Structural Characterization of Composites Based on Butadiene Rubber and Expanded Perlite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
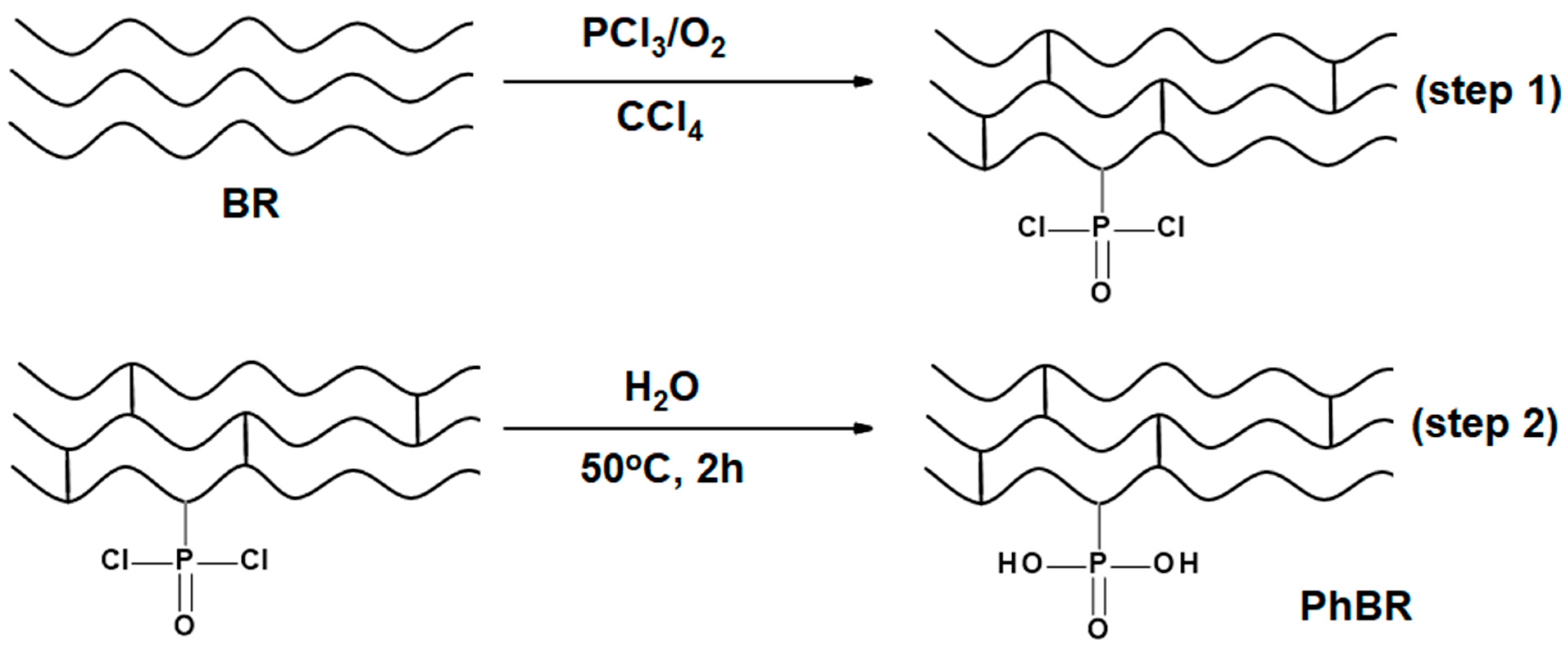
2.2.1. Synthesis of the First Type of Composite
2.2.2. Synthesis of the PhEP
2.2.3. Synthesis of the Second Type of Composite
2.2.4. Synthesis of PhBR
2.2.5. Characterization Techniques
3. Results and Discussion
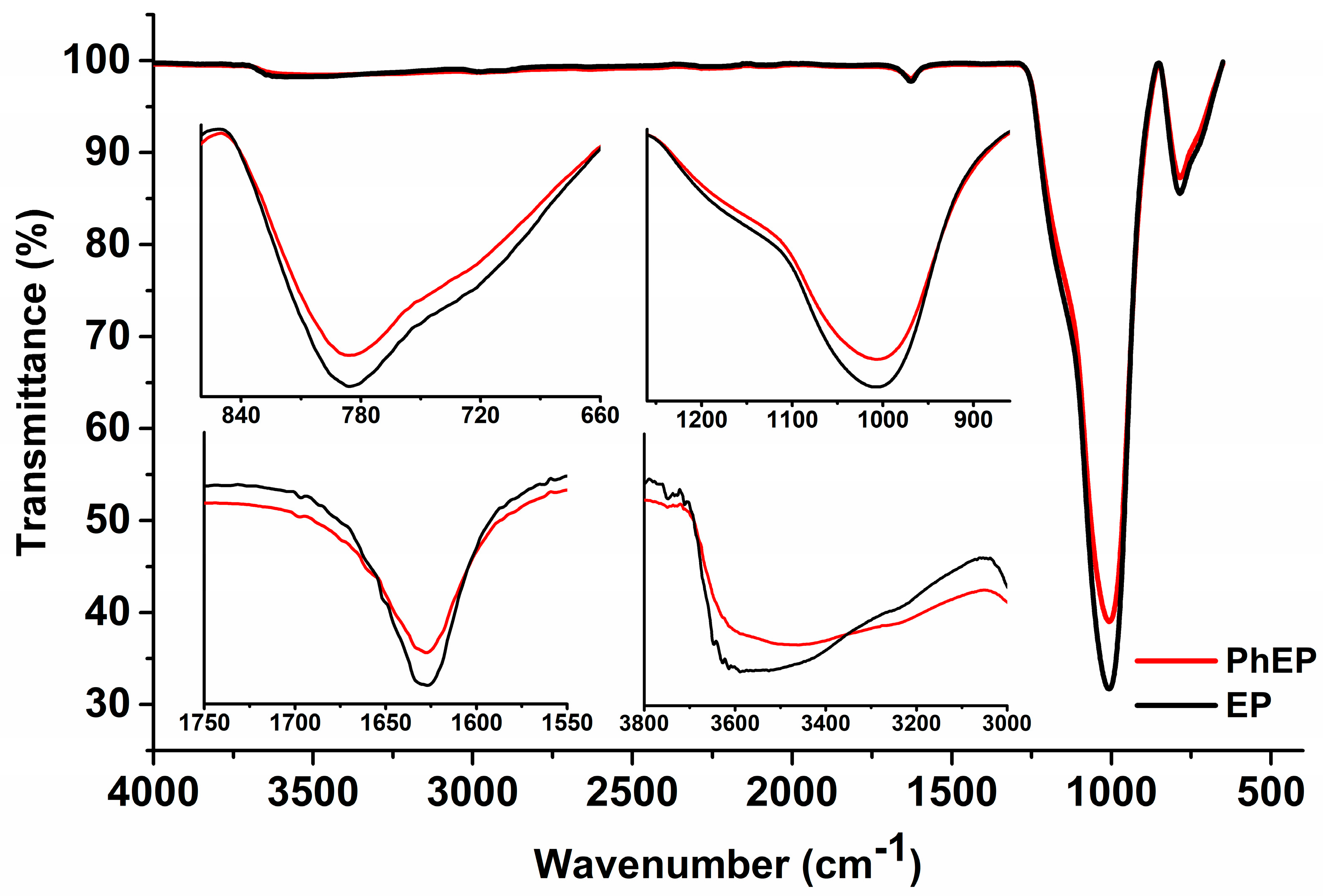
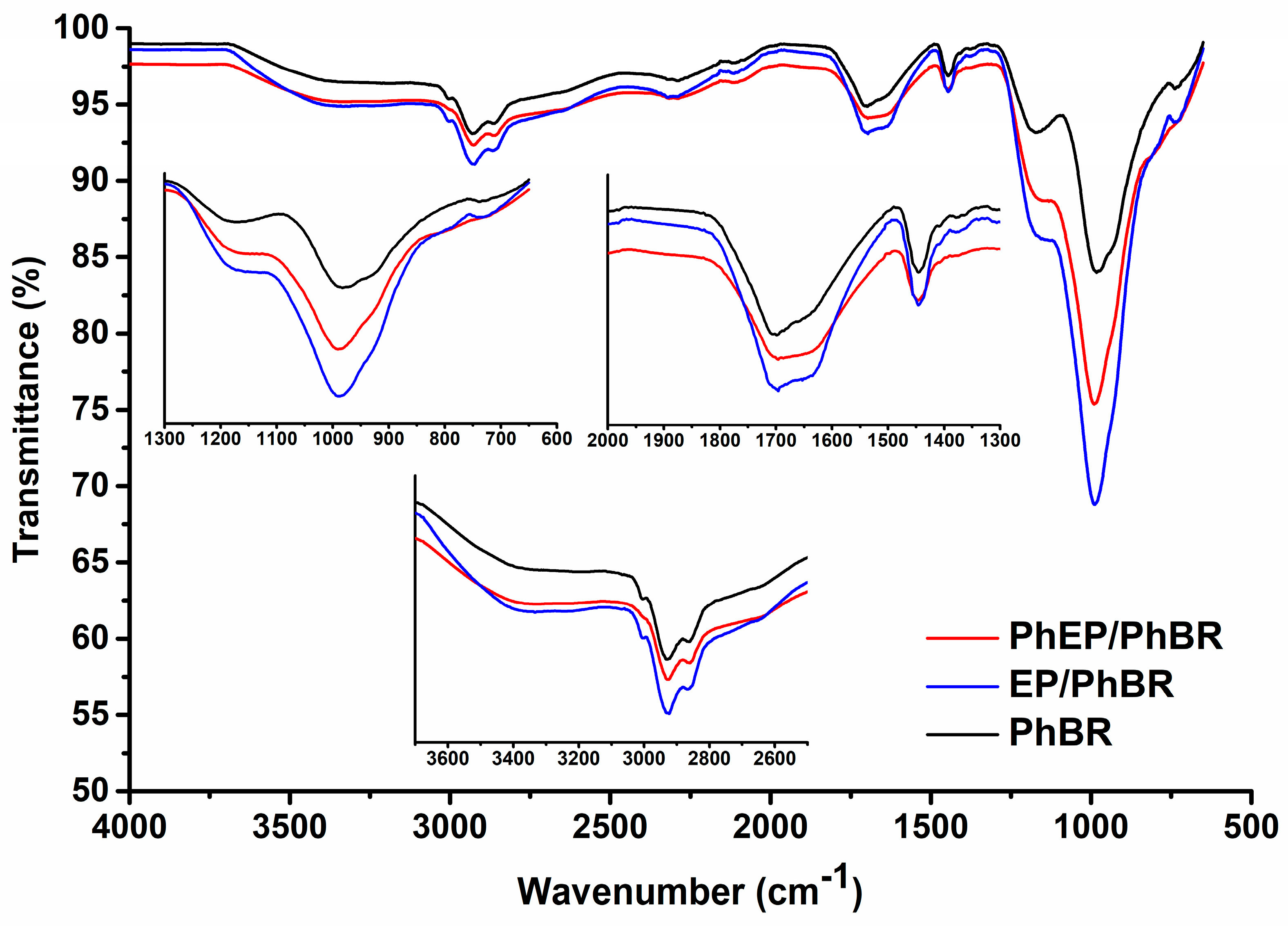
3.1. FTIR Spectroscopy
3.2. UV-Vis Spectroscopy
3.3. XRD Analysis
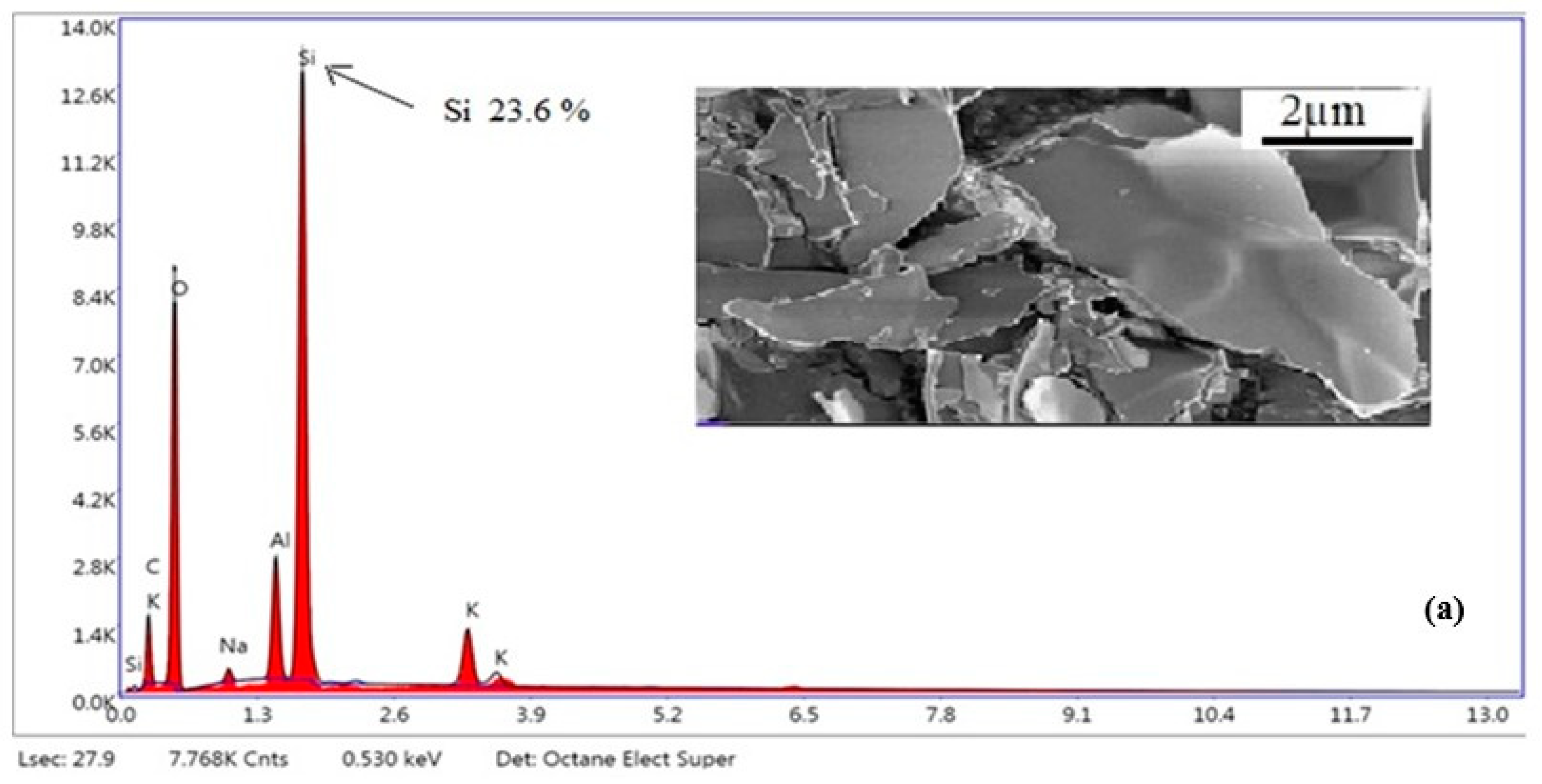
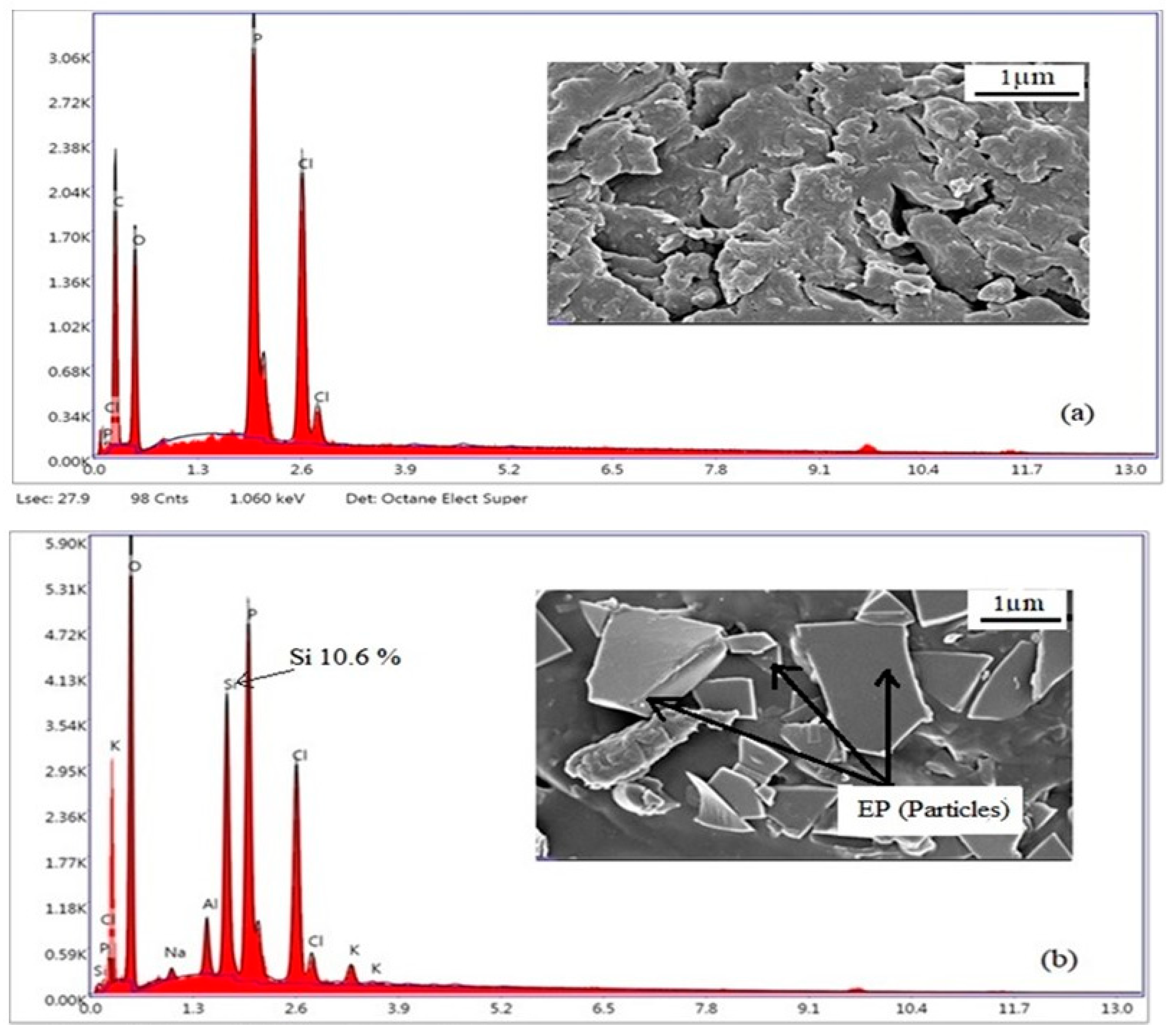
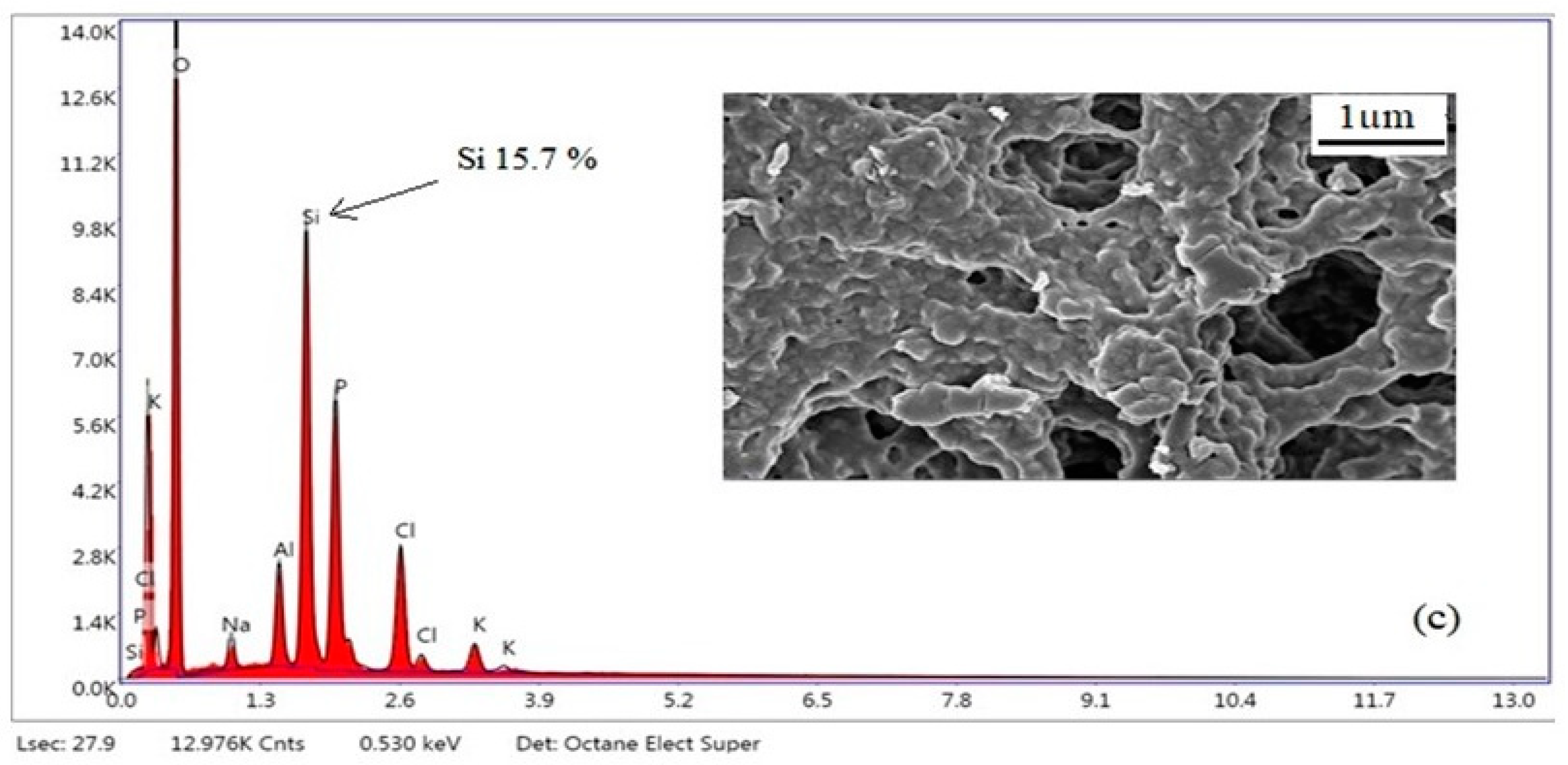
3.4. SEM-EDX Analysis
4. Conclusions
- -
- The proposed method for the synthesis of composites can be used for designing materials based on various mineral and industrial polymers, as well as their waste;
- -
- The chemical structure of the synthesized composites shows that these samples can be used for water purification.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samar, M.; Saxena, D.S. Study of Chemical of and Chysical Properties of Perlite and Its Application in India. Int. J. Sci. Technol. Manag. 2016, 5, 70–80. [Google Scholar]
- Gül, D. Characterization and Expansion Behaviour of Perlite. Master Thesis, Izmir Institute of Technology, Urla, Turkey, 2016. [Google Scholar]
- Aksoy, O.; Alyamac Seydibeyoglu, E.; Mocan, M.; Sutcu, M.; Ozveren-Ucar, N.; Seydibeyoglu, M. Characterization of Perlite Powders from Izmir, Türkiye Region. Physicochem. Probl. Miner. Process. 2022, 58, 155277. [Google Scholar] [CrossRef]
- Różycka, A.; Pichór, W. Effect of Perlite Waste Addition on the Properties of Autoclaved Aerated Concrete. Constr. Build. Mater. 2016, 120, 65–71. [Google Scholar] [CrossRef]
- Kotwica, Ł.; Pichór, W.; Kapeluszna, E.; Różycka, A. Utilization of Waste Expanded Perlite as New Effective Supplementary Cementitious Material. J. Clean. Prod. 2017, 140, 1344–1352. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Jing, Q.; Liu, P. Synthesis of Composite Insulation Materials—Expanded Perlite Filled with Silica Aerogel. J. Porous Mater. 2018, 25, 373–382. [Google Scholar] [CrossRef]
- Xu, H.; Jia, W.; Ren, S.; Wang, J. Novel and Recyclable Demulsifier of Expanded Perlite Grafted by Magnetic Nanoparticles for Oil Separation from Emulsified Oil Wastewaters. Chem. Eng. J. 2018, 337, 10–18. [Google Scholar] [CrossRef]
- Guo, F.; Aryana, S.; Han, Y.; Jiao, Y. A Review of the Synthesis and Applications of Polymer–Nanoclay Composites. Appl. Sci. 2018, 8, 1696. [Google Scholar] [CrossRef]
- Raji, M.; Nekhlaoui, S.; El Hassani, I.-E.E.A.; Essassi, E.M.; Essabir, H.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. Utilization of Volcanic Amorphous Aluminosilicate Rocks (Perlite) as Alternative Materials in Lightweight Composites. Compos. Part B Eng. 2019, 165, 47–54. [Google Scholar] [CrossRef]
- Krzyzak, A.; Kucharczyk, W.; Gaska, J.; Szczepaniak, R. Ablative Test of Composites with Epoxy Resin and Expanded Perlite. Compos. Struct. 2018, 202, 978–987. [Google Scholar] [CrossRef]
- Abir, A.; Faruk, M.; Arifuzzaman, M. Novel Expanded Perlite Based Composite Using Recycled Expanded Polystyrene for Building Material Applications. In Proceedings of the International Conference on Mechanical, Industrial and Energy Engineering 2020(ICMIEE20), Southfield, MI, USA, 19–21 December 2020. [Google Scholar]
- Atagür, M.; Sarikanat, M.; Uysalman, T.; Polat, O.; Elbeyli, İ.Y.; Seki, Y.; Sever, K. Mechanical, Thermal, and Viscoelastic Investigations on Expanded Perlite–Filled High-Density Polyethylene Composite. J. Elastomers Plast. 2018, 50, 747–761. [Google Scholar] [CrossRef]
- Ghorbankhan, A.; Nakhaei, M.R.; Safarpour, P. Fracture Behavior, Microstructure, and Mechanical Properties of PA6/NBR Nanocomposites. Polym. Compos. 2022, 43, 6696–6708. [Google Scholar] [CrossRef]
- Akkaya, R.; Akkaya, B. New Low-Cost Composite Adsorbent Synthesis and Characterization. Desalin. Water Treat. 2013, 51, 3497–3504. [Google Scholar] [CrossRef]
- Akkaya, R. Synthesis and Characterization of a New Low-Cost Composite for the Adsorption of Rare Earth Ions from Aqueous Solutions. Chem. Eng. J. 2012, 200–202, 186–191. [Google Scholar] [CrossRef]
- Alosmanov, R.; Wolski, K.; Matuschek, G.; Magerramov, A.; Azizov, A.; Zimmermann, R.; Aliyev, E.; Zapotoczny, S. Effect of Functional Groups on the Thermal Degradation of Phosphorus- and Phosphorus/Nitrogen-Containing Functional Polymers. J. Therm. Anal. Calorim. 2017, 130, 799–812. [Google Scholar] [CrossRef]
- Aliyeva, S.; Alosmanov, R.; Buniyatzadeh, I.; Eyvazova, G.; Azizov, A.; Maharramov, A. Functionalized Graphene Nanoplatelets/Modified Polybutadiene Hybrid Composite. Colloid Polym. Sci. 2019, 297, 1529–1540. [Google Scholar] [CrossRef]
- Aliyeva, S.; Maharramov, A.; Azizov, A.; Alosmanov, R.; Buniyatzadeh, I.; Eyvazova, G. Phosphorus-Containing Polybutadiene Rubber–Bentonite Hybrid Composite for the Removal of Rhodamine 6G from Water. Anal. Lett. 2016, 49, 2347–2364. [Google Scholar] [CrossRef]
- Alosmanov, R.M.; Azizov, A.A.; Magerramov, A.M. NMR Spectroscopic Study of Phosphorus-Containing Polymer Sorbent. Russ. J. Gen. Chem. 2011, 81, 1477–1479. [Google Scholar] [CrossRef]
- Jahanshahi, R.; Akhlaghinia, B. Expanded Perlite: An Inexpensive Natural Efficient Heterogeneous Catalyst for the Green and Highly Accelerated Solvent-Free Synthesis of 5-Substituted-1H-Tetrazoles Using [Bmim]N3 and Nitriles. RSC Adv. 2015, 5, 104087–104094. [Google Scholar] [CrossRef]
- Almeida, J.M.F.; Silva, I.N.; Damasceno Junior, E.; Fernandes, N.S. Modification of Expanded Perlite with Orthophenanthroline for Formation of Active Sites for Acid Dyes: Preparation and Characterization. Periód. Tchê Quím. 2018, 15, 338–346. [Google Scholar] [CrossRef]
- Alosmanov, R.; Buniyat-zadeh, I.; Soylak, M.; Shukurov, A.; Aliyeva, S.; Turp, S.; Guliyeva, G. Design, Structural Characteristic and Antibacterial Performance of Silver-Containing Cotton Fiber Nanocomposite. Bioengineering 2022, 9, 770. [Google Scholar] [CrossRef]
- Wheelwright, W.; Cooney, R.P.; Ray, S.; Zujovic, Z.; De Silva, K. Ultra-High Surface Area Nano-Porous Silica from Expanded Perlite: Formation and Characterization. Ceram. Int. 2017, 43, 11495–11504. [Google Scholar] [CrossRef]
- Alghadi, A.M.; Tirkes, S.; Tayfun, U. Mechanical, Thermo-Mechanical and Morphological Characterization of ABS Based Composites Loaded with Perlite Mineral. Mater. Res. Express 2020, 7, 015301. [Google Scholar] [CrossRef]
- Malpani, S.K.; Goyal, D.; Chinnam, S.; Sharma, S.K.; Katara, S.; Rani, A. Vapor Phase Alkylation of Isomeric Cresols with Tert-Butyl Alcohol over Perlite Supported Sulfated Zirconia Catalyst. Sustainability 2022, 14, 5149. [Google Scholar] [CrossRef]
- Aubert, T.; Golovatenko, A.A.; Samoli, M.; Lermusiaux, L.; Zinn, T.; Abécassis, B.; Rodina, A.V.; Hens, Z. General Expression for the Size-Dependent Optical Properties of Quantum Dots. Nano Lett. 2022, 22, 1778–1785. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Yang, Z. Synthesis, Structural Characterization and Evaluation of a Novel Floating Metal-Free Photocatalyst Based on g-C3N4 Grafted Expanded Perlite for the Degradation of Dyes. Mater. Technol. 2018, 33, 1–9. [Google Scholar] [CrossRef]
- Abbas, M.; Hachemaoui, A.; Yahiaoui, A.; Mourad, A.-H.I.; Belfedal, A.; Cherupurakal, N. Chemical Synthesis of Nanocomposites via In-Situ Polymerization of Aniline and Iodoaniline Using Exchanged Montmorillonite. Polym. Polym. Compos. 2021, 29, 982–991. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cui, C.Q. Electrochemical Copolymerization of Aniline and Metanilic Acid. J. Electroanal. Chem. 1996, 403, 109–116. [Google Scholar] [CrossRef]
- Hsieh, T.-H.; Ho, L.-C.; Wang, Y.-Z.; Ho, K.-S.; Tsai, C.-H.; Hung, L.-F. New Inverse Emulsion-Polymerized Iron/Polyaniline Composites for Permanent, Highly Magnetic Iron Compounds via Calcination. Polymers 2021, 13, 3240. [Google Scholar] [CrossRef]
- Mohamed Saat, A.; Alaauldin, S.; Kamil, M.S.; Zainal Azaim, F.Z.; Johan, M.R. The Optical Properties of Polyvinyl Alcohol (PVA), Phosphorylated Polyvinyl Alcohol (PPVA), and Phosphorylated Polyvinyl Alcohol—Aluminum Phosphate (PPVA-AlPO4) Nanocomposites: Effect of Phosphate Groups. In Design in Maritime Engineering; Ismail, A., Dahalan, W.M., Öchsner, A., Eds.; Advanced Structured Materials; Springer International Publishing: Cham, Switzerland, 2022; Volume 167, pp. 179–187. ISBN 978-3-030-89987-5. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Surendra, B.S.; Nagaswarupa, H.P.; Anantharaju, K.S.; Anil Kumar, M.R.; Nagabhushana, H.; Shetty, K. Acid Activation of Bentonite Clay under Microwave Irradiation: Characterization, Cyclic Voltammetry and Photocatalytic Activity. Mater. Today Proc. 2018, 5, 22643–22651. [Google Scholar] [CrossRef]
- Mohamed Saat, A.; Johan, M.R. Effect of Phosphoric Acid Concentration on the Optical Properties of Partially Phosphorylated PVA Complexes. Int. J. Polym. Sci. 2014, 2014, 495875. [Google Scholar] [CrossRef]
- Saufi, H.; El Alouani, M.; Alehyen, S.; El Achouri, M.; Aride, J.; Taibi, M. Photocatalytic Degradation of Methylene Blue from Aqueous Medium onto Perlite-Based Geopolymer. Int. J. Chem. Eng. 2020, 2020, 9498349. [Google Scholar] [CrossRef]
- Papadopoulos, A.P.; Bar-Tal, A.; Silber, A.; Saha, U.K.; Raviv, M. Inorganic and Synthetic Organic Components of Soilless Culture and Potting Mixes. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2008; pp. 505–543. ISBN 978-0-444-52975-6. [Google Scholar]
- Buga, M.-R.; Spinu-Zaulet, A.A.; Ungureanu, C.G.; Mitran, R.-A.; Vasile, E.; Florea, M.; Neatu, F. Carbon-Coated SiO2 Composites as Promising Anode Material for Li-Ion Batteries. Molecules 2021, 26, 4531. [Google Scholar] [CrossRef]
- Sun, J.; Xu, Z.; Li, W.; Shen, X. Effect of Nano-SiO2 on the Early Hydration of Alite-Sulphoaluminate Cement. Nanomaterials 2017, 7, 102. [Google Scholar] [CrossRef]
- Matmin, J.; Affendi, I.; Endud, S. Direct-Continuous Preparation of Nanostructured Titania-Silica Using Surfactant-Free Non-Scaffold Rice Starch Template. Nanomaterials 2018, 8, 514. [Google Scholar] [CrossRef]










| EP | Expanded perlite |
| PhChEP | Phosphochlorinated expanded perlite |
| PhEP | Phosphorylated expanded perlite |
| BR | Butadiene rubber |
| PhBR | Phosphorylated butadiene rubber |
| EP/PhBR | Expanded perlite/phosphorylated butadiene rubber |
| PhEP/PhBR | Phosphorylated expanded perlite/phosphorylated butadiene rubber |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edres, N.; Buniyat-zadeh, I.; Turp, S.M.; Soylak, M.; Aliyeva, S.; Binnetova, N.; Guliyeva, N.; Mammadyarova, S.; Alosmanov, R. Structural Characterization of Composites Based on Butadiene Rubber and Expanded Perlite. J. Compos. Sci. 2023, 7, 487. https://doi.org/10.3390/jcs7120487
Edres N, Buniyat-zadeh I, Turp SM, Soylak M, Aliyeva S, Binnetova N, Guliyeva N, Mammadyarova S, Alosmanov R. Structural Characterization of Composites Based on Butadiene Rubber and Expanded Perlite. Journal of Composites Science. 2023; 7(12):487. https://doi.org/10.3390/jcs7120487
Chicago/Turabian StyleEdres, Nada, Irada Buniyat-zadeh, Sinan Mehmet Turp, Mustafa Soylak, Solmaz Aliyeva, Nurlana Binnetova, Naila Guliyeva, Sevinj Mammadyarova, and Rasim Alosmanov. 2023. "Structural Characterization of Composites Based on Butadiene Rubber and Expanded Perlite" Journal of Composites Science 7, no. 12: 487. https://doi.org/10.3390/jcs7120487
APA StyleEdres, N., Buniyat-zadeh, I., Turp, S. M., Soylak, M., Aliyeva, S., Binnetova, N., Guliyeva, N., Mammadyarova, S., & Alosmanov, R. (2023). Structural Characterization of Composites Based on Butadiene Rubber and Expanded Perlite. Journal of Composites Science, 7(12), 487. https://doi.org/10.3390/jcs7120487







