Abstract
The effect of the process temperature and the initial concentration of Mg, Ca, Sr, Ba, Zn, Cd, and Hg 2-ethylhexanoates as catalysts on the productivity and selectivity of the oxidation stage of cumene is studied in the technological chain for the production of polymer composites from cumene; “production of phenol by cumene method (stage 1 is cumene oxidation to cumene hydroperoxide, stage 2 is decomposition of cumene hydroperoxide into phenol and acetone) → production of precursors from phenol → production of polymers from precursors → production of composites from polymers”. A criterion has been introduced that reflects the productivity of cumene oxidation at the moment of reaching the maximum concentration of cumene hydroperoxide, which takes into account the cumene conversion and selectivity achieved in this case in the shortest possible time using the selectivity comparable with the selectivity of a non-catalytic process. It has been shown that the achievement of the maximum value of this criterion, among all the considered catalysts, is ensured by Mg 2-ethylhexanoate at its relatively low initial concentration (1 mmol/L) under conditions of moderately-high process temperatures (393–413 K).
1. Introduction
Epoxy resins [1,2], phenol-formaldehyde resins [3,4], polycarbonates [5,6], polysulfones [7,8], and polyamides [9,10] are in high demand as polymer matrices for composites. All of these oligomers and polymers are united by the fact that phenol is the feed material for their synthesis [11]. The global consumption of phenol is currently as follows [11]:
(1) About 44% is used for the production of bisphenol A (a condensation product of phenol and acetone (I)), which, in turn, is a feed for the production of epoxy resins (II), polycarbonates (III), and polysulfones (IV);
(2) About 30% is used for the production of phenol-formaldehyde resins (obtaining phenolic novolac (V) and resol (VI));
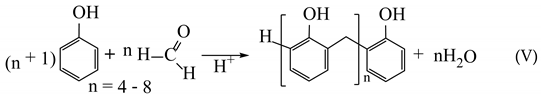
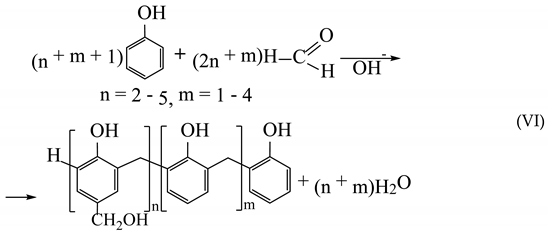
(3) About 12% of phenol is used for the production of polyamides (in particular, polyamide 6 (VII) and polyamide 66 (VIII)):
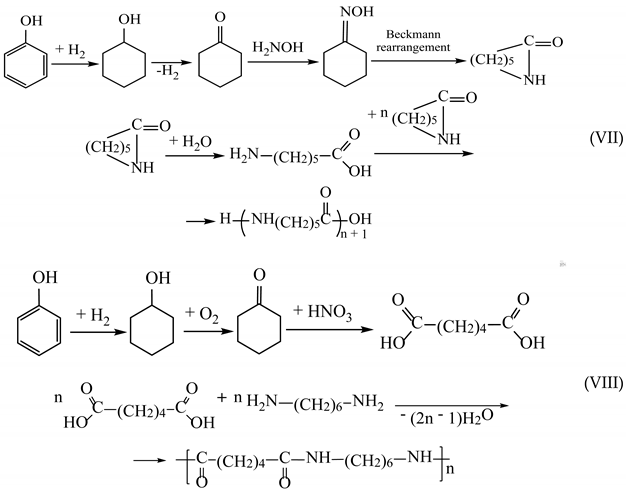
(4) About 14% is used for the production of other chemical compounds (antioxidants, non-ionic surfactants, cresols, drugs, antiseptics, and pesticides).
A total of 95% of the global production of phenol falls on the cumene method (Hock process) [11,12]. This process consists of two main stages:
(1) Cumene oxidation (the cumene obtained by alkylation of benzene with propylene) with air oxygen to obtain cumene hydroperoxide;
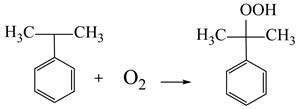
(2) The decomposition of cumene hydroperoxide into phenol and acetone (catalyst is sulfuric acid):
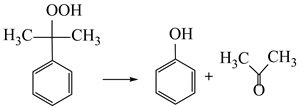
Thus, the technological chain for the production of polymer composites from cumene is as follows: production of phenol by cumene method → production of precursors from phenol → production of polymers from precursors → production of composites from polymers. Obviously, one of the factors affecting the productivity of the first stage of this technological chain is the productivity of cumene oxidation. As a rule, cumene oxidation is carried out in bubble columns at a temperature of 353–403 K and a pressure of 0.1–0.7 MPa; in this case, a sufficiently high selectivity (85–90%) is achieved at the cumene conversion of 10–25% [13]. The task of increasing the productivity of the cumene oxidation stage is complicated by the interdependence of cumene conversion and selectivity [14]. Therefore, research of catalysts is underway that aims increase the productivity of this stage while maintaining the value of selectivity. The ionic liquids [15,16,17], surfactants [18], carbon nanotubes [19,20], and Fe [21], Cu [22,23], Co [24,25], Mn [24], Ni [21], Ru [26], and Au и Ag [27] transition metal compounds are studied as catalysts for the oxidation of alkylaromatic hydrocarbons, and cumene in particular.
When using ionic liquids based on imidazole, a cumene conversion of 16.7% and the selectivity of 87.7% are achieved [15]. The ionic liquid N-hydroxyphthalimide acts as a highly-efficient universal catalyst in hydrocarbons oxidation [17,28,29]. In particular, the cumene conversion is 25–30% and the selectivity is 90–95% [28] in cumene oxidation at 353–393 K.
The authors of [19,20] highlight the high catalytic activity of carbon nanotubes (CNTs) in cumene oxidation. The catalytic properties of carbon nanotubes are associated with their ability to accelerate cumene hydroperoxide decomposition [19,20]. In addition, the “CNT-air oxygen” adduct is formed through cumene oxidation in the presence of carbon nanotubes, while the oxygen in the adduct reacts with cumene, generating free radicals [30]. At a sufficiently low temperature (353 K), a cumene conversion of 24.1% and the selectivity of 88.4% are achieved [30].
The use of ionic liquids and carbon nanotubes as catalysts makes it possible to lower the cumene oxidation temperature (down to 353–363 K); however, the time to achieve a cumene conversion of ~20% will be rather long (8–9 h [20]).
Over the past 15 years, the effect of surfactants on the processes of hydrocarbon oxidation has been intensively studied [18]. Thus, in cumene, which is oxidized in the presence of cationic surfactants by air oxygen, the resulting hydroperoxides form mixed micelles with molecules of surfactants, where the accelerated decomposition of cumene hydroperoxide into radicals occurs, followed by the formation of hydrophilic products: water, dimethylphenylcarbinol, and acetophenone [18]. The formation of these species affects the structure and size of mixed micelles and, unfortunately, reduces their selectivity [18].
Catalysts based on the metal nanoparticles Ag and Au [27], and Cu [31], have proven themselves to be useful in cumene oxidation.
One of the most active catalysts for the liquid phase oxidation of alkylaromatic hydrocarbons are transition metal compounds [21,22,23,24,25,26]. Ni [21], Cu [22,23], Mn [24], Co [24,25], and Ru [26] compounds have been studied as catalysts for alkylaromatic hydrocarbons oxidation. Despite the high activity of transition metal compounds in alkylaromatic hydrocarbons oxidation, the practical implementation of their use is being restrained [14]. This is due to the fact that, by accelerating the alkylaromatic hydrocarbons oxidation into target products (hydroperoxides), the transition metal compounds cause a noticeable hydroperoxides decomposition with the formation of a large amount of side chemical compounds [14].
The potential possibilities of intensifying the processes of alkylaromatic hydrocarbons oxidation using catalysts have not been limited. For instance, carboxylates [32], naphthenates [33], and stearates [34] of non-transition metals, which are quite simply synthesized and readily soluble in hydrocarbons, are fairly effective homogeneous catalysts for the oxidation of liquid-phase alkylaromatic hydrocarbons.
Despite a large number of works on the catalytic oxidation of cumene, the metal-based catalysts with the configuration of the upper filled electron sublevel s2 have not been given due attention (these are metals of the 2nd and 12th groups with a maximum oxidation state of +2). The lack of interest in these metals is probably due to the fact that they are not capable of valence transformations, and, most likely, their catalytic activity would be noticeably lower than that of transition metals exhibiting various oxidation states, including higher than +2. However, metals incapable of valence transformations can catalyze cumene oxidation through the formation of intermediate adducts with the species of the reaction mixture [35]. Therefore, the purpose of this work was to study the effect of metals of the 2nd and 12th groups in the composition of 2-ethylhexanoates on the productivity and selectivity of cumene oxidation. To achieve this purpose, in this work, we created a kinetic model of the process, verified it according to the experimental data obtained, and carried out computational experiments on the model, in which we estimated the effect of the process temperature and the initial concentration of the catalyst on the productivity and selectivity of the process carried out in industrial conditions.
2. Materials and Methods
2.1. Materials
In the experiments, the following substances were used:
(1) Cumene produced by PJSC Kazanorgsintez;
(2) Cumene hydroperoxide produced by PJSC Kazanorgsintez (25% wt. cumene hydroperoxide and 75% wt. cumene), which underwent distillation until 99.4% wt. cumene hydroperoxide;
(3) Air oxygen as an oxidizing agent;
(4) Mg, Ca, Sr, Ba, Zn, Cd, and Hg 2-ethylhexanoates are the catalysts for cumene oxidation and cumene hydroperoxide decomposition. They were obtained by reaction of 2-ethylhexanoic acid with metal oxides/hydroxides in boiling benzene; the water formed in the reaction was separated in a Dean–Stark trap; for complete involvement in the reaction of 2-ethylhexanoic acid, a small (1–2% wt.) excess of metal oxide/hydroxide was used; after separation of excess metal oxide/hydroxide and distillation of benzene, the catalysts were washed with distilled water; the content of metal 2-ethylhexanoates in the obtained samples was 99.12–99.74% wt.;
(5) Chlorobenzene is a solvent in the decomposition of cumene hydroperoxide.
2.2. Cumene Oxidation
The content of metal 2-ethylhexanoates in the obtained preparations was determined by complexometric titration and was 99.12–99.74% wt. In Table 1, the characteristic vibration frequencies are shown in IR spectra of metal carboxylates, which include Mg, Ca, Sr, Ba, Zn, Cd, and Hg 2-ethylhexanoates

Table 1.
Characteristic oscillation frequencies in IR spectra of metal carboxylates (M is metal) [36,37].
Cumene ([RH]0 = 6.44 mol/L) mixed with a catalyst ([Cat]0 = 5 mmol/L) was filled into a bubble column (diameter 36 mm, length 500 mm); thereafter, the cumene was oxidized with air at a temperature 383 or 393 K and atmospheric pressure. The air, preliminarily treated from mechanical impurities and dried, was continuously supplied by a compressor (flow rate G = 0.6 L/min) down the bubble column. The distribution of air in the liquid was carried out using a distribution grid (hole diameter was no more than 1 mm) located at the bottom of the bubble column. Samples of the reaction mixture were taken every hour through a valve on the side of the bubble column.
The catalytic cumene oxidation was carried out for 6 h, the samples were taken every hour. The experiment (cumene oxidation for 6 h) was checked for reproducibility by repeating it 3–4 times. The content of cumene hydroperoxide in the samples was determined by the iodometric method (the content of cumene hydroperoxide was determined in three parallel samples, the data were averaged). The composition of the oxidation products was determined by chromatography (the analysis of the oxidation reaction mass was carried out in three parallel samples, the data were averaged). Quantitative analysis by chromatography (Chromatec Crystal 5000 chromatograph, manufacturer is JSC SDO “CHROMATEC”, Yoshkar-Ola, Russia; capillary column Sol Gel Wax (polyethylene glycol) with a polar phase) was carried out by the method of internal normalization with correction factors. Cumene was used as a standard substance (correction factor K = 1).
2.3. Decomposition of Cumene Hydroperoxide
Glass ampoules were filled with a mixture of cumene hydroperoxide ([ROOH]0 = 1 or 1.5 mol/L) with catalyst ([Cat]0 = 5 mmol/L), sealed, then immersed in a thermostat heated to the required temperature, and the shaking device was turned on. At certain intervals, the ampoules were removed one at a time, and the reaction was stopped by cooling the ampoules in ice water; the composition of the reaction mixture was analyzed in the same way as in the case of cumene oxidation (see Section 2.2).
2.4. Preparation of Samples for Chromatographic Analysis
The unconverted hydroperoxide contained in the samples, being decomposed in the chromatograph column, distorted the results of the analysis. To avoid this error, samples containing cumene hydroperoxide were treated with a reducing agent, by an excess of triphenylphosphine P(C6H5)3 (Ph3P), before chromatographic analysis. In the presence of cumene hydroperoxide and triphenylphosphine, dimethylphenylkabinol and triphenylphosphine oxide are formed:
C6H5C(CH3)2OOH + Ph3P = C6H5C(CH3)2OH + Ph3P=O
The molar ratio of reagents C6H5C(CH3)2OOH:Ph3P = 1:1.5. The amount of dimethylphenylcarbinol (DMFC) released in this case was subsequently taken into account when calculating the chromatograms.
3. Results
The general kinetic scheme of cumene oxidation and cumene hydroperoxide decomposition in the presence of Mg, Ca, Sr, Ba, Zn, Cd, or Hg 2-ethylhexanoate consists of Reactions (1)–(47). Reactions (1)–(44) of this scheme were taken from [32], where the authors made the general kinetic scheme proposed by us for cumene oxidation and cumene hydroperoxide decomposition in the presence of Zn, Cd, or Hg 2-ethylhexanoate. It is reasonable to assume that Reactions (1)–(44) also run both in cumene oxidation and in cumene hydroperoxide decomposition in the presence of Mg, Ca, Sr, or Ba 2-ethylhexanoate.
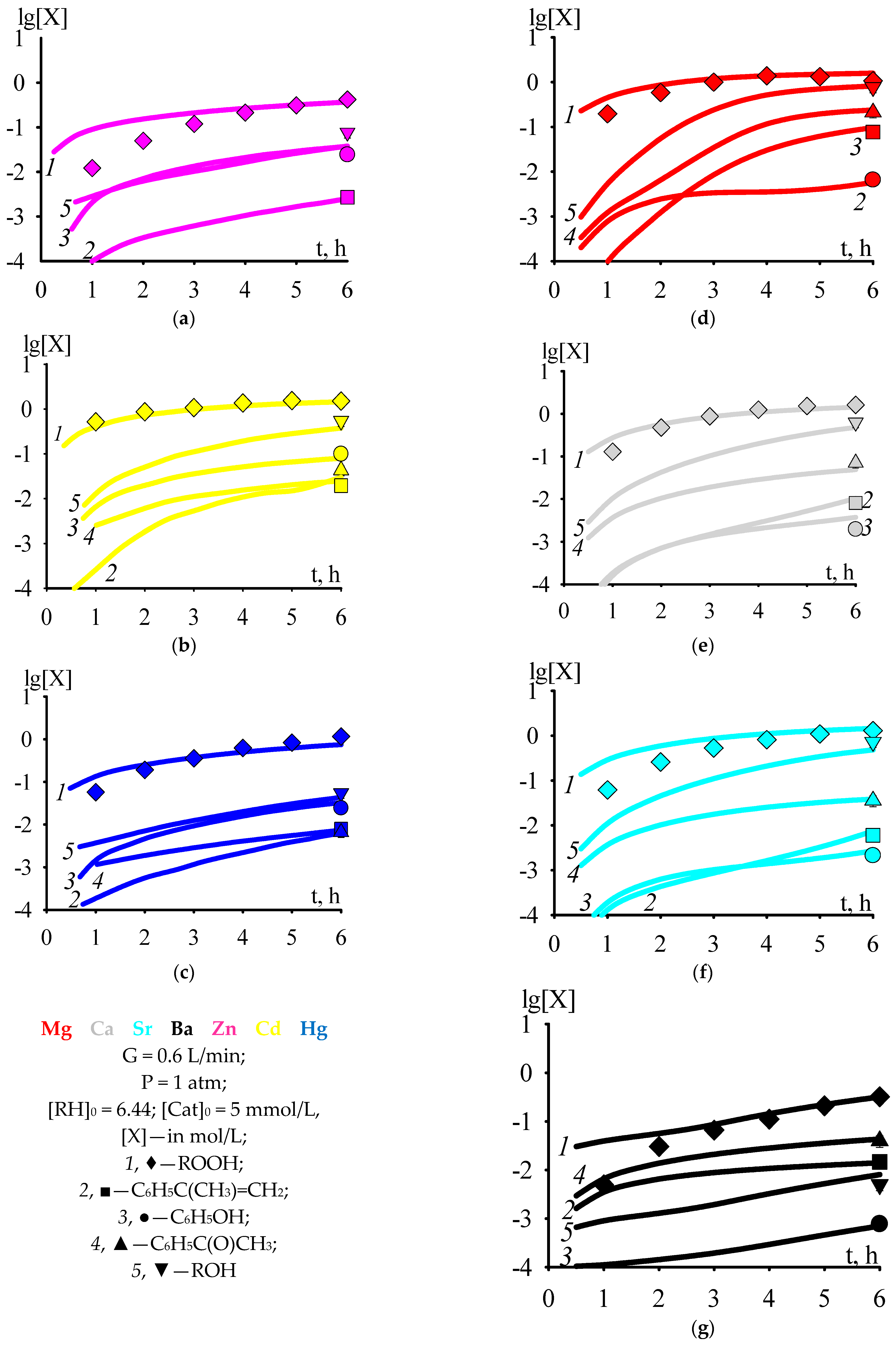
According to the kinetic scheme of cumene oxidation and cumene hydroperoxide decomposition in the presence of Zn, Cd, or Hg 2-ethylhexanoate [32], the formation of acetophenone runs without the participation of a catalyst, according to Reactions (20) and (43). The formation of acetophenone without the participation of a cumene oxidation catalyst was shown experimentally in [38]. However, when cumene oxidation runs in the presence of Mg, Ca, Sr, or Ba 2-ethylhexanoate, the acetophenone is formed by several orders of magnitude more (Figure 1b,c lg[C6H5C(O)CH3] ≤ −2 at t = 6 h) than in cumene oxidation in the presence of Zn, Cd, or Hg 2-ethylhexanoate (Figure 1d–g lg[C6H5C(O)CH3] > −2 at t = 6 h). To take this factor into account, we assumed that ROH·Cat adduct is capable of decomposing into acetophenone, thereby increasing the rate of accumulation of acetophenone, and introduced Reaction (45) into the general kinetic scheme of cumene oxidation and cumene hydroperoxide decomposition in the presence of Mg, Ca, Sr, Ba, Zn, Cd, or Hg 2-ethylhexanoate. In addition, the general kinetic scheme of cumene oxidation and cumene hydroperoxide decomposition in the presence of Mg, Ca, Sr, Ba, Zn, Cd, or Hg 2-ethylhexanoate was supplemented with Reaction (46) [39] and the reaction of catalyst deactivation with phenol (47). A reaction similar to Reaction (47) can run during the synthesis of [CaLi6(μ3-OPh)8(thf)6] from CaI2 and LiOPh, described in [40].
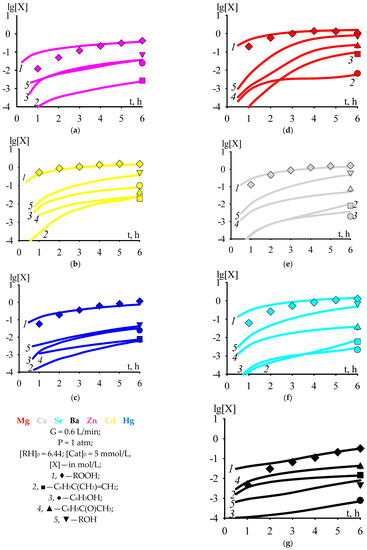
Figure 1.
The dependencies of the concentrations of cumene hydroperoxide and by-products in cumene oxidation versus time; T = 383 (a–c), 393 (d–g) K.
Formation of intermediate adducts:
Chain initiation:
Chain propagation:
Chain termination:
Molecular reactions:
In the above equations, RH is cumene (C6H5CH(CH3)2), Cat is catalyst, RH·Cat is a “cumene-catalyst” intermediate adduct, ROOH is cumene hydroperoxide, ROOH·Cat is a “cumene hydroperoxide-catalyst” intermediate adduct, ROH is dimethylphenylcarbinol, ROH·Cat is a “dimethylphenylcarbinol-catalyst” intermediate adduct, HC(O)H is formaldehyde, HC(O)H·Cat is a “formaldehyde-catalyst” intermediate adduct, O2 is oxygen, R• is cumyl radical, •OOH is hydrogen peroxide radical, RO• is cumyloxyl radical, •OH is hydroxyl radical, ROO• is cumyl peroxyl radical, H2O is water, C6H5C(O)CH3 is acetophenone, CH3• is methyl radical, CH3OO• is methyl peroxyl radical, CH3OOH is methyl hydroperoxide, is benzyloxy radical, RR is dicumyl, ROOR is dicumyl peroxide, CH3OH is methanol, C6H5C(CH3)=CH2 is α-methylstyrene, C6H5OOR is benzylcumonoate, HCOOH is formic acid, C6H5OH is phenol, CH3C(O)CH3 is acetone, ROOH·HCOOH is a “cumene hydroperoxide-formic acid” intermediate adduct, Cat’ is deactivated catalyst, CH4 is methane, C6H5C(CH3)(O)(CH2) is 2-methyl-2-phenyloxirane, (C6H5O)2Me is metal phenolate, H3C(CH2)3CH(C2H5)COOH is 2-ethylhexanoic acid, and Me is metal (Mg, Ca, Sr, Ba, Zn, Cd, and Hg).
Based on the scheme consisting of Reactions (1)–(47), the kinetic model was written as a system of nonlinear differential equations, which describes, according to the mass action law, the rate of change of the concentrations of all of the reaction mixture species (see Supplementary Materials S1). A procedure for solving the system of differential equations has been added to Supplementary Materials S1
The reaction rate coefficients in the kinetic model were set as functions of temperature according to the Arrhenius equation [41]. Here, A is a pre-exponential factor (it has the dimension of the corresponding reaction rate coefficient: 1/s for a monomolecular reaction; L/(mol s) for a bimolecular reaction; E is an activation energy, J/mol; R = 8.31 J/(mol K), T is a temperature, K. To determine the values of A and E, the inverse kinetic problem was solved. The inverse kinetic problem is the task of determining model parameters for which the discrepancy between the data calculated by use of the model and the experimental data are minimal [38]. The inverse kinetic problem was solved by the method of direct search of the zero order [42]. A comparison of the experimental data and data calculated by the kinetic model with the found values of A and E for each reaction rate coefficient (Table 2 and Table 3) is shown in Figure 1 and in Table 4. The kinetic model satisfactorily described the laboratory experimental data on cumene oxidation and cumene hydroperoxide decomposition in the presence of Mg, Ca, Sr, Ba, Zn, Cd, or Hg 2-ethylhexanoate within an average relative error of 25%. It should be noted that the kinetic models, especially those made on the basis of the radical chain mechanism, are overparametrized, as a rule. This is due to the difficulty of measuring the concentrations of intermediates. In our case, these intermediates are all kinds of radicals. Under the conditions of impossibility of accurate measurement of their very low concentration (of the order of 10−6 mol/L), the values of the parameters of the Arrhenius dependences of the rate coefficients A and E can also not be determined exactly, but only in a certain range. Therefore, the values given in Table 2 and 3 are just some of the possible values of the parameters A and E that can be found as a result of solving the inverse kinetic problem. Two-dimensional uncertainty regions of the significant rate coefficients of elementary reactions, within which the experimental data are described by the kinetic model within the average relative error of the experiment 25%, are given in Supplementary Materials S2. As an example, when calculating uncertainty regions, the processes of cumene oxidation and cumene hydroperoxide decomposition in the presence of Mg 2-ethylhexanoate as a catalyst were considered.

Table 2.
Pre-exponential factor A in the Arrhenius equation .

Table 3.
Activation energy E in the Arrhenius equation .

Table 4.
Species concentrations (mol/L) of cumene hydroperoxide decomposition in the presence of catalyst.
Then, using the kinetic model, we carried out computational experiments simulating cumene oxidation under industrial conditions (the initial concentrations of species were set in accordance with the composition of a flow charge from [43]) (it should be noted that, under industrial conditions, cumene oxidation is carried out in a continuous stirred-tank reactor (CSTR), while, in our work, a batch reactor is modeled. CSTR modeling of cumene oxidation is beyond the scope of this work). The results of the computational experiments and their discussion are shown below.
4. Discussion
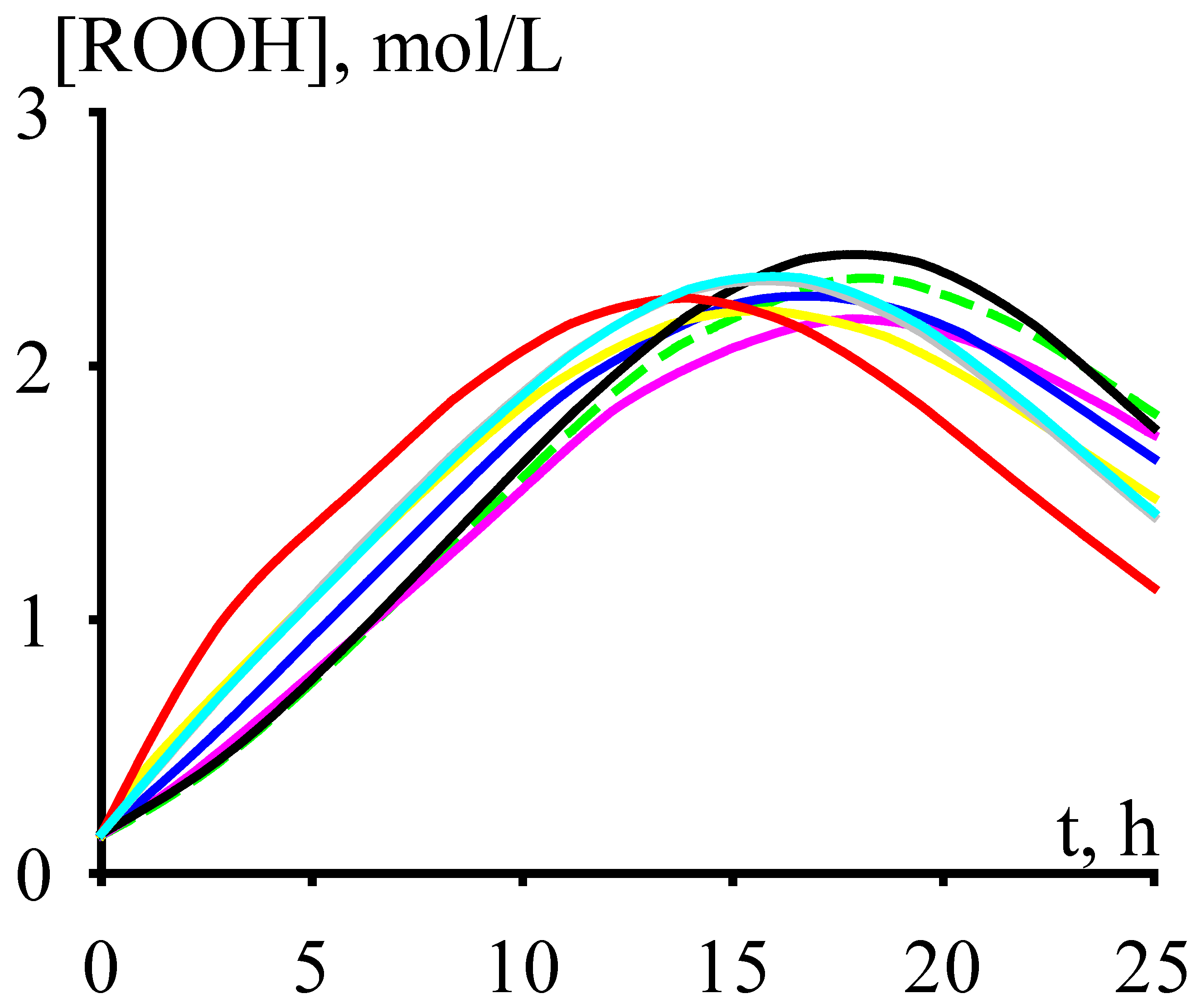
The concentration of cumene hydroperoxide accumulates over time up to a certain maximum value, after which it decreases (Figure 2) due to the prevalence of reactions leading to its decomposition (see Reactions (7)–(10), (18)–(19), (37)–(42), and (46) in the kinetic scheme).
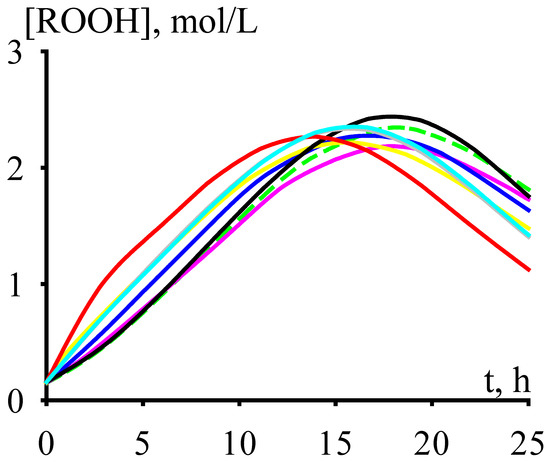
Figure 2.
The dependencies of cumene hydroperoxide accumulation versus time.
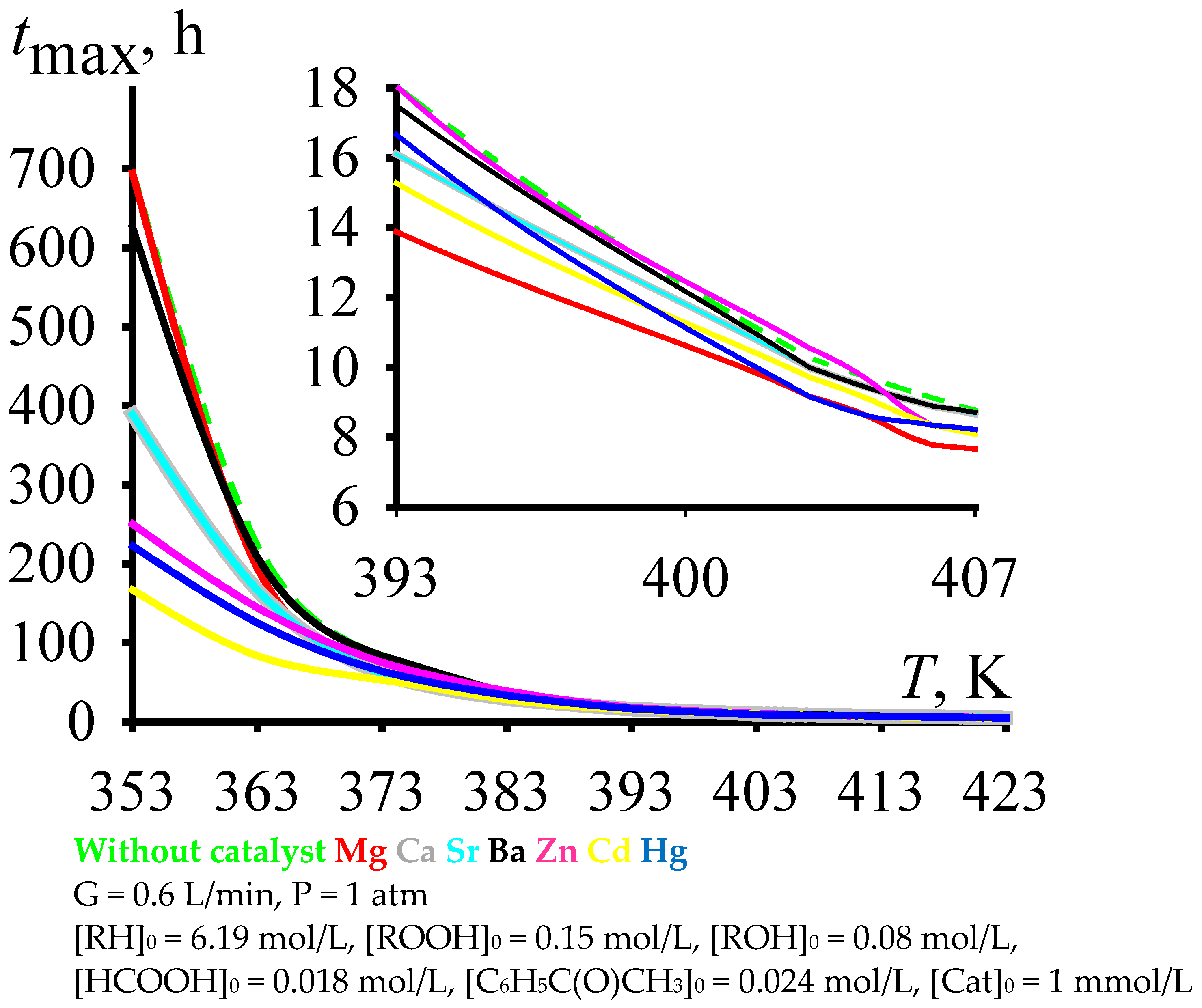
In this regard, it makes sense to carry out the cumene oxidation to this maximum concentration of cumene hydroperoxide. With an increase in the cumene oxidation temperature, the time to reach the maximum concentration of cumene hydroperoxide does decrease (Figure 3). Moreover, to ensure reasonable times for reaching the maximum concentration of cumene hydroperoxide, the cumene oxidation must be carried out at fairly high temperatures (under the process conditions shown in Figure 3, these are temperatures over 393 K).
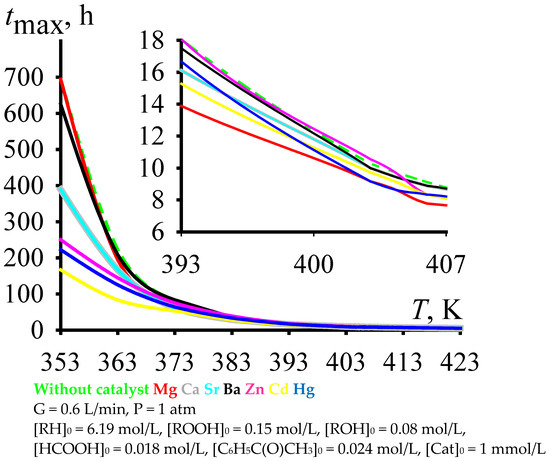
Figure 3.
Time of reaching the maximum cumene hydroperoxide concentration versus the temperature of cumene oxidation.
The following criterion was used as a criterion reflecting the productivity of cumene oxidation at the moment of reaching the maximum cumene hydroperoxide concentration, also taking into account the cumene conversion and selectivity achieved in this case:
where [ROOH]max is the maximum cumene hydroperoxide concentration, mol/L; tmax is the time of reaching the maximum cumene hydroperoxide concentration, h; [ROOH]0 is the initial cumene hydroperoxide concentration, mol/L; [RH]tmax is the cumene concentration at tmax, mol/L; [RH]0 is the initial cumene concentration, mol/L; 1 − ([RH]tmax/[RH]0) is the cumene conversion at tmax; ([ROOH]max − [ROOH]0)/([RH]0 − [RH]tmax) is the selectivity.
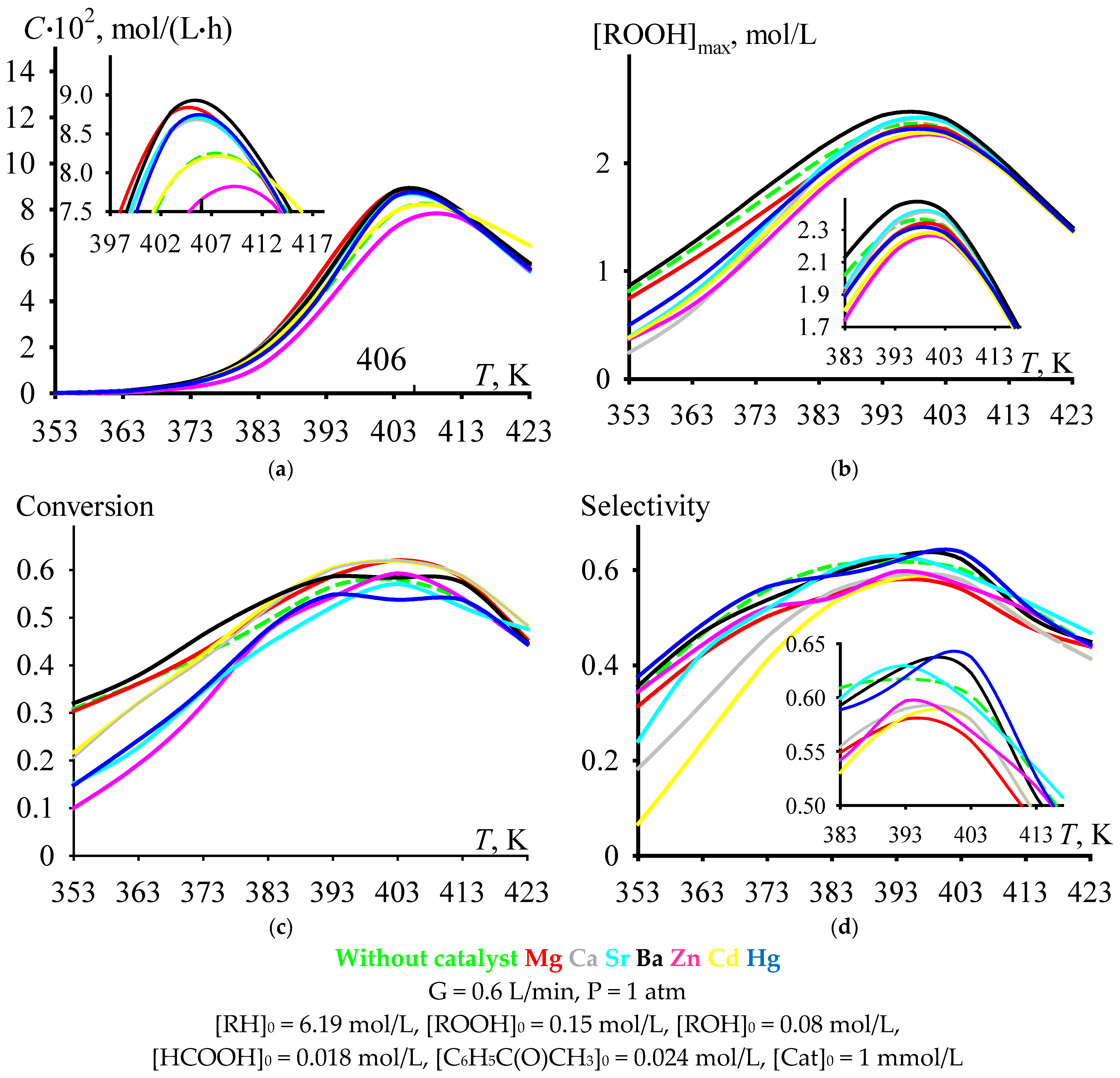
With an increase in the temperature of cumene oxidation, the C criterion increases to a maximum value, after which it decreases (Figure 4a). Under the process conditions shown in Figure 4, the maximum is observed at a temperature range near the value of 406 K, that is, in the range of moderately high temperatures (393–413 K). The dependencies of [ROOH]max (Figure 4b), cumene conversion at tmax (Figure 4c), and selectivity at tmax (Figure 4d) versus cumene oxidation temperature also have maximums in the same range. Moreover, it is rather problematic to unambiguously assess the effect of the catalyst on the ordinate of the maximum in the selected range of moderately high temperatures (Figure 4). Nevertheless, it can be noted that, in this temperature range, some of the catalysts provide selectivity at tmax, which is comparable to the value of the non-catalytic process, and some of the catalysts provide a lower selectivity at tmax compared to the value for the non-catalytic process. The selectivity values relate to each other as follows: Without catalyst ≈ Cd ≈ Ba ≈ Hg > Mg ≈ Ca ≈ Sr ≈ Zn (Figure 4d).
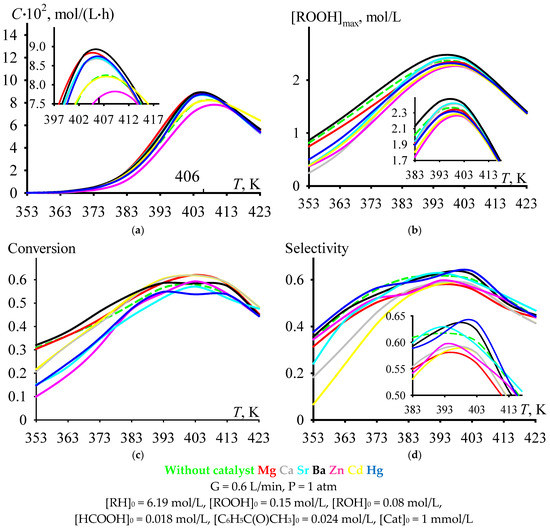
Figure 4.
The dependencies of criterion C (a), [ROOH]max (b), cumene conversion at tmax (c), and selectivity at tmax (d) versus cumene oxidation temperature.
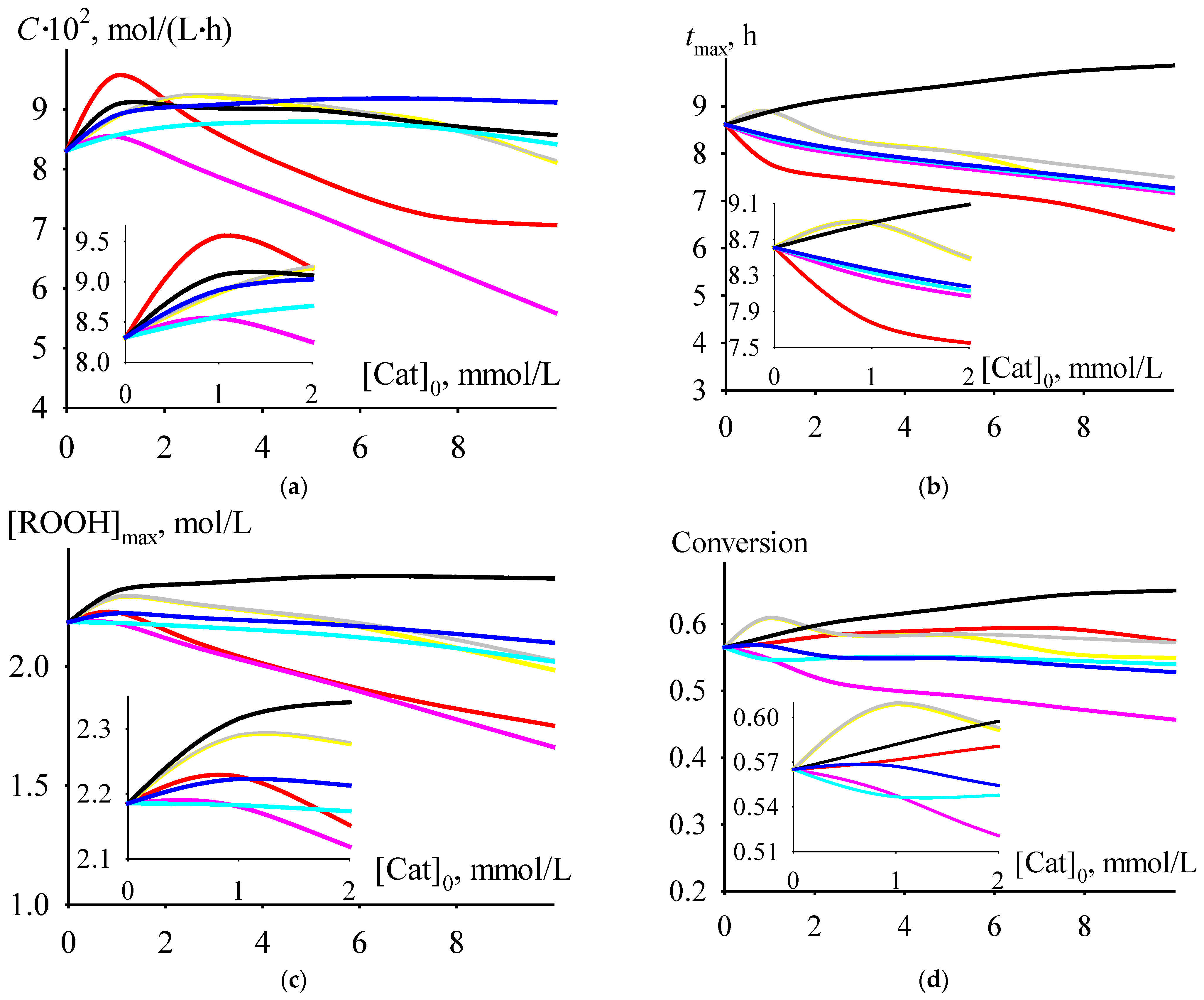
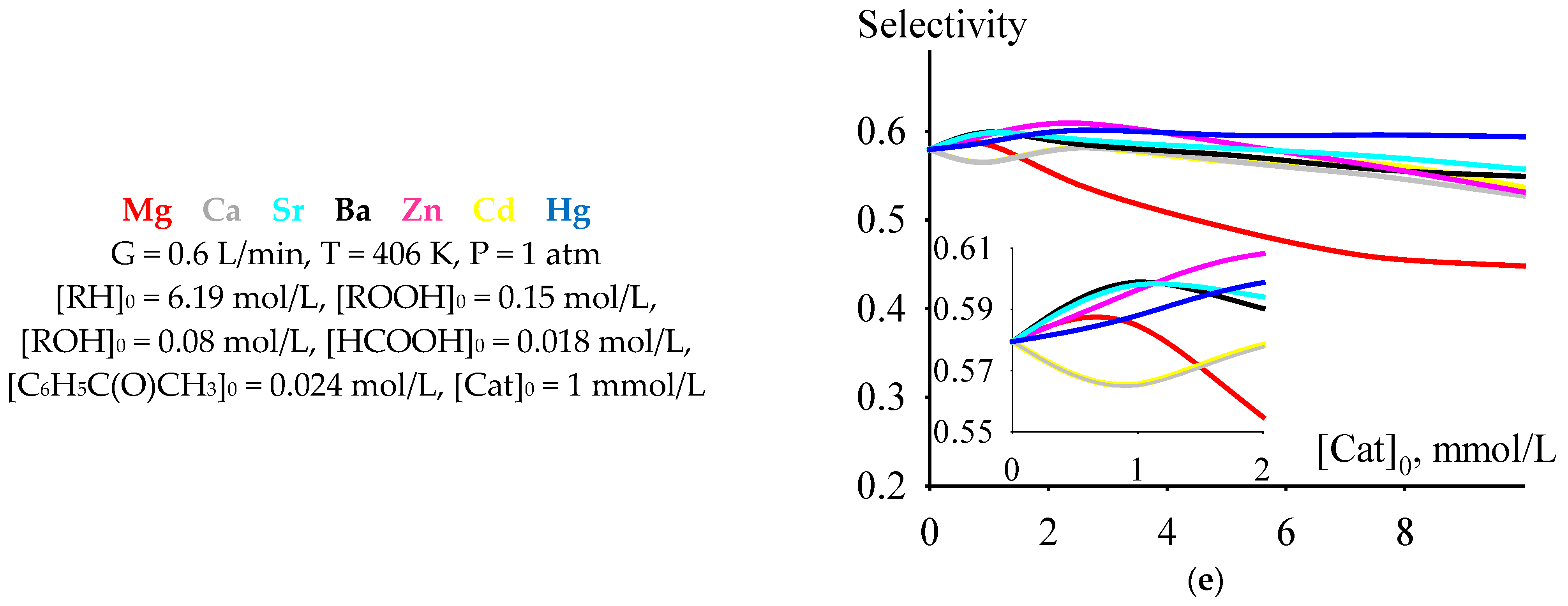
Among the dependencies of criterion C versus the initial concentrations of the catalysts under consideration at a temperature of 406 K, selected from the range of moderately high temperatures of 393–413 K, there is a dependence for cumene oxidation in the presence of Mg 2-ethylhexanoate. At initial catalyst concentrations less than 2 mmol/L, the values of criterion C are higher than for the process run in the presence of any of the other considered catalysts. Moreover, the maximum value of C is observed at an initial concentration of Mg 2-ethylhexanoate of 1 mmol/L (Figure 5a). At this initial concentration, the criterion C has a maximum or close to maximum value (with an increase in the initial catalyst concentration, the value of C after its maximum or close to maximum value decreases or, after a slight increase, it reaches a slightly decreasing plateau) and depends on the catalyst: Mg > Ba > Hg ≈ Ca ≈ Sr > Cd ≈ Zn. The time of reaching the maximum value of criterion C at an initial catalyst concentration of 1 mmol/L lines up in the series Ba ≈ Ca ≈ Sr > Hg ≈ Cd ≈ Zn > Mg (Figure 5b). The maximum concentration of cumene hydroperoxide (Figure 5c), cumene conversion (Figure 5d), and selectivity (Figure 5e) at an initial catalyst concentration of 1 mmol/L hardly depend on the catalyst and lie in the ranges of 2.2–2.3 mol/L (the value of 2.2 mol/L is typical for a non-catalytic process), 0.55–0.61, and 0.56–0.60, respectively.
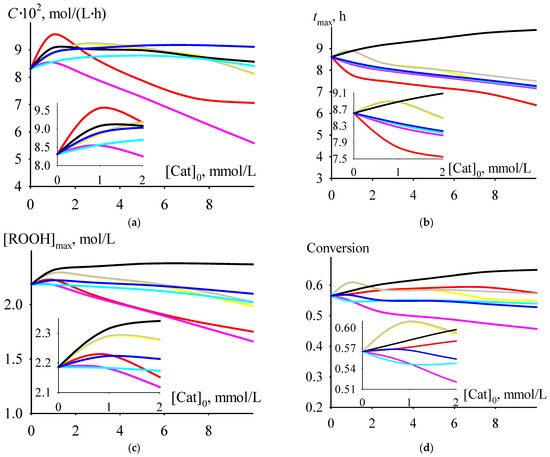
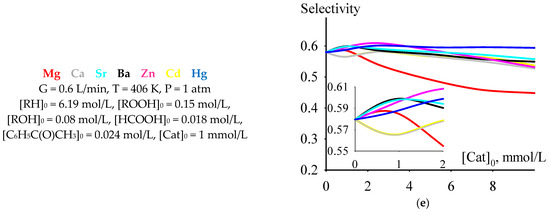
Figure 5.
The dependencies of criterion C (a), tmax (b), [ROOH]max (c), cumene conversion at tmax (d), and selectivity at tmax (e) versus initial catalyst concentration.
5. Conclusions
In the aspect of increasing the productivity of the first stage of the technological chain for the production of polymer composites from cumene, “production of phenol by the cumene method (stage 1 is cumene oxidation to cumene hydroperoxide, stage 2 is decomposition of cumene hydroperoxide into phenol and acetone) → production of precursors from phenol → production of polymers from precursors → production of composites from polymers,” considered the problem of establishing the effect of metals of the 2nd and 12th groups (Mg, Ca, Sr, Ba, Zn, Cd, and Hg) in the composition of 2-ethylhexanoates on the productivity and selectivity of the cumene oxidation stage. The problem was solved using kinetic simulation. The kinetic model was written on the basis of the kinetic scheme, which included the following reactions: formation of intermediate adducts “species—catalyst,” chain initiation, chain propagation, chain termination, and molecular reactions. It has the form of a system of nonlinear differential equations that describe, according to the mass action law, the rate of change concentrations of all species in the reaction mixture. The kinetic model was verified using experimental data: the model satisfactorily describes laboratory experimental data on cumene oxidation and cumene hydroperoxide decomposition within an average relative error of 25%. It should be noted that the values of A and E, for each reaction rate coefficient (Table 2 and Table 3), are just some of the possible values of the parameters A and E that can be found as a result of solving the inverse kinetic problem. Here, a criterion has been introduced that reflects the productivity of cumene oxidation at the moment of reaching the maximum concentration of cumene hydroperoxide, and it takes into account the cumene conversion and selectivity achieved in this case in the shortest possible time using the selectivity comparable with the selectivity of a non-catalytic process. According to the results of computational experiments on the kinetic model, it was shown that the achievement of the maximum value of this criterion among all the considered catalysts is ensured by Mg 2-ethylhexanoate at its relatively low initial concentration (1 mmol/L) under conditions of moderately high process temperatures (393–413 K).
Supplementary Materials
The following supplementary materials can be downloaded at: https://www.mdpi.com/article/10.3390/jcs7020070/s1.
Author Contributions
N.V.U.: Funding acquisition, Project administration, Conceptualization, Methodology, Data curation, Formal analysis, Writing—original draft, Writing—review & editing; D.A.S.: Investigation, Writing—original draft, Writing—review & editing; Y.L.L.: Investigation, Visualization; N.A.N.: Investigation, Visualization; K.A.T.: Methodology, Data curation, Formal analysis, Writing—original draft, Writing—review & editing; N.M.N.: Investigation; M.N.D.: Investigation; K.E.K.: Data curation; Y.O.M.: Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Russian Science Foundation No. 22-13-00461, https://rscf.ru/project/22-13-00461 (accessed on 9 June 2022).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parameswaranpillai, J.; Pulikkalparambil, H.; Rangappa, S.M. Epoxy Composites: Fabrication, Characterization and Applications; Wiley-VCH: Weinheim, Germany, 2021. [Google Scholar]
- Michelena, A.H.; Summerscales, J.; Graham-Jones, J.; Hall, W. Sustainable manufacture of natural fibre reinforced epoxy resin composites with coupling agent in the hardener. J. Compos. Sci. 2022, 6, 97. [Google Scholar] [CrossRef]
- Purse, M.; Edmund, G.; Hall, S.; Howlin, B.; Hamerton, I.; Till, S. Reactive molecular dynamics study of the thermal decomposition of phenolic resins. J. Compos. Sci. 2019, 3, 32. [Google Scholar] [CrossRef]
- Burmistr, M.V.; Boiko, V.S.; Lipko, E.O.; Gerasimenko, K.O.; Gomza, Y.P.; Vesnin, R.L.; Chernyayev, A.V.; Ananchenko, V.A.; Kovalenko, V.L. Antifriction and construction materials based on modified phenol-formaldehyde resins reinforced with mineral and synthetic fibrous fillers. Mech. Compos. Mater. 2014, 50, 213–222. [Google Scholar] [CrossRef]
- Abdeen, M.A.; Ayesh, A.S.; Ibrahim, S.; Khaldi, R.H. Preparation and physical characterization of conjugated polymer-polycarbonate polymer blends. J. Compos. Sci. 2013, 48, 1947–1957. [Google Scholar] [CrossRef]
- Brunella, V.; Rossatto, B.G.; Mastropasqua, C.; Cesano, F.; Scarano, D. Thermal/Electrical properties and texture of carbon black PC polymer composites near the electrical percolation threshold. J. Compos. Sci. 2021, 5, 212. [Google Scholar] [CrossRef]
- Khvatov, A.V.; Brevnov, P.N.; Shilkina, N.G.; Lomakin, S.M. Thermal and physical and mechanical properties of polysulfone composites with carbon nanotubes. Russ. J. Phys. Chem. B 2019, 13, 519–524. [Google Scholar] [CrossRef]
- Deberdeev, T.R.; Akhmetshina, A.I.; Karimova, L.K.; Ignat’eva, E.K.; Galikhmanov, N.R.; Grishin, S.V.; Berlin, A.A.; Deberdeev, R.Y. Aromatic polysulfones: Strategies of synthesis, properties, and application. Polym. Sci. Ser. D 2020, 13, 320–328. [Google Scholar] [CrossRef]
- Vaidya, U.; Janney, M.; Graham, K.; Ghossein, H.; Theodore, M. Mechanical response and processability of wet-laid recycled Carbon Fiber PE, PA66 and PET thermoplastic composites. J. Compos. Sci. 2022, 6, 198. [Google Scholar] [CrossRef]
- Kim, J.; Cho, D. Effects of alkali-treatment and feeding route of henequen fiber on the heat deflection temperature, mechanical, and impact properties of novel henequen Fiber/Polyamide 6 composites. J. Compos. Sci. 2022, 6, 89. [Google Scholar] [CrossRef]
- Global Cumene Market Size Study, by Production (Zeolite, Solid Phosphoric Acid, Aluminum Chloride), By Application (Phenol, Acetone, Others), and Regional Forecasts. Available online: https://www.giiresearch.com/report/bzc1090047-global-cumene-market-size-study-by-production.html (accessed on 9 June 2022).
- Hock, H.; Lang, S. Autoxydation von Kohlenwasserstoffen, IX. Mitteil.: Über Peroxyde von Benzol-Derivaten. Ber. Dtsch. Chem. Ges. 1944, 77, 257–264. [Google Scholar] [CrossRef]
- Weber, M.; Daldrup, J.-B.G.; Weber, M. Noncatalyzed Radical Chain Oxidation: Cumene Hydroperoxide. In Liquid Phase Aerobic Oxidation Catalysis; Stahl, S.S., Alsters, P.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 15–31. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; Taylor & Francis Group: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, J.; Chen, B.; Lei, Z. Process intensification on the selective catalytic oxidation of cumene with ionic liquids. Chem. Eng. Process. Process Intensif. 2018, 130, 88–92. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, G.; Huang, C.; Han, X.; Li, Y.; Chen, B. Selective oxidation of cumene to the equivalent amount of dimethylbenzyl alcohol and cumene hydroperoxide. Ind. Eng. Chem. Res. 2019, 58, 19785–19793. [Google Scholar] [CrossRef]
- Kuznetsova, N.I.; Babushkin, D.E.; Zudin, V.N.; Koscheeva, O.S.; Kuznetsova, L.I. Low-temperature oxidation of isopropylbenzene mediated by the system of NHPI, Fe (acac)3 and 1,10-phenanthroline. Catal. Commun. 2021, 149, 106218. [Google Scholar] [CrossRef]
- Kasaikina, O.T.; Potapova, N.V.; Krugovov, D.A.; Pisarenko, L.M. Catalysis of radical reactions in mixed micelles of surfactants with hydroperoxides. Kinet. Catal. 2017, 58, 556–562. [Google Scholar] [CrossRef]
- Mu, C.; Huang, K.; Cheng, T.; Wang, H.; Yu, H.; Peng, F. Ni foams decorated with carbon nanotubes as catalytic stirrers for aerobic oxidation of cumene. Chem. Eng. J. 2016, 306, 806–815. [Google Scholar] [CrossRef]
- Mu, C.; Cao, Y.; Wang, H.; Yu, H.; Peng, F. A kinetics study on cumene oxidation catalyzed by carbon nanotubes: Effect of N-doping. Chem. Eng. Sci. 2018, 177, 391–398. [Google Scholar] [CrossRef]
- Matienko, L.I.; Binyukov, V.I.; Mil, E.M.; Zaikov, G.E. Supramolecular macrostructures in the mechanisms of catalysis with nickel or iron heteroligand complexes. Curr. Organocatalysis. 2019, 6, 36–43. [Google Scholar] [CrossRef]
- Wang, F.; Jia, S.; Li, D.; Yu, B.; Zhang, L.; Liu, Y.; Han, X.; Zhang, R.; Wu, S. Self-template synthesis of CuO@Cu3(BTC)2 composite and its application in cumene oxidation. Mater. Lett. 2016, 164, 72–75. [Google Scholar] [CrossRef]
- Turovskij, N.; Raksha, E.; Berestneva, Y.; Eresko, A. Anion effect on the cumene hydroperoxide decomposition in the presence of Cu(II) 1,10-phenanthrolinates. J. Organomet. Chem. 2020, 922, 121371. [Google Scholar] [CrossRef]
- Nowacka, A.; Briantais, P.; Prestipino, C.; Llabrés i Xamena, F.X. Selective aerobic oxidation of cumene to cumene hydroperoxide over mono- and bimetallic trimesate metal-organic frameworks prepared by a facile “green” aqueous synthesis. ACS Sustain. Chem. Eng. 2019, 7, 7708–7715. [Google Scholar] [CrossRef]
- Nowacka, A.; Vismara, R.; Mercuri, G.; Moroni, M.; Palomino, M.; Domasevitch, K.V.; Di Nicola, C.; Pettinari, C.; Giambastiani, G.; Llabrés i Xamena, F.X.; et al. Cobalt (II) bipyrazolate metal-organic frameworks as heterogeneous catalysts in cumene aerobic oxidation: A tag-dependent selectivity. Inorg. Chem. 2020, 59, 8161–8172. [Google Scholar] [CrossRef]
- Bryant, J.R.; Matsuo, T.; Mayer, J.M. Cumene oxidation by cis-[RuIV(bpy)2(py)(O)]2+, revisited. Inorg. Chem. 2004, 43, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Crites, C.-O.L.; Hallett-Tapley, G.L.; Frenette, M.; González-Béjar, M.; Netto-Ferreira, J.C.; Scaiano, J.C. Insights into the mechanism of cumene peroxidation using supported gold and silver nanoparticles. ACS Catal. 2013, 3, 2062–2071. [Google Scholar] [CrossRef]
- Sapunov, V.N.; Koshel’, G.N.; Rumyantseva, Y.B.; Kurganova, E.A.; Kukushkina, N.D. The role of N-hydroxyphthalimide in the reaction mechanism of liquid-phase oxidation of p-cumene. Petr. Chem. 2013, 53, 171–176. [Google Scholar] [CrossRef]
- Hermans, I.; Vereecken, L.; Jacobs, P.A.; Peeters, J. Mechanism of the catalytic oxidation of hydrocarbons by N-hydroxyphthalimide: A theoretical study. Chem. Comm. 2004, 9, 1140–1141. [Google Scholar] [CrossRef]
- Liao, S.; Peng, F.; Yu, H.; Wang, H. Carbon nanotubes as catalyst for the aerobic oxidation of cumene to cumene hydroperoxide. Appl. Catal. A General. 2014, 478, 1–8. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; Ji, H.; Wu, B.; Zeng, X. Cumene liquid oxidation to cumene hydroperoxide over CuO nanoparticle with molecular oxygen under mild condition. J. Nat. Gas Chem. 2007, 16, 393–398. [Google Scholar] [CrossRef]
- Ulitin, N.V.; Kharlampidi, K.E.; Tereshchenko, K.A.; Novikov, N.A.; Shiyan, D.A.; Nurmurodov, T.S.; Nurullina, N.M.; Ziyatdinov, N.N.; Miroshkin, N.P. The cumene oxidation and cumene hydroperoxide decomposition in the presence of Zn, Cd or Hg 2-ethylhexanoate: Kinetic model and analysis of its sensitivity. Mol. Catal. 2021, 515, 111886. [Google Scholar] [CrossRef]
- Nurullina, N.M.; Batyrshin, N.N.; Kharlampidi, K.E. Effect of zinc-subgroup metal salts on the formation of hydroperoxide during the oxidation of cumene. Petrol. Chem. 2009, 49, 405–409. [Google Scholar] [CrossRef]
- Perkel, A.L.; Voronina, S.G.; Borkina, G.G. Liquid-phase oxidation of cyclohexane. Elementary steps in the developed process, reactivity, catalysis, and problems of conversion and selectivity. Rus. Chem. Bull. 2018, 67, 1747–1758. [Google Scholar] [CrossRef]
- Masters, C. Homogeneous Transition-Metal Catalysis; Chapman and Hall: London, UK, 1981. [Google Scholar]
- Ferenc, W.; Bocian, B.; Walkow-Dziewulska, A. Spectroscopic, magnetic and thermal behaviour of the 2, 3, 4-trimethoxybenzoates of heavy lanthanides(III) and yttrium(III). J. Serb. Chem. Soc. 2004, 69, 195–204. [Google Scholar] [CrossRef]
- Ferenc, W.; Walkow-Dziewulska, A.; Sadowsky, P.; Chrusciel, J. Spectral, thermal and magnetic characterization of 2, 3-dimethoxybenzoates of Co(II), Ni(II) and Cu(II). J. Serb. Chem. Soc. 2005, 70, 833–842. [Google Scholar] [CrossRef]
- Kharlampidi, K.E.; Tereshchenko, K.A.; Nurmurodov, T.S.; Shiyan, D.A.; Miroshkin, N.P.; Ziyatdinov, N.N.; Ziganshina, A.S.; Nurullina, N.M.; Khursan, S.L.; Ulitin, N.V. The kinetic modeling of cumene oxidation taking into account oxygen mass transfer. Chem. Eng. J. 2020, 392, 123811. [Google Scholar] [CrossRef]
- Dumbre, D.K.; Choudhary, V.R.; Patil, N.S.; Uphade, B.S.; Bhargava, S.K. Calcium oxide supported gold nanoparticles as catalysts for the selective epoxidation of styrene by t-butyl hydroperoxide. J. Colloid Interface Sci. 2014, 415, 111–116. [Google Scholar] [CrossRef]
- Maudez, W.; Häussinger, D.; Fromm, K.M. Substitution reactions on CaI2: Synthesis of mixed metal lithium-calcium-phenolates, and cluster transformation as a function of solvent. Z. Anorg. Allg. Chem. 2006, 632, 2295–2298. [Google Scholar] [CrossRef]
- Rodríguez-Díaz, J.M.; Santos-Martín, M.T. Study of the best designs for modifications of the Arrhenius equation. Chemom. Intell. Lab. Syst. 2009, 95, 199–208. [Google Scholar] [CrossRef]
- Dolan, E.D.; Lewis, R.M.; Torczon, V. On the local convergence of pattern search. SIAM J. Optim. 2003, 14, 567–583. [Google Scholar] [CrossRef]
- Kharlampidi, K.E.; Nurmurodov, T.S.; Ulitin, N.V.; Tereshchenko, K.A.; Miroshkin, N.P.; Shiyan, D.A.; Novikov, N.A.; Stoyanov, O.V.; Ziyatdinov, N.N.; Lapteva, T.V.; et al. Design of cumene oxidation process. Chem. Eng. Process. 2021, 161, 108314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).