Elstner, M.; Porezag, D.; Jungnickel, G.; Elsner, J.; Haugk, M.; Frauenheim, T.; Suhai, S.; Seifert, G. Self-consistent-charge density-functional tight-binding method for simulation of complex materials properties. Phys. Rev. B 1998, 58, 7260. [Google Scholar] [CrossRef]
- Frauenheim, T.; Seifert, G.; Elstner, M.; Niehaus, T.; Köhler, C.; Amkreutz, M.; Sternberg, M.; Hajnal, Z.; Di Carlo, A.; Suhai, S. Atomistic simulations of complex materials: Ground-state and excited-state properties. J. Phys. Condens. Matter 2002, 14, 3015. [Google Scholar] [CrossRef]
- Timsorn, K.; Wongchoosuk, C. Adsorption of NO2, HCN, HCHO and CO on pristine and amine functionalized boron nitride nanotubes by self-consistent charge density functional tight-binding method. Mater. Res. Express 2020, 7, 055005. [Google Scholar] [CrossRef]
- Stock, B.G.; Bezugly, V.; Kunstmann, J.; Cuniberti, G.; Frauenheim, T.; Niehaus, T.A. SCC-DFTB parametrization for boron and boranes. J. Chem. Theory Comput. 2012, 8, 1153–1163. [Google Scholar] [CrossRef] [PubMed]
- Gahrouei, M.M.; Vlastos, N.; D’Souza, R.; Odogwu, E.C.; Oliveira, L.D.S. Benchmark investigation of SCC-DFTB against standard and hybrid DFT to model electronic properties in two-dimensional MOFs for thermoelectric applications. J. Chem. Theory Comput. 2024, 20, 3976–3992. [Google Scholar] [CrossRef] [PubMed]
- Timsorn, K.; Wongchoosuk, C. Inkjet printing of room-temperature gas sensors for identification of formalin contamination in squids. J. Mater. Sci. Mater. Electron. 2019, 30, 4782–4791. [Google Scholar] [CrossRef]
- Marutaphan, A.; Seekaew, Y.; Wongchoosuk, C. Self-consistent charge density functional tight-binding study of poly(3,4-ethylenedioxythiphene): Poly(styrenesulfonate) ammonia gas sensor. Nanoscale Res. Lett. 2017, 12, 90. [Google Scholar] [CrossRef]
- Arunragsa, S.; Seekaew, Y.; Pon-on, W.; Wongchoosuk, C. Hydroxyl edge-functionalized graphene quantum dots for gas-sensing applications. Diamond Relat. Mater. 2020, 105, 107790. [Google Scholar] [CrossRef]
- Novotný, M.; Domίnguez-Gutiérrez, F.J.; Krstić, P. A computational study of hydrogen detection by borophene. J. Mater. Chem. C 2017, 5, 5426–5433. [Google Scholar] [CrossRef]
- Lukose, B.; Kuc, A.; Frenzel, J.; Heine, T. On the reticular construction concept of covalent organic frameworks. Beilstein J. Nanotechnol. 2010, 1, 60–70. [Google Scholar] [CrossRef]
- Gemming, S.; Enyashin, A.; Andrey, N.; Frenzel, J.; Seifert, G. Adsorption of nucleotides on the rutile (110) surface. Int. J. Mater. Res. 2010, 101, 758–764. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, R.; Zhang, D. Adsorption of formaldehyde molecule on the pristine and silicon-doped boron nitride nanotubes. Chem. Phys. Lett. 2008, 467, 131–135. [Google Scholar] [CrossRef]
- Zhai, H.J.; Kiran, B.; Li, J.; Wang, L.S. Hydrocarbon analogues of boron clusters-planarity aromaticity and antiaromaticity. Nat. Mater. 2003, 2, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, A.N.; Boldyrev, A.I.; Zhai, H.J.; Wang, L.S. All-boron aromatic clusters as potential new inorganic ligands and building blocks in chemistry. Coord. Chem. Rev. 2006, 250, 2811–2866. [Google Scholar] [CrossRef]
- Qin, X.; Yan, W.; Li, D.; Zhang, Z.; Chen, S. A first-principles study of gas molecule adsorption on carbon-, nitrogen-, and oxygen-doped two-dimensional borophene. Adv. Cond. Matter Phys. 2021, 2021, 3760631. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Yan, W.; Qin, X. Effect of doping on the photoelectric properties of borophene. Adv. Cond. Matter Phys. 2021, 3718040, 7. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z. Effect of Co-doping on the photoelectric properties of the novel two-dimensional material borophene. Int. J. Opt. 2023, 1603014, 9. [Google Scholar] [CrossRef]
- Kistanov, A.A.; Cai, Y.; Zhou, K.; Srikanth, N.; Dmitriev, S.V.; Zhang, Y.W. Exploring the charge localization and band gap opening of borophene: A first-principles study. Nanoscale 2018, 10, 1403. [Google Scholar] [CrossRef]


| Structure | EHOMO (eV) | ELUMO (eV) | EF (eV) | Eg (eV) |
|---|---|---|---|---|
| −6.18 | −3.46 | −6.15 | 2.72 | |
| −5.98 | −5.30 | −5.64 | 0.68 |
| System | Orientation | Adsorption Site | Distance (Å) | Ead (eV) | Q (e) | EHOMO (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|---|---|---|---|---|
| -NO2 | parallel | B-N | 1.23 | −5.80 | 0.273 | −5.81 | −4.31 | 1.50 |
| B-O | 2.25 | −4.39 | 0.218 | −5.94 | −4.99 | 0.95 | ||
| perpendicular | B-N | 2.97 | −4.39 | 0.218 | −5.94 | −4.99 | 0.95 | |
| B-O | 3.00 | −6.52 | 0.166 | −5.59 | −4.56 | 1.03 | ||
| -CO | parallel | B-C | 1.01 | −8.47 | −0.164 | −5.42 | −4.41 | 1.01 |
| B-O | 1.12 | −6.82 | −0.317 | −6.12 | −5.30 | 0.82 | ||
| perpendicular | B-C | 2.41 | −2.04 | 0.370 | −5.61 | −3.44 | 2.17 | |
| B-O | 1.04 | −0.72 | 0.341 | −5.65 | −3.39 | 2.26 | ||
| -CO2 | parallel | B-C | 1.75 | −6.66 | 0.131 | −5.26 | −4.49 | 0.77 |
| B-O | 1.72 | −3.93 | −0.027 | −5.83 | −4.61 | 1.22 | ||
| perpendicular | B-C | 2.20 | −0.61 | 0.470 | −5.07 | −2.63 | 2.44 | |
| B-O | 1.34 | −0.60 | 0.467 | −5.09 | −2.88 | 2.21 | ||
| -NH3 | parallel | B-N | 3.00 | −1.77 | 0.421 | −5.16 | −2.77 | 2.39 |
| B-H | 3.58 | −1.78 | 0.420 | −5.14 | −2.90 | 2.24 | ||
| perpendicular | B-N | 1.00 | −4.06 | 0.083 | −5.22 | −4.21 | 1.01 | |
| B-H | 2.50 | −1.78 | 0.420 | −5.14 | −2.90 | 2.24 |
| System | Orientation | Adsorption Site | Distance (Å) | Ead (eV) | Q (e) | EHOMO (eV) | ELUMO (eV) | Eg (eV) |
|---|---|---|---|---|---|---|---|---|
| -NO2 | parallel | N-N | 1.00 | −11.64 | 0.021 | −5.04 | −4.55 | 0.49 |
| N-O | 2.52 | −8.67 | 0.036 | −5.24 | −4.29 | 0.95 | ||
| perpendicular | N-N | 1.15 | −6.99 | 0.014 | −5.35 | −4.52 | 0.83 | |
| N-O | 3.10 | −8.67 | 0.036 | −5.24 | −4.29 | 0.95 | ||
| -CO | parallel | N-C | 1.15 | −12.08 | 0.105 | −5.17 | −4.56 | 0.61 |
| N-O | 1.34 | −7.86 | −0.117 | −5.42 | −4.82 | 0.60 | ||
| perpendicular | N-C | 1.65 | −4.28 | 0.166 | −6.03 | −5.71 | 0.32 | |
| N-O | 1.80 | −4.60 | 0.147 | −5.62 | −4.79 | 0.83 | ||
| -CO2 | parallel | N-C | 1.25 | −16.57 | −0.086 | −5.92 | −3.88 | 2.04 |
| N-O | 1.20 | −16.11 | −0.466 | −5.80 | −4.58 | 1.22 | ||
| perpendicular | N-C | 2.51 | −6.57 | 0.201 | −4.98 | −4.18 | 0.80 | |
| N-O | 1.00 | −3.23 | 0.115 | −5.80 | −4.90 | 0.90 | ||
| -NH3 | parallel | N-H | 1.02 | −6.34 | 0.121 | −5.92 | −4.81 | 1.11 |
| N-N | 1.14 | −6.70 | 0.039 | −5.90 | −5.00 | 0.90 | ||
| perpendicular | N-H | 1.57 | −7.22 | 0.116 | −5.41 | −4.19 | 1.22 | |
| N-N | 1.05 | −6.80 | −0.049 | −5.90 | −4.86 | 1.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timsorn, K.; Wongchoosuk, C. Nitrogen-Doped Borophene Quantum Dots: A Novel Sensing Material for the Detection of Hazardous Environmental Gases. J. Compos. Sci. 2024, 8, 397. https://doi.org/10.3390/jcs8100397
Timsorn K, Wongchoosuk C. Nitrogen-Doped Borophene Quantum Dots: A Novel Sensing Material for the Detection of Hazardous Environmental Gases. Journal of Composites Science. 2024; 8(10):397. https://doi.org/10.3390/jcs8100397
Chicago/Turabian StyleTimsorn, Kriengkri, and Chatchawal Wongchoosuk. 2024. "Nitrogen-Doped Borophene Quantum Dots: A Novel Sensing Material for the Detection of Hazardous Environmental Gases" Journal of Composites Science 8, no. 10: 397. https://doi.org/10.3390/jcs8100397
APA StyleTimsorn, K., & Wongchoosuk, C. (2024). Nitrogen-Doped Borophene Quantum Dots: A Novel Sensing Material for the Detection of Hazardous Environmental Gases. Journal of Composites Science, 8(10), 397. https://doi.org/10.3390/jcs8100397







