Nanocellulose as an Avenue for Drug Delivery Applications: A Mini-Review
Abstract
1. Introduction
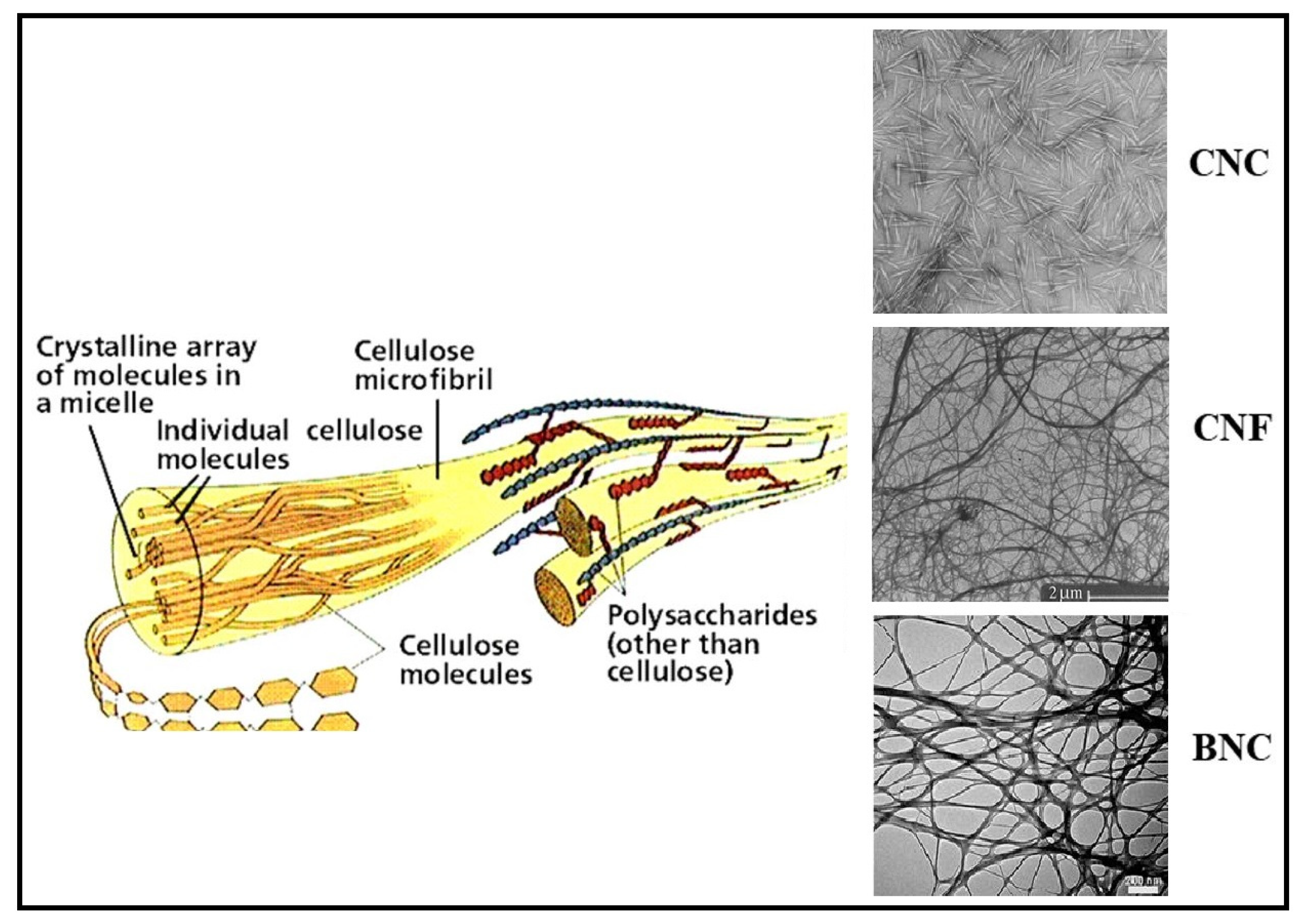
2. Nanocellulose
2.1. Cellulose Nanocrystals (CNC)
2.2. Cellulose Nanofibrils (CNF)
2.3. Bacterial Nanocellulose (BNC)
3. Surface Modification of Nanocellulose for Drug Delivery
4. Applications of Nanocellulose in Drug Delivery
4.1. Oral Drug Delivery
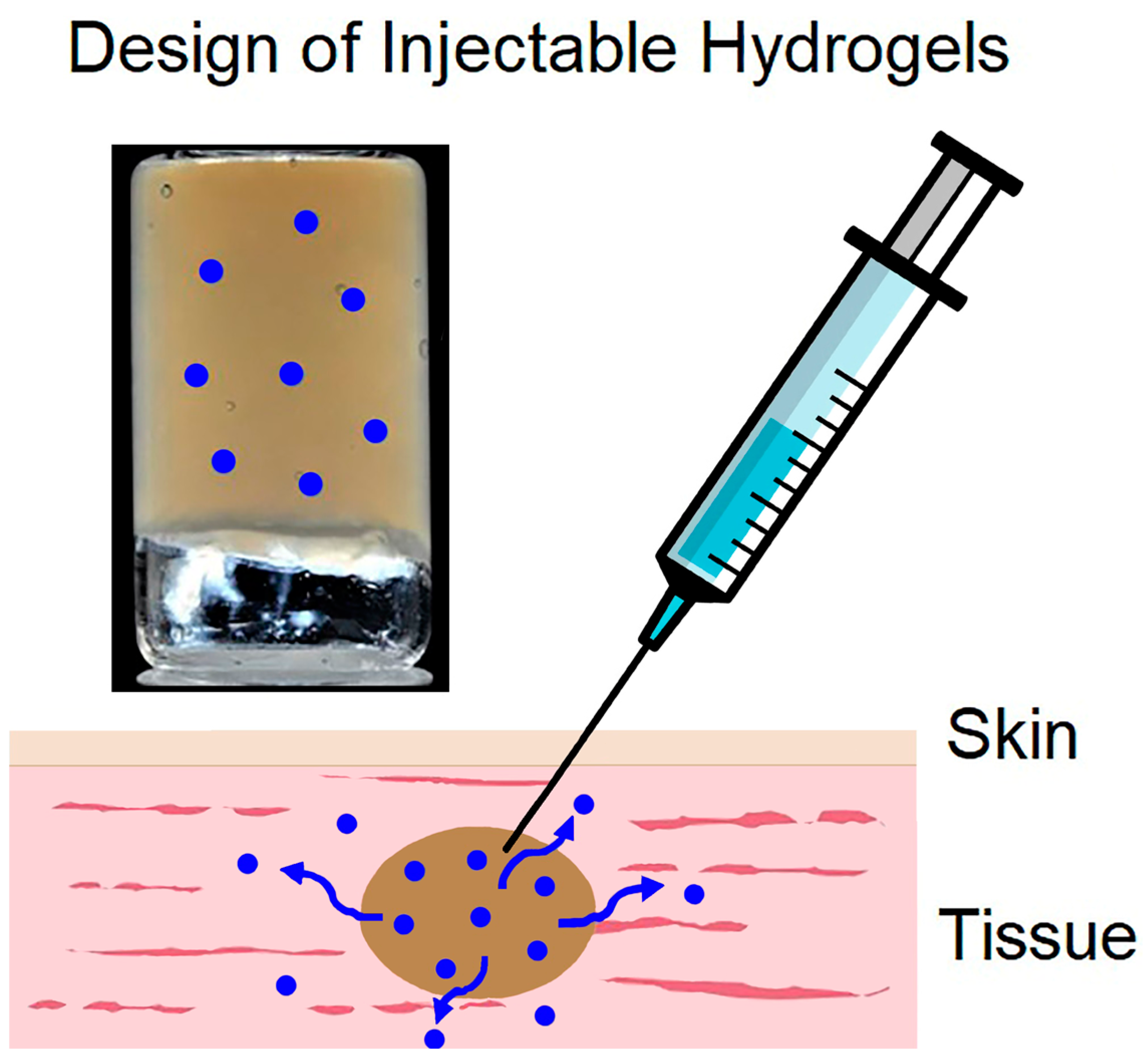
4.2. Local Application
4.3. Transdermal Delivery
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raghav, N.; Sharma, M.R.; Kennedy, J.F. Nanocellulose: A mini-review on types and use in drug delivery systems. Carbohydrate Polymer Technologies and Applications. Carbohydr. Polym. Technol. Appl. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of various lignocellulosic biomass for the production of nanocellulose: A comparative study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Panchal, P.; Ogunsona, E.; Mekonnen, T. Trends in advanced functional material applications of nanocellulose. Processes 2018, 7, 10. [Google Scholar] [CrossRef]
- Dhali, K.; Ghasemlou, M.; Daver, F.; Cass, P.; Adhikari, B. A review of nanocellulose as a new material towards environmental sustainability. Sci. Total Environ. 2021, 775, 145871. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, S., Dufresne, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; pp. 1–49. [Google Scholar]
- Kumar Gupta, P.; Sai Raghunath, S.; Venkatesh Prasanna, D.; Venkat, P.; Shree, V.; Chithananthan, C.; Choudhary, S.; Surender, K.; Geetha, K. An Update on Overview of Cellulose, Its Structure and Applications. Cellulose 2019, 201, 84727. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose Iα from Synchrotron X-ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- Huo, Y.; Liu, Y.; Xia, M.; Du, H.; Lin, Z.; Li, B.; Liu, H. Nanocellulose-based composite materials used in drug delivery systems. Polymers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Sanga Pachuau, L. A mini review on plant-based nanocellulose: Production, sources, modifications and its potential in drug delivery applications. Mini Rev. Med. Chem. 2015, 15, 543–552. [Google Scholar] [CrossRef]
- Gumrah Dumanli, A. Nanocellulose and its composites for biomedical applications. Curr. Med. Chem. 2017, 24, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Salimi, S.; Sotudeh-Gharebagh, R.; Zarghami, R.; Chan, S.Y.; Yuen, K.H. Production of nanocellulose and its applications in drug delivery: A critical review. ACS Sustain. Chem. Eng. 2019, 7, 15800–15827. [Google Scholar] [CrossRef]
- Khine, Y.Y.; Stenzel, M.H. Surface modified cellulose nanomaterials: A source of non-spherical nanoparticles for drug delivery. Mater. Horiz. 2020, 7, 1727–1758. [Google Scholar] [CrossRef]
- Lee, K.Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27. [Google Scholar] [CrossRef]
- Chu, Y.; Sun, Y.; Wu, W.; Xiao, H. Dispersion Properties of Nanocellulose: A Review. Carbohydr. Polym. 2020, 250, 116892. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today Adv. 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392–425. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.T. Nanocellulose, a versatile platform: From the delivery of active molecules to tissue engineering applications. Bioact. Mater. 2022, 9, 566–589. [Google Scholar] [CrossRef]
- Hernandez, C.C.; dos Santos Rosa, D. Extraction of cellulose nanowhiskers: Natural fibers source, methodology and application. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Mendez-Vilas, A., Solano, A., Eds.; Formatex Research Center S.L.: Badajoz, Spain, 2016; pp. 232–242. [Google Scholar]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and antimicrobial cellulose film from ginger nanofiber. Food Hydrocoll. 2020, 98, 105266. [Google Scholar] [CrossRef]
- Deb Dutta, S.; Patel, D.K.; Ganguly, K.; Lim, K.T. Isolation and characterization of cellulose nanocrystals from coffee grounds for tissue engineering. Mater. Lett. 2021, 287, 129311. [Google Scholar] [CrossRef]
- Lubis, R.; Wirjosentono, B.; Septevani, A.A. Preparation, characterization and antimicrobial activity of grafted cellulose fiber from durian rind waste. Colloids Surf. A Physicochem. Eng. Asp. 2020, 604, 125311. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Wang, S.; Ma, L.; Yu, Y.; Dai, H.; Zhang, Y. Extraction and comparison of cellulose nanocrystals from lemon (Citrus limon) seeds using sulfuric acid hydrolysis and oxidation methods. Carbohydr. Polym. 2020, 238, 116180. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, H.; He, Y.; Fei, X.; Peng, L. Preparation and characterization of carboxymethyl cellulose-based composite films reinforced by cellulose nanocrystals derived from pea hull waste for food packaging applications. Int. J. Biol. Macromol. 2020, 164, 4104–4112. [Google Scholar] [CrossRef]
- Onkarappa, H.S.; Prakash, G.K.; Pujar, G.H.; Rajith Kumar, C.R.; Latha, M.S.; Betageri, V.S. Hevea brasiliensis mediated synthesis of nanocellulose: Effect of preparation methods on morphology and properties. Int. J. Biol. Macromol. 2020, 160, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Abraham, E.; Deepa, B.; Pothen, L.A.; Cintil, J.; Thomas, S.; John, M.J.; Anandjiwala, R.; Narine, S.S. Environmental friendly method for the extraction of coir fibre and isolation of nanofibre. Carbohydr. Polym. 2013, 92, 1477–1483. [Google Scholar] [CrossRef]
- Borrega, M.; Orelma, H. Cellulose Nanofibril (CNF) Films and Xylan from Hot Water Extracted Birch Kraft Pulps. Appl. Sci. 2019, 9, 3436. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Ameram, N.; Muhammad, S.; Yusof, N.A.A.N.; Ishak, S.; Ali, A.; Shoparwe, N.F.; Ter, T.P. Chemical composition in sugarcane bagasse: Delignification with sodium hydroxide. Malays. J. Fundam. Appl. Sci. 2019, 15, 232–236. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Q.; Hirth, K.; Baez, C.; Agarwal, U.P.; Zhu, J.Y. Tailoring the yield and characteristics of wood cellulose nanocrystals (CNC) using concentrated acid hydrolysis. Cellulose 2015, 22, 153–1762. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Liu, S.; Tang, J. Bacterial cellulose nanofibril-based pickering emulsions: Recent trend and applications in the food industry. Foods 2022, 11, 4064. [Google Scholar] [CrossRef]
- Dufresne, A.; Dupeyre, D.; Vignon, M.R. Cellulose microfibrils from potato tuber cells: Processing and characterization of starch-cellulose microfibril composites. J. Appl. Polym. Sci. 2000, 76, 2080–2092. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Asrofi, M. Production of Nanocellulose from Pineapple Leaf Fibers via High-Shear Homogenization and Ultrasonication. Fibers 2018, 6, 28. [Google Scholar] [CrossRef]
- Gonçalves, A.P.; Oliveira, E.; Mattedi, S.; José, N.M. Separation of cellulose nanowhiskers from microcrystalline cellulose with an aqueous protic ionic liquid based on ammonium and hydrogensulphate. Sep. Purif. Technol. 2018, 196, 200–207. [Google Scholar] [CrossRef]
- Li, C.; Zhao, Z.K. Efficient Acid-Catalyzed Hydrolysis of Cellulose in Ionic Liquid. Adv. Synth. Catal. 2007, 349, 1847–1850. [Google Scholar] [CrossRef]
- Chen, Y.W.; Lee, H.V.; Abd Hamid, S.B. Investigation of optimal conditions for production of highly crystalline nanocellulose with increased yield via novel Cr(III)-catalyzed hydrolysis: Response surface methodology. Carbohydr. Polym. 2017, 178, 57–68. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. Cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Fall, A.B.; Lindström, S.B.; Sundman, O.; Ödberg, L.; Wågberg, L. Colloidal stability of aqueous nanofibrillated cellulose dispersions. Langmuir 2011, 27, 11332–11338. [Google Scholar] [CrossRef]
- Pääkko, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic hydrolysis combined with mechanical shearing and highpressure homogenization for nanoscale cellulose fibrils and strong gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- de Nooy, A.E.J.; Besemer, A.C.; van Bekkum, H. Highly selective tempo mediated oxidation of primary alcohol groups in polysaccharides. Recl. Des Trav. Chim. Des Pays-Bas 1994, 113, 165–166. [Google Scholar] [CrossRef]
- Sanchez-Salvador, J.L.; Balea, A.; Monte, M.C.; Negro, C.; Miller, M.; Olson, J.; Blanco, A. Comparison of mechanical and chemical nanocellulose as additives to reinforce recycled cardboard. Sci. Rep. 2020, 10, 3778. [Google Scholar] [CrossRef]
- Abol-Fotouh, D.; Hassan, M.A.; Shokry, H.; Roig, A.; Azab, M.S.; Kashyout, A.E.H.B. Bacterial nanocellulose from agro-industrial wastes: Low-cost and enhanced production by Komagataeibacter saccharivorans MD1. Sci. Rep. 2020, 10, 3491. [Google Scholar] [CrossRef]
- Jeon, S.; Yoo, Y.M.; Park, J.W.; Kim, H.J.; Hyun, J. Electrical conductivity and optical transparency of bacterial cellulose based composite by static and agitated methods. Curr. Appl. Phys. 2014, 14, 1621–1624. [Google Scholar] [CrossRef]
- Tyagi, N.; Suresh, S. Production of cellulose from sugarcane molasses using Gluconacetobacter intermedius SNT-1: Optimization & characterization. J. Clean. Prod. 2016, 112, 71–80. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Bacterial cellulose composites: Synthetic strategies and multiple applications in bio-medical and electro-conductive fields. Biotechnol. J. 2015, 10, 1847–1861. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Tan, T.H.; Lee, H.V.; Yehya Dabdawb, W.A.; Hamid, S.B.B.O.A.A. A review of nanocellulose in the drug-delivery system. Mater. Biomed. Eng. 2019, 1, 131–164. [Google Scholar] [CrossRef]
- Xue, Y.; Mou, Z.; Xiao, H. Nanocellulose as a sustainable biomass material: Structure, properties, present status and future prospects in biomedical applications. Nanoscale 2017, 9, 14758–14781. [Google Scholar] [CrossRef]
- Mateo, S.; Peinado, S.; Morillas-Gutiérrez, F.; La Rubia, M.D.; Moya, A.J. Nanocellulose from agricultural wastes: Products and applications—A review. Processes 2021, 9, 1594. [Google Scholar] [CrossRef]
- Negro, C.; MartÃn, A.B.; Sanchez-Salvador, J.L.; Campano, C.; Fuente, E.; Monte, M.C.; Blanco, A. Nanocellulose and its potential use for sustainable industrial applications. Lat. Am. Appl. Res. 2020, 50, 59–64. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2015, 132, 41719. [Google Scholar] [CrossRef]
- Knight, P.E.; Podczeck, F.; Newton, J.M. The rheological properties of modified microcrystalline cellulose containing high levels of model drugs. J. Pharm. Sci. 2009, 98, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Hare, J.I.; Lammers, T.; Ashford, M.B.; Puri, S.; Storm, G.; Barry, S.T. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv. Drug Deliv. Rev. 2017, 108, 25–38. [Google Scholar] [CrossRef]
- Siqueira, G.; Bras, J.; Dufresne, A. Cellulosic Bionanocomposites: A Review of Preparation, Properties and Applications. Polymers 2010, 2, 728–765. [Google Scholar] [CrossRef]
- Peng, B.L.; Dhar, N.; Liu, H.L.; Tam, K.C. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–1206. [Google Scholar] [CrossRef]
- Gupta, R.D.; Raghav, N. Nano-crystalline cellulose: Preparation, modification and usage as sustained release drug delivery excipient for some non-steroidal anti-inflammatory drugs. Int. J. Biol. Macromol. 2020, 147, 921–930. [Google Scholar] [CrossRef]
- Zainuddin, N.; Ahmad, I.; Kargarzadeh, H.; Ramli, S. Hydrophobic kenaf nanocrystalline cellulose for the binding of curcumin. Carbohydr. Polym. 2017, 163, 261–269. [Google Scholar] [CrossRef]
- Zainuddin, N.; Ahmad, I.; Zulfakar, M.H.; Kargarzadeh, H.; Ramli, S. Cetyltrimethylammonium bromide-nanocrystalline cellulose (CTAB-NCC) based microemulsions for enhancement of topical delivery of curcumin. Carbohydr. Polym. 2021, 254, 117401. [Google Scholar] [CrossRef]
- Putro, J.N.; Ismadji, S.; Gunarto, C.; Soetaredjo, F.E.; Ju, Y.H. Effect of natural and synthetic surfactants on polysaccharide nanoparticles: Hydrophobic drug loading, release, and cytotoxic studies. Colloids Surf. 2019, 578, 123618. [Google Scholar] [CrossRef]
- Kupnik, K.; Primožič, M.; Kokol, V.; Leitgeb, M. Nanocellulose in drug delivery and antimicrobially active materials. Polymers 2020, 12, 2825. [Google Scholar] [CrossRef]
- Liu, S.; Qamar, S.A.; Qamar, M.; Basharat, K.; Bilal, M. Engineered nanocellulose-based hydrogels for smart drug delivery applications. Int. J. Biol. Macromol. 2021, 181, 275–290. [Google Scholar] [CrossRef]
- Nicu, R.; Ciolacu, F.; Ciolacu, D.E. Advanced functional materials based on nanocellulose for pharmaceutical/medical applications. Pharmaceutics 2021, 13, 1125. [Google Scholar] [CrossRef]
- Lou, J.; Duan, H.; Qin, Q.; Teng, Z.; Gan, F.; Zhou, X.; Zhou, X. Advances in oral drug delivery systems: Challenges and opportunities. Pharmaceutics 2023, 15, 484. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and recent progress in oral drug delivery systems for biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. Int. J. Pharm. Sci. Res. 2016, 7, 2274. [Google Scholar] [CrossRef]
- Sawynok, J. Topical Analgesics. In Clinical Pain Management: A Practical Guide; Lynch, M.E., Craig, K.D., Peng, P.W.H., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2010; pp. 135–141. [Google Scholar]
- Ji, T.; Kohane, D.S. Nanoscale systems for local drug delivery. Nano Today 2019, 28, 100765. [Google Scholar] [CrossRef]
- Letchford, K.; Jackson, J.K.; Wasserman, B.Z.; Ye, L.; Hamad, W.Y.; Burt, H.M. The use of nanocrystalline cellulose for the binding and controlled release of drugs. Int. J. Nanomed. 2011, 6, 321–330. [Google Scholar] [CrossRef]
- Plackett, D.; Letchford, K.; Jackson, J.; Burt, H. A review of nanocellulose as a novel vehicle for drug delivery. Nord. Pulp. Pap. Res. J. 2014, 29, 105–118. [Google Scholar] [CrossRef]
- Mohanta, V.; Madras, G.; Patil, S. Layer-by-layer assembled thin films and microcapsules of nanocrystalline cellulose for hydrophobic drug delivery. ACS Appl. Mater. Interfaces 2014, 6, 20093–20101. [Google Scholar] [CrossRef]
- Emara, L.H.; El-Ashmawy, A.A.; Taha, N.F.; El-Shaffei, K.A.; Mahdey, E.S.M.; El-kholly, H.K. Nano-crystalline cellulose as a novel tablet excipient for improving solubility and dissolution of Meloxicam. J. Appl. Pharm. Sci. 2016, 6, 32–43. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, P.L.; Zhao, S.W.; Guo, Y.R.; Li, L.; Yuan, F.L.; Pan, Q.J. Cellulose nanofiber induced self-assembly of zinc oxide nanoparticles: Theoretical and experimental study on interfacial interaction. Carbohydr. Polym. 2018, 195, 525–533. [Google Scholar] [CrossRef]
- Patil, M.D.; Patil, V.D.; Sapre, A.A.; Ambone, T.S.; Torris, A.T.; Shukla, P.G.; Shanmuganathan, K. Tuning Controlled Release Behavior of Starch Granules Using Nanofibrillated Cellulose Derived from Waste Sugarcane Bagasse. ACS Sustain. Chem. Eng. 2018, 6, 9208–9217. [Google Scholar] [CrossRef]
- Thomas, D.; Latha, M.S.; Thomas, K.K. Synthesis and in vitro evaluation of alginate-cellulose nanocrystal hybrid nanoparticles for the controlled oral delivery of rifampicin. J. Drug Deliv. Sci. Technol. 2018, 46, 392–399. [Google Scholar] [CrossRef]
- Supramaniam, J.; Adnan, R.; Mohd Kaus, N.H.; Bushra, R. Magnetic nanocellulose alginate hydrogel beads as potential drug delivery system. Int. J. Biol. Macromol. 2018, 118, 640–648. [Google Scholar] [CrossRef]
- Hivechi, A.; Bahrami, S.H.; Siegel, R.A. Drug release and biodegradability of electrospun cellulose nanocrystal reinforced polycaprolactone. Mater. Sci. Eng. 2019, 94, 929–937. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Manjusha, V.; Chithra Sekhar, V. A new biodegradable nanocellulose-based drug delivery system for pH-controlled delivery of curcumin. Int. J. Biol. Macromol. 2021, 183, 2044–2054. [Google Scholar] [CrossRef]
- Valo, H.; Arola, S.; Laaksonen, P.; Torkkeli, M.; Peltonen, L.; Linder, M.B.; Serimaa, R.; Kuga, S.; Hirvonen, J.; Laaksonen, T. Drug release from nanoparticles embedded in four different nanofibrillar cellulose aerogels. Eur. J. Pharm. Sci. 2013, 50, 69–77. [Google Scholar] [CrossRef]
- Shi, X.; Zheng, Y.; Wang, G.; Lin, Q.; Fan, J. PH- and electro-response characteristics of bacterial cellulose nanofiber/sodium alginate hybrid hydrogels for dual controlled drug delivery. RSC Adv. 2014, 4, 47056–47065. [Google Scholar] [CrossRef]
- Ahmad, N.; Amin, M.C.I.M.; Mahali, S.M.; Ismail, I.; Chuang, V.T.G. Biocompatible and mucoadhesive bacterial cellulose-g-poly(acrylicacid) hydrogels for oral protein delivery. Mol. Pharm. 2014, 11, 4130–4142. [Google Scholar] [CrossRef]
- Laurén, P.; Paukkonen, H.; Lipiäinen, T.; Dong, Y.; Oksanen, T.; Räikkönen, H.; Ehlers, H.; Laaksonen, P.; Yliperttula, M.; Laaksonen, T. Pectin and Mucin Enhance the Bioadhesion of Drug Loaded Nanofibrillated Cellulose Films. Pharm. Res. 2018, 35, 145. [Google Scholar] [CrossRef]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef]
- Bertsch, P.; Schneider, L.; Bovone, G.; Tibbitt, M.W.; Fischer, P.; Gstöhl, S. Injectable biocompatible hydrogels from cellulose nanocrystals for locally targeted sustained drug release. ACS Appl. Mater. Interfaces 2019, 11, 38578–38585. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Mota, J.P.; de Almeida, T.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical drug delivery systems based on bacterial nanocellulose: Accelerated stability testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef]
- Bacakova, L.; Pajorova, J.; Bacakova, M.; Skogberg, A.; Kallio, P.; Kolarova, K.; Svorcik, V. Versatile application of nanocellulose: From industry to skin tissue engineering and wound healing. Nanomaterials 2019, 9, 164. [Google Scholar] [CrossRef]
- Abba, M.; Ibrahim, Z.; Chong, C.S.; Zawawi, N.A.; Kadir, M.R.A.; Yusof, A.H.M.; Razak, S.I.A. Transdermal Delivery of Crocin Using Bacterial Nanocellulose Membrane. Fibers Polym. 2019, 20, 2025–2031. [Google Scholar] [CrossRef]
- Sarkar, G.; Orasugh, J.T.; Saha, N.R.; Roy, I.; Bhattacharyya, A.; Chattopadhyay, A.K.; Rana, D.; Chattopadhyay, D. Cellulose nanofibrils/chitosan based transdermal drug delivery vehicle for controlled release of ketorolac tromethamine. New J. Chem. 2017, 41, 15312–15319. [Google Scholar] [CrossRef]
- Alkhatib, Y.; Dewaldt, M.; Moritz, S.; Nitzsche, R.; Kralisch, D.; Fischer, D. Controlled extended octenidine release from a bacterial nanocellulose / Poloxamer hybrid system. Eur. J. Pharm. Biopharm. 2016, 1, 164–176. [Google Scholar] [CrossRef]
- Medhi, P.; Olatunji, O.; Nayak, A.; Uppuluri, C.T.; Olsson, R.T.; Nalluri, B.N.; Das, D.B. Lidocaine-loaded fish scale-nanocellulose biopolymer composite microneedles. Aaps Pharmscitech 2017, 18, 1488–1494. [Google Scholar] [CrossRef]
- Plappert, S.F.; Liebner, F.W.; Konnerth, J.; Nedelec, J.M. Anisotropic nanocellulose gel–membranes for drug delivery: Tailoring structure and interface by sequential periodate–chlorite oxidation. Carbohyd. Polym. 2019, 226, 115306. [Google Scholar] [CrossRef]
- Balla, E.D.; Bikiaris, N.D.; Nanaki, S.G.; Papoulia, C.; Chrissafis, K.; Klonos, P.A.; Kyritsis, A.; Kostoglou, M.; Zamboulis, A.; Papageorgiou, G.Z. Chloramphenicol loaded sponges based on PVA/nanocellulose nanocomposites for topical wound delivery. J. Compos. Sci. 2021, 5, 208. [Google Scholar] [CrossRef]
- Solomevich, S.O.; Dmitruk, E.I.; Bychkovsky, P.M.; Nebytov, A.E.; Yurkshtovich, T.L.; Golub, N.V. Fabrication of oxidized bacterial cellulose by nitrogen dioxide in chloroform/cyclohexane as a highly loaded drug carrier for sustained release of cisplatin. Carbohydr. Polym. 2020, 248, 116745. [Google Scholar] [CrossRef]
- Saïdi, L.; Vilela, C.; Oliveira, H.; Silvestre, A.J.; Freire, C.S. Poly (N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohydr. Polym. 2017, 169, 357–365. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, H.; Xu, G.; Hou, X.; He, H.; Wang, S. A biocompatible cellulose-nanofiber-based multifunctional material for Fe3+ detection and drug delivery. J. Mater. Chem. 2020, 8, 11796–11804. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
| Nanocellulose in Drug Delivery | |||
|---|---|---|---|
| Route | Advantages | Disadvantages | References |
| Oral |
|
| [66,67] |
| Transdermal |
|
| [68] |
| Topical/Local |
|
| [69,70] |
| Type of Nanocellulose | Delivery Method | Delivery Route | Drug | References |
|---|---|---|---|---|
| Spray-dried CNF | Encapsulation Drug release | Oral | Acetaminophen | [81] |
| Magnetic CNC | Colonic release | Oral | Ibuprofen | [78] |
| CNF | Colon-specific drug delivery | Oral | Methotrexate | [63] |
| CNF | Pseudo-Fickian diffusion | Topical | Chloramphenicol | [94] |
| BNC | Controlled drug release | Topical | Octenidine | [91] |
| BNC | Controlled cutaneous drug release | Transdermal | Caffeine, Ibuprofen, Lidocaine, Diclofenac | [87] |
| BNC | Controlled pH-responsive drug release | Localized drug delivery | Cisplatin | [95] |
| BNC | Controlled pH-sensitive drug delivery | Transdermal, oral delivery | Diclofenac | [96] |
| CNF | pH-sensitive controlled drug delivery | Transdermal | Doxorubicin | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varghese, R.T.; Cherian, R.M.; Chirayil, C.J.; Antony, T.; Kargarzadeh, H.; Thomas, S. Nanocellulose as an Avenue for Drug Delivery Applications: A Mini-Review. J. Compos. Sci. 2023, 7, 210. https://doi.org/10.3390/jcs7060210
Varghese RT, Cherian RM, Chirayil CJ, Antony T, Kargarzadeh H, Thomas S. Nanocellulose as an Avenue for Drug Delivery Applications: A Mini-Review. Journal of Composites Science. 2023; 7(6):210. https://doi.org/10.3390/jcs7060210
Chicago/Turabian StyleVarghese, Rini Thresia, Reeba Mary Cherian, Cintil Jose Chirayil, Tijo Antony, Hanieh Kargarzadeh, and Sabu Thomas. 2023. "Nanocellulose as an Avenue for Drug Delivery Applications: A Mini-Review" Journal of Composites Science 7, no. 6: 210. https://doi.org/10.3390/jcs7060210
APA StyleVarghese, R. T., Cherian, R. M., Chirayil, C. J., Antony, T., Kargarzadeh, H., & Thomas, S. (2023). Nanocellulose as an Avenue for Drug Delivery Applications: A Mini-Review. Journal of Composites Science, 7(6), 210. https://doi.org/10.3390/jcs7060210