Synthesis of BaSnO3 as a Highly Dispersed Additive for the Preparation of Proton-Conducting Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the Precursor BaSn(OH)6
2.3. Characterization
3. Results and Discussion
3.1. Synthesis of Precursor BaSn(OH)6
3.2. Characteristics of Intermediate Products of Thermolysis and Final Sample of Barium Stannate
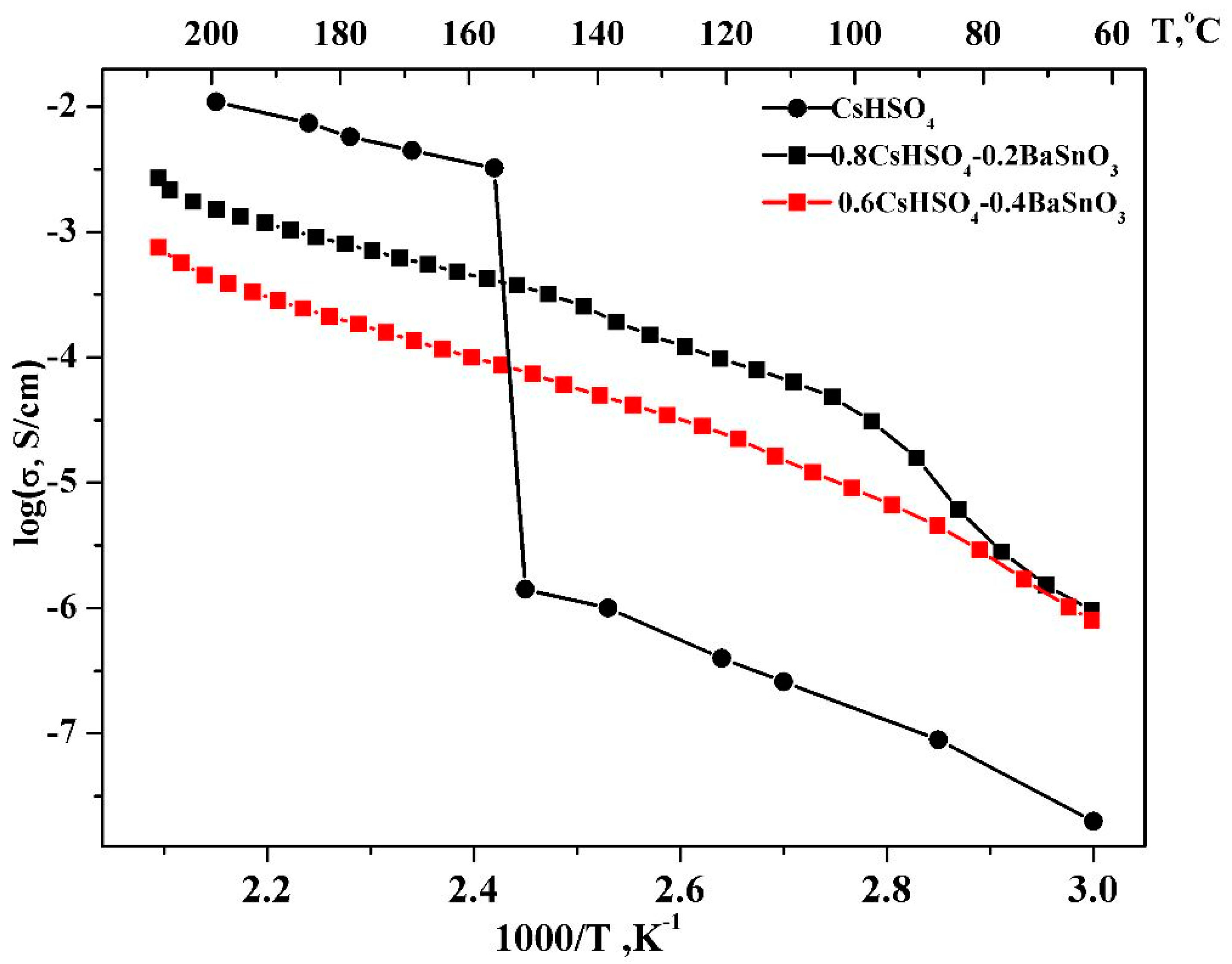
3.3. Study of Transport Properties of Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, D.H.; Qu, L.; Kim, D.W.; Kang, S.B.; Park, J.-S.; Cho, I.S. Role of cations in the photovoltaic performance optimization of ternary stannates, MSnO3 (M = Ca, Sr, and Ba) and N2SnO4 (N = Ca, Sr, Ba, and Zn). Ceram. Int. 2023, 49, 32015–32023. [Google Scholar] [CrossRef]
- Chahib, S.; Leroy, G.; Duponchel, B.; Poupin, C.; Ez-zahraouy, H.; Fasquelle, D. Investigation of structural, morphological, and dielectric properties of BaSnO3 ceramics and thin films prepared by sol-gel method. Ceram. Int. 2023, 49, 17542–17553. [Google Scholar] [CrossRef]
- Muraleedharan, S.; Ashok, A.M. Nitrogen doping for effective enhancement of optoelectronic performance of ASnO3 (A-Ca, Sr, Ba) transparent conducting oxides deposited by doctor blade method. Optik 2023, 287, 171096. [Google Scholar] [CrossRef]
- Khatun, M.; Mitra, P.; Mukherjee, S. Effect of band gap and particle size on photocatalytic degradation of NiSnO3 nanopowder for some conventional organic dyes. Hybrid Adv. 2023, 4, 100079. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Zhang, Q.; Wang, H.; Xu, G.; Wang, X. Influence of La doping on the ethanol gas sensing properties of CdSnO3 micro-cubes. Sens. Actuators B 2023, 394, 134447. [Google Scholar] [CrossRef]
- Marikutsa, A.; Dobrovolskii, A.A.; Rumyantseva, M.N.; Mikhaylov, A.A.; Medvedev, A.G.; Lev, O.; Prikhodchenko, P.V. Improved H2S sensitivity of nanosized BaSnO3 obtained by hydrogen peroxide assisted sol-gel processing. J. Alloys Compd. 2023, 944, 169141. [Google Scholar] [CrossRef]
- Venkatesh, G.; Suganesh, R.; Jayaprakash, J.; Srinivasan, M.; Prabu, K.M. Perovskite type BaSnO3-reduced graphene oxide nanocomposite for photocatalytic decolourization of organic dye pollutant. Chem. Phys. Lett. 2022, 787, 139237. [Google Scholar] [CrossRef]
- Bogdan, T.V.; Krasnikov, P.A.; Smirnov, A.V.; Koklin, A.E.; Mashchenko, N.V.; Bogdan, V.I. Utilization of Acetone, by-product of cumene process for phenol production, via BaSnO3-catalyzed aldol condensation. Dokl. Phys. Chem. 2022, 507, 147–152. [Google Scholar] [CrossRef]
- Salem, F.Z.; Ahmed, M.A.; Sadek, M.A.; Elmahgary, M.G. Novel hydrogen-doped SrSnO3 perovskite with excellent optoelectronic properties as a potential photocatalyst for water splitting. Int. J. Hydrogen Energy 2022, 47, 18321–18333. [Google Scholar] [CrossRef]
- Ashiq, M.G.B.; Mahmood, Q.; Haq, B.U.; Flemban, T.H.; Kattan, N.A.; Alshahrani, T.; Laref, A. The study of electronics, optoelectronics, thermoelectric, and mechanical properties of Zn/CdSnO3 perovskites. Mater. Sci. Semicond. Process. 2022, 137, 106229. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Lu, W.; Fouad, H.; Mourad, A.-H.I.; Akhtar, M.S.; Guo, W. Hydrothermal synthesis of hollow CoSnO3 nanocubes for highly response and selective ethanol gas sensing. Mater. Lett. 2022, 316, 132056. [Google Scholar] [CrossRef]
- Sanchela, A.V.; Wei, M.; Cho, H.J.; Ohta, H. Optoelectronic properties of transparent oxide semiconductor ASnO3 (A = Ba, Sr, and Ca) epitaxial films and thin film transistors. J. Sci. Technol. A 2022, 40, 020803. [Google Scholar] [CrossRef]
- Qi, C.; Zhang, C.; Yang, Z. Constructing heterointerface of crystalline Au nanoparticles and amorphous porous CoSnO3 nanocubes for sensitive electrochemical detection of glucose. Microchem. J. 2022, 183, 108039. [Google Scholar] [CrossRef]
- Tao, F.; Li, F.; Huang, J.; Xue, Z.; Yu, C.; Cai, Z.; Pei, L. A General Hydrothermal Growth and Photocatalytic Performance of Barium Tin Hydroxide/Tin Dioxide Nanorods. Cryst. Res. Technol. 2021, 57, 2100156. [Google Scholar] [CrossRef]
- Sá, B.S.; Zito, C.A.; Perfecto, T.M.; Volanti, D.P. Porous ZnSnO3 nanocubes as a triethylamine sensor. Sens. Actuators B 2021, 338, 129869. [Google Scholar] [CrossRef]
- Ochoa-Munoz, Y.H.; Rodriguez-Paez, J.E.; de Gutierrez, R.M. Structural and optical study of perovskite nanoparticles MSnO3 (M = Ba, Zn, Ca) obtained by a wet chemical route. Mater. Chem. Phys. 2021, 266, 124557. [Google Scholar] [CrossRef]
- Wang, J.; Luo, B. Optical and electronic properties in amorphous BaSnO3 thin films. Phys. B Condens. Matter. 2021, 601, 412586. [Google Scholar] [CrossRef]
- Kramer, J.W.; Kelly, B.; Manivannan, V. Synthesis of MSn(OH)6 (where M = Mg, Ca, Zn, Mn, or Cu) materials at room temperature. Cent. Eur. J. Chem. 2010, 8, 65–69. [Google Scholar] [CrossRef]
- Chu, X.; Gan, Z.; Bai, L.; Dong, Y.; Rumyantseva, M.N. The acetic acid vapor sensing properties of BaSnO3 microtubes prepared by electrospinning method. Mater. Sci. Eng. B 2020, 259, 114606. [Google Scholar] [CrossRef]
- Arora, I.; Praveen, K. Effect of annealing temperature on structure-property correlations in Zn2SnO4 nanostructured films for optoelectronics. Mater. Res. Express 2020, 7, 035023. [Google Scholar] [CrossRef]
- Debata, S.; Banerjee, S.; Chakraborty, S.; Sharma, P.K. Template assisted hydrothermal synthesis of CoSnO3 hollow microspheres for electrocatalytic oxygen evolution reaction. Int. J. Hydrogen Energy 2019, 44, 21623–21636. [Google Scholar] [CrossRef]
- Gomez-Solis, C.; Oliva, J.; Diaz-Torres, L.A.; Bernal-Alvarado, J.; Reyes-Zamudio, V.; Abidov, A.; Torres-Martinez, L.M. Efficient photocatalytic activity of MSnO3 (M: Ca, Ba, Sr) stannates for photoreduction of 4-nitrophenol and hydrogen production under UV light irradiation. J. Photochem. Photobiol. A Chem. 2019, 371, 365–373. [Google Scholar] [CrossRef]
- Rembeza, S.I.; Belousov, S.A.; Kosheleva, N.N.; Rembeza, E.S.; Svistova, T.V.; Suvaci, E.; Özel, E.; Tuncolu, G.; Açiksari, C. Amorphous films of ternary zinc and tin oxides for transparent electronics. Tech. Phys. Lett. 2018, 44, 984–987. [Google Scholar] [CrossRef]
- Sahmi, A.; Laib, R.; Omeiri, S.; Bensadok, K.; Trari, M. Photoelectrochemical properties of the perovskite BaSnO3 synthetized by chemical route. Application to electro-photocatalytic mineralization of ibuprofen. J. Photochem. Photobiol. A Chem. 2018, 364, 443–448. [Google Scholar] [CrossRef]
- Honorio, L.M.C.; Santos, M.V.B.; Da Silva Filho, E.C.; Osajima, J.A.; Maia, A.S.; Santos, I.M.G. Alkaline Earth Stannates Applied in Photocatalysis: Prospection and Review of Literature. Ceram. 2018, 64, 559–569. [Google Scholar] [CrossRef]
- Inagaki, M.; Kuroishi, T.; Yamashita, Y.; Urata, M. Syntheses of MSn(OH)6 by coprecipitation and of MSnO3 by thermal decomposition (M = Mg, Co, Zn, Mn, Cd, Ca, Sr, Ba). Z. Anorg. Allg. Chem. 1985, 527, 193–202. [Google Scholar] [CrossRef]
- Jayathilaka, W.A.D.M.; Chinnappan, A.; Ji, D.; Ghosh, R.; Tran, T.Q.; Ramakrishna, S. Facile and Scalable Electrospun Nanofiber-Based Alternative Current Electroluminescence (ACEL) Device. ACS Appl. Electron. Mater. 2021, 3, 267–276. [Google Scholar] [CrossRef]
- Kohan, M.; Mahmoudi, T.; Wang, Y.; Im, Y.H.; Hahn, Y.-B. SnO2/BaSnO3 electron transport materials for stable and efficient perovskite solar cells. Appl. Surf. Sci. 2023, 613, 156068. [Google Scholar] [CrossRef]
- Zvonareva, I.A.; Medvedev, D.A. Proton-conducting barium stannate for high-temperature purposes: A brief review. J. Eur. Ceram. Soc. 2023, 43, 198–207. [Google Scholar] [CrossRef]
- Antonio, J.E.; Rosas-Huerta, J.L.; Cervantes, J.M.; León-Flores, J.; Romero, M.; Carvajal, E.; Escamilla, R. Li/Na atoms’ substitution effects on the structural, electronic, and mechanical properties of the CaSnO3 perovskite for battery applications. Comp. Mater. Sci. 2023, 219, 112006. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.; Yang, H.; Feng, X.; Bai, Y.; Wu, C. CoSnO3/C nanocubes with oxygen vacancy as high-capacity cathode materials for rechargeable aluminum batteries. Green Energy Environ. 2023, 8, 883–892. [Google Scholar] [CrossRef]
- Roy, A.; Ghosh, P.; Devi, P.S.; Sundaram, S.; Mallick, T.K. Efficient carbon counter electrodes for BaSnO3-based dye-sensitized solar cells. Mater. Today Proc. 2021, 45, 3685–3691. [Google Scholar] [CrossRef]
- Wei, J.L.; Jin, X.Y.; Yu, M.C.; Wang, L.; Guo, Y.H.; Dong, S.T.; Zhang, Y.M. Electrospun ZnSnO3/C nanofibers as an anode material for lithium-ion batteries. J. Electron. Mater. 2021, 50, 4945–4953. [Google Scholar] [CrossRef]
- Sim, C.K.; Majid, S.R.; Mahmood, N.Z. ZnSnO3/mesoporous biocarbon composite towards sustainable electrode material for energy storage device. Microchem. J. 2021, 164, 105968. [Google Scholar] [CrossRef]
- Purushothamreddy, N.; Kovendhan, M.; Dileep, R.K.; Veerappan, G.; Kumar, S.K.; Joseph, D.P. Synthesis and characterization of nanostructured La-doped BaSnO3 for dye-sensitized solar cell application. Mater. Chem. Phys. 2020, 250, 123137. [Google Scholar] [CrossRef]
- Roy, A.; Das, P.P.; Selvaraj, P.; Devi, P.S.; Sundaram, S. Template free synthesis of CdSnO3 micro-cuboids for dye sensitized solar cells. J. Photochem. Photob. A Chem. 2019, 380, 111824. [Google Scholar] [CrossRef]
- Cheng, H.; Koh, K.L.P.; Liu, P.; Thang, T.Q.; Duong, H.M. Continuous self-assembly of carbon nanotube thin films and their composites for supercapacitors. Colloids Surf. A Physicochem. Eng. Aspects. 2015, 481, 626–632. [Google Scholar] [CrossRef]
- Veerappan, G.; Yoo, S.; Zhang, K.; Ma, M.; Kang, B.; Park, J.H. High-reversible capacity of Perovskite BaSnO3/rGO composite for Lithium-Ion Battery Anodes. Electrochim. Acta 2016, 214, 31–37. [Google Scholar] [CrossRef]
- Jena, H.; Kutty, K.V.G.; Kutty, T.R.N. Ionic transport and structural investigations on MSn(OH)6 (M = Ba, Ca, Mg, Co, Zn, Fe, Mn) hydroxide perovskites synthesized by wet sonochemical methods. Mater. Chem. Phys. 2004, 88, 167–179. [Google Scholar] [CrossRef]
- Wang, Y.; Li, D.; Liu, Y.; Zhang, J. Fabrication of novel rugby-like ZnSnO3/reduced graphene oxide composites as a high-performance anode material for lithium-ion batteries. Mater. Lett. 2016, 167, 222–225. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Uvarov, N.F.; Lavrova, G.V.; Hairetdinov, E.F. Composite protonic solid electrolytes in the CsHSO4-SiO2 system. Solid State Ionics 1996, 90, 161–167. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Lavrova, G.V. Influence of dispersed TiO2 on protonic conductivity of CsHSO4. Solid State Ionics 1998, 106, 137–141. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Lavrova, G.V.; Simonova, L.G. The influence of heterogeneous dopant porous structure on the properties of protonic solid electrolyte in the CsHSO4-SiO2 system. Solid State Ionics 1999, 118, 317–323. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Lavrova, G.V. The investigation of disordered phases in nanocomposite proton electrolytes based on MeHSO4 (Me = Rb, Cs, K). Solid State Ionics 2001, 145, 197–204. [Google Scholar] [CrossRef]
- Mohammad, N.; Mohamad, A.B.; Kadhum, A.A.H.; Shyuan, L.K. Conductivity and thermal stability of solid acid composites CsH2PO4/NaH2PO4/SiO2. Malays. J. Analytic. Sci. 2016, 20, 633–641. [Google Scholar] [CrossRef]
- Navarrete, L.; Yoo, C.-Y.; Serra, J.M. Comparative Study of Epoxy-CsH2PO4 Composite Electrolytes and Porous Metal Based Electrocatalysts for Solid Acid Electrochemical Cells. Membranes 2021, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Ponomareva, V.G.; Lavrova, G.V. Controlling the proton transport properties of solid acids via structural and microstructural modification. J. Solid State Electrochem. 2011, 15, 213–221. [Google Scholar] [CrossRef]
- Anfimova, T.; Jensen, A.H.; Christensen, E.; Jensen, J.O.; Bjerrum, N.J.; Li, Q. CsH2PO4/NdPO4 composites as proton conducting electrolytes for intermediate temperature fuel cells. J. Electrochem. Soc. 2015, 162, 436–441. [Google Scholar] [CrossRef]
- Aparnev, A.I.; Loginov, A.V.; Uvarov, N.F.; Ponomareva, V.G.; Bagryantseva, I.N.; Manakhov, A.A.; Al-Qasim, A.S.; Golovakhin, V.V.; Bannov, A.G. Novel Highly Dispersed Additive for Proton-Conducting Composites. Appl. Sci. 2023, 13, 5038. [Google Scholar] [CrossRef]
- Gusev, A.I. Nanocrystalline Materials: Methods of Preparation and Properties; Ural Branch of the Russian Academy of Sciences: Yekaterinburg, Russia, 1998; 198p. [Google Scholar]
- Yong, Z.J. A general sonochemical Approach to Rapid Synthesis of 1D Single-crystalline MSn(OH)6 (M = Ba, Ca, Sr) Nanostructures. Adv. Mater. Res. 2011, 295–297, 1554–1559. [Google Scholar] [CrossRef]
- Wang, G.; Bai, J.; Shan, C.; Zhang, D.; Lu, N.; Liu, Q.; Zhou, Z.; Wang, S.; Liu, C. Synthesis and ethanol gas sensing properties of mesoporous perovskite-type BaSnO3 nanoparticles interconnected network. Mater. Lett. 2017, 205, 169–172. [Google Scholar] [CrossRef]
- Loginov, A.V.; Aparnev, A.I.; Uvarov, N.F. Synthesis of SrSnO3/SnO2 Composites via Thermal Decomposition of a Precursor. Inorg. Mater. 2022, 58, 420–424. [Google Scholar] [CrossRef]
- Haase, P.; Christensen, H.G.; Nielsen, U.G.; Koch, C.B.; Galazka, Z.; Majzlan, J. Stability and solubility of members of tin perovskites in the schoenfliesite subgroup, □2(BSn4+)(OH,O)6 (B = Ca, Fe3+, Mg, Mn2+, Zn, Cu). Chem. Thermod. Therm. Anal. 2021, 1–2, 100005. [Google Scholar] [CrossRef]
- Stanulis, A.; Sakirzanovas, S.; Van Bael, M.; Kareiva, A. Sol–gel (combustion) synthesis and characterization of different alkaline earth metal (Ca, Sr, Ba) stannates. J. Sol-Gel Sci. Technol. 2012, 64, 643–652. [Google Scholar] [CrossRef]
- Hu, X.; Lv, G.; Jia, Z.; Jiang, J.; Xiao, T.; Yuan, M.; Tang, Y. A general sonochemical approach to rapid synthesis of 1D single-crystalline MSn(OH)6 (M = Ba, Ca, Sr) nanostructures. Appl. Surf. Sci. 2011, 257, 9008–9013. [Google Scholar] [CrossRef]
- Medvedev, A.G.; Mikhaylov, A.A.; Shames, A.I.; Ilyukhin, A.B.; Churakov, A.V.; Grishanov, D.A.; Mel’nik, E.A.; Tripol’skaya, T.A.; Lev, O.; Prikhodchenko, P.V. Identification of barium hydroxo-hydroperoxostannate precursor for low-temperature formation of perovskite barium stannate. Inorg. Chem. 2020, 59, 18358–18365. [Google Scholar] [CrossRef] [PubMed]
- Azad, A.-M.; Hashim, M.; Baptist, S.; Badri, A.; Haq, A.U. Phase evolution and microstructural development in sol-gel derived MSnO3 (M = Ca, Sr and Ba). J. Mater. Sci. 2000, 35, 5475–5483. [Google Scholar] [CrossRef]
- Loginov, A.V.; Aparnev, A.I.; Uvarov, N.F. Nanocomposites Prepared via Thermal Decomposition of Calcium Hydroxystannate CaSn(OH)6. Inorg. Mater. 2022, 58, 814–821. [Google Scholar] [CrossRef]
- Lavrova, G.V.; Shutova, E.S.; Ponomareva, V.G.; Dunyushkina, L.A. Proton conductivity and interphase interaction in CsH2PO4-SrZrO3 composites. Russ. J. Electrochem. 2013, 49, 718–724. [Google Scholar] [CrossRef]
- Wang, Y.; Chesnaud, A.; Bevillon, E.; Dezanneau, G. Properties of Y-doped BaSnO3 proton conductors. Solid State Ionics 2012, 214, 45–55. [Google Scholar] [CrossRef]
- Prakash, A.; Xu, P.; Faghaninia, A.; Shukla, S.; Ager, J.W.; Lo, C.S.; Jalan, B. Wide bandgap BaSnO3 films with room temperature conductivity exceeding 104 S cm−1. Nat. Commun. 2017, 8, 15167. [Google Scholar] [CrossRef]
- Geneste, G.; Dezanneau, G. Competition between elastic and chemical effects in the doping, defect association, and hydration of barium stannate. Solid State Ionics 2017, 308, 121–132. [Google Scholar] [CrossRef]
- Buscaglia, M.T.; Leoni, M.; Viviani, M.; Buscaglia, V.; Martinelli, A.; Testino, A.; Nanni, P. Synthesis and characterization of BaSn(OH)6 and BaSnO3 acicular particles. J. Mater. Res. 2003, 18, 560–566. [Google Scholar] [CrossRef]
- Wu, X.; Lv, G.; Hu, X.; Tang, Y. A Two-Step Method to Synthesize BaSn(OH)6 Crystalline Nanorods and Their Thermal Decomposition to Barium Stannate. J. Nanomater. 2012, 2012, 912731. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. A SEM study of nanosized metal films and metal nanoparticles obtained by magnetron sputtering. Russ. Chem. Bull. 2011, 60, 2602–2607. [Google Scholar] [CrossRef]
- Kachala, V.V.; Khemchyan, L.L.; Kashin, A.S.; Orlov, N.V.; Grachev, A.A.; Zalesskiy, S.S.; Ananikov, V.P. Target-oriented analysis of gaseous, liquid and solid chemical systems by mass spectrometry, nuclear magnetic resonance spectroscopy and electron microscopy. Russ. Chem. Rev. 2013, 82, 648–685. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density. In Particle Technology Series; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2004; 365p. [Google Scholar] [CrossRef]
- Karnaukhov, A.P. Adsorption. In The Texture of Dispersed and Porous Materials; Nauka: Novosibirsk, Russia, 1999; 470p. [Google Scholar]
- Cerda, J.; Arbiol, J.; Dezanneau, G.; Diaz, R.; Morante, J.R. Perovskite-Type BaSnO3 Powders for High Temperature Gas Sensor Applications. Sens. Actuators B. 2002, 84, 21–25. [Google Scholar] [CrossRef]
- Ahmed, J.; Blakely, C.K.; Bruno, S.R.; Poltavets, V.V. Synthesis of MSnO3 (M = Ba, Sr) nanoparticles by reverse micelle method and particle size distribution analysis by whole powder pattern modeling. Mater. Res. Bull. 2012, 47, 2282–2287. [Google Scholar] [CrossRef]
- Lu, W.; Schmidt, H. Lyothermal synthesis of nanocrystalline BaSnO3 powders. Ceram. Inter. 2008, 34, 645–649. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies Contents: Tables and Charts; Wiley: Chichester, NY, USA, 2001; 347p. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; Wiley: New York, NY, USA, 1997; Volume 479. [Google Scholar]
- Amalric-Popescu, D.; Bozon-Verduraz, F. Infrared studies on SnO2 and Pd/SnO2. Catal. Today 2001, 70, 139–154. [Google Scholar] [CrossRef]
- Shukla, S.S. Synthesis and Dispersion of Barium Stannate Nanopowders. 2011. 28p. Available online: https://core.ac.uk/reader/53187964 (accessed on 1 October 2020).
- Baranov, A.I. Crystals with disordered hydrogen-bond networks and superprotonic conductivity. Review. Crystallogr. Rep. 2003, 48, 1012–1037. [Google Scholar] [CrossRef]




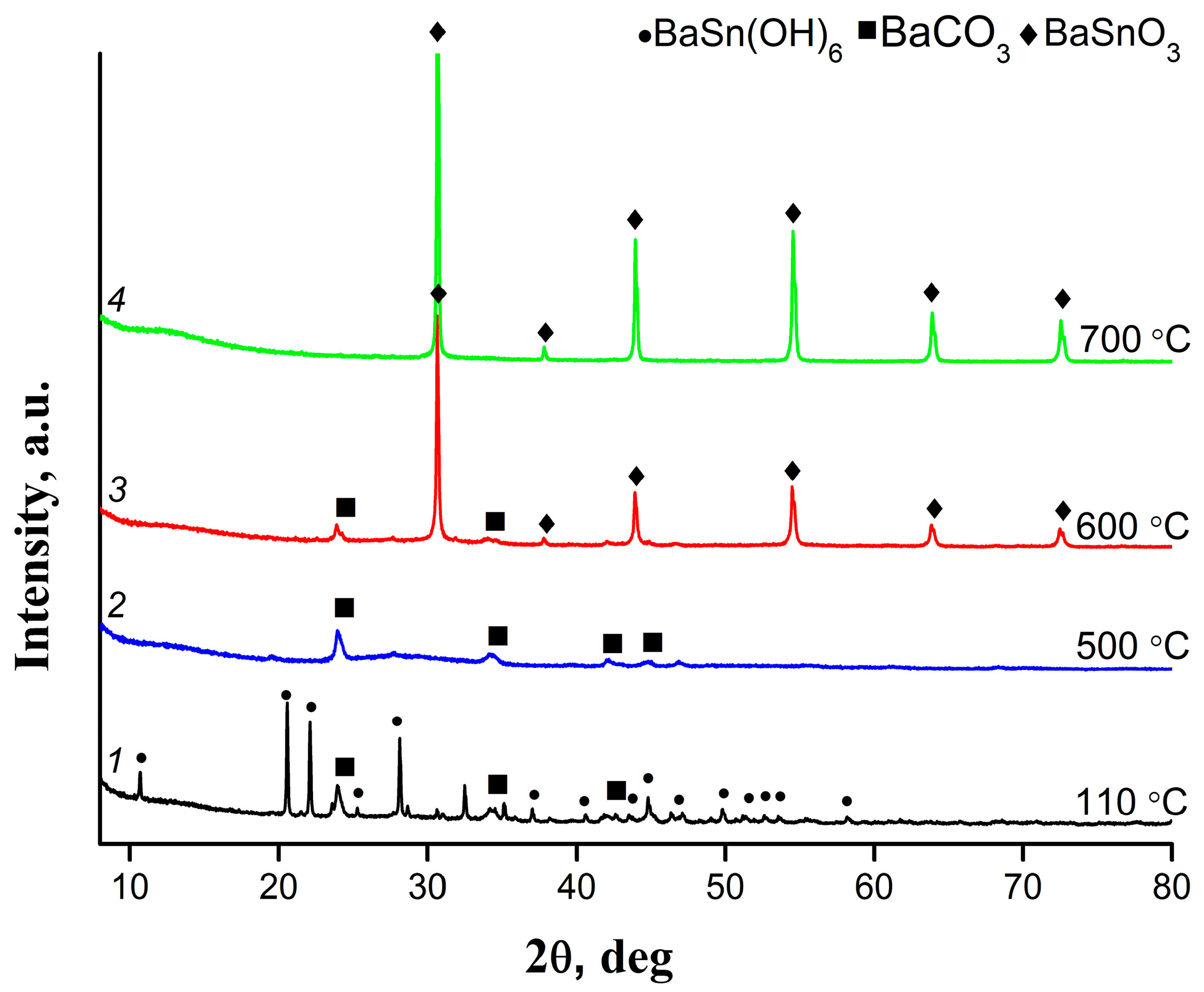
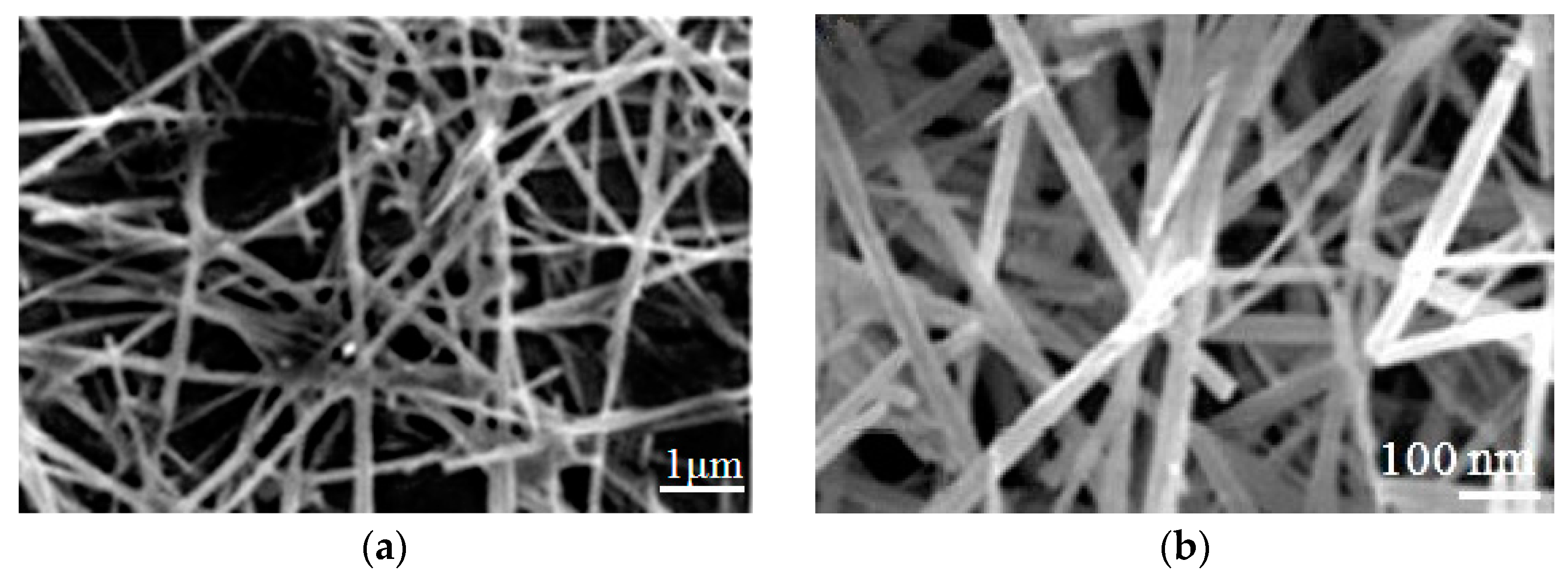
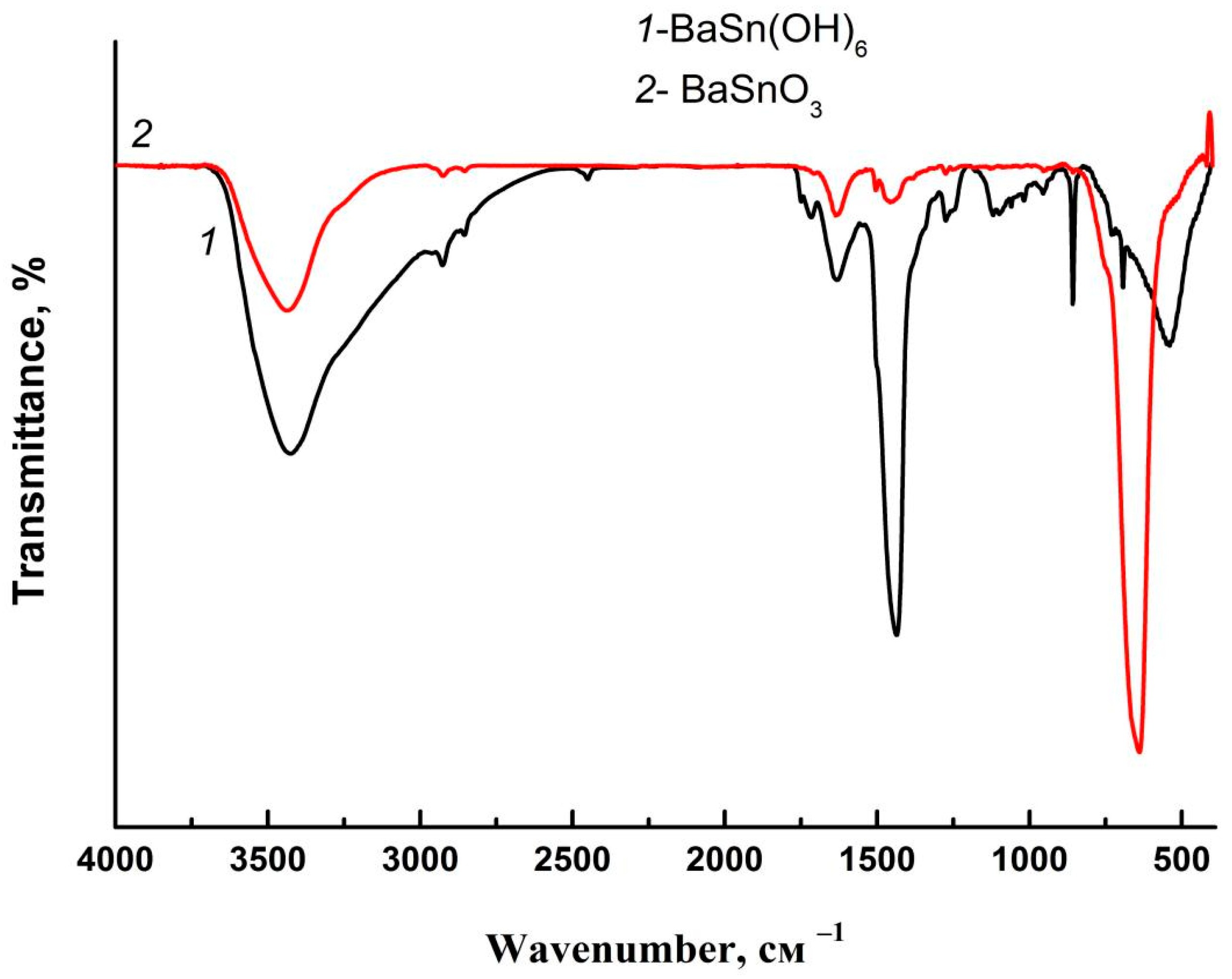

| Heating Temperature, °C | 110 | 500 | 600 | 700 |
|---|---|---|---|---|
| Phase composition | BaSn(OH)6, BaCO3, amorphous phase SnO2 × xH2O | BaCO3, amorphous phases BaSnO3 and SnO2 | BaSnO3, BaCO3 (traces) | BaSnO3 |
| Specific surface area, m2·g−1 | 59 | 43 | 23 | 15 |
| Pore size, nm | 4.4 | 5.8 | 3.3 | 2.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loginov, A.V.; Aparnev, A.I.; Uvarov, N.F.; Ponomareva, V.G.; Bannov, A.G. Synthesis of BaSnO3 as a Highly Dispersed Additive for the Preparation of Proton-Conducting Composites. J. Compos. Sci. 2023, 7, 469. https://doi.org/10.3390/jcs7110469
Loginov AV, Aparnev AI, Uvarov NF, Ponomareva VG, Bannov AG. Synthesis of BaSnO3 as a Highly Dispersed Additive for the Preparation of Proton-Conducting Composites. Journal of Composites Science. 2023; 7(11):469. https://doi.org/10.3390/jcs7110469
Chicago/Turabian StyleLoginov, Anton V., Alexander I. Aparnev, Nikolai F. Uvarov, Valentina G. Ponomareva, and Alexander G. Bannov. 2023. "Synthesis of BaSnO3 as a Highly Dispersed Additive for the Preparation of Proton-Conducting Composites" Journal of Composites Science 7, no. 11: 469. https://doi.org/10.3390/jcs7110469
APA StyleLoginov, A. V., Aparnev, A. I., Uvarov, N. F., Ponomareva, V. G., & Bannov, A. G. (2023). Synthesis of BaSnO3 as a Highly Dispersed Additive for the Preparation of Proton-Conducting Composites. Journal of Composites Science, 7(11), 469. https://doi.org/10.3390/jcs7110469







