Recent Advances on The Applications of Phase Change Materials in Cold Thermal Energy Storage: A Critical Review
Abstract
:1. Introduction
2. Conceptual Challenges of Using PCMs in Different Applications of CTES
- It is critical to select the appropriate PCM for a given application. PCMs are classified as organic, inorganic, or eutectic mixes, with varying melting and freezing points and latent heat capacities. Choosing a PCM that meets the temperature demands of the application while preserving stability and reliability might be difficult;
- To minimise corrosion problems in low-temperature applications, the construction materials of the container used to hold diverse eutectic PCMs for thermal energy storage must be considered;
- To minimise leakage and assure compatibility with current equipment, PCMs are often enclosed within containers. Finding acceptable encapsulating materials that are PCM-compatible, thermally conductive, and chemically stable might be difficult. Furthermore, the encapsulation technique should not interfere with heat transfer during phase change;
- The rate of heat transmission during the charging (melting) and discharging (solidification) processes determines the effectiveness of PCM-based thermal storage devices. PCMs have a lower and poor thermal conductivity than traditional materials such as metals. This might result in poorer heat transfer rates and might require the use of higher thermal conductivity structures or composites, complicating the process of design and production even more. In this regard, high heat transfer rates can be difficult to achieve, particularly for large-scale applications, because they may necessitate sophisticated heat exchanger designs and adequate interaction with current systems;
- During phase transitions, some PCMs experience considerable volume changes, which can cause mechanical stress and distortion of the incarceration structures. Controlling these volume variations in order to prevent system damage across several phase change cycles necessitates careful design and research;
- During their lifetime, PCMs are predicted to go through several phase change cycles. A crucial problem is guaranteeing the PCM’s stability and strength across many cycles without a substantial drop in effectiveness.
3. Applications of PCM-Based CTES and Imperative Improvements
3.1. Building
3.2. Air Conditioning and Refrigeration

3.3. Food Storage
3.4. Cold Chain Applications
3.5. Other Applications
4. Critical Evaluation on Utilising PCMs in Different CTES Applications and Nominal Improvements
- Organic, inorganic, and eutectic PCMs (binary and ternary) all have benefits and are used in CTES applications. Basically, the decision between them is influenced by criteria such as the needed temperature range, heat transfer efficiency, cost considerations, safety regulations, and the unique needs of the application. However, it should be noted that eutectic PCMs provide optimum advantages that make them suited for a wide range of applications in a variety of applications. For instance, eutectic PCMs have a stable and clearly stated melting point, allowing them to move through a sudden phase change at a given temperature. This property assures consistent and reliable thermal behaviour, making them perfect for specific applications requiring exact temperature control. Furthermore, eutectic PCMs possess considerable latent heat capacities, which means they can store and release a large quantity of thermal energy during the phase transition process. This characteristic enables them to deliver optimal temperature regulation and thermal buffering by effectively storing thermal energy. Compared to organic and inorganic PCMs, eutectic PCMs have higher thermal conductivity. This allows for faster heat transmission during the phase transition process, making them suited for applications that demand rapid heating or cooling cycles. Eutectic PCMs have been designed to sustain several phase change cycles without deterioration. Their capacity to withstand multiple melting and solidification cycles without deterioration in performance makes them suitable for long-term applications. These characteristics also contribute to their popularity and adaptability. This can contribute to a diverse selection of sectors employing eutectic PCMs including building and construction, energy storage, HVAC systems, thermal regulation in electronics, cold chain management, and thermal comfort enhancement in clothing and textiles. New eutectic PCM formulations and their uses may arise as research and technology advance in the future;
- Incorporating eutectic phase transition materials into wall boards, concrete, gypsum, flooring, and other building materials reduces energy expenditure while increasing thermal comfort. Many examples of successful implementation of PCMs for cold storage in buildings were described in [37,38,39,40,41,42,43]. Apparently, macro-encapsulation provides an effective, secure, and straightforward means of using eutectic PCM, among numerous integration approaches, and its potential uses have received a lot of attention in recent years;
- Because organic eutectic PCMs are often non-toxic, non-corrosive, less chemically reacted, pose fewer environmental concerns, and have customized melting temperature and enthalpy, they are suitable for thermal energy storage in electronics and structures such as portable cooling units and temperature-controlled packaging. However, it should be noted that each organic eutectic PMC has a specific melting point and latent melting enthalpy and, therefore, it would suit a unique application only [85];
- When a specific, narrow temperature range must be implemented for an application, eutectic PCMs are frequently preferred. Their fast phase change enables precise temperature control. Organic PCMs, on the other hand, may be preferable when a wider range of cooling temperatures is appropriate. However, eutectic PCMs have low heat conductivity and leakage during the phase transition, which severely limits their application. Therefore, advanced research was conducted to develop shape-stabilized composites in addition to enhancing their thermal conductivity. One plausible option was to add metallic nanoparticles and carbon-based materials of high thermal conductivity to eutectic PCMs in addition to mitigating the leaking of PCMs. Also, the dispersion of nanoparticles in specific mass fractions and an increase in the mass fraction of nanoparticles can significantly improve the heat transfer characteristics of eutectic PCMs, leading to a reduction in solidification and melting time. Other colleagues were focused on adding different conductive materials such as expanded graphite, carbon nanotube TiO2, and ZnO. Detailed examples of the most advanced methods were elucidated by Veerakumar and Sreekumar (2015) [27], Dong et al. (2022) [43], Zheng et al. [44], Said and Hassan (2018) [46], Song et al. (2019) [57], Xiaofeng and Xuelai (2020) [58], and Hussain et al. [66]. Most importantly, the characteristics of higher thermal conductivity of eutectic PCMs compared to organic PCMs have allowed for faster heat transfer during the phase change process. In turn, this paved the way towards the implication of eutectic PCMs in several applications that necessitate rapid cooling or temperature stabilization.
5. Conclusions
- Cold storage in buildings: Smaller PCM ball diameter and faster chilled water flow rate increase freezing speed;
- Air conditioning systems: Phase change cold storage has a capacity that is 1.5 times greater than ice cold storage and offers better overall performance. Also, when the inlet air temperature of the phase change material air conditioner is 35 °C, the PCM-equipped air conditioner outperforms the conventional unit by 14%, 13%, and 12%at inlet velocities of 0.96 m/s, 1.2 m/s, and 1.44 m/s, respectively;
- Refrigeration systems: With three fins and a nanoparticle concentration of ϕ = 6 vol.%, the melting rate can be increased to its maximum potential, resulting in a 33.5% reduction in the time of the overall melting process;
- Cold chain applications: As the temperature rises and the mass fraction of microcapsules increases, the thermal conductivity of the latent heat functional fluid drops. Furthermore, higher values of coil pitch/tank height, coil spacing/tank width, plate area/maximum plate area and plate thickness/pipe diameter result in higher charging rates. Full-thickness plates (plate area/maximum plate area = 1 and plate thickness/pipe diameter = 0.0081) increase the time-averaged charge rate by 18%;
- Cold storage applications: Adding 4% fumed silica stops PCM leakage. Silica and graphene improve PCM nucleation and minimise super-cooling;
- Finned heat exchanger: The cooling capacity, cooling time, and average performance of the system are 80.8%, 69.7% and 15.9% greater than those of pure water, respectively;
- Solar cold storage: The PCM package with many fins has a larger energy storage capacity and a larger fusion heat flux.
6. Recommendations for Future Research Directions
- Increasing the heat storage density of PCMs is vital when developing a more efficient structure of cold chain transportation and refrigeration equipment;
- It will be an interesting future research direction to study the phase transition behaviour of aqueous solutions including other forms of carbon nano-fillers;
- In the context of cold thermal energy storage systems, it is important to study the thermal effects of many different radial configurations, geometries, initial temperatures, heat transfer fluid temperatures, and heat transfer coefficients;
- Incorporating super-cooling and extending the prior approaches to various container forms and boundary circumstances are possible future advances;
- A special focus is needed on the low charging rate and device design methodology for future commercial application;
- It is rare to find an accessible paper on the subject of corrosion analysis of eutectic PCM containers. As a result, greater research into novel eutectic PCMs and corrosion studies of container materials is proposed.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PCMs | Phase change materials |
| CTES | Cold thermal energy storage |
| TES | Thermal energy storage |
| AC | Air conditioning |
| SEM | Scanning electronic microscopy |
| DSC | Differential scanning calorimetry |
| MCNTs | Multiwall carbon nanotubes |
| FT-IR | Fourier Transform Infrared spectroscopy |
| HTF | Heat transfer fluid |
| NCPCMs | Nanocomposite phase change materials |
| PBTES | Packed bed thermal energy storage |
| LAES | Liquid air energy storage |
| LHTES | Latent heat thermal energy storage |
| GHX | Ground heat exchanger |
| ANSYS | Analytical system |
References
- BP p.l.c. (Formerly The British Petroleum Company p.l.c and BP Amoco p.l.c). BP statistical review of world energy. Br. Petrol. 2010. [Google Scholar]
- Stritih, U.; Charvat, P.; Koželj, R.; Klimes, L.; Osterman, E.; Ostry, M.; Butala, V. PCM thermal energy storage in solar heating of ventilation air—Experimental and numerical investigations. Sustain. Cities Soc. 2018, 37, 104–115. [Google Scholar] [CrossRef]
- Nkwetta, D.N.; Vouillamoz, P.-E.; Haghighat, F.; El Mankibi, M.; Moreau, A.; Desai, K. Phase change materials in hot water tank for shifting peak power demand. Sol. Energy 2014, 107, 628–635. [Google Scholar] [CrossRef]
- Li, G.; Qian, S.; Lee, H.; Hwang, Y. Radermacher, Experimental investigation of energy and exergy performance of short term adsorption heat storage for residential application. Energy 2014, 65, 675–691. [Google Scholar] [CrossRef]
- Li, G.; Hwang, Y.; Radermacher, R. Experimental investigation on energy and exergy performance of adsorption cold storage for space cooling application. Int. J. Refrig. 2014, 44, 23–35. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, Y.; Cao, X.; Du, Y.; Zhang, Z.; Gui, Y. Latent Heat Thermal Energy Storage Systems with Solid-Liquid Phase Change Materials: A Review. Adv. Eng. Mater. 2018, 20, 1700753. [Google Scholar] [CrossRef]
- Li, G. Energy and exergy performance assessments for latent heat thermal energy storage systems. Renew. Sustain. Energy Rev. 2015, 51, 926–954. [Google Scholar] [CrossRef]
- Qiu, X.; Song, G.; Chu, X.; Li, X.; Tang, G. Microencapsulated n-alkane with p(n-butyl methacrylate-co-methacrylic acid) shell as phase change materials for thermal energy storage. Sol. Energy 2013, 91, 212–220. [Google Scholar] [CrossRef]
- Krupa, I.; Nógellová, Z.; Špitalský, Z.; Malíková, M.; Sobolčiak, P.; Abdelrazeq, H.W.; Ouederni, M.; Karkri, M.; Janigová, I.; Al-Maadeed, M.A.S. Positive influence of expanded graphite on the physical behavior of phase change materials based on linear low-density polyethylene and paraffin wax. Thermochim. Acta 2015, 614, 218–225. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Han, P.; Tang, G.; Song, G. Fabrication, thermal property and thermal reliability of microencapsulated paraffin with ethyl methacrylate-based copolymer shell. J. Therm. Anal. Calorim. 2016, 124, 1291–1299. [Google Scholar] [CrossRef]
- Puertas, A.M.; Romero-Cano, M.S.; Nieves, F.J.D.L.; Rosiek, S.; Batlles, F.J. Simulations of Melting of Encapsulated CaCl2·6H2O for Thermal Energy Storage Technologies. Energies 2017, 10, 568. [Google Scholar] [CrossRef]
- Smaisim, G.F.; Abed, A.M.; Hadrawi, S.K.; Shamel, A. Modeling and Thermodynamic Analysis of Solar Collector Cogeneration for Residential Building Energy Supply. J. Eng. 2022, 2022, 6280334. [Google Scholar] [CrossRef]
- Stritih, U.; Osterman, E.; Evliya, H.; Butala, V.; Paksoy, H. Exploiting solar energy potential through thermal energy storage in Slovenia and Turkey. Renew. Sustain. Energy Rev. 2013, 25, 442–461. [Google Scholar] [CrossRef]
- Smaisim, G.F.; Al-Madhhachi, H.; Abed, A.M. Study the thermal management of Li-ion batteries using looped heat pipes with different nanofluids. Case Stud. Therm. Eng. 2022, 37, 102227. [Google Scholar] [CrossRef]
- Alharbi, K.A.M.; Smaisim, G.F.; Sajadi, S.M.; Fagiry, M.A.; Aybar, H.; Elkhatib, S.E. Numerical study of lozenge, triangular and rectangular arrangements of lithium-ion batteries in their thermal management in a cooled-air cooling system. J. Energy Storage 2022, 52, 104786. [Google Scholar] [CrossRef]
- Li, Z.X.; Al-Rashed, A.A.; Rostamzadeh, M.; Kalbasi, R.; Shahsavar, A.; Afrand, M. Heat transfer reduction in buildings by embedding phase change material in multi-layer walls: Effects of repositioning, thermophysical properties and thickness of PCM. Energy Convers. Manag. 2019, 195, 43–56. [Google Scholar] [CrossRef]
- Tian, M.-W.; Smaisim, G.F.; Yan, S.-R.; Sajadi, S.M.; Mahmoud, M.Z.; Aybar, H.; Abed, A.M. Economic cost and efficiency analysis of a lithium-ion battery pack with the circular and elliptical cavities filled with phase change materials. J. Energy Storage 2022, 52, 104794. [Google Scholar] [CrossRef]
- Wu, W.; Smaisim, G.F.; Sajadi, S.M.; Fagiry, M.A.; Li, Z.; Shamseldin, M.A.; Aybar, H. Impact of phase change material-based heatsinks on lithium-ion battery thermal management: A comprehensive review. J. Energy Storage 2022, 52, 104874. [Google Scholar] [CrossRef]
- Tian, M.-W.; Abed, A.M.; Yan, S.-R.; Sajadi, S.M.; Mahmoud, M.Z.; Aybar, H.; Smaisim, G.F. Economic cost and numerical evaluation of cooling of a cylindrical lithium-ion battery pack using air and phase change materials. J. Energy Storage 2022, 52, 104925. [Google Scholar] [CrossRef]
- Jiang, Y.; Smaisim, G.F.; Mahmoud, M.Z.; Li, Z.; Aybar, H.; Abed, A.M. Simultaneous numerical investigation of the passive use of phase-change materials and the active use of a nanofluid inside a rectangular duct in the thermal management of lithium-ion batteries. J. Power Sources 2022, 541, 231610. [Google Scholar] [CrossRef]
- Hai, T.; Abidi, A.; Wang, L.; Abed, A.M.; Mahmoud, M.Z.; El Din, E.M.T.; Smaisim, G.F. Simulation of solar thermal panel systems with nanofluid flow and PCM for energy consumption management of buildings. J. Build. Eng. 2022, 58, 104981. [Google Scholar] [CrossRef]
- Dhaidan, N.S.; Kokz, S.A.; Rashid, F.L.; Hussein, A.K.; Younis, O.; Al-Mousawi, F.N. Review of solidification of phase change materials dispersed with nanoparticles in different containers. J. Energy Storage 2022, 51, 104271. [Google Scholar] [CrossRef]
- Rashid, F.L.; Hussein, A.K.; Malekshah, E.H.; Abderrahmane, A.; Guedri, K.; Younis, O. Review of Heat Transfer Analysis in Different Cavity Geometries with and without Nanofluids. Nanomaterials 2022, 12, 2481. [Google Scholar] [CrossRef] [PubMed]
- Rai, U.; Pandey, P. Solidification and thermal behaviour of binary organic eutectic and monotectic; succinonitrile–pyrene system. J. Cryst. Growth 2003, 249, 301–308. [Google Scholar] [CrossRef]
- Baskin, D.G. Fixation and Tissue Processing in Immunohistochemistry. In Pathobiology of Human Disease; Elsevier: San Diego, CA, USA, 2014; pp. 3797–3806. [Google Scholar]
- Nartowska, E.; Styś-Maniara, M.; Kozłowski, K. The Potential Environmental and Social Influence of the Inorganic Salt Hydrates Used as a Phase Change Material for Thermal Energy Storage in Solar Installations. Int. J. Environ. Res. Public Health 2023, 20, 1331. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, C.; Sreekumar, A. Phase change material based cold thermal energy storage: Materials, techniques and applications—A review. Int. J. Refrig. 2016, 67, 271–289. [Google Scholar] [CrossRef]
- Nie, B.; Palacios, A.; Zou, B.; Liu, J.; Zhang, T.; Li, Y. Review on phase change materials for cold thermal energy storage applications. Renew. Sustain. Energy Rev. 2020, 134, 110340. [Google Scholar] [CrossRef]
- Selvnes, H.; Allouche, Y.; Manescu, R.I.; Hafner, A. Review on cold thermal energy storage applied to refrigeration systems using phase change materials. Therm. Sci. Eng. Prog. 2020, 22, 100807. [Google Scholar] [CrossRef]
- Radouane, N. A Comprehensive Review of Composite Phase Change Materials (cPCMs) for Thermal Management Applications, Including Manufacturing Processes, Performance, and Applications. Energies 2022, 15, 8271. [Google Scholar] [CrossRef]
- Oró, E.; de Gracia, A.; Castell, A.; Farid, M.M.; Cabeza, L.F. Review on phase change materials (PCMs) for cold thermal energy storage applications. Appl. Energy 2012, 99, 513–533. [Google Scholar] [CrossRef]
- Khan, M.I.; Asfand, F.; Al-Ghamdi, S.G. Progress in research and development of phase change materials for thermal energy storage in concentrated solar power. Appl. Therm. Eng. 2023, 219, 119546. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Al-Sulaiman, F.A.; Ibrahim, N.I.; Zahir, H.; Al-Ahmed, A.; Saidur, R.; Yılbaş, B.S.; Sahin, A.Z. A review on current status and challenges of inorganic phase change materials for thermal energy storage systems. Renew. Sustain. Energy Rev. 2016, 70, 1072–1089. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Du, K.; Calautit, J.; Wang, Z.; Wu, Y.; Liu, H. A review of the applications of phase change materials in cooling, heating and power generation in different temperature ranges. Appl. Energy 2018, 220, 242–273. [Google Scholar] [CrossRef]
- Comodi, G.; Carducci, F.; Nagarajan, B.; Romagnoli, A. Application of cold thermal energy storage (CTES) for building demand management in hot climates. Appl. Therm. Eng. 2016, 103, 1186–1195. [Google Scholar] [CrossRef]
- Zou, T.; Fu, W.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Preparation and performance of form-stable TBAB hydrate/SiO2 composite PCM for cold energy storage. Int. J. Refrig. 2019, 101, 117–124. [Google Scholar] [CrossRef]
- Philip, N.; Dheep, G.R.; Sreekumar, A. Cold thermal energy storage with lauryl alcohol and cetyl alcohol eutectic mixture: Thermophysical studies and experimental investigation. J. Energy Storage 2020, 27, 101060. [Google Scholar] [CrossRef]
- Jebasingh, B.E.; Arasu, A.V. Characterisation and stability analysis of eutectic fatty acid as a low cost cold energy storage phase change material. J. Energy Storage 2020, 31, 101708. [Google Scholar] [CrossRef]
- Alkhwildi, A.; Elhashmi, R.; Chiasson, A. Parametric modeling and simulation of Low temperature energy storage for cold-climate multi-family residences using a geothermal heat pump system with integrated phase change material storage tank. Geothermics 2020, 86, 101864. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, W.; Fu, S.; Zhang, H. Study of a novel ceramsite-based shape-stabilized composite phase change material (PCM) for energy conservation in buildings. Constr. Build. Mater. 2020, 246, 118479. [Google Scholar] [CrossRef]
- Wang, T.; Zhou, X.; Liu, Z.; Wang, J.; Zhang, Y.; Pan, W.-P. Preparation of energy storage materials working at 20–25 °C as a cold source for long-term stable operation. Appl. Therm. Eng. 2020, 183, 116220. [Google Scholar] [CrossRef]
- Dong, X.; Gao, G.; Zhao, X.; Qiu, Z.; Li, C.; Zhang, J.; Zheng, P. Investigation on heat transfer and phase transition in phase change material (PCM) balls and cold energy storage tank. J. Energy Storage 2022, 50, 104695. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, W.; Liang, F. A review about phase change material cold storage system applied to solar-powered air-conditioning system. Adv. Mech. Eng. 2017, 9, 1687814017705844. [Google Scholar] [CrossRef]
- Jiang, J.-F.; Li, S.-F.; Liu, Z.-H. Study on heat transfer and cold storage characteristics of a falling film type of cold energy regenerator with PCM. Appl. Therm. Eng. 2018, 143, 676–687. [Google Scholar] [CrossRef]
- Said, M.; Hassan, H. Parametric study on the effect of using cold thermal storage energy of phase change material on the performance of air-conditioning unit. Appl. Energy 2018, 230, 1380–1402. [Google Scholar] [CrossRef]
- Sunxi, Z.; Xuelai, Z.; Sheng, L.; Yuyang, L.; Xiaofeng, X. Performance study on expand graphite/organic composite phase change material for cold thermal energy storage. Energy Procedia 2019, 158, 5305–5310. [Google Scholar] [CrossRef]
- Xie, N.; Li, Z.; Gao, X.; Fang, Y.; Zhang, Z. Preparation and performance of modified expanded graphite/eutectic salt composite phase change cold storage material. Int. J. Refrig. 2020, 110, 178–186. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Mariappan, V.; Anish, R.; Sarafoji, P.; Reddy, M.J.B. Experimental study on the charging and discharging behaviour of capric–lauric acid/oleic acid mixture in a cold thermal energy storage system for cold storage applications. Mater. Today Proc. 2021, 46, 10022–10029. [Google Scholar] [CrossRef]
- Ghodrati, A.; Zahedi, R.; Ahmadi, A. Analysis of cold thermal energy storage using phase change materials in freezers. J. Energy Storage 2022, 51, 104433. [Google Scholar] [CrossRef]
- Selvnes, H.; Allouche, Y.; Hafner, A.; Schlemminger, C.; Tolstorebrov, I. Cold thermal energy storage for industrial CO2 refrigeration systems using phase change material: An experimental study. Appl. Therm. Eng. 2022, 212, 118543. [Google Scholar] [CrossRef]
- Zheng, H.; Tian, G.; Zhao, Y.; Xin, X.; Yang, C.; Cao, L.; Ma, Y. Experimental study on the preparation and cool storage performance of a phase change micro-capsule cold storage material. Energy Build. 2022, 262, 111999. [Google Scholar] [CrossRef]
- Liu, Z.; Lou, F.; Qi, X.; Wang, Q.; Zhao, B.; Yan, J.; Shen, Y. Experimental study on cold storage phase-change materials and quick-freezing plate in household refrigerators. J. Food Process. Eng. 2019, 42, e13279. [Google Scholar] [CrossRef]
- Tas, C.E.; Unal, H. Thermally buffering polyethylene/halloysite/phase change material nanocomposite packaging films for cold storage of foods. J. Food Eng. 2020, 292, 110351. [Google Scholar] [CrossRef]
- Zhan, D.; Zhao, L.; Yu, Q.; Zhang, Y.; Wang, Y.; Li, G.; Lu, G.; Zhan, D.; Li, M. Phase change material for the cold storage of perishable products: From material preparation to material evaluation. J. Mol. Liq. 2021, 342, 117455. [Google Scholar] [CrossRef]
- Du, J.; Nie, B.; Zhang, Y.; Du, Z.; Wang, L.; Ding, Y. Cooling performance of a thermal energy storage-based portable box for cold chain applications. J. Energy Storage 2020, 28, 101238. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, N.; Jing, Y.; Cao, X.; Yuan, Y.; Haghighat, F. Experimental and numerical investigation on dodecane/expanded graphite shape-stabilized phase change material for cold energy storage. Energy 2019, 189, 116175. [Google Scholar] [CrossRef]
- Xiaofeng, X.; Xuelai, Z. Simulation and experimental investigation of a multi-temperature insulation box with phase change materials for cold storage. J. Food Eng. 2020, 292, 110286. [Google Scholar] [CrossRef]
- Liu, K.; He, Z.; Lin, P.; Zhao, X.; Chen, Q.; Su, H.; Luo, Y.; Wu, H.; Sheng, X.; Chen, Y. Highly-efficient cold energy storage enabled by brine phase change material gels towards smart cold chain logistics. J. Energy Storage 2022, 52, 104828. [Google Scholar] [CrossRef]
- Ma, K.; Zhang, X.; Ji, J.; Han, L. Development, characterization and modification of mannitol-water based nanocomposite phase change materials for cold storage. Colloids Surfaces A: Physicochem. Eng. Asp. 2022, 650, 129571. [Google Scholar] [CrossRef]
- Lin, N.; Li, C.; Zhang, D.; Li, Y.; Chen, J. Emerging phase change cold storage materials derived from sodium sulfate decahydrate. Energy 2022, 245, 123294. [Google Scholar] [CrossRef]
- Chang, Y.; Sun, Z. Synthesis and thermal properties of n-tetradecane phase change microcapsules for cold storage. J. Energy Storage 2022, 52, 104959. [Google Scholar] [CrossRef]
- Ikutegbe, C.A.; Al-Shannaq, R.; Farid, M.M. Microencapsulation of low melting phase change materials for cold storage applications. Appl. Energy 2022, 321, 119347. [Google Scholar] [CrossRef]
- Afsharpanah, F.; Pakzad, K.; Ajarostaghi, S.S.M.; Arıcı, M. Assessment of the charging performance in a cold thermal energy storage container with two rows of serpentine tubes and extended surfaces. J. Energy Storage 2022, 51, 104464. [Google Scholar] [CrossRef]
- Browne, M.C.; Norton, B.; McCormack, S.J. Heat retention of a photovoltaic/thermal collector with PCM. Sol. Energy 2016, 133, 533–548. [Google Scholar] [CrossRef]
- Hussain, S.I.; Dinesh, R.; Roseline, A.A.; Dhivya, S.; Kalaiselvam, S. Enhanced thermal performance and study the influence of sub cooling on activated carbon dispersed eutectic PCM for cold storage applications. Energy Build. 2017, 143, 17–24. [Google Scholar] [CrossRef]
- Sze, J.Y.; Mu, C.; Romagnoli, A.; Li, Y. Non-eutectic Phase Change Materials for Cold Thermal Energy Storage. Energy Procedia 2017, 143, 656–661. [Google Scholar] [CrossRef]
- Yu, C.; Yang, S.H.; Pak, S.Y.; Youn, J.R.; Song, Y.S. Graphene embedded form stable phase change materials for drawing the thermo-electric energy harvesting. Energy Convers. Manag. 2018, 169, 88–96. [Google Scholar] [CrossRef]
- Huang, Y.; She, X.; Li, C.; Li, Y.; Ding, Y. Evaluation of thermal performance in cold storage applications using EG-water based nano-composite PCMs. Energy Procedia 2019, 158, 4840–4845. [Google Scholar] [CrossRef]
- Talukdar, S.; Afroz, H.M.M.; Hossain, A.; Aziz, M.; Hossain, M. Heat transfer enhancement of charging and discharging of phase change materials and size optimization of a latent thermal energy storage system for solar cold storage application. J. Energy Storage 2019, 24, 100797. [Google Scholar] [CrossRef]
- Dhivya, S.; Hussain, S.I.; Sheela, S.J.; Kalaiselvam, S. Experimental study on microcapsules of Ag doped ZnO nanomaterials enhanced Oleic-Myristic acid eutectic PCM for thermal energy storage. Thermochim. Acta 2019, 671, 70–82. [Google Scholar] [CrossRef]
- Zou, T.; Liang, X.; Wang, S.; Gao, X.; Zhang, Z.; Fang, Y. Effect of expanded graphite size on performances of modified CaCl2·6H2O phase change material for cold energy storage. Microporous Mesoporous Mater. 2020, 305, 110403. [Google Scholar] [CrossRef]
- Borri, E.; Sze, J.Y.; Tafone, A.; Romagnoli, A.; Li, Y.; Comodi, G. Experimental and numerical characterization of sub-zero phase change materials for cold thermal energy storage. Appl. Energy 2020, 275, 115131. [Google Scholar] [CrossRef]
- Rakkappan, S.R.; Sivan, S.; Naarendharan, M.; Sudhir, P.S.; Preetham, D.S. Experimental Investigation on Enhanced Energy Storage Characteristics of Spherically Encapsulated 1-Decanol/Expanded Graphite Composite for Cold Storage System. J. Energy Storage 2021, 41, 102941. [Google Scholar] [CrossRef]
- Nie, B.; Chen, J.; Du, Z.; Li, Y.; Zhang, T.; Cong, L.; Zou, B.; Ding, Y. Thermal performance enhancement of a phase change material (PCM) based portable box for cold chain applications. J. Energy Storage 2021, 40, 102707. [Google Scholar] [CrossRef]
- Tafone, A.; Borri, E.; Cabeza, L.F.; Romagnoli, A. Innovative cryogenic Phase Change Material (PCM) based cold thermal energy storage for Liquid Air Energy Storage (LAES)—Numerical dynamic modelling and experimental study of a packed bed unit. Appl. Energy 2021, 301, 117417. [Google Scholar] [CrossRef]
- Rezaei, H.; Ghomsheh, M.J.; Kowsary, F.; Ahmadi, P. Performance assessment of a range-extended electric vehicle under real driving conditions using novel PCM-based HVAC system. Sustain. Energy Technol. Assessments 2021, 47, 101527. [Google Scholar] [CrossRef]
- Sarafoji, P.; Mariappan, V.; Anish, R.; Karthikeyan, K.; Kalidoss, P. Characterization and thermal properties of Lauryl alcohol—Capric acid with CuO and TiO2 nanoparticles as phase change material for cold storage system. Mater. Lett. 2022, 316, 132052. [Google Scholar] [CrossRef]
- Wang, F.; Xia, X.; Lv, Y.; Cheng, C.; Yang, L.; Zhang, L.; Zhao, J. Heat transfer, energy conversion, and efficiency during cold discharge of a novel tetrabutylammonium bromide hydrate cold storage system. Appl. Therm. Eng. 2022, 211, 118462. [Google Scholar] [CrossRef]
- Mousavi, S.B.; Ahmadi, P.; Hanafizadeh, P.; Khanmohammadi, S. Dynamic simulation and techno-economic analysis of liquid air energy storage with cascade phase change materials as a cold storage system. J. Energy Storage 2022, 50, 104179. [Google Scholar] [CrossRef]
- Wu, J.; Chen, Q.; Zhang, Y.; Sun, K. A novel design of discrete heat and cold sources for improving the thermal performance of latent heat thermal energy storage unit. J. Energy Storage 2022, 50, 104199. [Google Scholar] [CrossRef]
- Laouer, A.; Teggar, M.; Tunçbilek, E.; Arıcı, M.; Hachani, L.; Ismail, K.A. Melting of hybrid nano-enhanced phase change material in an inclined finned rectangular cavity for cold energy storage. J. Energy Storage 2022, 50, 104185. [Google Scholar] [CrossRef]
- Feng, J.; Ling, Z.; Huang, J.; Fang, X.; Zhang, Z. Experimental research and numerical simulation of the thermal performance of a tube-fin cold energy storage unit using water/modified expanded graphite as the phase change material. Energy Storage Sav. 2022, 1, 71–79. [Google Scholar] [CrossRef]
- Liu, G.; Li, Q.; Wu, J.; Xie, R.; Zou, Y.; Marson, A.; Scipioni, A.; Manzardo, A. Improving system performance of the refrigeration unit using phase change material (PCM) for transport refrigerated vehicles: An experimental investigation in South China. J. Energy Storage 2022, 51, 104435. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, R.; Ansu, A.; Goyal, R.; Sarı, A.; Tyagi, V. A comprehensive review on development of eutectic organic phase change materials and their composites for low and medium range thermal energy storage applications. Sol. Energy Mater. Sol. Cells 2021, 223, 110955. [Google Scholar] [CrossRef]
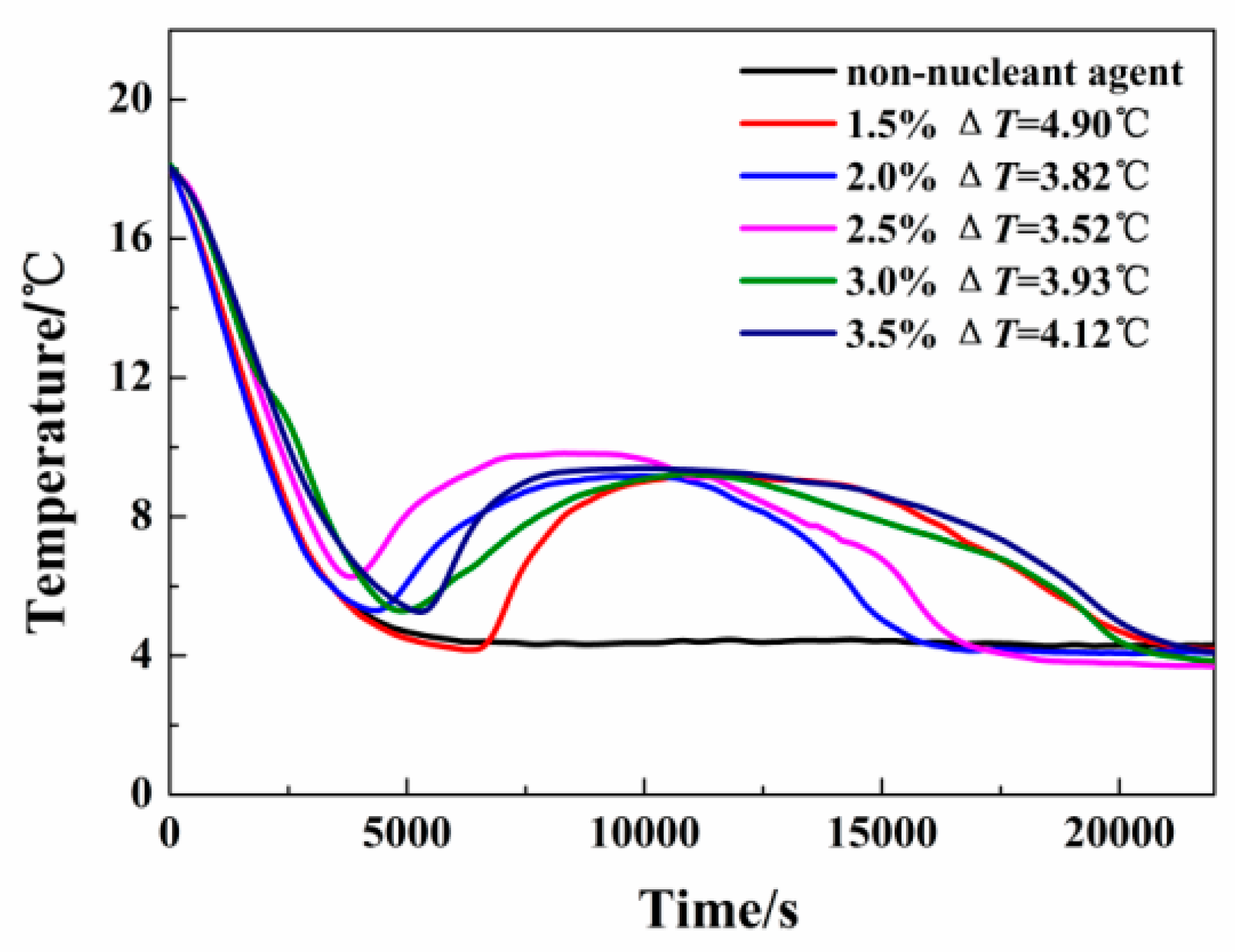
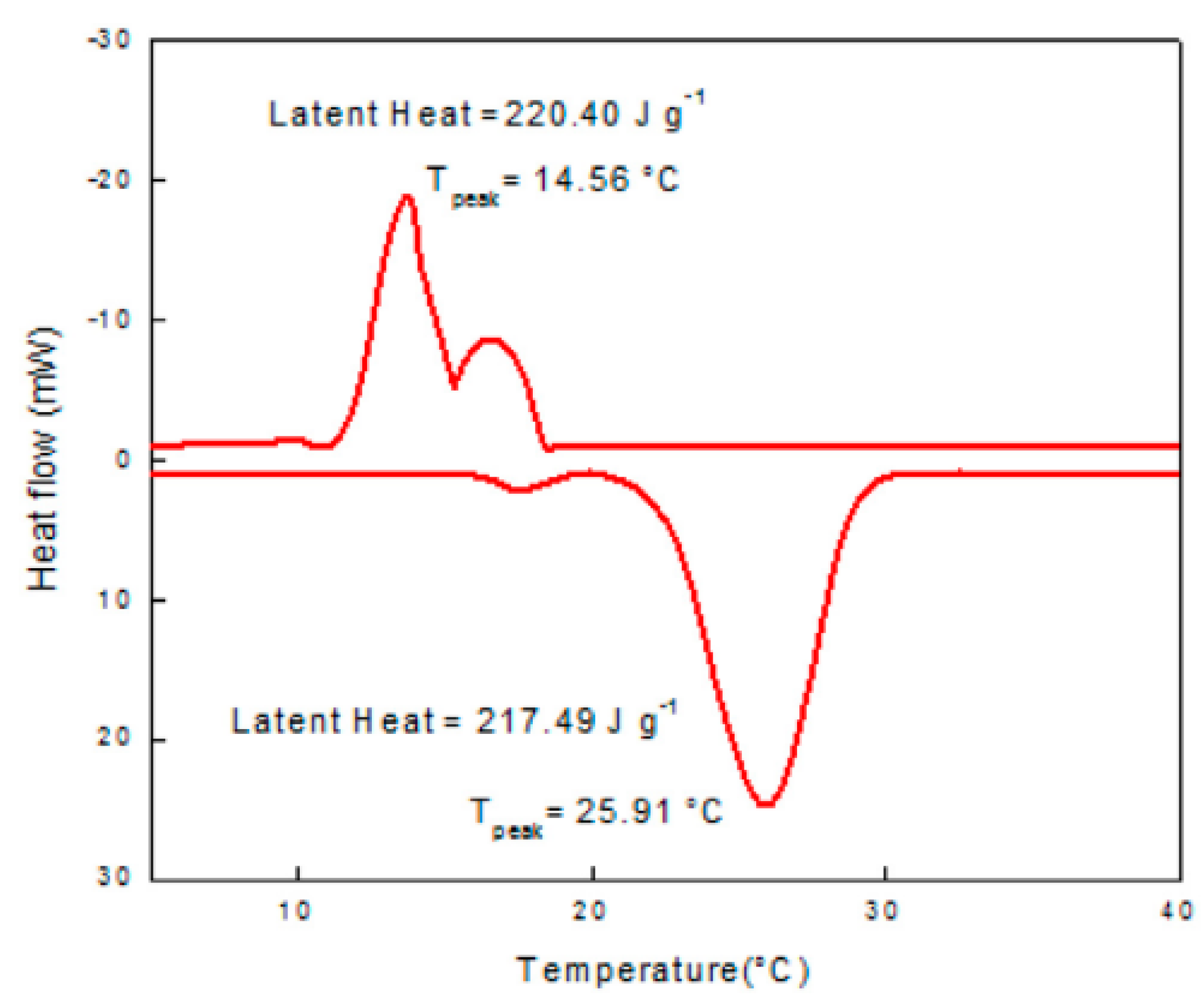

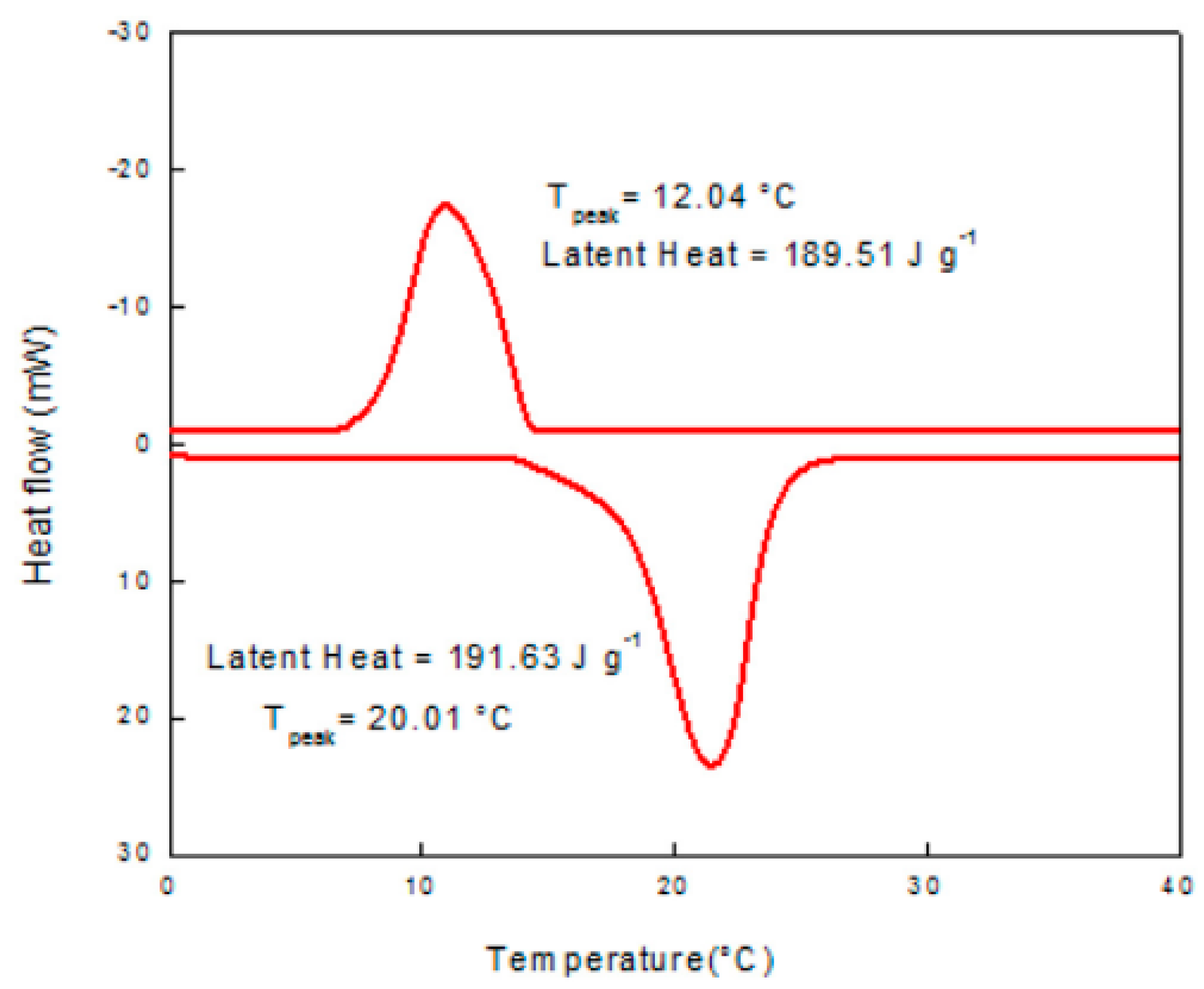


















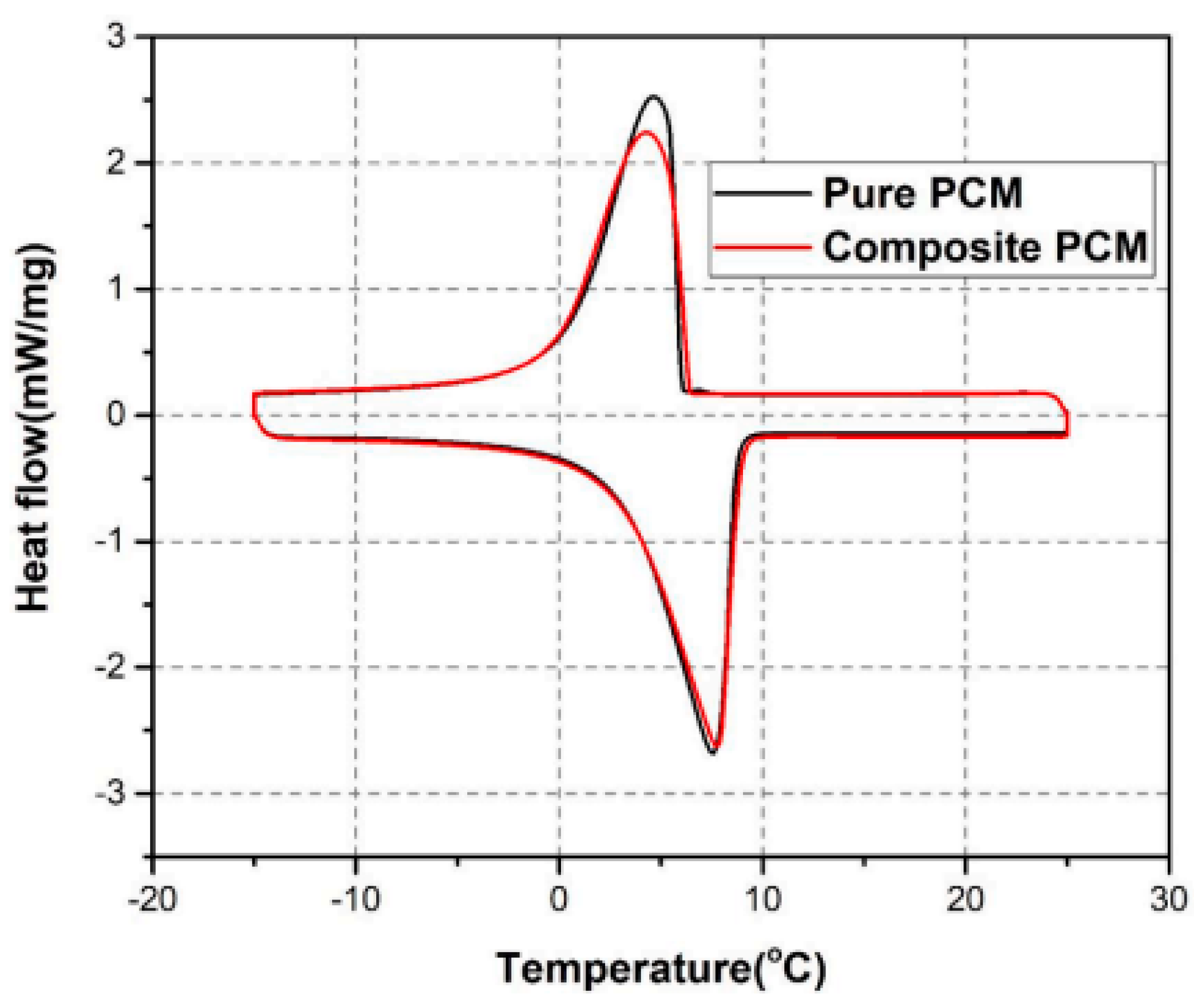
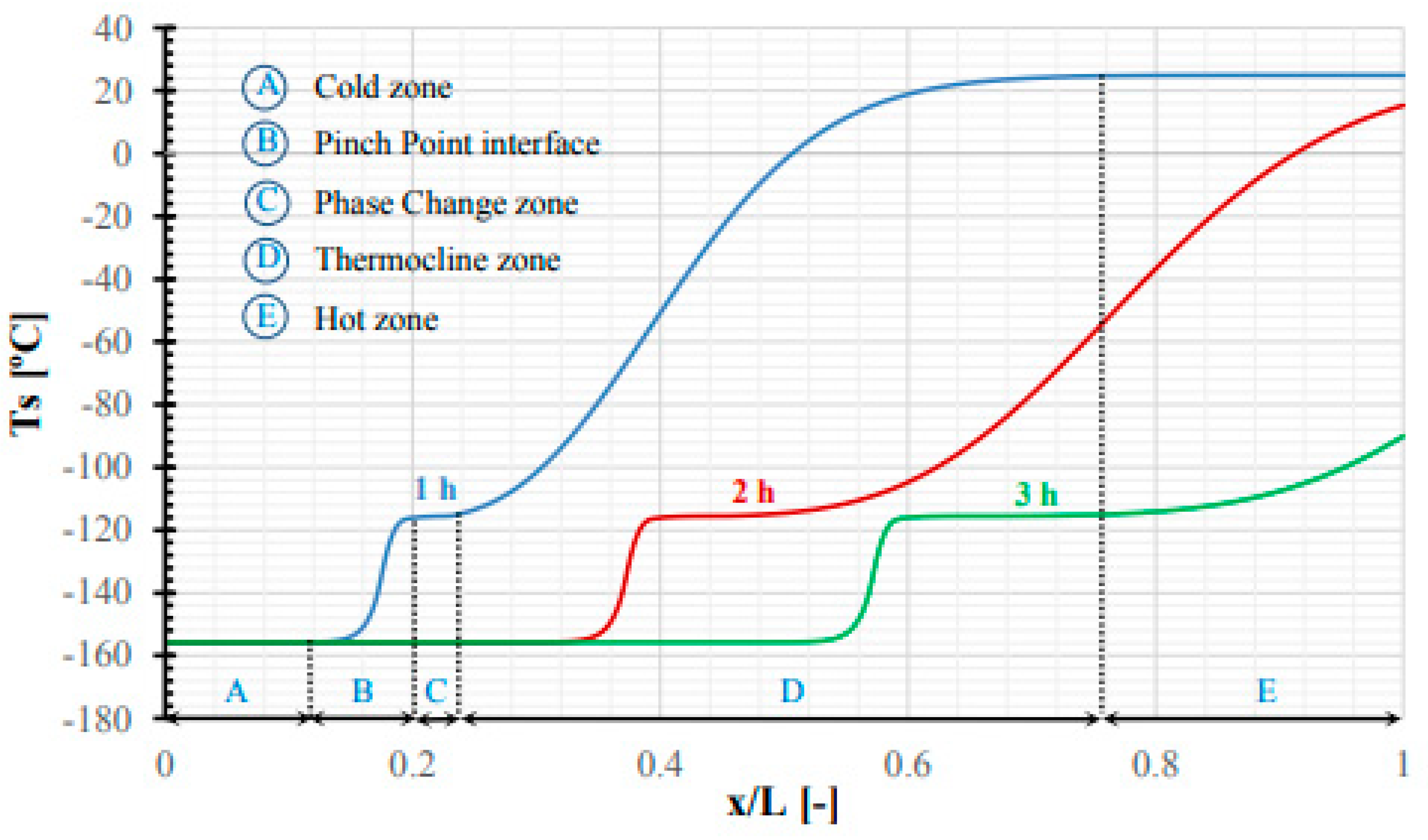
| Authors (Year) [Reference] | Configuration/Composition | Study Type | Studied Parameters | Highlighted Results/Findings |
|---|---|---|---|---|
| Zou et al. (2019) [37] | TBAB hydrate/fumed silica (SiO2) composite PCM. | Experimental | Effect of Na2HPO4·12H2O content on the super-cooling degree of TBAB hydrate and the nucleating agent on crystalline behaviour of TBAB hydrate. | The form-stable performance of the composite PCM with 30 percent SiO2 was outstanding, and there was no liquid leakage at temperatures over the melting point (8.33 °C). The latent heat of the composite PCM was 134 J/g, and the super-cooling degree was only 2.01 °C. |
| Philip et al. (2020) [38] | A eutectic mixture of lauryl alcohol and cetyl alcohol, with the ratio being 80:20. | Experimental | Effect of mixing lauryl alcohol and cetyl alcohol. | The 80:20 eutectic composition of lauryl alcohol and cetyl alcohol with melting temperature of 20.01 °C and latent heat of 191.63 J/g is fit for use in cold thermal energy storage. |
| Jebasingh et al. (2020) [39] | A mass ratio of 85:15 between capric acid (CA) and myristic acid (MA), both of which belong to the class of organic fatty acids. | Experimental | Effect of the mixing ratio on latent heat capacity, thermal conductivity, and stability. | The phase change temperature was found to be 20.86 °C, and the latent heat capacity was found to be 156.99 J/g. |
| Alkhwildi et al. (2020) [40] | Low- to moderate-temperature salt hydrate phase change material. | Experimental and Numerical | Tank volume, melt temperature, and GHX size. | The lowest PCM tank capacity was attained with a temperature of 27 °C for all of the GHX setups. |
| Yang et al. [41] | A binary eutectic PCM of lauryl alcohol, stearic acid, and nanoparticles denoted as LA–SA/Al2O3. | Experimental | The influence of mass fraction of LA–SA/Al2O3 on the mechanical properties. | The best ratio of LA–SA/Al2O3 is 82 wt.% LA + 18 wt.% SA with 0.5 wt% Al2O3 nanoparticles, with a melting temperature of 21.3 °C and a latent heat of 205.9 kJ/kg. |
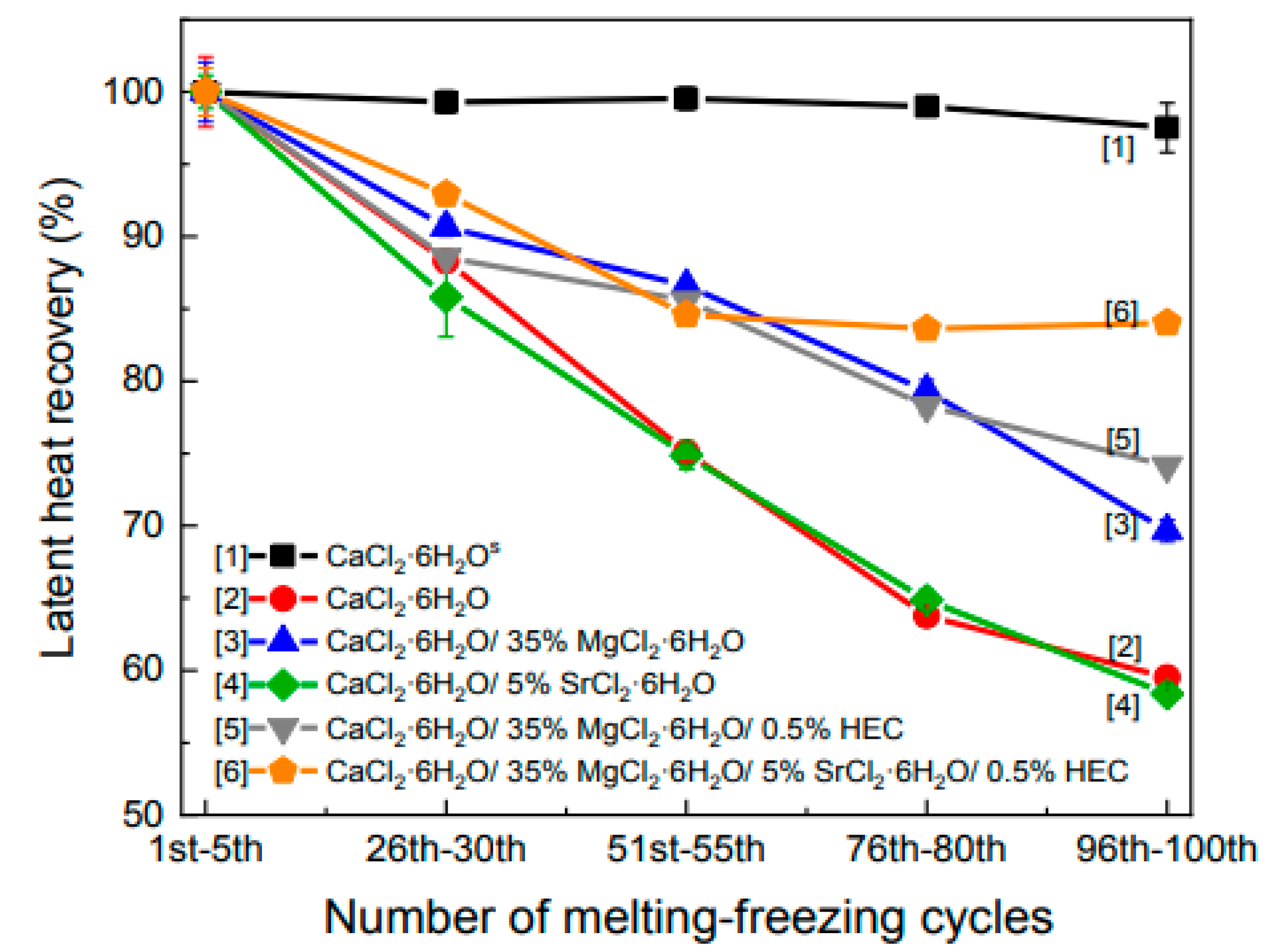
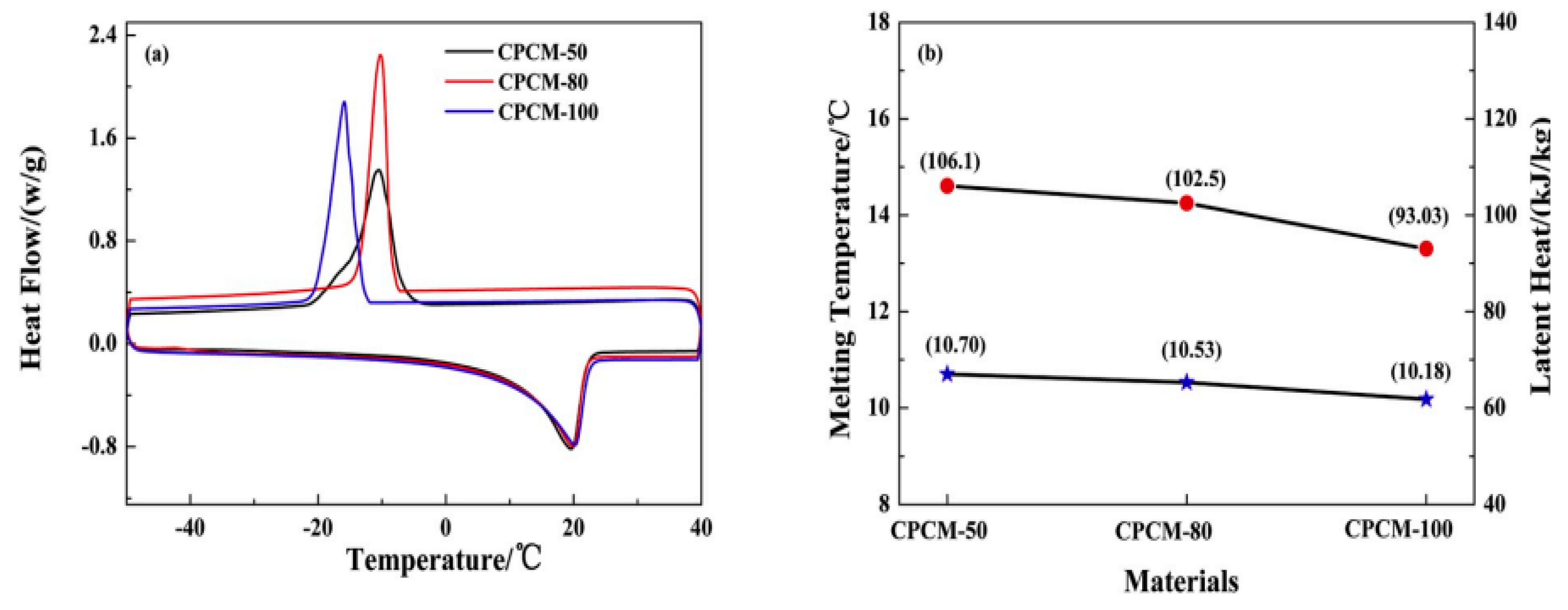
| Wang et al. (2021) [42] | SrCl2.6H2O was added to PCMs (CPCMs) of the CaCl2.6H2O/MgCl2.6H2O binary salt system in order to lower the super-cooling degree of inorganic PCM. | Experimental | Effect of adding MgCl2 6H2O and SrCl2 6H2O. | At a temperature of 23 °C, the phase transition temperature of the PCM was achieved by combining 35% MgCl2.6H2O, 5% SrCl2.6H2O, and 0.50% HEC in a solution that was balanced by CaCl2.6H2O. |
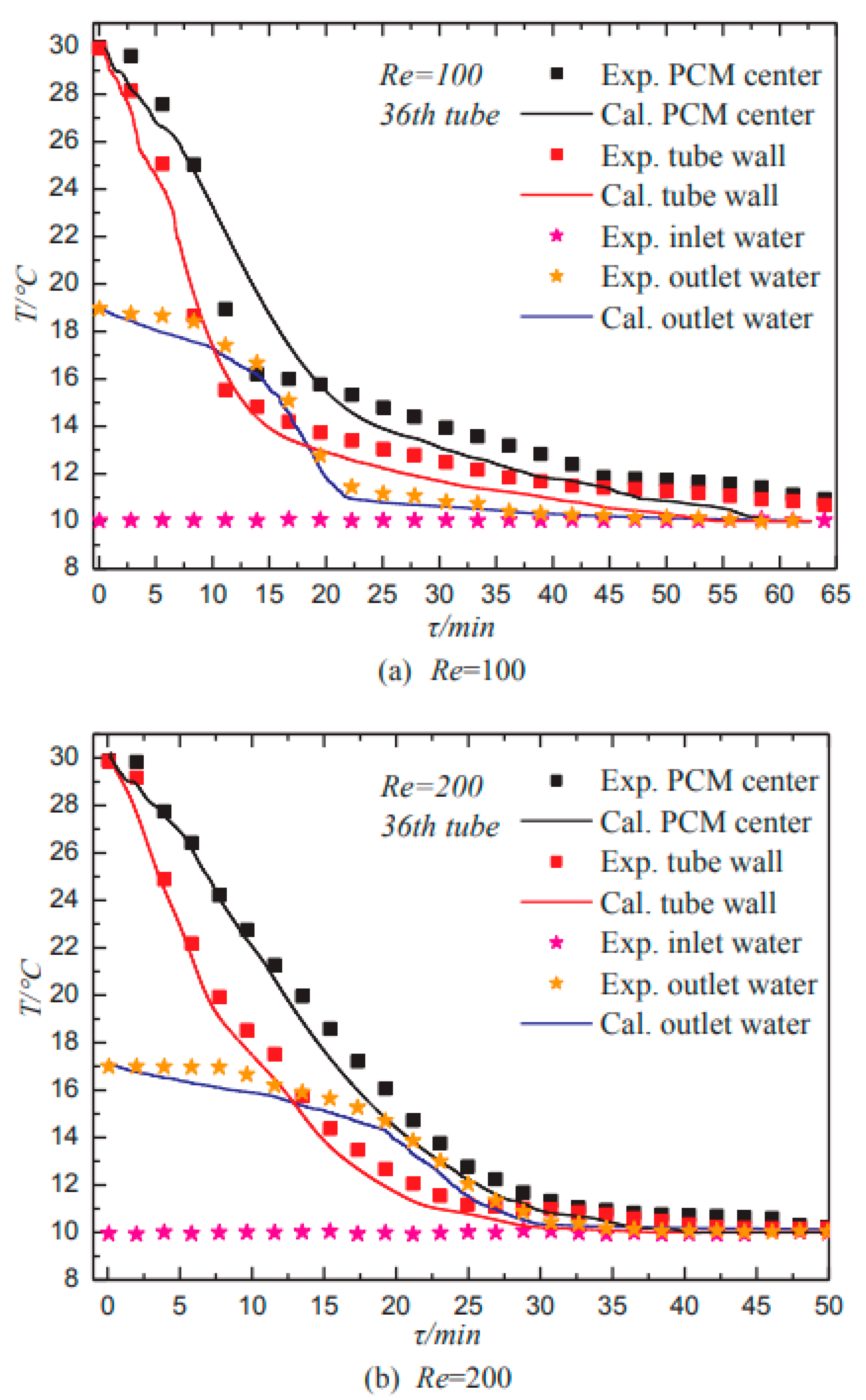
| Dong et al. (2022) [43] | Cold energy storage tank filled with multiple PCM balls. | Experimental and Numerical | Ball diameter of PCM and flow rate of chilled water. | A lower PCM ball diameter allows for a higher freezing rate to be obtained, which may be accomplished by increasing the flow velocity of cold water. |
| Authors (Year) [Reference] | Configuration/Composition | Study Type | Studied Parameters | Highlighted Results/Findings |
|---|---|---|---|---|
| Jiang et al. (2018) [45] | Mixture of decanoic acid, lauric acid, and oleic acid. | Experimental and Numerical | Pumping power and film Reynolds number. | The cold energy regenerator has excellent heat transfer performance as well as cold storage properties while using a minimal amount of pumping power. |
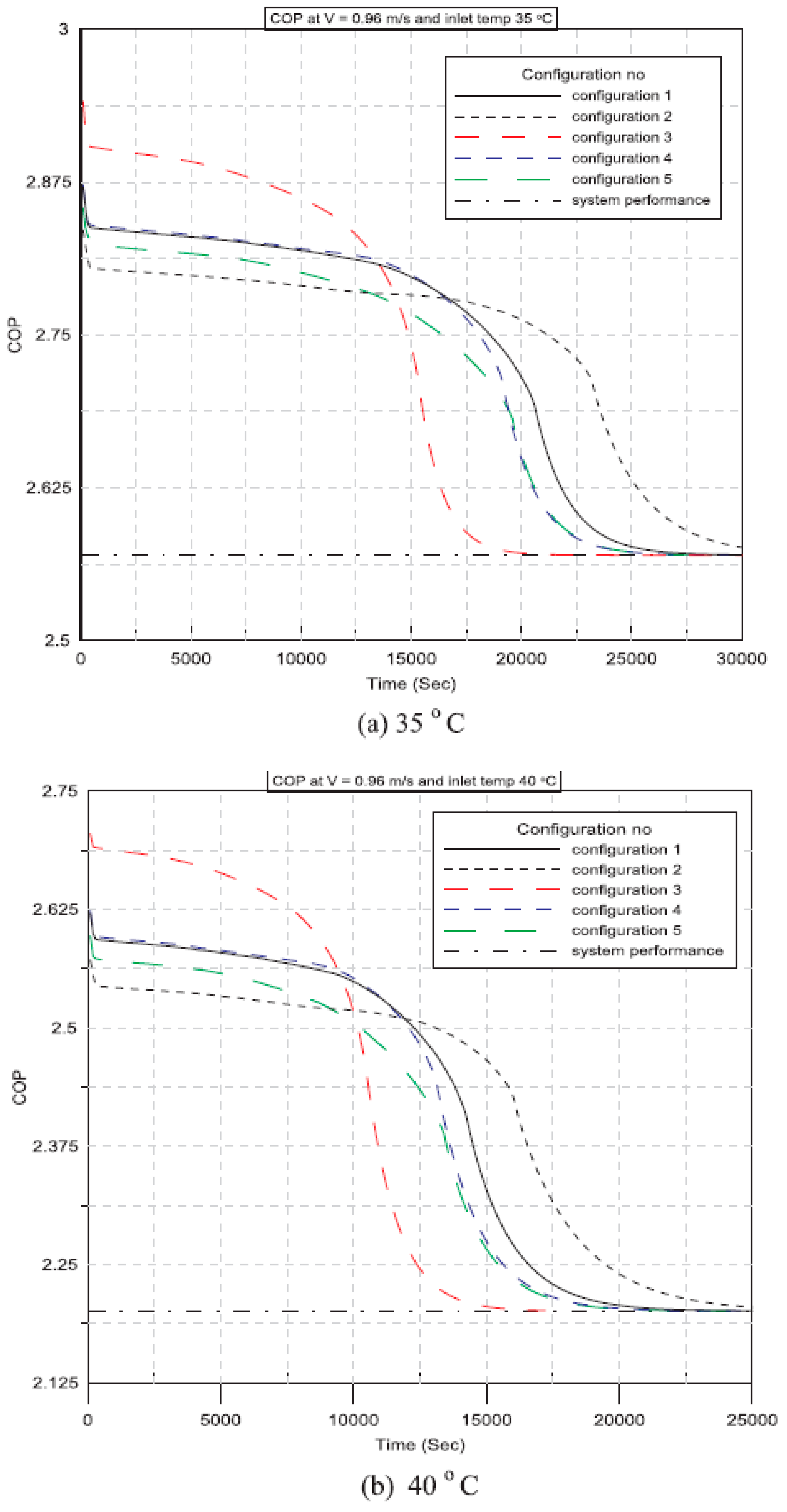
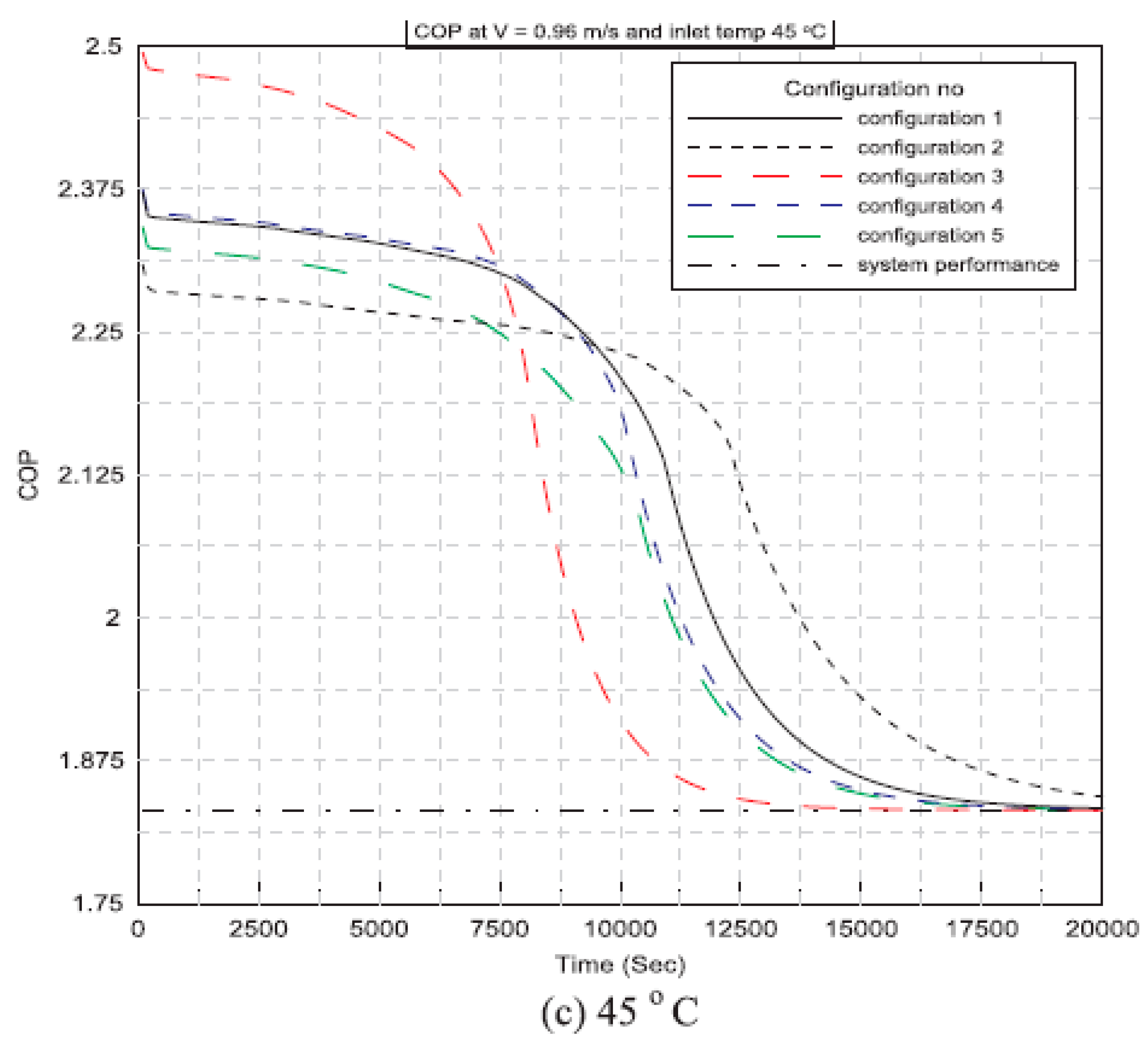
| Said et al. (2018) [46] | Integrating plates of PCM with a condenser of an air conditioning unit. | Experimental | Configurations of PCM plates, as well as the velocity and temperature of the air that enters the PCM plates, are all taken into consideration. | At various configurations, the PCM-equipped air conditioner outperforms the conventional unit by 14%, 13%, and 12%at inlet velocities of 0.96 m/s, 1.2 m/s, and 1.44 m/s, respectively for an inlet air temperature of 35 °C. |
| Sunxi et al. (2019) [47] | PCM of OA–MA and EG with the ideal mass ratio of 93:7 (OA–MA:EG). | Experimental | Effect of adding EG into OA–MA. | The OA–MA/EG composite PCM demonstrated a high degree of thermal resilience when subjected to thermal cycle testing. |
| Xie et al. (2020) [48] | K2HPO4·3H2O–NaH2PO4·2H2O–Na2S2O3·5H2O–H2O eutectic salt/MEG composite PCM. | Experimental | Mass fraction and thermal cycles. | Tests with a thermal cycle duration of 400 times indicated that the composite PCM had outstanding thermal reliability. |
| Karthikeyan et al. (2021) [49] | Capric–lauric acid/oleic acid combination. | Experimental | The inlet temperature and flow rate of HTF. | When the flow rate of HTF during the discharging process was increased, the melting rate increased by 16 percentage points in comparison to lower flow rates. On the other hand, despite the overall rise in flow rates, the charge rate did not shift considerably. When compared to the HTF intake temperature of −15 °C, the solidification of PCM at temperatures of −5 and −10 °C requires much more time. |
| Ghodrati et al. (2022) [50] | Two PCMs of water and ethylene glycol. | Experimental | Effect of using water and ethylene glycol as PCMs. | When water is used as the PCM for the first 100 min, the system only uses 63% of its total energy; however, when there is no PCM present, the system loses roughly 90.9% of its total energy after the first 100 min. When water is replaced with ethylene, the first 500 min of the reaction only results in the release of 35.97% of the system’s total potential energy. |
| Selvnes et al. (2022) [51] | Commercial PCM with phase change temperature of −9.6 ◦C. | Experimental | Effect of pitch of pillow plate heat exchanger on charging and discharging of PCM, refrigerant evaporation temperature. | The use of a plate pitch of 30 mm produced the greatest mean discharge rate and the highest total discharged energy throughout the course of the cycle with 9.79 kilowatts and 17.04 kilowatt hours, respectively. Charging time for the 30 mm design was risen by about 150% compared to when it was used at an evaporation temperature of −13 °C. |
| Zheng et al. (2022) [52] | To create phase change microcapsule suspensions with mass fractions of 5%, 10%, and 15%, PCMs were encapsulated and then combined with water and had strong thermal conductivity. | Experimental | Effect of adding wt.% of SDS, xanthan gum, and NaCl to deionized water. | 99.1% deionized water, 0.2% SDS, 0.2% xanthan gum, and 0.5% NaCl made the optimum base liquid. System cooling capacity dropped. Phase change cold storage offers 1.5 times the capacity of ice cold storage and performs better overall. |
| Authors (Year) [Reference] | Configuration/Composition | Study Type | Studied Parameters | Highlighted Results/Findings |
|---|---|---|---|---|
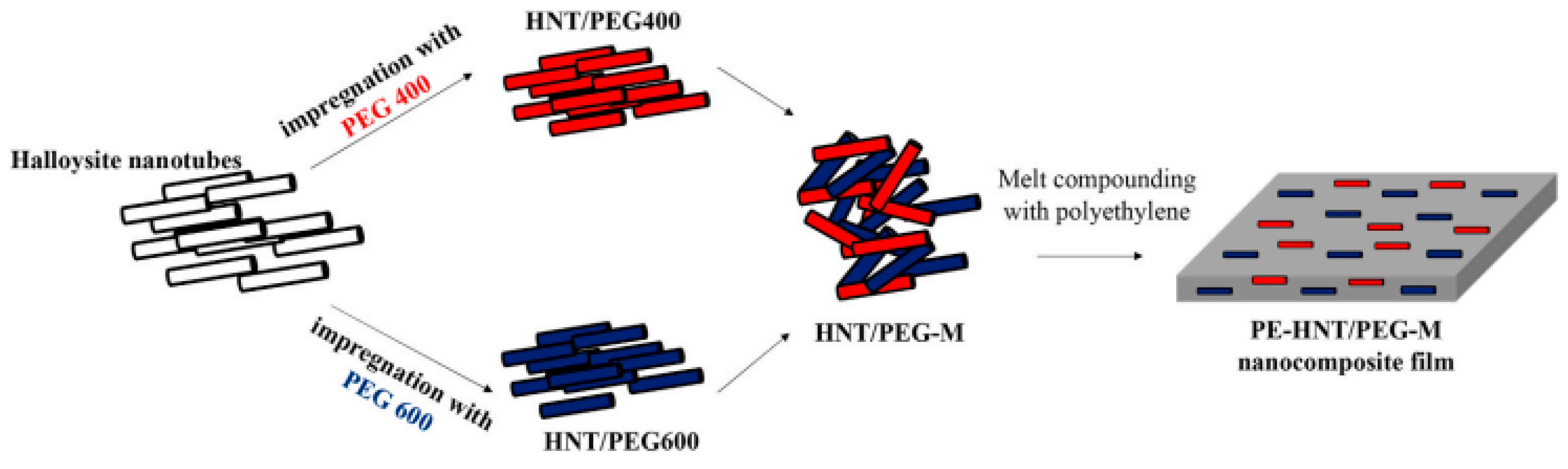
| Tas and Unal (2021) [54] | HNTs that have been impregnated with polymeric PCMs, as well as PEG400 and PEG600. | Experimental | PCM impregnation ratios. | Individual HNT/PEG400 and HNT/PEG600 nano-hybrids showed melting transitions between 22.0 °C and 6.4 °C and 6.0 °C and 19.0 °C, respectively. |
| Zhan et al. (2021) [55] | Adding edible additives (D-(+)-glucos, glycine, and D-sorbitol) to a 1% NaCl solution. | Experimental | The wt.% of glycine–1% NaCl solution. | After going through 50 cycles, the solution containing 5% glycine and 1% NaCl had a phase transition temperature of −6.72 °C and an activation energy (ΔH) of 275.79 J/g. Before and after cycling, there is not a discernible change in either the glycine concentration or the temperature at which the phase transition occurs in the solution that contains 5% glycine and 1% NaCl. |
| Authors (Year) [Reference] | Configuration/Composition | Study Type | Studied Parameters | Highlighted Results/Findings |
|---|---|---|---|---|
| Song et al. (2019) [57] | Dodecane as PCM and expanded graphite (EG) as the skeleton. | Experimental and Numerical | Effect of adding EG to dodecane. | The thermal conductivity of the dodecane/EG mixture is fifteen times greater than that of the dodecane alone. |
| Xiaofeng and Xuelai (2020) [58] | PCM1: (n-octanoic acid–myristic acid composite) and PCM2: (potassium sorbate–water composite). | Experimental and Numerical | The temperature of the cold storage material. | The amount of time that the box spends cooling and the temperature that it maintains both fluctuate but always stay within the parameters of what is considered to be an acceptable range for the purpose of preserving the quality of perishable goods. |
| Liu et al. (2022) [59] | Brine phase change material gels (BPCMGs). | Experimental | Stored cold energy. | When the total amount of cold energy stored is 691,800 J, the cold storage durations for aquatic goods and biological samples reach a maximum of 21.44 h and 16.37 h, respectively. |
| Ma et al. (2022) [60] | Utilizing sodium dodecyl benzene sulfonate (SDBS), polyacrylamide (PAM), and guar gum (GG), as well as adding MgCl2, nano-copper oxide (nano-CuO), and covalently modified hydroxylated multiwall carbon nanotubes (MWCNT-OH) were also employed. | Experimental | Effect of adding MgCl2, MWCNT-OH, SDBS, PAM, and GG. | The highest thermal performance was achieved by the phase change system consisting of mannitol/MgCl2@MWCNT-OH/PAM. This system’s thermal conductivity of 0.685 W/m K rose by 18.16%, and the amount of time spent in cold storage was cut by 57.3%. |
| Lin et al. (2022) [61] | PCM is derived from sodium sulphate decahydrate with the addition of KCl and NH4Cl, carboxymethyl cellulose (CMC), and borax (B). | Experimental | Effect of adding KCl and NH4Cl, CMC, and B to PCM. | Analyses of the composites’ microstructure as well as their chemical structures revealed that the produced materials have high levels of chemical compatibility. |
| Chang et al. (2022) [62] | Microcapsules of poly (urea-formaldehyde) (UF), where n-tetradecane is used as core material and SDS as an emulsifier. | Experimental | Temperature and mass fraction of microcapsules. | Latent heat functional fluid thermal conductivity rises with temperature and decreases with microcapsule mass fraction. Temperature or microcapsule fraction enhances its viscosity. |
| Ikutegbe et al. (2022) [63] | Microencapsulated PCM (m-PCM). | Experimental | Polymerisation time and optimum core–shell mass ratio. | Above 440 °C, the microcapsules began to decompose, whereas PT6 began to entirely evaporate at 240 °C. After 30 days of heating at 40 °C, the synthesised m-PCMs lost just 0.6% of their initial mass, which stabilized after 8 days of heating at that temperature. |
| Afsharpanah et al. (2022) [64] | A small ice container unit in the form of a cuboid, with two rows of serpentine tubes and connecting plates. | Numerical | The ratio of the serpentine tube pitch length to the container height (γ1), the ratio of the serpentine tube row distance to the container width (γ2), the ratio of the serpentine tube diameter to the container diagonal length (γ3), the ratio of the plate area to the maximum plate area (γ4), and the ratio of the plate thickness to the tube diameter (γ5). | Enhanced charging rates are the result of higher values for γ1, γ2, γ4, and γ5 and lower values for γ3. It was also discovered that using full-thickness plates (with a γ4 value of 1 and a γ5 value of 0.0081) results in an 18% boost in the time-averaged charging rate. |
| Authors (Year) [Reference] | Configuration/Composition | Study type | Studied Parameters | Highlighted Results/Findings |
|---|---|---|---|---|
| Browne et al. (2016) [65] | Novel PV/T/PCM system. | Experimental | Influence of using PCM. | Water temperature was roughly 5.5 °C higher than that of a PV/T system without PCM. |
| Hussain et al. (2017) [66] | PCM in a base fluid that contains 0.1 weight percent of activated carbon. | Experimental | Effect of adding highly porous activated carbon (AC) nano-sheets. | When compared to pure eutectic, the highest time savings was 54%, and the heat transfer rate increased linearly from 0.02 wt.% to 0.1 wt.% for nano-dispersed PCM. |
| Sze et al. (2017) [67] | Aqueous solutions of ethylene glycol and ethanol, with a stable nano-filler consisting of graphene oxide powder at a concentration of 1% by weight. | Experimental | Effect of adding graphene oxide on super-cooling, melting, and latent heat of fusion. | After adding 1 wt.% graphene oxide, super-cooling degrees for 49.5 wt.% ethylene glycol dropped by 61% from 9 °C to 3 °C. Melt beginning and peak temperatures are now −53 °C and −36 °C, respectively. The latent heat of fusions was reduced by 12% for 50% ethylene glycol and 6% for 60% ethanol. |
| Yu et al. (2018) [68] | An energy harvesting system was developed by integrating two PCMs (PEG and 1-TD) with N- and P-type semiconductor while utilising graphene nano-platelets as a supporter and promoting the shape stability. | Experimental and Numerical | The electric current measured from the energy harvesting device was studied for variable environmental temperatures. | The maximum harvesting current was 10 mA for both the heating and cooling processes, and the harvesting fields were maintained for 1900 and 850 s, respectively. |
| Huang et al. (2019) [69] | After adding EG of varying concentrations to the water, MCNT at concentrations of 0.0625%, 0.125%, 0.25%, and 0.5% was then distributed into each of the EG-water-based fluids. | Experimental | EG concentration and particle volume fraction. | Due to the very low overall concentration of MCNT, the addition of MCNT has a negligible impact on the latent heat and melting point of the material. Additionally, the quantity of MCNT that is concentrated in EG–water-based fluids causes a rise in the thermal conductivity of the fluids. |
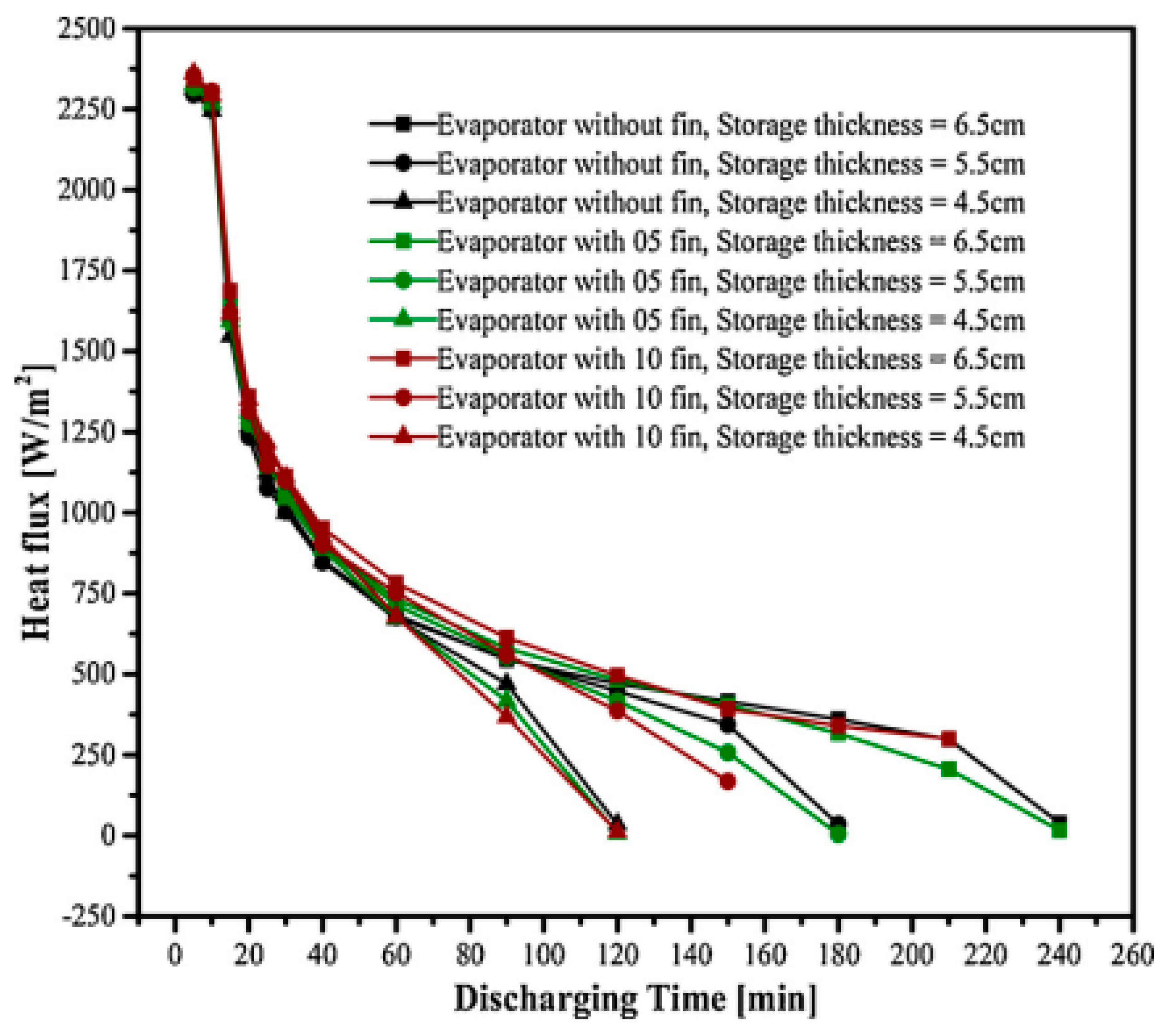
| Talukdar et al. (2019) [70] | PCM pack occupied by PCM acts as a heat exchanger. | Experimental and Numerical | Effect of longitudinal aluminium fins inside the PCM pack and thickness of PCM pack. | The PCM pack with a thickness of 6.5 cm and a greater number of fins was found to have a larger energy storage capacity as well as a higher heat flow during the melting process. |
| Dhivya et al. [71] | Microcapsules of silver (Ag)-doped ZnO nanoparticles with different mass fractions distributed in eutectic PCM containing oleic/myristic acid. | Experimental | Explore the effect of the weight percentage of nanoparticles added to PCMs for improving the thermal conductivity. | The thermal conductivity increases by 2.78%, 27.17%, 37.56%, and 48.62%, due to adding 0.05 wt.%, 0.1 wt.%, 0.15 wt.%, and 0.2 wt.%, respectively. |
| Zou et al. (2020) [72] | EG added to CaCl2 6H2O PCM. | Experimental | Mass fraction and size of EG. | The impact of EG-50 in maintaining the form was deemed to be superior to that of EG80 and EG-100. The disparity in thermal performances of CPCMs was brought on by the dissimilarities in the sizes of their EG components. |
| Borri et al. (2020) [73] | Aqueous sodium chloride, aqueous ethylene glycol, and decane. | Experimental and Numerical | Type of PCM. | According to the trials, aqueous alcohol showed the greatest level of agreement. |
| Rakkappan et al. (2021) [74] | Composite made of graphite that has been expanded with 1-decanol (CPCM). | Experimental | Macro-encapsulated diameter and wall temperature. | The optimal wall temperature for charging is determined to be −3 °C, whereas the optimal wall temperature for discharging is found to be 13 °C. Both the charging and discharging rates are higher with larger diameters. |
| Nie et al. (2021) [75] | Paraffin-based PCM (RT 5), fumed silica, and graphene. | Experimental | wt.% of graphene and fumed silica. | The composite’s heat conductivity rose by 55.4% when graphene at a weight fraction of 1% was added to it. The PCM leakage was able to be stopped with the addition of 4 weight percent of fumed silica. By using silica and graphene, one may improve the nucleation process of phase change kinetics and lower the amount of PCM super-cooling that occurs. |
| Tafone et al. (2021) [76] | Novel cryogenic HGCS packed bed filled with PCM. | Experimental and Numerical | Effect of introducing a PCM in the HGCS. | The incorporation of a PCM into the HGCS helps to reduce the thermocline effect that is seen in the SH configuration. This results in the following advantages over SH design: a longer discharge phase and lower specific consumption (0.272 kWhe/kgLA as opposed to 0.330 kWhe/kgLA). |
| Rezaei et al. (2021) [77] | A novel HVAC system made up of a heat pump and an outside heat exchanger that runs parallel to a shell-and-tube heat exchanger that uses N-hexadecane PCM and R134a as the refrigerant in the tubes. | Experimental and Numerical | Effect of the specific melting point of N-hexadecane PCM on compressor’s power consumption and overall performance of the vehicle | In comparison to traditional heat pump systems, the system that is suggested enhanced the vehicle’s range by 19% at 10 °C and by 11% at 0 °C, according to the results. |
| Sarafoji et al. (2022) [78] | In the beginning, the PCM mixture comprised a binary mixture of lauryl alcohol and capric acid (LA–CA) with 53:47 wt.%; afterwards, TiO2 and CuO nanoparticles were added to the mixture at 0.25 wt.%. | Experimental | Effect of weight % lauryl alcohol–capric acid and the TiO2 and CuO nanoparticles. | The thermal conductivity of the suggested LA–CA/CuO PCM is increased by 17.56% with the incorporation of nanoparticles. The PCM has a melting temperature of 8.7 °C and a latent heat of 159.1 J/g. Even after 1000 cycles, the thermal and chemical stability of the suggested LA–CA/CuO PCM remains outstanding. This is due to the PCM’s high copper content. |
| Wang et al. (2022) [79] | Tetrabutylammonium bromide (TBAB) hydrate cold storage system. | Experimental | Gas disturbance, cold charge temperature, and flow rate. | Gas disturbance forced convection, increased cold discharge capacity, and shortened discharge time by a factor of three. Increasing flow rate and reducing cold charge temperature enhanced cold discharge capacity. Under diverse settings, cold discharge efficiency was 83%. |
| Mousavi et al. (2022) [80] | System for the storage of liquid air energy (LAES), which makes use of a packed bed for thermal energy storage (PBTES). | Numerical | Cycles of the system. | The system with a general efficacy of 42.5% reaches the equilibrium state after about 22 cycles, and transient behaviour causes the performance of the system to decline by around 5.9% in comparison to the optimum cycle. |
| Wu et al. (2022) [81] | LHTES unit that has separate heat and cold sources, including symmetrical and staggered layouts with side-heated and bottom-heated LHTES applications. | Numerical | Arrangements of heat and cold sources. | The side-heated and symmetrical arrangement reduced the heat storage and release cycle by 49.9%, advanced the steady-state period by 46.6%, raised the steady-state liquid percentage by 4.2%, and enhanced the heat transfer rate by 22.3%. The separate source configuration did not increase bottom-heated LHTES performance. |
| Laouer et al. (2022) [82] | Enhancing the melting process of PCM (water) in an inclined rectangular container by adding fins and hybrid nanoparticles made of copper and aluminium oxide. | Numerical | Number of fins, fin length ratio, and nanoparticle volume fraction. | When compared to a ratio of W/H equal to 0.25, the rate of melting is about two times greater when the fin length ratio is W/H = 0.75. In the case of three fins and a nanoparticle concentration of ϕ= 6 vol.%, the melting rate may be increased to its maximum potential, resulting in a time reduction in the full melting process by 33.5%. |
| Feng et al. (2022) [83] | Water and modified expanded graphite (MEG). | Experimental and Numerical | Heat exchanger fin spacing and wt.% water/MEG. | Heat exchanger fin spacing did not affect the cold energy storage unit, but tube pass spacing did. The system’s cooling capacity, cooling duration, and average power were 80.8%, 69.7%, and 15.9% greater than those of pure water, respectively. |
| Liu et al. (2022) [84] | A ground-breaking phase change cold storage unit (PCCSU) was developed as a mobile refrigeration unit for the transport of refrigerated vehicles | Experimental | In a hot summer with an average ambient temperature of 29 °C, the average air temperature of the inner compartment could be maintained at 12.3 °C for 16.6 h, 14.5 °C for 14.7 h, and 16.5 °C for 10 h. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashid, F.L.; Al-Obaidi, M.A.; Dulaimi, A.; Bernardo, L.F.A.; Redha, Z.A.A.; Hoshi, H.A.; Mahood, H.B.; Hashim, A. Recent Advances on The Applications of Phase Change Materials in Cold Thermal Energy Storage: A Critical Review. J. Compos. Sci. 2023, 7, 338. https://doi.org/10.3390/jcs7080338
Rashid FL, Al-Obaidi MA, Dulaimi A, Bernardo LFA, Redha ZAA, Hoshi HA, Mahood HB, Hashim A. Recent Advances on The Applications of Phase Change Materials in Cold Thermal Energy Storage: A Critical Review. Journal of Composites Science. 2023; 7(8):338. https://doi.org/10.3390/jcs7080338
Chicago/Turabian StyleRashid, Farhan Lafta, Mudhar A. Al-Obaidi, Anmar Dulaimi, Luís Filipe Almeida Bernardo, Zeina Ali Abdul Redha, Hisham A. Hoshi, Hameed B. Mahood, and Ahmed Hashim. 2023. "Recent Advances on The Applications of Phase Change Materials in Cold Thermal Energy Storage: A Critical Review" Journal of Composites Science 7, no. 8: 338. https://doi.org/10.3390/jcs7080338
APA StyleRashid, F. L., Al-Obaidi, M. A., Dulaimi, A., Bernardo, L. F. A., Redha, Z. A. A., Hoshi, H. A., Mahood, H. B., & Hashim, A. (2023). Recent Advances on The Applications of Phase Change Materials in Cold Thermal Energy Storage: A Critical Review. Journal of Composites Science, 7(8), 338. https://doi.org/10.3390/jcs7080338










