Synthesis of Chitosan Capped Zinc Sulphide Nanoparticle Composites as an Antibacterial Agent for Liquid Handwash Disinfectant Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Nanocomposites
2.3. Characterization
2.4. Formulation of a Liquid Handwash System
2.5. Antibacterial Activity Test
2.6. Quality Assessment
3. Results and Discussion
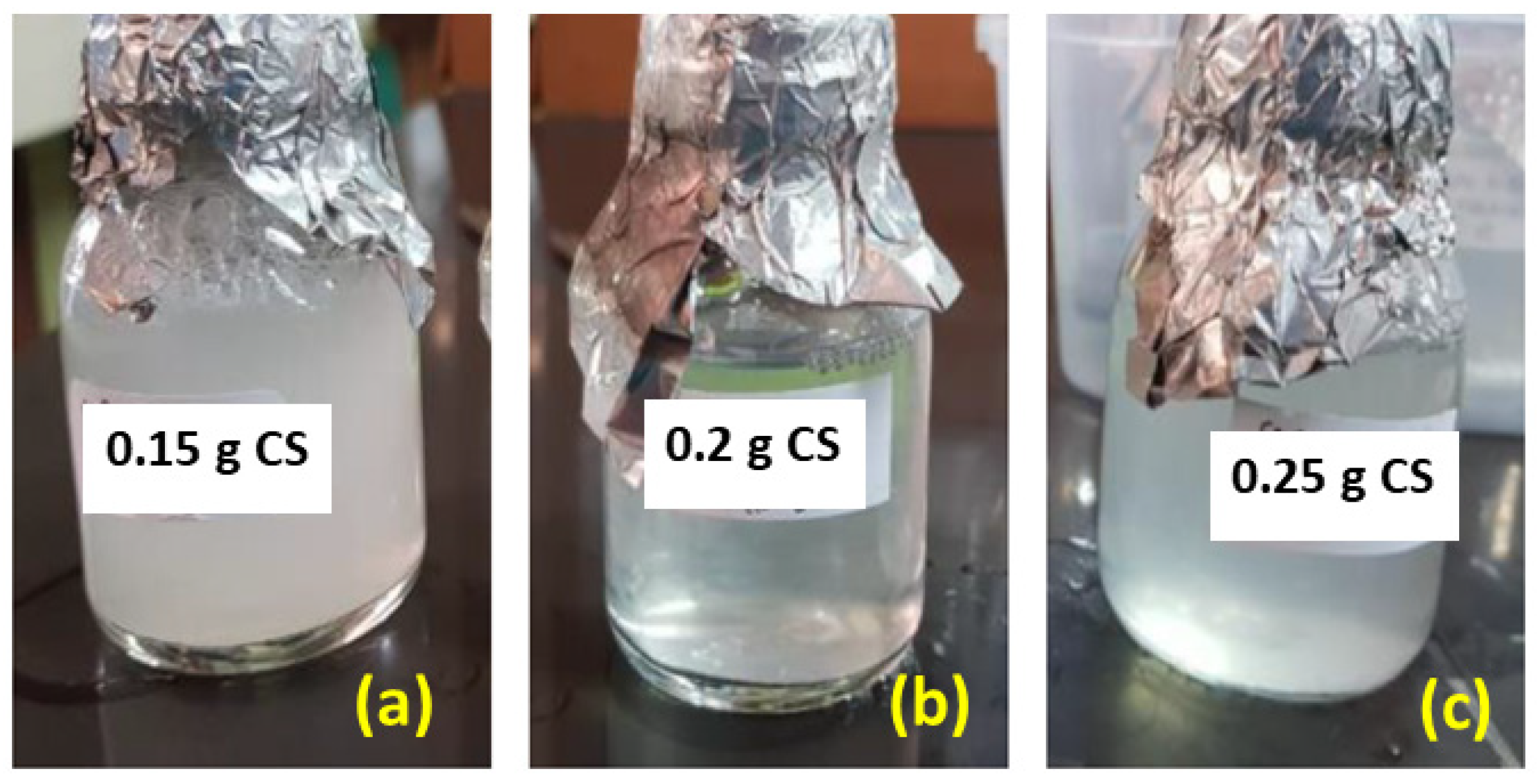
3.1. Preparation of CS-ZnS Nanocomposites
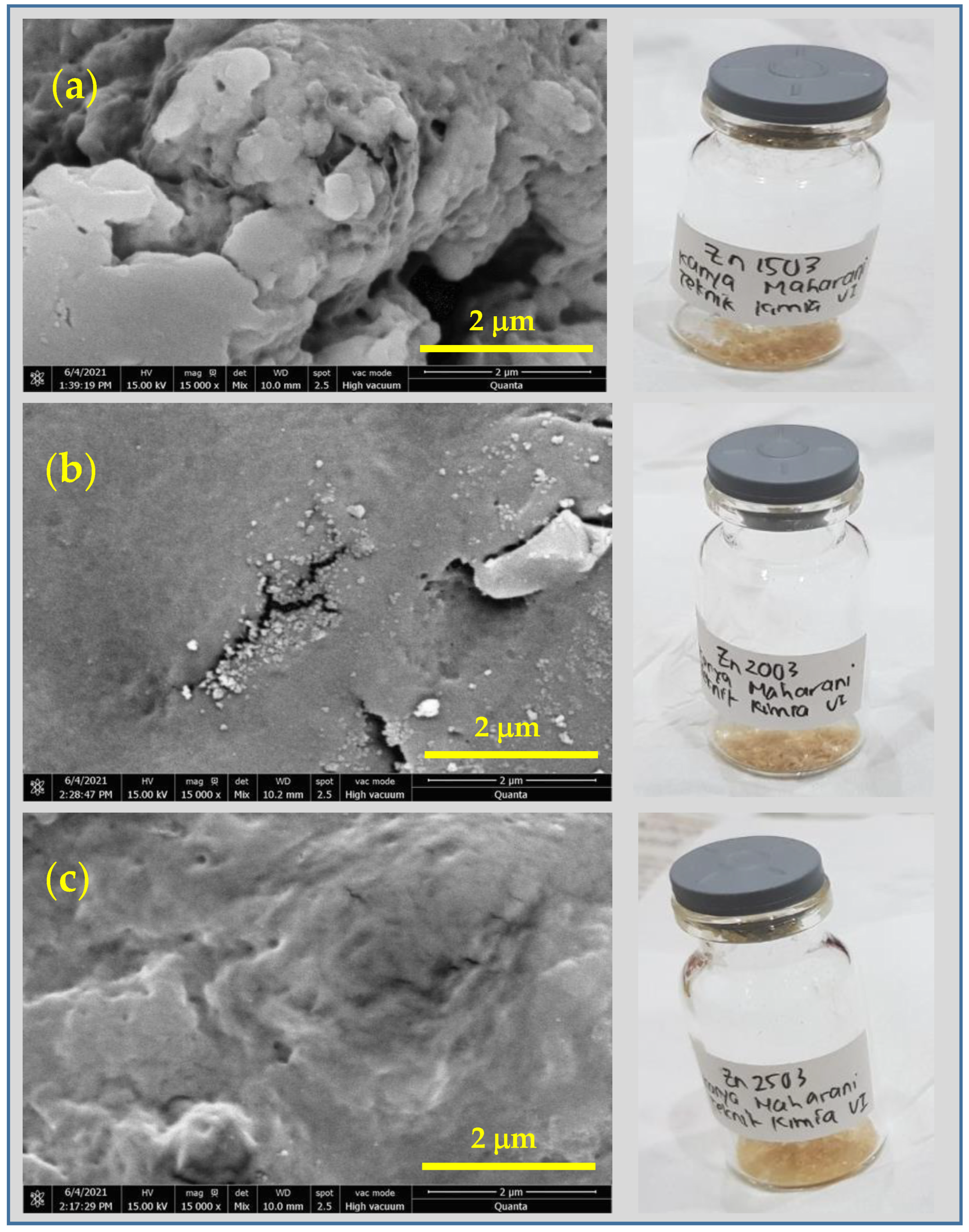
3.2. SEM-EDS Results of CS-ZnS NCs
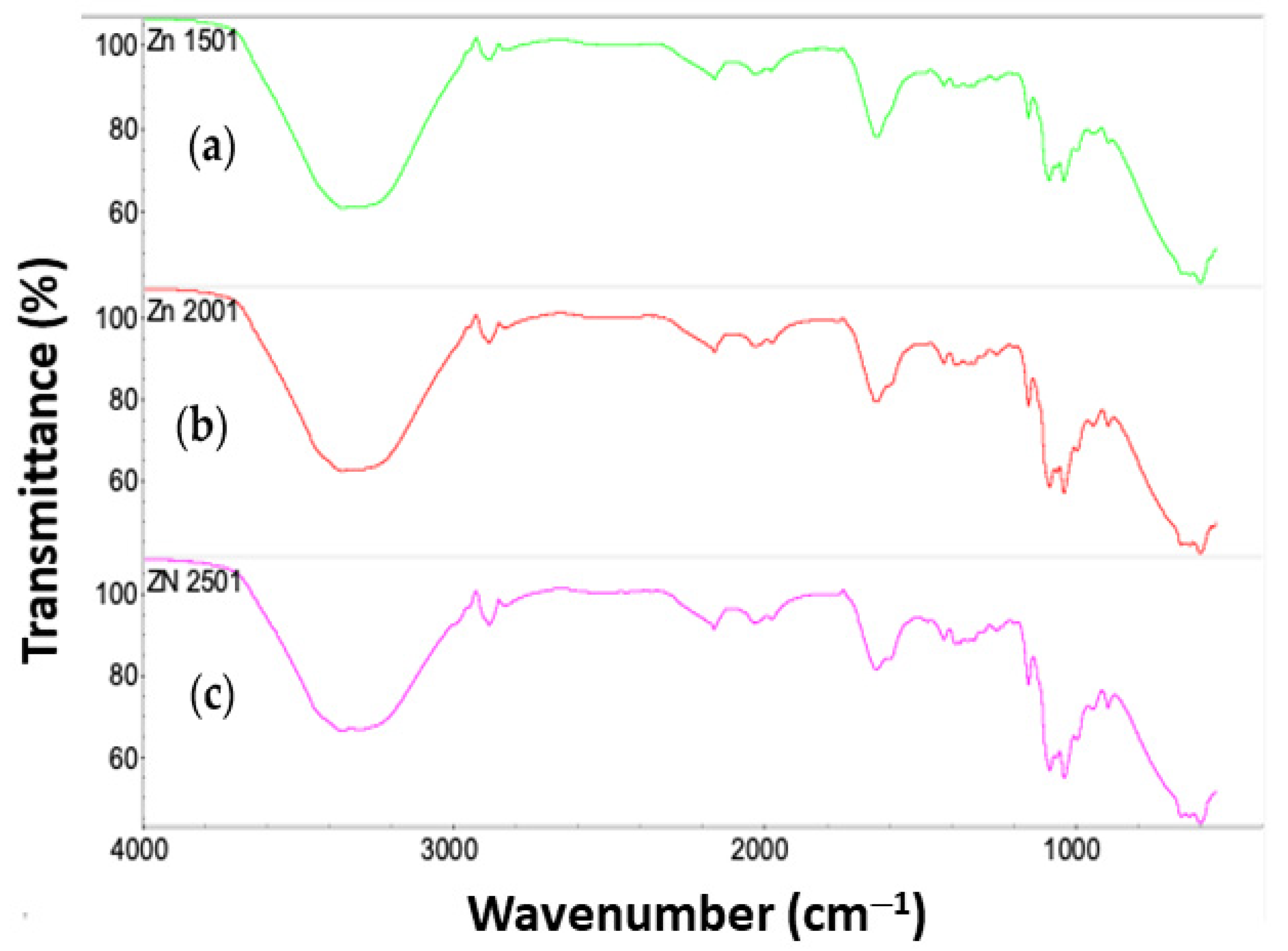
3.3. FTIR Analysis of CS-ZnS NCs
3.4. XRD Analysis of CS-ZnS NCs
3.5. Preparation of Liquid Handwash Containing CS-ZnS NCs
3.6. Antibacterial Activity of CS-ZnS LHs
3.7. Formulation of Liquid Handwash and Quality Assessment of the CS-ZnS Systems
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- SNI 2588:2017; Sabun Cair Pembersih Tangan (Liquid Handwash). Badan Standardisasi Nasional (National Standardization Agency): Jakarta, Indonesia, 2017.
- Longe, J. How Products Are Made: An Illustrated Guide to Product Manufacturing, 4th ed.; Gale Research: Detroit, MI, USA, 1998. [Google Scholar]
- Ullah, H.; Ali, S. Classification of Antibacterial Agents and Their Functions. In Antibacterial Agents; Kumavath, R.N., Ed.; IntechOpen: Kerala, India, 2017. [Google Scholar] [CrossRef]
- Kusrini, E.; Safira, A.I.; Usman, A.; Prasetyanto, E.A.; Nugrahaningtyas, K.D.; Santosa, S.J.; Wilson, L.D. Nanocomposites of Terbium Sulfide Nanoparticles with a Chitosan Capping Agent for Antibacterial Applications. J. Compos. Sci. 2023, 7, 39. [Google Scholar] [CrossRef]
- Lim, M.; Shahri, N.; Taha, H.; Mahadi, A.; Kusrini, E.; Lim, J.; Usman, A. Biocompatible chitin-encapsulated CdS quantum dots: Fabrication and antibacterial screening. Carbohydr. Polym. 2021, 260, 117806. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Lee, H.O.; Santiago, K.C.; Pelzer, M.; Kuti, A.; Jenrette, E.; Bahoura, M. Rapid Microwave Synthesis of Tunable Cadmium Selenide (CdSe) Quantum Dots for Optoelectronic Applications. J. Nanomater. 2020, 2020, 5056875. [Google Scholar] [CrossRef]
- Li, G.P.; Zhai, J.F.; Li, D.; Fang, X.N.; Jiang, H.; Dong, Q.Z.; Wang, E.K. One-pot synthesis of monodispersed ZnS nanospheres with high antibacterial activity. J. Mater. Chem. 2010, 20, 9215–9219. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. Antimicrobial activity of CdS and Ag2S quantum dots immobilized on poly(amidoamine) grafted carbon nanotubes. Colloids Surf. B: Biointerfaces 2012, 100, 215–221. [Google Scholar] [CrossRef]
- Li, H.; Li, M.; Wan, Y.S.; Lelkes, P.I.; Shih, W.H. Cytotoxicity Test of Water Soluble ZnS and CdS Quantum Dots. J. Nanosci. Nanotechnol. 2011, 11, 3543–3551. [Google Scholar] [CrossRef] [PubMed]
- Geng, B.Y.; Liu, X.W.; Du, O.B.; Wei, X.W.; Zhang, L.D. Structure and optical properties of periodically twinned ZnS nanowires. Appl. Phys. Lett. 2006, 88, 163104. [Google Scholar] [CrossRef]
- Wang, L.; Dai, J.; Liu, X.; Zhu, Z.; Huang, X.; Wu, P. Morphology-controlling synthesis of ZnS through a hydrothermal/solvothermal method. Ceram. Int. 2012, 38, 1873–1878. [Google Scholar] [CrossRef]
- Li, Z.; Chen, P.; Xu, X.; Ye, X.; Wang, J. Preparation of chitosan–sodium alginate microcapsules containing ZnS nanoparticles and its effect on the drug release. Mater. Sci. Eng. C 2009, 7, 2250–2253. [Google Scholar] [CrossRef]
- Mamiyev, Z.Q.; Balayeva, N.O. Optical and structural studies of ZnS nanoparticles synthesized via chemical in situ technique. Chem. Phys. Lett. 2016, 646, 69–74. [Google Scholar] [CrossRef]
- Donne, A.L.; Jana, S.K.; Banerjee, S.; Basu, S.; Binetti, S. Optimized luminescence properties of Mn doped ZnS nanoparticles for photovoltaic applications. J. Appl. Phys. 2013, 113, 014903. [Google Scholar] [CrossRef]
- La Porta, F.D.A.; Nogueira, A.E.; Gracia, L.; Pereira, W.S.; Botelho, G.; Mulinari, T.A.; Andrés, J.; Longo, E. An experimental and theoretical investigation on the optical and photocatalytic properties of ZnS nanoparticles. J. Phys. Chem. Solids 2017, 103, 179–189. [Google Scholar] [CrossRef]
- Mamiyev, Z.; Balayeva, N.O. Metal Sulfide Photocatalysts for Hydrogen Generation: A Review of Recent Advances. Catalysts 2022, 12, 1316. [Google Scholar] [CrossRef]
- Sarangi, B.; Mishra, S.P.; Behera, N. Advances in green synthesis of ZnS nanoparticles: An overview. Mater. Sci. Semicond. Process. 2022, 147, 106723. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y. Formation of zinc sulfide nanorods and nanoparticles in ternary W/O microemulsions. J. Colloid Interface Sci. 2003, 259, 275–281. [Google Scholar] [CrossRef]
- Hoa, T.T.Q.; Vu, L.V.; Canh, T.D.; Long, N.N. Preparation of ZnS nanoparticles by hydrothermal method. J. Phys. Conf. Ser. 2009, 187, 012081. [Google Scholar] [CrossRef]
- Sánchez-Lopez, J.C.; Fernández, A. The gas-phase condensation method for the preparation of quantum-sized ZnS nanoparticles. Thin Solid Film. 1998, 317, 497–499. [Google Scholar] [CrossRef]
- Hedayati, K.; Zendehnam, A.; Hassanpour, F. Fabrication and characterization of zinc sulfide nanoparticles and nanocomposites prepared via a simple chemical precipitation method. J. Nanostruct. 2016, 6, 207–212. [Google Scholar] [CrossRef]
- Lenggoro, I.W.; Okuyama, K.; de la Morat, J.F.; Tohge, N. Preparation of ZnS nanoparticles by electrospray pyrolysis. J. Aerosol Sci. 2000, 31, 121–136. [Google Scholar] [CrossRef]
- Rahimi-Nasarabadi, M. Electrochemical synthesis and characterization of zinc sulfide nanoparticles. J. Nanostruct. 2014, 4, 211–216. [Google Scholar] [CrossRef]
- Thottoli, A.K.; Achuthanunni, A.K. Effect of polyvinyl alcohol concentration on the ZnS nanoparticles and wet chemical synthesis of wurtzite ZnS nanoparticles. J. Nanostruct. Chem. 2013, 3, 31. [Google Scholar] [CrossRef]
- Wang, L.P.; Hong, G.Y. A new preparation of zinc sulfide nanoparticles by solid state method at low temperature. Mater. Res. Bull. 2000, 35, 695–701. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, L.; Wang, R. Creation of ZnS nanoparticles by laser ablation in water. Appl. Phys. A 2016, 122, 104. [Google Scholar] [CrossRef]
- Biswas, S.; Kar, S.; Chaudhuri, S. Synthesis and characterization of zinc sulfide nanostructures. Synth. React. Inorg. Metal-Org. Nano-Metal Chem. 2006, 36, 33–36. [Google Scholar] [CrossRef]
- Wang, G.Z.; Geng, B.Y.; Huang, X.M.; Wang, Y.W.; Li, G.H.; Zhang, L.D. A convenient ultrasonic irradiation technique for in situ synthesis of zinc sulfide nanocrystallites at room temperature. Appl. Phys. Mater. Sci. Process. 2003, 77, 933–936. [Google Scholar] [CrossRef]
- Tolia, J.V.; Chakraborty, M.; Murthy, Z.V.P. Photocatalytic degradation of malachite green dye using doped and undoped ZnS nanoparticles. Pol. J. Chem. Technol. 2012, 14, 16–21. [Google Scholar] [CrossRef]
- Lalithadevi, B.; Rao, K.M.; Ramananda, D. Investigations on structural and optical properties of starch capped ZnS nanoparticles synthesized by microwave irradiation method. Chem. Phys. Lett. 2018, 700, 74–79. [Google Scholar] [CrossRef]
- Yun, Y.-H.; Youn, H.-G.; Shin, J.-Y.; Yoon, S.-D. Preparation of functional chitosan-based nanocomposite films containing ZnS nanoparticles. Int. J. Biol. Macromol. 2017, 104, 1150–1157. [Google Scholar] [CrossRef]
- Bahri, S.; Homaei, A.; Mosaddegh, E. Zinc sulfide-chitosan hybrid nanoparticles as a robust surface for immobilization of Sillago sihama α-amylase. Colloids Surf. B Biointerfaces 2022, 218, 112754. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, M.; Liu, H.; Zhang, S. Low-toxic Ag2S quantum dots for photoelectrochemical detection glucose and cancer cells. Biosens. Bioelectron. 2014, 56, 307–312. [Google Scholar] [CrossRef]
- Kumar, D.S.; Kumar, B.J.; Mahesh, H.M. Chapter 3: Quantum Nanostructures (QDs): An Overview. Synth. Inorg. Nanomater. 2018, 59–88. [Google Scholar] [CrossRef]
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.; Zarghami, N.; Kouhi, M. Quantum dots: Synthesis, bioapplications, and toxicity. Nanoscale Res. Lett. 2012, 7, 480. [Google Scholar] [CrossRef]
- Green, M. The nature of quantum dot capping ligands. J. Mater. Chem. 2010, 20, 5797. [Google Scholar]
- Şenel, S.; McClure, S. Potential applications of chitosan in veterinary medicine. Adv. Drug Deliv. Rev. 2004, 56, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Parvaneh, I.; Samira, S.; Mohsen, N. Characterization of ZnS nanoparticles synthesized by co-precipitation method. Chin. Phys. B 2015, 24, 046104. [Google Scholar]
- Afsheen, S.; Naseer, H.; Iqbal, T.; Abrar, M.; Bashir, A.; Ijaz, M. Synthesis and characterization of metal sulphide nanoparticles to investigate the effect of nanoparticles on germination of soybean and wheat seeds. Mater. Chem. Phys. 2020, 252, 123216. [Google Scholar] [CrossRef]
- Tamrakar, R.; Ramrakhiani, M.; Chandra, B. Effect of Capping Agent Concentration on Photophysical Properties of Zinc Sulfide Nanocrystals. Open Nanosci. J. 2008, 2, 12–16. [Google Scholar] [CrossRef]
- Luyk, E. Sputter Coating for SEM: How This Sample Preparation Technique Assists Your Imaging [Internet]. ThermoFisher. 2019. Available online: https://www.thermofisher.com/blog/microscopy/sputter-coating-for-sem-how-this-sample-preparation-technique-assists-your-imaging/ (accessed on 6 November 2022).
- Rave Scientific. Proper Target Material Selection When Coating Samples Using an SEM Sputter Coater [Internet]. Rave Scientific. 2021. Available online: https://ravescientific.com/education/37-proper-target-material-selection-when-coating-samples-using-an-sem-sputter-coater (accessed on 6 November 2022).
- AbdElhady, M.M. Preparation and Characterization of Chitosan/Zinc Oxide Nanoparticles for Imparting Antimicrobial and UV Protection to Cotton Fabric. Int. J. Carbohydr. Chem. 2012, 2012, 840591. [Google Scholar] [CrossRef]
- Liu, L.; Dai, J.; Zhao, T.; Guo, S.; Hou, D.; Zhang, P. A novel Zn(II) dithiocarbamate/ZnS nanocomposite for highly efficient Cr6+ removal from aqueous solutions. RSC Adv. 2017, 7, 35075–35085. [Google Scholar] [CrossRef]
- Zamiri, R.; Abbastabar Ahangar, H.; Zakaria, A.; Zamiri, G.; Shabani, M.; Singh, B.; Ferreira, J. The structural and optical constants of Ag2S semiconductor nanostructure in the Far-Infrared. Chem. Cent. J. 2015, 9, 28. [Google Scholar] [CrossRef]
- Mansur, H.; Mansur, A. Nano-photocatalysts based on ZnS quantum dots/chitosan for the photodegradation of dye pollutants. IOP Conf. Ser. Mater. Sci. Eng. 2015, 76, 012003. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Kumar, S.; Dutta, P.; Koh, J. A physico-chemical and biological study of novel chitosan–chloroquinoline derivative for biomedical applications. Int. J. Biol. Macromol. 2011, 49, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Gul, Z.; Akbar, A.; Leghari, S.K. Elucidating therapeutic and biological potential of berberis baluchistanica ahrendt bark, leaf, and root extracts. Front. Microbiol. 2022, 13, 823673. [Google Scholar] [CrossRef]
- Giridhar, M.; Naik, H.S.B.; Prabhakar, M.C.; Naik, M.M.; Ballesh, N.; Mahesh, M.C. Synthesis, characterization and antibacterial activity of water-soluble dye-capped zinc sulphide nanoparticles from waste Zn–C battery. Bull. Mater. Sci. 2021, 44, 6. [Google Scholar] [CrossRef]
- Omar, M.; Sanif, M.; Ali, N.; Hamid, M.; Taha, H.; Mahadi, A. Synthesis of Schiff Base Encapsulated ZnS Nanoparticles: Characterization and Antibacterial Screening. Int. J. Technol. 2020, 11, 1309. [Google Scholar] [CrossRef]
- Tarun, J.; Susan, V.; Susan, J.; Suria, J.; Criton, S. Evaluation of pH of bathing soaps and shampoos for skin and hair care. Indian J. Dermatol. 2014, 59, 442. [Google Scholar] [CrossRef]
- Usman, A.; Kusrini, E.; Widiantoro, A.B.; Hardiya, E.; Abdullah, N.A.; Yulizar, Y. Fabrication of Chitosan Nanoparticles Containing Samarium Ion Potentially Applicable for Fluorescence Detection and Energy Transfer. Int. J. Technol. 2018, 9, 1112–1120. [Google Scholar] [CrossRef]
- Kusrini, E.; Shiong, N.S.; Harahap, Y.; Yulizar, Y.; Dianursanti Arbianti, R.; Pudjiastuti, A.R. Effects of Monocarboxylic Acids and Potassium Persulfate on Preparation of Chitosan Nanoparticles. Int. J. Technol. 2015, 6, 11–21. [Google Scholar] [CrossRef]
- Kusrini, E.; Ayuningtyas, K.; Mawarni, D.P.; Wilson, L.D.; Sufyan, M.; Rahman, A.; Prasetyanto, Y.E.A.; Usman, A. Micro-structured Materials for the Removal of Heavy Metals using a Natural Polymer Composite. Int. J. Technol. 2021, 12, 275–286. [Google Scholar] [CrossRef]
- Kusrini, E.; Wu, S.; Susanto, B.H.; Lukita, M.; Gozan, M.; Hans, M.D.; Rahman, A.; Degirmenci, V.; Usman, A. Simultaneous Absorption and Adsorption Processes for Biogas Purification using Ca(OH)2 Solution and Activated Clinoptilolite Zeolite/Chitosan Composites. Int. J. Technol. 2019, 10, 1243–1250. [Google Scholar] [CrossRef]






| Component | Composition (wt.%) |
|---|---|
| Solvent (distilled water) | 78 |
| Primary surfactant (ALS) | 10 |
| Primary surfactant (SDS) | 10 |
| Secondary surfactant (LG) | 1 |
| Antibacterial agent (CS-ZnS NCs) | 1 |
| Weight Content (%) | |||
|---|---|---|---|
| Element | CS-ZnS NCs | ||
| 0.15 g CS | 0.2 g CS | 0.25 g CS | |
| C | 48.6% | 52.3% | 55.4% |
| O | 34.0% | 29.6% | 30.0% |
| N | 11.1% | 10.5% | 7.6% |
| Zn | 6.3% | 7.6% | 7.0% |
| S | ≈0% | ≈0% | ≈0% |
| Liquid Handwash Solutions | Average Zone of Inhibition Diameter (mm) | |
|---|---|---|
| S. aureus | E. coli | |
| CS-ZnS LHs with 0.15 g CS | 16.9 | 0.00 |
| CS-ZnS LHs with 0.2 g CS | 18.3 | 0.00 |
| CS-ZnS LHs with 0.25 g CS | 19.1 | 0.00 |
| Tetracycline as control | 22.7 | 17.6 |
| Sample | Inhibition Zone Diameter (mm) | |
|---|---|---|
| E. coli | S. aureus | |
| Zn(NO3)2 | 22.1 | 32 |
| Eu(NO3)3 | 9.4 | 14.2 |
| Tb(NO3)3 | 11.0 | 8.2 |
| CS | - | 6.8 |
| Tetracycline (positive control) | 18.3 | 9.4 |
| . | CS-ZnS Liquid Handwash Formulations | |||
|---|---|---|---|---|
| Parameter | Requirement | 0.15 g CS | 0.2 g CS | 0.25 g CS |
| pH | 4–10 | 6.97 | 6.95 | 6.90 |
| Ethanol-insoluble matter | >10% | 0.011% | 0.01% | 0.01% |
| Total active ingredients | <0.5% | 99.33% | 99.32% | 99.31 |
| Free fatty acid | <1% | 0.009% | 0.01% | 0.01% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusrini, E.; Wilson, L.D.; Padmosoedarso, K.M.; Mawarni, D.P.; Sufyan, M.; Usman, A. Synthesis of Chitosan Capped Zinc Sulphide Nanoparticle Composites as an Antibacterial Agent for Liquid Handwash Disinfectant Applications. J. Compos. Sci. 2023, 7, 52. https://doi.org/10.3390/jcs7020052
Kusrini E, Wilson LD, Padmosoedarso KM, Mawarni DP, Sufyan M, Usman A. Synthesis of Chitosan Capped Zinc Sulphide Nanoparticle Composites as an Antibacterial Agent for Liquid Handwash Disinfectant Applications. Journal of Composites Science. 2023; 7(2):52. https://doi.org/10.3390/jcs7020052
Chicago/Turabian StyleKusrini, Eny, Lee D. Wilson, Kanya Maharani Padmosoedarso, Dias Puspitaning Mawarni, Muhammad Sufyan, and Anwar Usman. 2023. "Synthesis of Chitosan Capped Zinc Sulphide Nanoparticle Composites as an Antibacterial Agent for Liquid Handwash Disinfectant Applications" Journal of Composites Science 7, no. 2: 52. https://doi.org/10.3390/jcs7020052
APA StyleKusrini, E., Wilson, L. D., Padmosoedarso, K. M., Mawarni, D. P., Sufyan, M., & Usman, A. (2023). Synthesis of Chitosan Capped Zinc Sulphide Nanoparticle Composites as an Antibacterial Agent for Liquid Handwash Disinfectant Applications. Journal of Composites Science, 7(2), 52. https://doi.org/10.3390/jcs7020052









