Nonwoven Electrospun Membranes as Tissue Scaffolds: Practices, Problems, and Future Directions
Abstract
:1. Introduction
2. Types of Tissue
- Connective;
- Muscle;
- Nervous;
- Epithelial.
2.1. Connective Tissue
2.2. Muscle Tissue
2.3. Nervous Tissue
2.4. Epithelial Tissue
3. Biomimetic from ECM and Polymeric Nanofibers
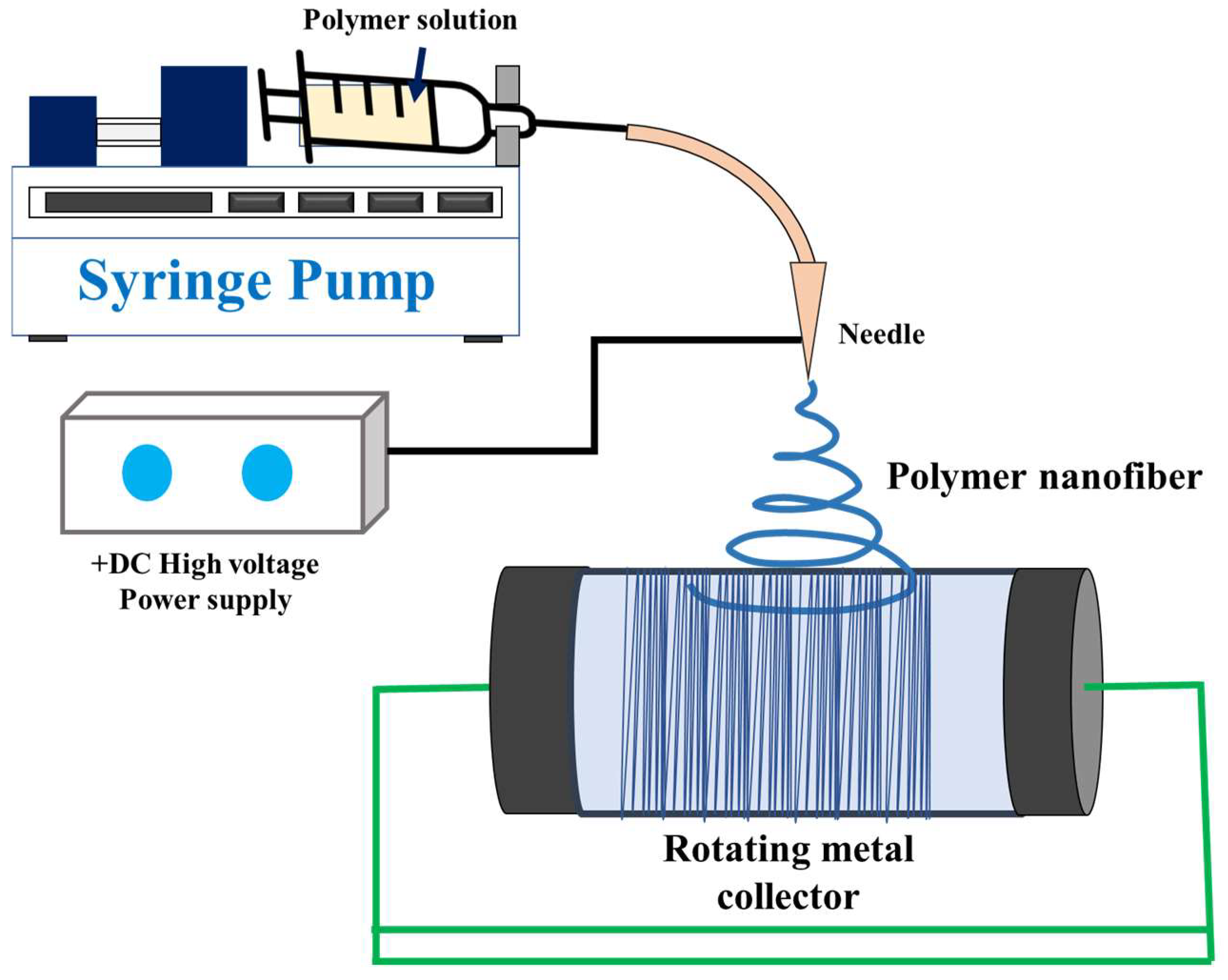
4. Background of Electrospinning
5. Electrospinning Process and Membrane Morphology
6. Problem in the Electrospinning Process
6.1. Low Productivity
6.2. Solvent Toxicity
6.3. Dense Compact Fibers
7. Recent Advancement of Electrospinning
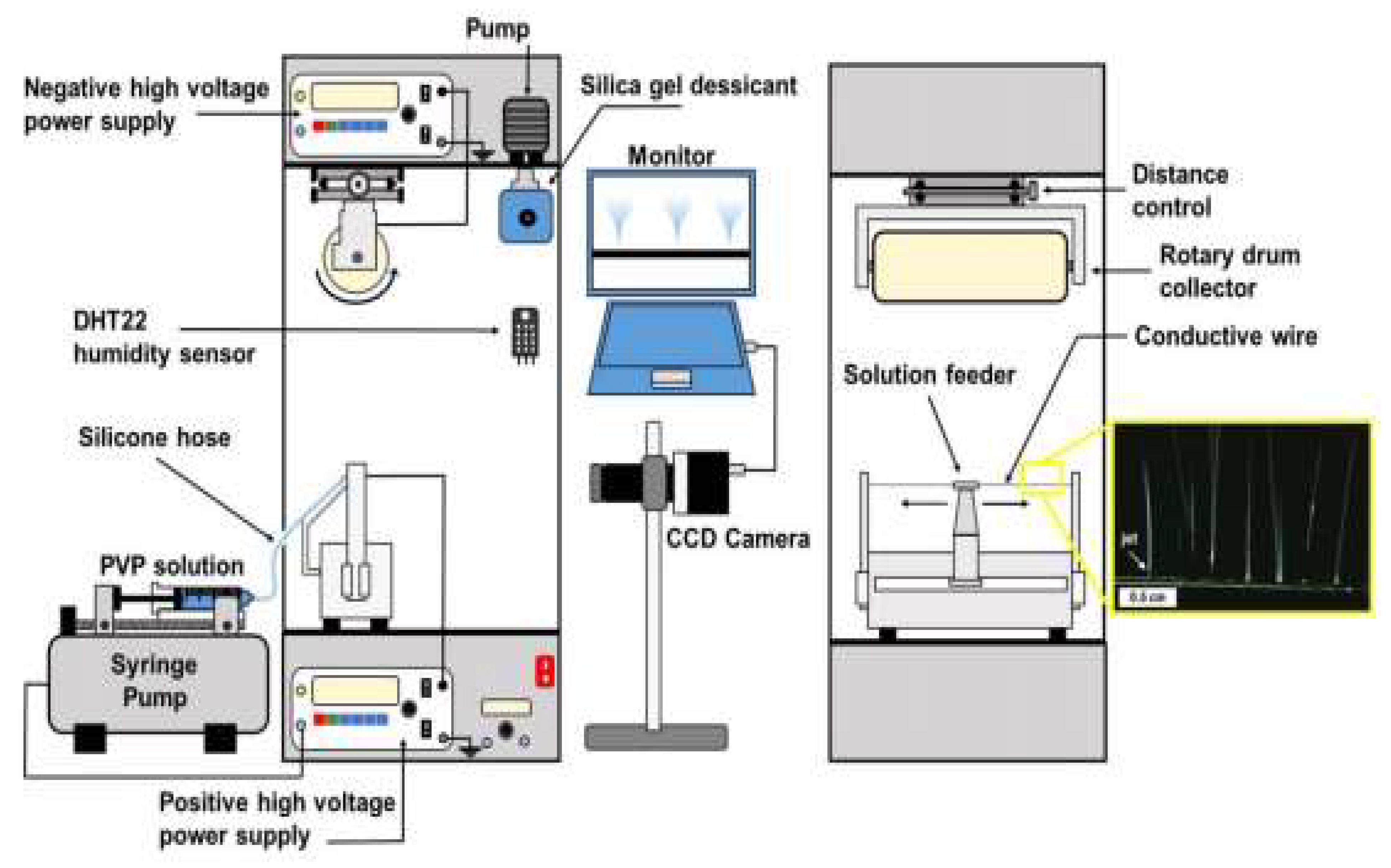
7.1. Advancement in Electrospinning Machine
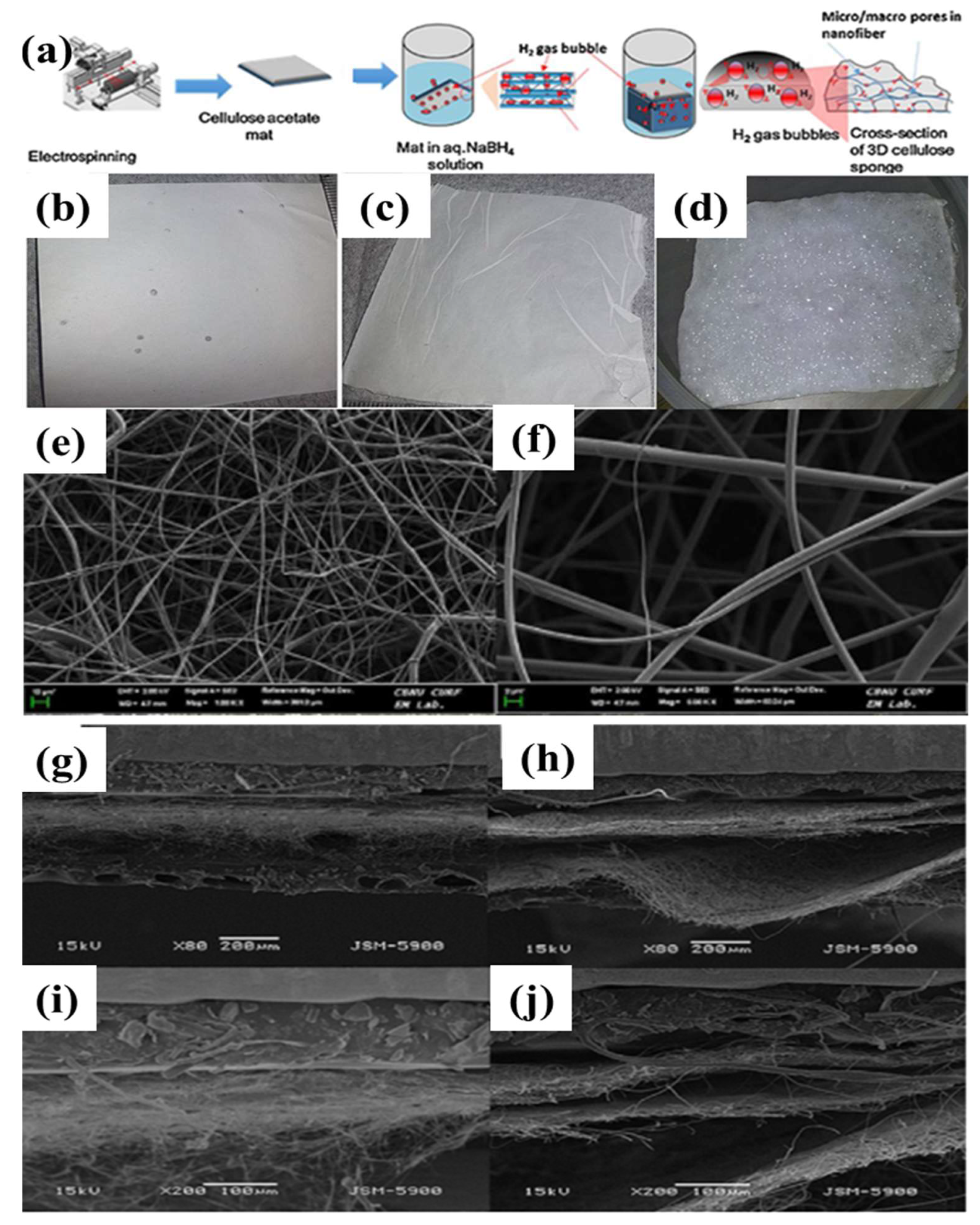
7.2. Post-Electrospinning Process for Membrane Modification
7.3. Replacement of Toxic Organic Solvent by Green Solvent
8. Essential Oils as Green Solvent
8.1. Lavender EO
8.2. Eucalyptus EO
8.3. Lemon EO
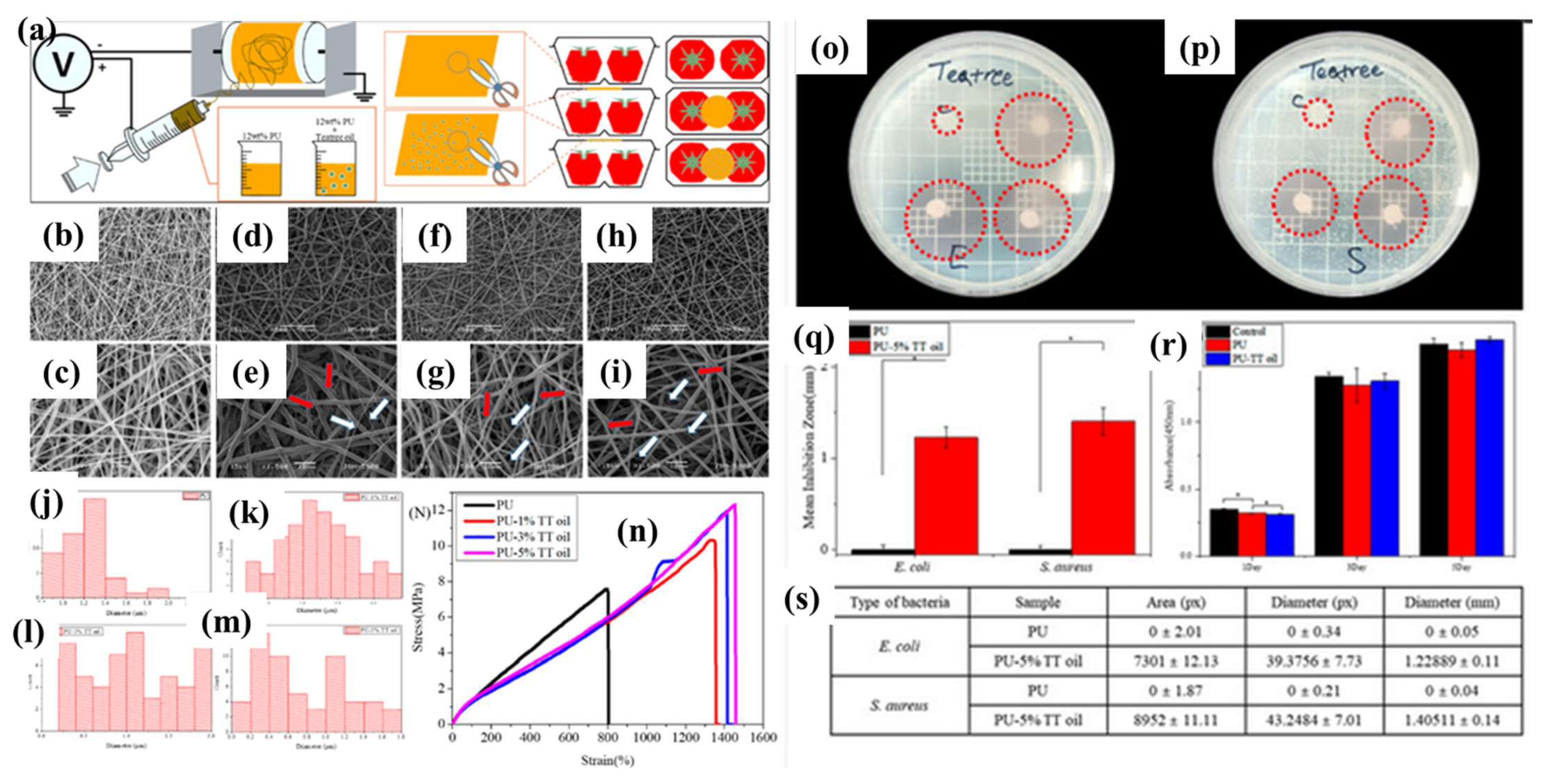
8.4. Tea Tree EO
8.5. Clove Oil
8.6. Cinnamon Oil
8.7. Wintergreen Oil
9. Significance of Essential Oils in Nanofiber Formation
9.1. Enhanced Antimicrobial Properties
9.2. Bioactive and Biocompatible
9.3. Improved Mechanical Properties
9.4. Controlled Release of Active Compounds
9.5. Aromatherapy and Wellness
9.6. Natural and Sustainable Materials
9.7. Food Packaging and Preservation
9.8. EOs as Next Generation Green Solvent
10. Conclusions and Future Remark
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pant, B.; Park, M.; Ojha, G.P.; Kim, D.-U.; Kim, H.-Y.; Park, S.-J. Electrospun salicylic acid/polyurethane composite nanofibers for biomedical applications. Int. J. Polym. Mater. Polym. Biomater. 2018, 67, 739–744. [Google Scholar] [CrossRef]
- Bhatt, L.R.; Khanal, S.; Koirala, A.R.; Pant, H.R. Preparation and surface morphology of herbal based polylactide microspheres. Mater. Lett. 2019, 235, 157–160. [Google Scholar] [CrossRef]
- Joshi, M.K.; Pant, H.R.; Tiwari, A.P.; Maharjan, B.; Liao, N.; Kim, H.J.; Park, C.H.; Kim, C.S. Three-dimensional cellulose sponge: Fabrication, characterization, biomimetic mineralization, and in vitro cell infiltration. Carbohydr. Polym. 2016, 136, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, D.; Adhi, P.; Nasir, M. Design and Development of a Control System for Nanofiber Electrospinning. Mechatron. Electr. Power Veh. Technol. 2013, 4, 641–644. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Sheikh, F.A.; Gómez-Cerezo, N.; Alneairi, A.; Luqman, M.; Pant, H.R.; Ivanovski, S. A review of protein adsorption and bioactivity characteristics of poly ε-caprolactone scaffolds in regenerative medicine. Eur. Polym. J. 2022, 162, 110892. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Pant, H.R.; Mousa, H.M.; Lee, J.; Kim, H.J.; Park, C.H.; Kim, C.S. Synthesis of high porous electrospun hollow TiO2 nanofibers for bone tissue engineering application. J. Ind. Eng. Chem. 2016, 35, 75–82. [Google Scholar] [CrossRef]
- Seo, Y.-A.; Pant, H.R.; Nirmala, R.; Lee, J.-H.; Song, K.G.; Kim, H.Y. Fabrication of highly porous poly (ε-caprolactone) microfibers via electrospinning. J. Porous Mater. 2012, 19, 217–223. [Google Scholar] [CrossRef]
- Choi, W.; Gu, J.E.; Park, S.H.; Kim, S.; Bang, J.; Baek, K.Y.; Park, B.; Lee, J.S.; Chan, E.P.; Lee, J.H. Tailor-Made Polyamide Membranes for Water Desalination. ACS Nano 2015, 9, 345. [Google Scholar] [CrossRef]
- Elmarghany, M.R.; El-Shazly, A.H.; Rajabzadeh, S.; Salem, M.S.; Shouman, M.A.; Nabil Sabry, M.; Matsuyama, H.; Nady, N. Triple-Layer Nanocomposite Membrane Prepared by Electrospinning Based on Modified PES with Carbon Nanotubes for Membrane Distillation Applications. Membranes 2020, 10, 15. [Google Scholar] [CrossRef]
- Essalhi, M.; Khayet, M.; Ismail, N.; Sundman, O.; Tavajohi, N. Improvement of Nanostructured Electrospun Membranes for Desalination by Membrane Distillation Technology. Desalination 2021, 510, 115086. [Google Scholar] [CrossRef]
- Eykens, L.; De Sitter, K.; Dotremont, C.; Pinoy, L.; Van der Bruggen, B. Membrane Synthesis for Membrane Distillation: A Review. Sep. Purif. Technol. 2017, 182, 36. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, A.A. MXene-Embedded Electrospun Polymeric Nanofibers for Biomedical Applications: Recent Advances. Micromachines 2023, 14, 1477. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.R.; Risal, P.; Park, C.H.; Tijing, L.D.; Jeong, Y.J.; Kim, C.S. Core–shell structured electrospun biomimetic composite nanofibers of calcium lactate/nylon-6 for tissue engineering. Chem. Eng. J. 2013, 221, 90–98. [Google Scholar] [CrossRef]
- García, J.M.; García, F.C.; Serna, F.; de la Peña, J.L. High-Performance Aromatic Polyamides. Prog. Polym. Sci. 2010, 35, 623. [Google Scholar] [CrossRef]
- Pant, H.R.; Kim, C.S. Biomimetic synthesis of hollow calcium phosphate nanospheres on core–shell structured electrospun calcium lactate/nylon-6 nanofibers. Mater. Lett. 2013, 92, 90–93. [Google Scholar] [CrossRef]
- Guo, F.; Servi, A.; Liu, A.; Gleason, K.K.; Rutledge, G.C. Desalination by Membrane Distillation Using Electrospun Polyamide Fiber Membranes with Surface Fluorination by Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2015, 7, 8225. [Google Scholar] [CrossRef]
- Chodankar, N.R.; Pham, H.D.; Nanjundan, A.K.; Fernando, J.F.S.; Jayaramulu, K.; Golberg, D.; Han, Y.-K.; Dubal, D.P. True Meaning of Pseudocapacitors and Their Performance Metrics: Asymmetric versus Hybrid Supercapacitors. Small 2020, 16, 2002806. [Google Scholar] [CrossRef]
- Ke, H.; Feldman, E.; Guzman, P.; Cole, J.; Wei, Q.; Chu, B.; Alkhudhiri, A.; Alrasheed, R.; Hsiao, B.S. Electrospun Polystyrene Nanofibrous Membranes for Direct Contact Membrane Distillation. J. Membr. Sci. 2016, 515, 86. [Google Scholar] [CrossRef]
- Fuchs, A.; Youssef, A.; Seher, A.; Hartmann, S.; Brands, R.C.; Müller-Richter, U.D.A.; Kübler, A.C.; Linz, C. A new multilayered membrane for tissue engineering of oral hard- and soft tissue by means of melt electrospinning writing and film casting—An in vitro study. J. Cranio-Maxillofac. Surg. 2019, 47, 695–703. [Google Scholar] [CrossRef]
- Pant, H.R.; Risal, P.; Park, C.H.; Tijing, L.D.; Jeong, Y.J.; Kim, C.S. Synthesis, characterization, and mineralization of polyamide-6/calcium lactate composite nanofibers for bone tissue engineering. Colloids Surf. B Biointerfaces 2013, 102, 152–157. [Google Scholar] [CrossRef]
- Kishan, A.P.; Cosgriff-Hernandez, E.M. Recent advancements in electrospinning design for tissue engineering applications: A review. J. Biomed. Mater. Res. Part A 2017, 105, 2892–2905. [Google Scholar] [CrossRef] [PubMed]
- Joo Kim, H.; Raj Pant, H.; Hee Kim, J.; Jung Choi, N.; Sang Kim, C. Fabrication of multifunctional TiO2–fly ash/polyurethane nanocomposite membrane via electrospinning. Ceram. Int. 2014, 40, 3023–3029. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Khenoussi, N.; Schacher, L.; Adolphe, D. Nanofiber production: Study and development of electrospinning device. Exp. Tech. 2012, 36, 32–39. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Yang, Y.; Wang, X.; Zhu, M.; Hsiao, B.S. Dual-Biomimetic Superhydrophobic Electrospun Polystyrene Nanofibrous Membranes for Membrane Distillation. ACS Appl. Mater. Interfaces 2014, 6, 2423. [Google Scholar] [CrossRef] [PubMed]
- Alghoraibi, I.; Alomari, S. Different Methods for Nanofiber Design and Fabrication. In Handbook of Nanofibers; Barhoum, A., Bechelany, M., Makhlouf, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–46. [Google Scholar]
- Liu, Y.; Park, M.; Shin, H.K.; Pant, B.; Park, S.J.; Kim, H.Y. Preparation and Characterization of Chitosan-Based Nanofibers by Ecofriendly Electrospinning. Mater. Lett. 2014, 132, 23. [Google Scholar] [CrossRef]
- Lu, X.; Si, Y.; Zhang, S.; Yu, J.; Ding, B. In Situ Synthesis of Mechanically Robust, Transparent Nanofiber-Reinforced Hydrogels for Highly Sensitive Multiple Sensing. Adv. Funct. Mater. 2021, 31, 2103117. [Google Scholar] [CrossRef]
- Moatmed, S.M.; Khedr, M.H.; El-dek, S.I.; Kim, H.Y.; El-Deen, A.G. Highly Efficient and Reusable Superhydrophobic/Superoleophilic Polystyrene@ Fe3O4 Nanofiber Membrane for High-Performance Oil/Water Separation. J. Environ. Chem. Eng. 2019, 7, 103508. [Google Scholar] [CrossRef]
- Altinbasak, I.; Jijie, R.; Barras, A.; Golba, B.; Sanyal, R.; Bouckaert, J.; Drider, D.; Bilyy, R.; Dumych, T.; Paryzhak, S.; et al. Reduced Graphene-Oxide-Embedded Polymeric Nanofiber Mats: An “On-Demand” Photothermally Triggered Antibiotic Release Platform. ACS Appl. Mater. Interfaces 2018, 10, 41098–41106. [Google Scholar] [CrossRef]
- Putra, N.E.; Mirzaali, M.J.; Apachitei, I.; Zhou, J.; Zadpoor, A.A. Multi-material additive manufacturing technologies for Ti-, Mg-, and Fe-based biomaterials for bone substitution. Acta Biomater. 2020, 109, 1–20. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Zhou, T.; Liu, Y.; Leng, J. Shape Memory Polymer Nanofibers and Their Composites: Electrospinning, Structure, Performance, and Applications. Front. Mater. 2015, 2, 62. [Google Scholar] [CrossRef]
- Beregoi, M.; Evanghelidis, A.; Diculescu, V.C.; Iovu, H.; Enculescu, I. Polypyrrole Actuator Based on Electrospun Microribbons. ACS Appl. Mater. Interfaces 2017, 9, 38068–38075. [Google Scholar] [CrossRef] [PubMed]
- Firenzuoli, F.; Jaitak, V.; Horvath, G.; Bassolé, I.H.; Setzer, W.N.; Gori, L. Essential oils: New perspectives in human health and wellness. Evid.-Based Complement. Altern. Med. 2014, 2014, 467363. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.R.; Pant, B.; Sharma, R.K.; Amarjargal, A.; Kim, H.J.; Park, C.H.; Tijing, L.D.; Kim, C.S. Antibacterial and photocatalytic properties of Ag/TiO2/ZnO nano-flowers prepared by facile one-pot hydrothermal process. Ceram. Int. 2013, 39, 1503–1510. [Google Scholar] [CrossRef]
- Chifiriuc, M.C.; Ficai, A.; Grumezescu, A.M.; Ditu, L.-M.; Popa, M.; Iordache, C.; Holban, A.M.; Beresteanu, Ş.V.G.; Grigore, R.; Lazar, V. Chapter 1—Soft tissue engineering and microbial infections: Challenges and perspectives. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 1–29. [Google Scholar] [CrossRef]
- Stecco, C.; Hammer, W.; Vleeming, A.; De Caro, R. 1—Connective Tissues. In Functional Atlas of the Human Fascial System; Stecco, C., Hammer, W., Vleeming, A., De Caro, R., Eds.; Churchill Livingstone: London, UK, 2015; pp. 1–20. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Layton, C. 12—Connective and other mesenchymal tissues with their stains. In Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Suvarna, S.K., Layton, C., Bancroft, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 153–175. [Google Scholar] [CrossRef]
- Watkins, J. 10.01—Biomechanics of Musculoskeletal Adaptation. In Comprehensive Biomedical Physics; Brahme, A., Ed.; Elsevier: Oxford, UK, 2014; pp. 1–37. [Google Scholar] [CrossRef]
- Gillies, A.R.; Lieber, R.L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011, 44, 318–331. [Google Scholar] [CrossRef]
- Calvert, J.W.; Lefer, D.J. Chapter 6—Overview of Cardiac Muscle Physiology. In Muscle; Hill, J.A., Olson, E.N., Eds.; Academic Press: Boston/Waltham, MA, USA, 2012; pp. 57–66. [Google Scholar] [CrossRef]
- Bertrand, L.; Horman, S.; Beauloye, C. Chapter 12—Glucose Uptake and Its Consequence on Cardiomyocyte Function. In Glucose Intake and Utilization in Pre-Diabetes and Diabetes; Watson, R.R., Dokken, B.B., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 147–155. [Google Scholar] [CrossRef]
- Kwee, B.J.; Mooney, D.J. Biomaterials for skeletal muscle tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef]
- Angevine, J.B. Nervous System, Organization of. In Encyclopedia of the Human Brain; Ramachandran, V.S., Ed.; Academic Press: New York, NY, USA, 2002; pp. 313–371. [Google Scholar] [CrossRef]
- McMillan, D.B.; Harris, R.J. Chapter F—Nervous Tissue. In An Atlas of Comparative Vertebrate Histology; McMillan, D.B., Harris, R.J., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 141–170. [Google Scholar] [CrossRef]
- Sontheimer, H. (Ed.) Introduction. In Diseases of the Nervous System; Academic Press: San Diego, CA, USA, 2015; pp. xiii–xviii. [Google Scholar] [CrossRef]
- Ganz, T. Epithelia: Not just physical barriers. Proc. Natl. Acad. Sci. USA 2002, 99, 3357–3358. [Google Scholar] [CrossRef]
- Guirao, B.; Rigaud, S.U.; Bosveld, F.; Bailles, A.; López-Gay, J.; Ishihara, S.; Sugimura, K.; Graner, F.; Bellaïche, Y. Unified quantitative characterization of epithelial tissue development. eLife 2015, 4, e08519. [Google Scholar] [CrossRef]
- Kaluzhny, Y.; Kinuthia, M.W.; Lapointe, A.M.; Truong, T.; Klausner, M.; Hayden, P. Oxidative stress in corneal injuries of different origin: Utilization of 3D human corneal epithelial tissue model. Exp. Eye Res. 2020, 190, 107867. [Google Scholar] [CrossRef]
- McLean, W.I.; Irvine, A.D. Disorders of keratinisation: From rare to common genetic diseases of skin and other epithelial tissues. Ulst. Med. J. 2007, 76, 72. [Google Scholar]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef]
- Türker, E.; Yildiz, Ü.H.; Yildiz, A.A. Biomimetic hybrid scaffold consisting of co-electrospun collagen and PLLCL for 3D cell culture. Int. J. Biol. Macromol. 2019, 139, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Stocco, T.D.; Antonioli, E.; Romagnolli, M.L.; Sousa, G.F.; Ferretti, M.; Lobo, A.O. Aligned biomimetic scaffolds based on carbon nanotubes-reinforced polymeric nanofibers for knee meniscus tissue engineering. Mater. Lett. 2020, 264, 127351. [Google Scholar] [CrossRef]
- Jing, X.; Li, H.; Mi, H.-Y.; Liu, Y.-J.; Tan, Y.-M. Fabrication of fluffy shish-kebab structured nanofibers by electrospinning, CO2 escaping foaming and controlled crystallization for biomimetic tissue engineering scaffolds. Chem. Eng. J. 2019, 372, 785–795. [Google Scholar] [CrossRef]
- Qian, Y.; Chen, H.; Xu, Y.; Yang, J.; Zhou, X.; Zhang, F.; Gu, N. The preosteoblast response of electrospinning PLGA/PCL nanofibers: Effects of biomimetic architecture and collagen I. Int. J. Nanomed. 2016, 11, 4157. [Google Scholar]
- Jia, W.; Li, M.; Kang, L.; Gu, G.; Guo, Z.; Chen, Z. Fabrication and comprehensive characterization of biomimetic extracellular matrix electrospun scaffold for vascular tissue engineering applications. J. Mater. Sci. 2019, 54, 10871–10883. [Google Scholar] [CrossRef]
- Chahal, S.; Kumar, A.; Hussian, F.S.J. Development of biomimetic electrospun polymeric biomaterials for bone tissue engineering. A review. J. Biomater. Sci. Polym. Ed. 2019, 30, 1308–1355. [Google Scholar] [CrossRef]
- Chantre, C.O.; Gonzalez, G.M.; Ahn, S.; Cera, L.; Campbell, P.H.; Hoerstrup, S.P.; Parker, K.K. Porous biomimetic hyaluronic acid and extracellular matrix protein Nanofiber scaffolds for accelerated cutaneous tissue repair. ACS Appl. Mater. Interfaces 2019, 11, 45498–45510. [Google Scholar] [CrossRef]
- Vashaghian, M.; Zaat, S.J.; Smit, T.H.; Roovers, J.P. Biomimetic implants for pelvic floor repair. Neurourol. Urodyn. 2018, 37, 566–580. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Li, Q.; Zou, Q.; Du, C. Biomimetic mineralization of carboxymethyl chitosan nanofibers with improved osteogenic activity in vitro and in vivo. Carbohydr. Polym. 2018, 195, 225–234. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Ruini, F.; Ceresa, C.; Gentile, P.; Varela, P.; Ferreira, A.M.; Fracchia, L.; Ciardelli, G. Nanostructured scaffold with biomimetic and antibacterial properties for wound healing produced by ‘green electrospinning’. Colloids Surf. B Biointerfaces 2018, 172, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Murugan, R.; Ramakrishna, S. Nano-featured scaffolds for tissue engineering: A review of spinning methodologies. Tissue Eng. 2006, 12, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Serio, F.; da Cruz, A.F.; Chandra, A.; Nobile, C.; Rossi, G.R.; D’Amone, E.; Gigli, G.; Del Mercato, L.L.; de Oliveira, C.C. Electrospun polyvinyl-alcohol/gum arabic nanofibers: Biomimetic platform for in vitro cell growth and cancer nanomedicine delivery. Int. J. Biol. Macromol. 2021, 188, 764–773. [Google Scholar] [CrossRef]
- Keirouz, A.; Wang, Z.; Reddy, V.S.; Nagy, Z.K.; Vass, P.; Buzgo, M.; Ramakrishna, S.; Radacsi, N. The History of Electrospinning: Past, Present, and Future Developments. Adv. Mater. Technol. 2023, 8, 2201723. [Google Scholar] [CrossRef]
- Tucker, N.; Stanger, J.J.; Staiger, M.P.; Razzaq, H.; Hofman, K. The history of the science and technology of electrospinning from 1600 to 1995. J. Eng. Fibers Fabr. 2012, 7 (Suppl. S2), 155892501200702S155892501200710. [Google Scholar] [CrossRef]
- Ojha, G.P.; Pant, B.; Acharya, J.; Park, M. Prussian Red Anions Immobilized Freestanding Three-Dimensional Porous Carbonaceous Networks: A New Avenue to Attain Capacitor- and Faradic-Type Electrodes in a Single Frame for 2.0 V Hybrid Supercapacitors. ACS Sustain. Chem. Eng. 2022, 10, 2994–3006. [Google Scholar] [CrossRef]
- Ojha, G.P.; Pant, B.; Acharya, J.; Park, M. An electrochemically reduced ultra-high mass loading three-dimensional carbon nanofiber network: A high energy density symmetric supercapacitor with a reproducible and stable cell voltage of 2.0 V. Nanoscale 2021, 13, 19537–19548. [Google Scholar] [CrossRef]
- Tijing, L.D.; Woo, Y.C.; Yao, M.; Ren, J.; Shon, H.K. 1.16 Electrospinning for Membrane Fabrication: Strategies and Applications. In Comprehensive Membrane Science and Engineering, 2nd ed.; Drioli, E., Giorno, L., Fontananova, E., Eds.; Elsevier: Oxford, UK, 2017; pp. 418–444. [Google Scholar] [CrossRef]
- Zheng, Y. 3—Fabrication on bioinspired surfaces. In Bioinspired Design of Materials Surfaces; Zheng, Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 99–146. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kim, H.-Y.; Park, M.; Park, S.-J. Fly-ash-incorporated electrospun zinc oxide nanofibers: Potential material for environmental remediation. Environ. Pollut. 2019, 245, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.; Ojha, G.P.; Kuk, Y.-S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties. Nanomaterials 2020, 10, 1960. [Google Scholar] [CrossRef]
- Pandey, P.; Thapa, K.; Ojha, G.P.; Seo, M.-K.; Shin, K.H.; Kim, S.-W.; Sohn, J.I. Metal-organic frameworks-based triboelectric nanogenerator powered visible light communication system for wireless human-machine interactions. Chem. Eng. J. 2023, 452, 139209. [Google Scholar] [CrossRef]
- Pant, B.; Prasad Ojha, G.; Acharya, J.; Park, M. Ag3PO4-TiO2-Carbon nanofiber Composite: An efficient Visible-light photocatalyst obtained from electrospinning and hydrothermal methods. Sep. Purif. Technol. 2021, 276, 119400. [Google Scholar] [CrossRef]
- Su, C.; Li, Y.; Dai, Y.; Gao, F.; Tang, K.; Cao, H. Fabrication of three-dimensional superhydrophobic membranes with high porosity via simultaneous electrospraying and electrospinning. Mater. Lett. 2016, 170, 67–71. [Google Scholar] [CrossRef]
- Lee, M.; Ojha, G.P.; Oh, H.J.; Kim, T.; Kim, H.Y. Copper//terbium dual metal organic frameworks incorporated side-by-side electrospun nanofibrous membrane: A novel tactics for an efficient adsorption of particulate matter and luminescence property. J. Colloid Interface Sci. 2020, 578, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Lasprilla-Botero, J.; Álvarez-Láinez, M.; Lagaron, J. The influence of electrospinning parameters and solvent selection on the morphology and diameter of polyimide nanofibers. Mater. Today Commun. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.R.; Manaf, O.; Sundaran, S.P.; Juraij, K.; Sujith, A. Chapter 8—Development of nanocomposite membranes by electrospun nanofibrous materials. In Nanocomposite Membranes for Water and Gas Separation; Sadrzadeh, M., Mohammadi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–218. [Google Scholar] [CrossRef]
- Jose Varghese, R.; Sakho, E.h.M.; Parani, S.; Thomas, S.; Oluwafemi, O.S.; Wu, J. Chapter 3—Introduction to nanomaterials: Synthesis and applications. In Nanomaterials for Solar Cell Applications; Thomas, S., Sakho, E.H.M., Kalarikkal, N., Oluwafemi, S.O., Wu, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 75–95. [Google Scholar] [CrossRef]
- Akdere, M.; Schneiders, T. 9—Modeling of the electrospinning process. In Advances in Modeling and Simulation in Textile Engineering; Akankwasa, N.T., Veit, D., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2021; pp. 237–253. [Google Scholar] [CrossRef]
- Davoodi, P.; Gill, E.L.; Wang, W.; Shery Huang, Y.Y. Chapter Two—Advances and innovations in electrospinning technology. In Biomedical Applications of Electrospinning and Electrospraying; Kasoju, N., Ye, H., Eds.; Woodhead Publishing: Sawston, Cambridge, UK, 2021; pp. 45–81. [Google Scholar] [CrossRef]
- Bambole, V.; Yakhmi, J.V. Chapter 14—Tissue engineering: Use of electrospinning technique for recreating physiological functions. In Nanobiomaterials in Soft Tissue Engineering; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 387–455. [Google Scholar] [CrossRef]
- Shin, M.; Awasthi, G.P.; Sharma, K.P.; Pandey, P.; Park, M.; Ojha, G.P.; Yu, C. Nanoarchitectonics of Three-Dimensional Carbon Nanofiber-Supported Hollow Copper Sulfide Spheres for Asymmetric Supercapacitor Applications. Int. J. Mol. Sci. 2023, 24, 9685. [Google Scholar] [CrossRef] [PubMed]
- Bui, H.; Pham, V.H.; Pham, V.D.; Hoang, T.H.C.; Pham, T.B.; Do, T.C.; Ngo, Q.M.; Nguyen, T.V. Determination of low solvent concentration by nano-porous silicon photonic sensors using volatile organic compound method. Environ. Technol. 2019, 40, 3403–3411. [Google Scholar] [CrossRef]
- Cremar, L.; Gutierrez, J.; Martinez, J.; Materon, L.; Gilkerson, R.; Xu, F.; Lozano, K. Development of antimicrobial chitosan based nanofiber dressings for wound healing applications. Nanomed. J. 2018, 5, 6–14. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Drug Delivery Applications of Core-Sheath Nanofibers Prepared by Coaxial Electrospinning: A Review. Pharmaceutics 2019, 11, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Cheng, Y.W.; Hsu, C.H.; Chien, H.S.; Tsou, S.Y. How to manipulate the electrospinning jet with controlled properties to obtain uniform fibers with the smallest diameter?—A brief discussion of solution electrospinning process. J. Polym. Res. 2011, 18, 111–123. [Google Scholar] [CrossRef]
- Deitzel, J.M.; Kleinmeyer, J.; Harris, D.; Beck Tan, N.C. The effect of processing variables on the morphology of electrospun nanofibers and textiles. Polymer 2001, 42, 261–272. [Google Scholar] [CrossRef]
- Angammana, C.J.; Jayaram, S.H. Analysis of the Effects of Solution Conductivity on Electrospinning Process and Fiber Morphology. IEEE Trans. Ind. Appl. 2011, 47, 1109–1117. [Google Scholar] [CrossRef]
- Bosworth, L.A.; Downes, S. Acetone, a Sustainable Solvent for Electrospinning Poly(ε-Caprolactone) Fibres: Effect of Varying Parameters and Solution Concentrations on Fibre Diameter. J. Polym. Environ. 2012, 20, 879–886. [Google Scholar] [CrossRef]
- Chang, S.; Fane, A.G. The effect of fibre diameter on filtration and flux distribution—Relevance to submerged hollow fibre modules. J. Membr. Sci. 2001, 184, 221–231. [Google Scholar] [CrossRef]
- Abdel-Hady, F.; Alzahrany, A.; Hamed, M. Experimental Validation of Upward Electrospinning Process. ISRN Nanotechnol. 2011, 2011, 851317. [Google Scholar] [CrossRef]
- De Vrieze, S.; Van Camp, T.; Nelvig, A.; Hagström, B.; Westbroek, P.; De Clerck, K. The effect of temperature and humidity on electrospinning. J. Mater. Sci. 2009, 44, 1357–1362. [Google Scholar] [CrossRef]
- Cai, Y.; Gevelber, M. The effect of relative humidity and evaporation rate on electrospinning: Fiber diameter and measurement for control implications. J. Mater. Sci. 2013, 48, 7812–7826. [Google Scholar] [CrossRef]
- Vass, P.; Szabó, E.; Domokos, A.; Hirsch, E.; Galata, D.; Farkas, B.; Démuth, B.; Andersen, S.K.; Vigh, T.; Verreck, G. Scale-up of electrospinning technology: Applications in the pharmaceutical industry. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1611. [Google Scholar] [CrossRef]
- Yalcinkaya, F.; Yalcinkaya, B.; Jirsak, O. Influence of salts on electrospinning of aqueous and nonaqueous polymer solutions. J. Nanomater. 2015, 2015, 134251. [Google Scholar] [CrossRef]
- Zhang, C.; Feng, F.; Zhang, H. Emulsion electrospinning: Fundamentals, food applications and prospects. Trends Food Sci. Technol. 2018, 80, 175–186. [Google Scholar] [CrossRef]
- Partheniadis, I.; Nikolakakis, I.; Laidmäe, I.; Heinämäki, J. A Mini-Review: Needleless Electrospinning of Nanofibers for Pharmaceutical and Biomedical Applications. Processes 2020, 8, 673. [Google Scholar] [CrossRef]
- Khan, W.S.; Asmatulu, R.; Ceylan, M.; Jabbarnia, A. Recent progress on conventional and non-conventional electrospinning processes. Fibers Polym. 2013, 14, 1235–1247. [Google Scholar] [CrossRef]
- Prahasti, G.; Zulfi, A.; Munir, M.M. Needleless electrospinning system with wire spinneret: An alternative way to control morphology, size, and productivity of nanofibers. Nano Express 2020, 1, 010046. [Google Scholar] [CrossRef]
- Duan, G.; Greiner, A. Air-blowing-assisted coaxial electrospinning toward high productivity of Core/sheath and hollow fibers. Macromol. Mater. Eng. 2019, 304, 1800669. [Google Scholar] [CrossRef]
- Nasouri, K.; Shoushtari, A.M.; Mojtahedi, M.R.M. Thermodynamic studies on polyvinylpyrrolidone solution systems used for fabrication of electrospun nanostructures: Effects of the solvent. Adv. Polym. Technol. 2015, 34, 1–8. [Google Scholar] [CrossRef]
- Nikmaram, N.; Roohinejad, S.; Hashemi, S.; Koubaa, M.; Barba, F.J.; Abbaspourrad, A.; Greiner, R. Emulsion-based systems for fabrication of electrospun nanofibers: Food, pharmaceutical and biomedical applications. RSC Adv. 2017, 7, 28951–28964. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of electrospun nanofibers for biomedical and dental applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Gao, Y.; Zhai, Y. Electrospun nanofibers as a wound dressing for treating diabetic foot ulcer. Asian J. Pharm. Sci. 2019, 14, 130–143. [Google Scholar] [CrossRef]
- Lu, T.-D.; Chen, B.-Z.; Wang, J.; Jia, T.-Z.; Cao, X.-L.; Wang, Y.; Xing, W.; Lau, C.H.; Sun, S.-P. Electrospun nanofiber substrates that enhance polar solvent separation from organic compounds in thin-film composites. J. Mater. Chem. A 2018, 6, 15047–15056. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, M.; Jiang, Z.; Jiang, S.; Zhang, Q.; Xiong, R.; Huang, C. Green electrospun nanofibers and their application in air filtration. Macromol. Mater. Eng. 2018, 303, 1800336. [Google Scholar] [CrossRef]
- Wang, L.; Ryan, A.J. 1—Introduction to electrospinning. In Electrospinning for Tissue Regeneration; Bosworth, L.A., Downes, S., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 3–33. [Google Scholar] [CrossRef]
- Mouthuy, P.A.; Ye, H. 5.04—Biomaterials: Electrospinning. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, NJ, USA, 2011; pp. 23–36. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271–292. [Google Scholar] [CrossRef]
- Campuzano, S.; Pelling, A.E. Scaffolds for 3D Cell Culture and Cellular Agriculture Applications Derived From Non-animal Sources. Front. Sustain. Food Syst. 2019, 3, 38. [Google Scholar] [CrossRef]
- Luo, Y.; Engelmayr, G.; Auguste, D.T.; da Silva Ferreira, L.; Karp, J.M.; Saigal, R.; Langer, R. Chapter 24—3D Scaffolds. In Principles of Tissue Engineering, 4th ed.; Lanza, R., Langer, R., Vacanti, J., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 475–494. [Google Scholar] [CrossRef]
- Amith, V.; Sridhar, R.; Angadi, G.; Murthy, H.N. Recent Advancement in Electrospun nanofibrous mats with emphasis on their applications. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1065, 012008. [Google Scholar]
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 174–189. [Google Scholar] [CrossRef]
- Patel, S.; Patel, G. A Review and Analysis on Recent Advancements in Bubble Electrospinning Technology for Nanofiber Production. Recent Pat. Nanotechnol. 2019, 13, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, M.; Tiraferri, A.; Musteata, V.E.; Chisca, S.; Sougrat, R.; Huang, L.B.; Nunes, S.P.; Barboiu, M. Biomimetic Artificial Water Channel Membranes for Enhanced Desalination. Nat. Nanotechnol. 2021, 16, 190. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Assadpour, E.; Tabarestani, H.S.; Falsafi, S.R.; Jafari, S.M. Electrospinning approach for nanoencapsulation of bioactive compounds; recent advances and innovations. Trends Food Sci. Technol. 2020, 100, 190–209. [Google Scholar] [CrossRef]
- Sagitha, P.; Reshmi, C.; Sundaran, S.P.; Sujith, A. Recent advances in post-modification strategies of polymeric electrospun membranes. Eur. Polym. J. 2018, 105, 227–249. [Google Scholar] [CrossRef]
- Shaulsky, E.; Nejati, S.; Boo, C.; Perreault, F.; Osuji, C.O.; Elimelech, M. Post-fabrication modification of electrospun nanofiber mats with polymer coating for membrane distillation applications. J. Membr. Sci. 2017, 530, 158–165. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.-S.; Li, C.-W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Chen, H.; Huang, M.; Liu, Y.; Meng, L.; Ma, M. Functionalized electrospun nanofiber membranes for water treatment: A review. Sci. Total Environ. 2020, 739, 139944. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Membrane Modification. In Nanotechnology in Membrane Processes; Springer: New York, NY, USA, 2021; pp. 135–170. [Google Scholar]
- Shen, W.; Ao, F.; Ge, X.; Ning, Y.; Wang, L.; Ren, H.; Fan, G. Effects of solvents on electrospun fibers and the biological application of different hydrophilic electrospun mats. Mater. Today Commun. 2022, 30, 103093. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Soni, T.G.; Shah, D.O. A review on therapeutic contact lenses for ocular drug delivery. Drug Deliv. 2016, 23, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Soylak, M. Chapter 5—Type of green solvents used in separation and preconcentration methods. In New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species; Soylak, M., Yilmaz, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 207–266. [Google Scholar] [CrossRef]
- Avossa, J.; Herwig, G.; Toncelli, C.; Itel, F.; Rossi, R.M. Electrospinning based on benign solvents: Current definitions, implications and strategies. Green Chem. 2022, 24, 2347–2375. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Ncube, S.; Chimuka, L. Chapter Twelve—Green chemistry features in molecularly imprinted polymers preparation process. In Comprehensive Analytical Chemistry; Marć, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 86, pp. 337–364. [Google Scholar]
- Zhang, J.; Lin, T.; Wang, X. 4—Carbon and polymer nanofiber reinforcements in polymer matrix composites: Processing and applications. In Functional Nanofibers and Their Applications; Wei, Q., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2012; pp. 55–70. [Google Scholar] [CrossRef]
- Rather, A.H.; Wani, T.U.; Khan, R.S.; Pant, B.; Park, M.; Sheikh, F.A. Prospects of Polymeric Nanofibers Loaded with Essential Oils for Biomedical and Food-Packaging Applications. Int. J. Mol. Sci. 2021, 22, 4017. [Google Scholar] [CrossRef]
- Ko, F.K.; Wan, Y. Introduction to Nanofiber Materials; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar]
- Padron, S.; Fuentes, A.; Caruntu, D.; Lozano, K. Experimental study of nanofiber production through forcespinning. J. Appl. Phys. 2013, 113, 024318. [Google Scholar] [CrossRef]
- Zheng, L.; Sekerková, G.; Vranich, K.; Tilney, L.G.; Mugnaini, E.; Bartles, J.R.J.C. The deaf jerker mouse has a mutation in the gene encoding the espin actin-bundling proteins of hair cell stereocilia and lacks espins. Cell 2000, 102, 377–385. [Google Scholar] [CrossRef]
- Bernards, D.A.; Lance, K.D.; Ciaccio, N.A.; Desai, T.A. Nanostructured thin film polymer devices for constant-rate protein delivery. Nano Lett. 2012, 12, 5355–5361. [Google Scholar] [CrossRef]
- Ji, W.; Yang, F.; Ma, J.; Bouma, M.J.; Boerman, O.C.; Chen, Z.; van den Beucken, J.J.; Jansen, J.A. Incorporation of stromal cell-derived factor-1α in PCL/gelatin electrospun membranes for guided bone regeneration. Biomaterials 2013, 34, 735–745. [Google Scholar] [CrossRef]
- Schlesinger, E.; Johengen, D.; Luecke, E.; Rothrock, G.; McGowan, I.; van der Straten, A.; Desai, T. A tunable, biodegradable, thin-film polymer device as a long-acting implant delivering tenofovir alafenamide fumarate for HIV pre-exposure prophylaxis. Pharm. Res. 2016, 33, 1649–1656. [Google Scholar] [CrossRef]
- Sodha, S.; Wall, K.; Redenti, S.; Klassen, H.; Young, M.J.; Tao, S.L. Microfabrication of a three-dimensional polycaprolactone thin-film scaffold for retinal progenitor cell encapsulation. J. Biomater. Sci. Polym. Ed. 2011, 22, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Mani, M.P.; Mohd Faudzi, A.A.; Ramakrishna, S.; Ismail, A.F.; Jaganathan, S.K.; Tucker, N.; Rathanasamy, R. Sustainable electrospun materials with enhanced blood compatibility for wound healing applications—A mini review. Curr. Opin. Biomed. Eng. 2023, 27, 100457. [Google Scholar] [CrossRef]
- Pakolpakçıl, A.; Draczyński, Z.; Szulc, J.; Stawski, D.; Tarzyńska, N.; Bednarowicz, A.; Sikorski, D.; Hernandez, C.; Sztajnowski, S.; Krucińska, I.; et al. An In Vitro Study of Antibacterial Properties of Electrospun Hypericum perforatum Oil-Loaded Poly(lactic Acid) Nonwovens for Potential Biomedical Applications. Appl. Sci. 2021, 11, 8219. [Google Scholar] [CrossRef]
- Manikandan, A.; Mani, M.P.; Jaganathan, S.K.; Rajasekar, R.; Jagannath, M. Formation of functional nanofibrous electrospun polyurethane and murivenna oil with improved haemocompatibility for wound healing. Polym. Test. 2017, 61, 106–113. [Google Scholar] [CrossRef]
- Mani, M.P.; Jaganathan, S.K.; Khudzari, A.Z.M.; Prabhakaran, P. Development of advanced nanostructured polyurethane composites comprising hybrid fillers with enhanced properties for regenerative medicine. Polym. Test. 2019, 73, 12–20. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, J.; Ko, S.W.; Son, B.C.; Lee, J.H.; Kim, C.S.; Park, C.H. Fabrication of Antibacterial Nanofibrous Membrane Infused with Essential Oil Extracted from Tea Tree for Packaging Applications. Polymers 2020, 12, 125. [Google Scholar] [CrossRef]
- Milanesi, G.; Vigani, B.; Rossi, S.; Sandri, G.; Mele, E. Chitosan-Coated Poly(lactic acid) Nanofibres Loaded with Essential Oils for Wound Healing. Polymers 2021, 13, 2582. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Electrospun PCL membranes incorporated with biosynthesized silver nanoparticles as antibacterial wound dressings. Appl. Nanosci. 2016, 6, 337–344. [Google Scholar] [CrossRef]
- Bernards, D.A.; Bhisitkul, R.B.; Wynn, P.; Steedman, M.R.; Lee, O.-T.; Wong, F.; Thoongsuwan, S.; Desai, T.A. Ocular biocompatibility and structural integrity of micro-and nanostructured poly (caprolactone) films. J. Ocul. Pharmacol. Ther. 2013, 29, 249–257. [Google Scholar] [CrossRef]
- Phan, D.-N.; Khan, M.Q.; Nguyen, V.-C.; Vu-Manh, H.; Dao, A.-T.; Thanh Thao, P.; Nguyen, N.-M.; Le, V.-T.; Ullah, A.; Khatri, M.; et al. Investigation of Mechanical, Chemical, and Antibacterial Properties of Electrospun Cellulose-Based Scaffolds Containing Orange Essential Oil and Silver Nanoparticles. Polymers 2022, 14, 85. [Google Scholar] [CrossRef]
- Thapa, K.; Regmi, K.R.; Shah, D.; Sharma, R.K.; Panomsuwan, G.; Techapiesancharoenkij, R.; Pant, H.R. Residual solvent-assisted facile deposition of honeycomb-like silver nanoflakes on the surface of electrospun PAN nanofibers. Chem. Phys. Lett. 2022, 801, 139724. [Google Scholar] [CrossRef]
- Berechet, M.D.; Gaidau, C.; Miletic, A.; Pilic, B.; Râpă, M.; Stanca, M.; Ditu, L.M.; Constantinescu, R.; Lazea-Stoyanova, A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials 2020, 13, 1618. [Google Scholar] [CrossRef]
- Zhang, W.; Huang, C.; Kusmartseva, O.; Thomas, N.L.; Mele, E. Electrospinning of polylactic acid fibres containing tea tree and manuka oil. React. Funct. Polym. 2017, 117, 106–111. [Google Scholar] [CrossRef]
- Souza, M.A.; Oliveira, J.E.; Medeiros, E.S.; Glenn, G.M.; Mattoso, L.H.C. Controlled Release of Linalool Using Nanofibrous Membranes of Poly(lactic acid) Obtained by Electrospinning and Solution Blow Spinning: A Comparative Study. J. Nanosci. Nanotechnol. 2015, 15, 5628–5636. [Google Scholar] [CrossRef]
- Pokharel, P.; Pant, B.; Pokhrel, K.; Pant, H.R.; Lim, J.-g.; Lee, D.S.; Kim, H.-Y.; Choi, S. Effects of functional groups on the graphene sheet for improving the thermomechanical properties of polyurethane nanocomposites. Compos. Part B Eng. 2015, 78, 192–201. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Cantrell, C.L.; Astatkie, T.; Jeliazkova, E. Distillation Time Effect on Lavender Essential Oil Yield and Composition. J. Oleo Sci. 2013, 62, 195–199. [Google Scholar] [CrossRef]
- Park, C.-H.; Kang, S.-J.; Tijing, L.D.; Pant, H.R.; Kim, C.S. Inductive heating of electrospun Fe2O3/polyurethane composite mat under high-frequency magnetic field. Ceram. Int. 2013, 39, 9785–9790. [Google Scholar] [CrossRef]
- Tijing, L.D.; Park, C.-H.; Kang, S.-J.; Amarjargal, A.; Kim, T.-H.; Pant, H.R.; Kim, H.J.; Lee, D.H.; Kim, C.S. Improved mechanical properties of solution-cast silicone film reinforced with electrospun polyurethane nanofiber containing carbon nanotubes. Appl. Surf. Sci. 2013, 264, 453–458. [Google Scholar] [CrossRef]
- Tijing, L.D.; Amarjargal, A.; Jiang, Z.; Ruelo, M.T.G.; Park, C.-H.; Pant, H.R.; Kim, D.-W.; Lee, D.H.; Kim, C.S. Antibacterial tourmaline nanoparticles/polyurethane hybrid mat decorated with silver nanoparticles prepared by electrospinning and UV photoreduction. Curr. Appl. Phys. 2013, 13, 205–210. [Google Scholar] [CrossRef]
- Tijing, L.D.; Ruelo, M.T.G.; Amarjargal, A.; Pant, H.R.; Park, C.-H.; Kim, D.W.; Kim, C.S. Antibacterial and superhydrophilic electrospun polyurethane nanocomposite fibers containing tourmaline nanoparticles. Chem. Eng. J. 2012, 197, 41–48. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, W.; Jiang, Z.; Amarjargal, A.; Park, C.-H.; Pant, H.R.; Im, I.-T.; Kim, C.S. Two-nozzle electrospinning of (MWNT/PU)/PU nanofibrous composite mat with improved mechanical and thermal properties. Curr. Appl. Phys. 2013, 13, 1247–1255. [Google Scholar] [CrossRef]
- Park, C.-H.; Kim, E.K.; Tijing, L.D.; Amarjargal, A.; Pant, H.R.; Kim, C.S.; Shon, H.K. Preparation and characterization of LA/PCL composite fibers containing beta tricalcium phosphate (β-TCP) particles. Ceram. Int. 2014, 40, 5049–5054. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Pant, H.R.; Lim, J.K. Super-hydrophilic electrospun nylon-6/hydroxyapatite membrane for bone tissue engineering. Eur. Polym. J. 2013, 49, 1314–1321. [Google Scholar] [CrossRef]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential oils used in aromatherapy: A systemic review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Hung, T.T.; Ngan, L.T.M.; Viet, H.; Hoang, N.V.M.; Hieu, T.T. Chemical composition and anti-Helicobacter pylori activity of essential oil from fresh fruits of Litsea cubeba (Lour.) Pers. J. Essent. Oil Res. 2023, 35, 207–219. [Google Scholar] [CrossRef]
- Aghaei Afshar, A.; Sharififard, M.; Jahanifard, E.; Gorouhi, M.A.; Yousefi, S.; Shirani-Bidabadi, L.; Faraji, M.; Alizadeh, I. Application of plants as eco-friendly components against common bed bugs (Cimex lectularius L.): A systematic review of the literature. J. Essent. Oil Res. 2023, 35, 238–246. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, L.-L.; Wang, W.; Wang, G. Recent updates on bioactive properties of α-terpineol. J. Essent. Oil Res. 2023, 35, 274–288. [Google Scholar] [CrossRef]
- El Kharraf, S.; Farah, A.; El-Guendouz, S.; Lourenço, J.P.; Rosa Costa, A.M.; El Hadrami, E.M.; Machado, A.M.; Tavares, C.S.; Figueiredo, A.C.; Miguel, M.G. β-Cyclodextrin inclusion complexes of combined Moroccan Rosmarinus officinalis, Lavandula angustifolia and Citrus aurantium volatile oil: Production optimization and release kinetics in food models. J. Essent. Oil Res. 2023, 35, 247–261. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Falah, F.; Lavi Arab, F.; Vasiee, M.; Tabatabaee Yazdi, F. Chemical Composition and Antioxidant, Antimicrobial, and Antiproliferative Activities of Cinnamomum zeylanicum Bark Essential Oil. Evid.-Based Complement. Altern. Med. 2020, 2020, 5190603. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, H.; Lin, L.; Zhao, C.; Zhang, X.; Xiao, Z.; Li, C. Antibacterial activity and mechanism of cinnamon essential oil and its application in milk. JAPS J. Anim. Plant Sci. 2016, 26, 523–541. [Google Scholar]
- Ojha, P.K.; Poudel, D.K.; Dangol, S.; Rokaya, A.; Timsina, S.; Satyal, P.; Setzer, W.N. Volatile Constituent Analysis of Wintergreen Essential Oil and Comparison with Synthetic Methyl Salicylate for Authentication. Plants 2022, 11, 1090. [Google Scholar] [CrossRef]
- Cuchet, A.; Jame, P.; Anchisi, A.; Schiets, F.; Oberlin, C.; Lefèvre, J.-C.; Carénini, E.; Casabianca, H. Authentication of the naturalness of wintergreen (Gaultheria genus) essential oils by gas chromatography, isotope ratio mass spectrometry and radiocarbon assessment. Ind. Crops Prod. 2019, 142, 111873. [Google Scholar] [CrossRef]
- Fang, R.; Zweig, M.; Li, J.; Mirzababaei, J.; Simmonds, M.S.J. Diversity of volatile organic compounds in 14 rose cultivars. J. Essent. Oil Res. 2023, 35, 220–237. [Google Scholar] [CrossRef]
- Horzum, N.; Muñoz-Espí, R.; Hood, M.A.; Demir, M.M.; Crespy, D. 1. Green Electrospinning; De Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Osanloo, M.; Arish, J.; Sereshti, H. Developed methods for the preparation of electrospun nanofibers containing plant-derived oil or essential oil: A systematic review. Polym. Bull. 2019, 77, 6085–6104. [Google Scholar] [CrossRef]
- Li, H.; Yang, Y.; Hai, L. Chemical components, antimicrobial and antioxidant activities of essential oil from Artemisia kanashiroi in Northwest China. J. Essent. Oil Res. 2023, 35, 296–309. [Google Scholar] [CrossRef]
- Milenković, A.N.; Stanojević, J.S.; Troter, D.Z.; Pejčić, M.G.; Stojanović-Radić, Z.Z.; Cvetković, D.J.; Stanojević, L.P. Chemical composition, antimicrobial and antioxidant activities of essential oils isolated from black (Piper nigrum L.) and cubeb pepper (Piper cubeba L.) fruits from the Serbian market. J. Essent. Oil Res. 2023, 35, 262–273. [Google Scholar] [CrossRef]
- Motealleh, B.; Zahedi, P.; Rezaeian, I.; Moghimi, M.; Abdolghaffari, A.H.; Zarandi, M.A. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly(ɛ-caprolactone)/polystyrene blends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 977–987. [Google Scholar] [CrossRef]
- Khataei, S.; Al-Musawi, M.H.; Asadi, K.; Ramezani, S.; Abbasian, M.; Ghorbani, M. Effect of molecular weight and content of polyvinylpyrrolidone on cell proliferation, loading capacity and properties of electrospun green tea essential oil-incorporated polyamide-6/polyvinylpyrrolidone nanofibers. J. Drug Deliv. Sci. Technol. 2023, 82, 104310. [Google Scholar] [CrossRef]
- Punetha, A.; Kumar, D.; Chauhan, A.; Suryavanshi, P.; Padalia, R.C.; Upadhyay, R.K.; Venkatesha, K.T. Soil moisture stress induced changes in essential oil content and bioactive compounds in German chamomile (Chamomilla recutita L.). J. Essent. Oil Res. 2023, 35, 289–295. [Google Scholar] [CrossRef]
- Saleem, H.; Trabzon, L.; Kilic, A.; Zaidi, S.J. Recent Advances in Nanofibrous Membranes: Production and Applications in Water Treatment and Desalination. Desalination 2020, 478, 114178. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Li, Y.; Jiao, W.; Si, Y.; Yu, J.; Ding, B. Green-Solvent-Processed Fibrous Membranes with Water/Oil/Dust-Resistant and Breathable Performances for Protective Textiles. ACS Appl. Mater. Interfaces 2021, 13, 2081. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, X.; Gong, X.; Ding, M.; Yu, J.; Zhang, S.; Ding, B. Environmentally Friendly Polyamide Nanofiber Membranes with Interconnective Amphiphobic Channels for Seawater Desalination. ACS Appl. Mater. Interfaces 2022, 14, 35287–35296. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Mele, E. Effect of Antibacterial Plant Extracts on the Morphology of Electrospun Poly(Lactic Acid) Fibres. Materials 2018, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298. [Google Scholar] [CrossRef]
- Qi, Z.; Yu, H.; Chen, Y.; Zhu, M. Highly Porous Fibers Prepared by Electrospinning a Ternary System of Nonsolvent/Solvent/Poly(L-lactic acid). Mater. Lett. 2009, 63, 415. [Google Scholar] [CrossRef]
- Dodero, A.; Schlatter, G.; Hebraud, A.; Vicini, S.; Castellano, M.J.C.P. Polymer-free cyclodextrin and natural polymer-cyclodextrin electrospun nanofibers: A comprehensive review on current applications and future perspectives. Carbohydr. Polym. 2021, 264, 118042. [Google Scholar] [CrossRef]
- Kesici Güler, H.; Cengiz Çallıoğlu, F.; Sesli Çetin, E. Antibacterial PVP/cinnamon essential oil nanofibers by emulsion electrospinning. J. Text. Inst. 2019, 110, 302–310. [Google Scholar] [CrossRef]
- Stramarkou, M.; Oikonomopoulou, V.; Missirli, T.; Thanassoulia, I.; Krokida, M. Encapsulation of rosemary essential oil into biodegradable polymers for application in crop management. J. Polym. Environ. 2020, 28, 2161–2177. [Google Scholar] [CrossRef]
- Whaley, A.K.; Minakov, D.A.; Orlova, A.A.; Ponkratova, A.O.; Fock, E.; Rukoyatkina, N.; Gambaryan, S.; Luzhanin, V.G. Analysis of Empetrum nigrum L. lipophilic secondary metabolites, their metabolomic profiles and antioxidant activity. J. Essent. Oil Res. 2023, 35, 310–323. [Google Scholar] [CrossRef]
- Çallıoğlu, F.C.; Güler, H.K.; Çetin, E.S. Emulsion electrospinning of bicomponent poly (vinyl pyrrolidone)/gelatin nanofibers with thyme essential oil. Mater. Res. Express 2019, 6, 125013. [Google Scholar] [CrossRef]
- Mele, E. Electrospinning of essential oils. Polymers 2020, 12, 908. [Google Scholar] [CrossRef]
- Mori, C.L.D.O.; Passos, N.A.d.; Oliveira, J.E.; Altoé, T.F.; Mori, F.A.; Mattoso, L.H.C.; Scolforo, J.R.; Tonoli, G.H.D. Nanostructured polylactic acid/candeia essential oil mats obtained by electrospinning. J. Nanomater. 2015, 16, 33. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shavisi, N.; Karami, N.; Lorestani, R.; Dabirian, F.J.L. Electrospun carboxymethyl cellulose-gelatin nanofibrous films encapsulated with Mentha longifolia L. essential oil for active packaging of peeled giant freshwater prawn. LWT 2021, 152, 112322. [Google Scholar] [CrossRef]
- Sinsup, P.; Teeranachaideekul, V.; Makarasen, A.; Chuenchom, L.; Prajongtat, P.; Techasakul, S.; Yingyuad, P.; Dechtrirat, D.J.M. Zingiber cassumunar roxb. Essential oil-loaded electrospun poly (lactic acid)/poly (ethylene oxide) fiber blend membrane for antibacterial wound dressing application. Membranes 2021, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Mulmi, P.; Pant, H. Fabrication of Air Freshening Spongy Three Dimensional Electrospun Membrane. J. Inst. Eng. 2018, 14, 14. [Google Scholar] [CrossRef]
- Phaiju, S.; Mulmi, P.; Shahi, D.; Hwang, T.; Tiwari, A.; Joshi, R.; Pant, H.; Joshi, M. Antibacterial Cinnamon Essential Oil Incorporated Poly(Ɛ−Caprolactone) Nanofibrous Mats: New Platform for Biomedical Application. J. Inst. Sci. Technol. 2020, 25, 9–16. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, H.W.; Karuppanapandian, T.; Kim, W. Chitosan green tea polyphenol complex as a released control compound for wound healing. Chin. J. Traumatol. = Zhonghua Chuang Shang Za Zhi 2010, 13, 91–95. [Google Scholar]
- Ge, Y.; Tang, J.; Fu, H.; Fu, Y.; Wu, Y. Characteristics, Controlled-release and Antimicrobial Properties of Tea Tree Oil Liposomes-incorporated Chitosan-based Electrospun Nanofiber Mats. Fibers Polym. 2019, 20, 698–708. [Google Scholar] [CrossRef]
- Lucas-González, R.; Yilmaz, B.; Mousavi Khaneghah, A.; Hano, C.; Shariati, M.A.; Bangar, S.P.; Goksen, G.; Dhama, K.; Lorenzo, J.M. Cinnamon: An antimicrobial ingredient for active packaging. Food Packag. Shelf Life 2023, 35, 101026. [Google Scholar] [CrossRef]
- Partovi, R.; Talebi, F.; Babaei, A.; Sharifzadeh, A. Antimicrobial Activity of Polylactic Acid Film Incorporated With Marjoram and Clove Essential Oils on Microbial and Chemical Properties of Minced Beef During Refrigerated Storage. Int. J. Enteric Pathog. 2020, 8, 25–31. [Google Scholar] [CrossRef]
- Ahmadi, S.; Hivechi, A.; Bahrami, S.H.; Milan, P.B.; Ashraf, S.S. Cinnamon extract loaded electrospun chitosan/gelatin membrane with antibacterial activity. Int. J. Biol. Macromol. 2021, 173, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Liakos, I.L.; Holban, A.M.; Carzino, R.; Lauciello, S.; Grumezescu, A.M. Electrospun Fiber Pads of Cellulose Acetate and Essential Oils with Antimicrobial Activity. Nanomaterials 2017, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, Y.; Li, Y.; Li, Y.; Liu, H.; Yang, Z.; Wu, H.; Hu, Y. Formation of cinnamon essential oil/xanthan gum/chitosan composite microcapsules basing on Pickering emulsions. Colloid Polym. Sci. 2022, 300, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, K.; Kodam, K.M. Encapsulation of therapeutic lavender oil in an electrolyte assisted polyacrylonitrile nanofibres for antibacterial applications. RSC Adv. 2014, 4, 54892–54901. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mani, M.P.; Ismail, A.F.; Ayyar, M. Manufacturing and Characterization of Novel Electrospun Composite Comprising Polyurethane and Mustard Oil Scaffold with Enhanced Blood Compatibility. Polymers 2017, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Lee, S. Electrospun Nanofibrous Membranes with Essential Oils for Wound Dressing Applications. Fibers Polym. 2020, 21, 999–1012. [Google Scholar] [CrossRef]
- da Cruz, E.P.; Pires, J.B.; dos Santos, F.N.; Fonseca, L.M.; Radünz, M.; Dal Magro, J.; Gandra, E.A.; da Rosa Zavareze, E.; Dias, A.R.G. Encapsulation of lemongrass essential oil into cassava starch fibers for application as antifungal agents in bread. Food Hydrocoll. 2023, 145, 109105. [Google Scholar] [CrossRef]






| Parameters | Effect on Fiber Morphology |
|---|---|
| Solution (material) parameters | |
| Solvent vapor pressure | Increased porosity is associated with greater volatility [83]. |
| Polymeric concentration | Higher concentrations (within the optimal range) lead to an increase in fiber diameter [84]. |
| Solvent choice | The choice of solvent is crucial, as it can significantly affect the solubility and rheological properties of the spinning solution. Different solvents can lead to variations in fiber diameter and morphology [85]. |
| Solution viscosity | Higher viscosity (within the optimal range) results in an increase in fiber diameter. However, exceeding the critical viscosity value can lead to the formation of beaded or deformed nanofibers, and may even cause clogging of the spinneret [86]. |
| Solution surface tension | The surface tension of the spinning solution affects the ability of the solution to form a stable jet. A lower surface tension promotes the formation of thinner fibers, while a higher surface tension results in thicker fibers. Surfactants are sometimes added to adjust the surface tension and improve fiber formation [87]. |
| Solution conductivity | Increasing the conductivity leads to a decrease in fiber diameter, and higher conductivity can result in more pronounced bending instabilities, leading to the formation of non-uniform or beaded fibers [88]. |
| Processing (Operational) parameters | |
| Voltage | There is no definitive correlation between fiber diameter and voltage; however, it is commonly observed that increases in applied voltage cause a reduction in fiber diameter. Additionally, higher voltages may result in a higher probability of bead formation [89]. |
| Flow rate | Enhancement of the fiber diameter and the occurrence of bead formation are commonly observed at higher feed rates (above the minimum rate) [90]. |
| Needle-collector distance | Within the optimal range, the fiber diameter tends to decrease as the spinneret to the collector distances increases [91]. |
| Ambient (Environmental) parameters | |
| Temperature | Increasing the temperature generally leads to a decrease in fiber diameter [92]. |
| Humidity | Higher humidity levels tend to induce the formation of circular pores in the fibers [93]. |
| Essential Oils | BP (°C) | Main Component | Polymeric Solution | Application Areas |
|---|---|---|---|---|
| Green tea oil | 165 | Terpinene | Chitosan-PEO | Wound healing [193] |
| Chamomile oil | 161 | Terpenoids organic acids | PCL-PS | Wound healing [173] |
| Tea tree oil liposomes | 165 | Terpinene | Chitosan-PEO | Antimicrobial properties [194] |
| Cinnamon oil | 194–234 | Cinnamadehyde | PVA | Food packaging [195] |
| Clove oil | 251 | eugenol | PLA | Antimicrobial activity [196] |
| Cinnamon oil | 194–234 | Cinnamadehyde | Chitosan-Gelatin | Antibacterial activity [197] |
| Oregano oil | 239 | Thymol | Cellulose Acetate | Antimicrobial properties [198] |
| Cinnamon oil | 194–234 | Cinnamadehyde | Xanthan-chitosan | Antimicrobial properties [199] |
| Lavender oil | 204 | Linalool | Polyacrylonitrile | Antimicrobial properties [200] |
| Mustardoil | 170 | Erucic acid | Polyurethane | Blood compatibility test [201] |
| Blackpepper oil | 166 | Trans-aryophyllene | Polylactic acid | Wound healing [142] |
| Palmarosa | 100 | Geraniol | Polyvinyl Alcohol | Wound healing [202] |
| Lemongrass oil | 228 | Citral | Nylon-6,6 | Air freshener [203] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, D.; Bhatta, L.R.; Sharma, R.K.; Pant, B.; Park, M.; Ojha, G.P.; Pant, H.R. Nonwoven Electrospun Membranes as Tissue Scaffolds: Practices, Problems, and Future Directions. J. Compos. Sci. 2023, 7, 481. https://doi.org/10.3390/jcs7120481
Shah D, Bhatta LR, Sharma RK, Pant B, Park M, Ojha GP, Pant HR. Nonwoven Electrospun Membranes as Tissue Scaffolds: Practices, Problems, and Future Directions. Journal of Composites Science. 2023; 7(12):481. https://doi.org/10.3390/jcs7120481
Chicago/Turabian StyleShah, Dinesh, Lok Ranjan Bhatta, Ram Kumar Sharma, Bishweshwar Pant, Mira Park, Gunendra Prasad Ojha, and Hem Raj Pant. 2023. "Nonwoven Electrospun Membranes as Tissue Scaffolds: Practices, Problems, and Future Directions" Journal of Composites Science 7, no. 12: 481. https://doi.org/10.3390/jcs7120481
APA StyleShah, D., Bhatta, L. R., Sharma, R. K., Pant, B., Park, M., Ojha, G. P., & Pant, H. R. (2023). Nonwoven Electrospun Membranes as Tissue Scaffolds: Practices, Problems, and Future Directions. Journal of Composites Science, 7(12), 481. https://doi.org/10.3390/jcs7120481










