Thermal Decomposition Characteristics of PEO/LiBF4/LAGP Composite Electrolytes
Abstract
:1. Introduction
2. Materials and Experimental
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Balakrishnan, P.G.; Ramesh, R.; Kumar, T.P. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Wen, J.; Yu, Y.; Chen, C. A Review on lithium-ion batteries safety issues: Existing problems and possible solutions. Mater. Express 2012, 2, 197–212. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Zhao, X.; Chu, G.; Sun, J.; Chen, C. Thermal runaway caused fire and explosion of lithium ion battery. J. Power Sources 2012, 208, 210–224. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Ren, D.; He, X.; Ouyang, M. Mitigating Thermal Runaway of Lithium-Ion Batteries. Joule 2020, 4, 743–770. [Google Scholar] [CrossRef]
- Song, L.; Zheng, Y.; Xiao, Z.; Wang, C.; Long, T. Review on Thermal Runaway of Lithium-Ion Batteries for Electric Vehicles. J. Electron. Mater. 2022, 51, 30–46. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Singh, P. Review—Solid Electrolytes in Rechargeable Electrochemical Cells. J. Electrochem. Soc. 2015, 162, A2387–A2392. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Capuano, F.; Croce, F.; Scrosati, B. Composite polymer electrolytes. J. Electrochem. Soc. 1991, 138, 1918–1922. [Google Scholar] [CrossRef]
- Agrawal, R.C.; Pandey, G.P. Solid polymer electrolytes: Materials designing and all-solid-state battery applications: An review. J. Phys. D Appl. Phys. 2008, 41, 3715–3725. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.; Fan, L.; Nan, C.; Goodenough, J. PEO/garnet composite electrolytes for solid-state lithium batteries: From ‘ceramic-in-polymer’ to ‘polymer-in-ceramic’. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Li, H.; Chen, L.; Wu, F. Progress in thermal stability of all-solid-state-Li-ion batteries. InfoMat 2021, 3, 827–853. [Google Scholar] [CrossRef]
- Masoud, E.M.; El-Bellihi, A.-A.; Bayoumy, W.; Mousa, M. Organic–inorganic composite polymer electrolyte based on PEO–LiClO4 and nano-Al2O3 filler for lithium polymer batteries: Dielectric and transport properties. J. Alloy. Compd. 2013, 575, 223–228. [Google Scholar] [CrossRef]
- Choi, J.; Lee, C.-H.; Yu, J.-H.; Doh, C.-H.; Lee, S.-M. Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J. Power Sources 2015, 274, 458–463. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Z.; Chen, S.; Chen, B.; Yang, J.; Zhang, Q.; Ding, F.; Chen, Y.; Xu, X. A promising PEO/LAGP hybrid electrolyte prepared by a simple method for all-solid-state lithium batteries. Solid State Ion. 2016, 295, 65–71. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Z.; Chen, X.; Zhao, Y.; Xu, Q.; Long, P.; Chen, S.; Xu, X. A new composite solid electrolyte PEO/Li10GeP2S12/SN for all-solid-state lithium battery. Electrochim. Acta 2016, 210, 905–914. [Google Scholar] [CrossRef]
- Wang, W.; Yi, E.; Fici, A.J.; Laine, R.M.; Kieffer, J. Lithium ion conducting poly(ethylene oxide)-based solid electrolytes containing active or passive ceramic nanoparticles. J. Phys. Chem. C 2017, 121, 2563–2573. [Google Scholar] [CrossRef]
- Blake, A.J.; Kohlmeyer, R.R.; Hardin, J.O.; Carmona, E.A.; Maruyama, B.; Berrigan, J.D.; Huang, H.; Durstock, M.F. 3D printable ceramic-polymer electrolytes for flexible high-performance Li-ion batteries with enhanced thermal stability. Adv. Energy Mater. 2017, 7, 1602920. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Zhang, X.; Liu, T.; Lin, Y.; Li, L.; Shen, Y.; Nan, C. Lithium-salt-rich PEO/Li0.3La0.557TiO3 interpenetrating composite electrolyte with three-dimensional ceramic nano-backbone for all-solid-state lithium-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 24791–24798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, P.; Fang, Q.; Jing, M.; Shen, X.; Yang, L. A novel solid PEO/LLTO-nanowires polymer composite electrolyte for solid-state lithium-ion battery. Electrochim. Acta 2018, 292, 718–726. [Google Scholar] [CrossRef]
- Cha, J.H.; Didwal, P.N.; Kim, J.M.; Chang, D.R.; Park, C.-J. Poly(ethylene oxide)-based composite solid polymer electrolyte containing Li7La3Zr2O12 and poly(ethylene glycol) dimethyl ether. J. Membr. Sci. 2019, 595, 117538. [Google Scholar] [CrossRef]
- Xia, Y.; Fujieda, T.; Tatsumi, K.; Prosini, P.P.; Sakai, T. Thermal and electrochemical stability of cathode materials in solid polymer electrolyte. J. Power Sources 2001, 92, 234–243. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Dhanalakshmi, R.B.; Kathiresan, M.; Zhou, Y.; Stephan, A.M. The suppression of lithium dendrites by a triazine-based porous organic polymer-laden PEO-based electrolyte and its application for all-solid-state lithium batteries. Mater. Chem. Front. 2020, 4, 933–940. [Google Scholar] [CrossRef]
- Joost, M.; Kunz, M.; Jeong, S.; Schonhoff, M.; Winter, M.; Passerini, S. Ionic mobility in ternary polymer electrolytes for lithium-ion batteries. Electrochim. Acta 2012, 86, 330–338. [Google Scholar] [CrossRef]
- Cheng, S.H.-S.; He, K.-Q.; Liu, Y.; Zha, J.-W.; Kamruzzaman, M.d.; Ma, R.L.-W.; Dang, Z.-M.; Li, R.K.; Chung, C. Electrochemical performance of all-solid-state lithium batteries using inorganic lithium garnets particulate reinforced PEO/LiClO4 electrolyte. Electrochim. Acta 2017, 253, 430–438. [Google Scholar] [CrossRef]
- Piana, G.; Bella, F.; Geobaldo, F.; Meligrana, G.; Gerbaldi, C. PEO/LAGP hybrid solid polymer electrolytes for ambient temperature lithium batteries by solvent-free, “one pot” preparation. J. Energy Storage 2019, 26, 100947. [Google Scholar] [CrossRef]
- Thokchom, J.S.; Gupta, N.; Kumar, B. Superionic Conductivity in a Lithium Aluminum Germanium Phosphate Glass–Ceramic. J. Electrochem. Soc. 2008, 155, A915–A920. [Google Scholar] [CrossRef]
- Kumar, B.; Kumar, J.; Leese, R.; Fellner, J.P.; Rodrigues, S.J.; Abraham, K.M. A solid state rechargeable long cycle life lithium-air battery. J. Electrochem. Soc. 2009, 157, A50. [Google Scholar] [CrossRef]
- Chung, H.; Kang, B. Increase in grain boundary ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 by adding excess lithium. Solid State Ionics 2014, 263, 125–130. [Google Scholar] [CrossRef]
- Robinson, J.P.; Kichambare, P.D.; Deiner, J.L.; Miller, R.; Rottmayer, M.A.; Koenig, G.M., Jr. High temperature electrode-electrolyte interface formation between LiMn1.5Ni0.5O4 and Li1.4Al0.4Ge1.6(PO4)3. J. Am. Ceram. Soc. 2018, 101, 1087–1094. [Google Scholar] [CrossRef]
- Jung, Y.-C.; Lee, S.-M.; Choi, J.; Jang, S.S.; Kim, D.-W. All Solid-State Lithium Batteries Assembled with Hybrid Solid Electrolytes. J. Electrochem. Soc. 2015, 162, A704–A710. [Google Scholar] [CrossRef]
- Wang, C.; Yang, Y.; Liu, X.; Zhong, H.; Xu, H.; Xu, Z.; Shao, H.; Ding, F. Suppression of lithium dendrite formation by using LAGP-PEO (LiTFSI) composite solid electrolyte and lithium metal anode modified by PEO (LiTFSI) in all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2017, 9, 13694–13702. [Google Scholar] [CrossRef]
- Guo, Q.; Han, Y.; Wang, H.; Xiong, S.; Li, Y.; Liu, S.; Xie, K. New class of LAGP-based solid polymer composite electrolyte for efficient and safe solid-state lithium batteries. ACS Appl. Mater. Interfaces 2017, 9, 41837–41844. [Google Scholar] [CrossRef]
- Sung, B.-J.; Didwal, P.N.; Verma, R.; Nguyen, A.-G.; Chang, D.R.; Park, C.-J. Composite solid electrolyte comprising poly(propylene carbonate) and Li1.5Al0.5Ge1.5(PO4)3for long-life all-solid-state Li-ion batteries. Electrochim. Acta 2021, 392, 139007. [Google Scholar] [CrossRef]
- Lee, J.; Howell, T.; Rottmayer, M.; Boeckl, J.; Huang, H. Free-standing LAGP/PEO/LiTFSI composite electrolyte membranes for applications to flexible solid-state lithium-based batteries. J. Electrochem. Soc. 2019, 166, A416–A422. [Google Scholar] [CrossRef]
- Lee, J.; Rottmayer, M.; Huang, H. Impacts of Lithium Salts on the Thermal and Mechanical Characteristics in the Lithiated PEO/LAGP Composite Electrolytes. J. Compos. Sci. 2021, 6, 12. [Google Scholar] [CrossRef]
- Sircar, A.K.; Weissman, P.T.; Kumar, B.; Marsh, R. Evaluation of doped polyethylene oxide as solid electrolyte. Thermochim. Acta 1993, 226, 281–299. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, L.; Guo, Y. Thermal behavior and decomposition kinetics of six electrolyte salts by thermal analysis. J. Power Sources 2006, 156, 555–559. [Google Scholar] [CrossRef]
- Xuan, X.; Zhang, H.; Wang, J.; Wang‡, H. Vibrational Spectroscopic and Density Functional Studies on Ion Solvation and Association of Lithium Tetrafluorobrate in Acetonitrile. J. Phys. Chem. A 2004, 108, 7513–7521. [Google Scholar] [CrossRef]
- Foran, G.; Mankovsky, D.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. The Impact of Absorbed Solvent on the Performance of Solid Polymer Electrolytes for Use in Solid-State Lithium Batteries. iScience 2020, 23, 101597. [Google Scholar] [CrossRef] [PubMed]
- Commarieu, B.; Paolella, A.; Collin-Martin, S.; Gagnon, C.; Vijh, A.; Guerfi, A.; Zaghib, K. Solid-to-liquid transition of polycarbonate solid electrolytes in Li-metal batteries. J. Power Sources 2019, 436, 226852. [Google Scholar] [CrossRef]
- Chen, P.; Liang, X.; Wang, J.; Zhang, D.; Yang, S.; Wu, W.; Zhang, W.; Fan, X.; Zhang, D. PEO/PVDF-based gel polymer electrolyte by incorporating nano-TiO2 for electrochromic glass. J. Sol-Gel Sci. Technol. 2017, 81, 850–858. [Google Scholar] [CrossRef]
- Barroso-Bujans, F.; Fernandez-Alonso, F.; Cerveny, S.; Parker, S.F.; Alegría, A.; Colmenero, J. Polymers under extreme two-dimensional confinement: Poly(ethylene oxide) in graphite oxide. Soft Matter 2011, 7, 7173–7176. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; Silva, M.; Veiga, H.; Esperança, M.; Costa, M.; Smith, M.J. Synthesis and electrochemical characterization of aPEO-based polymer electrolytes. J. Solid State Electrochem. 2012, 16, 1623–1629. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Hu, Y. New insights into the compositional dependence of Li-ion transport in polymer–ceramic composite electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 4113. [Google Scholar] [CrossRef]
- Enotiadis, A.; Fernandes, N.J.; Becerra, N.A.; Zammarano, M.; Giannelis, E.P. Nanocomposite electrolytes for lithium batteries with reduced flammability. Electrochim. Acta 2018, 269, 76–82. [Google Scholar] [CrossRef]
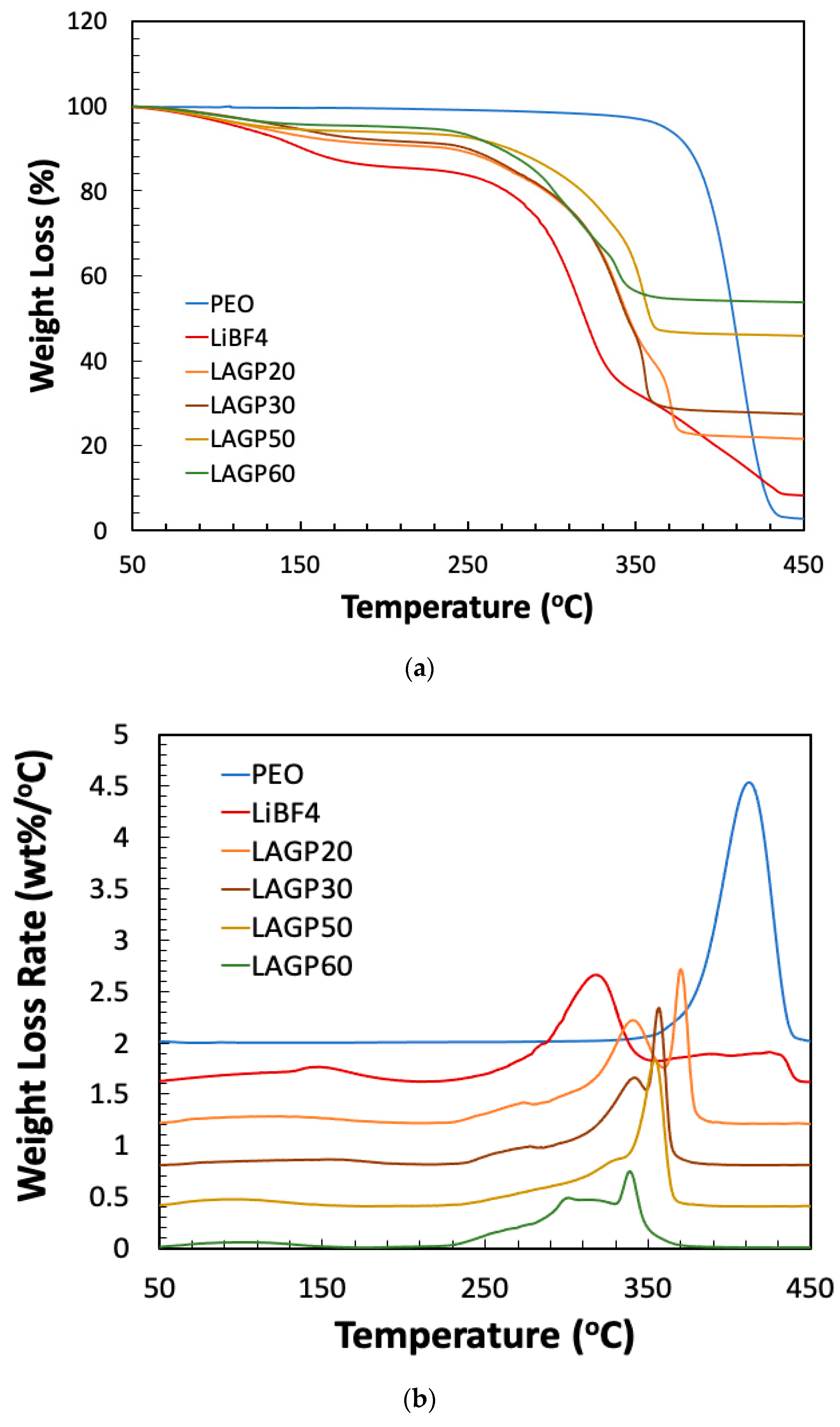


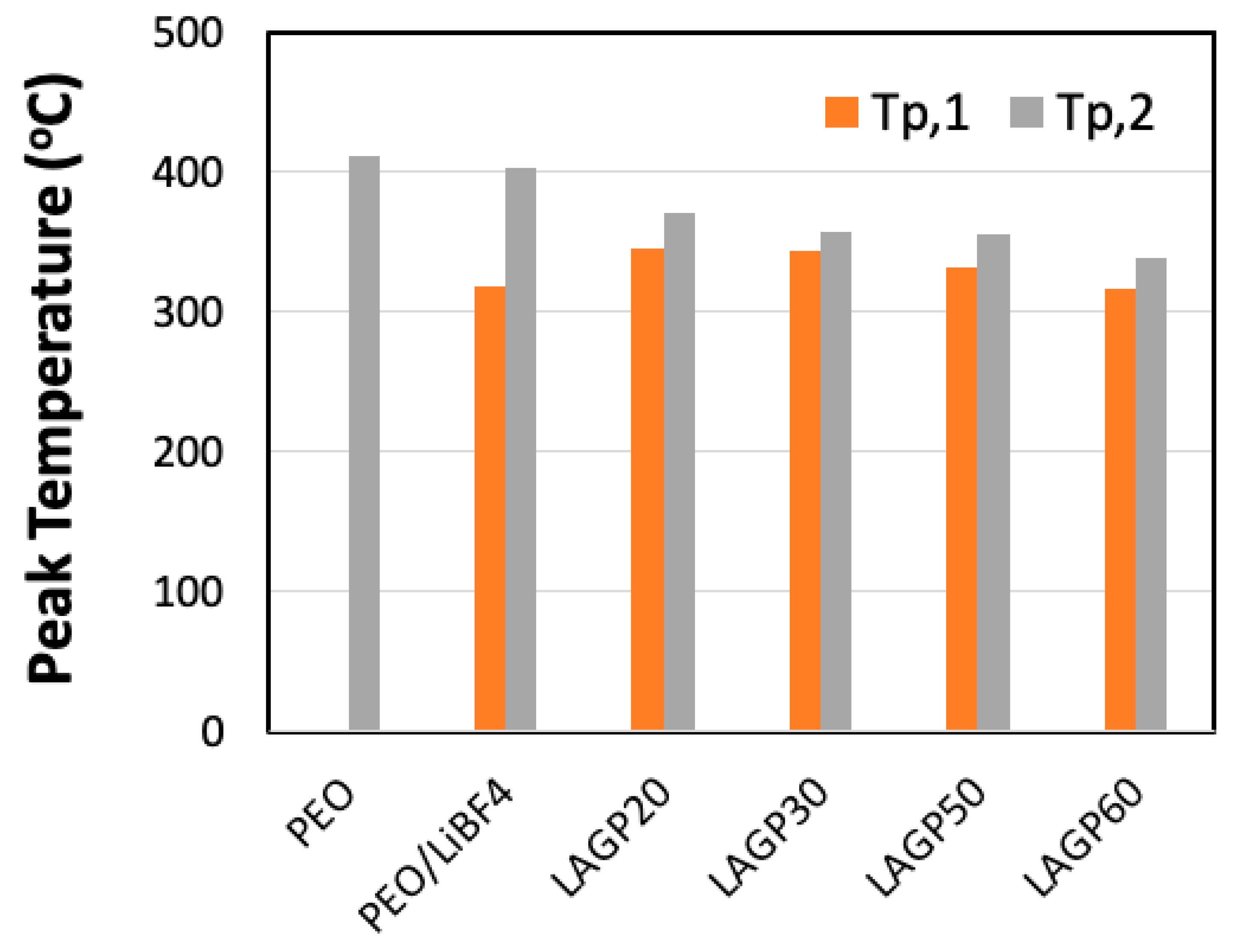

| Sample Name | LAGP (wt%) | PEO (wt%) | LiBF4 (wt%) | ||
|---|---|---|---|---|---|
| PEO | 0 | 100 | 0 | ||
| PEO/LiBF4 | 0 | 78.95 | 21.05 | 3.75 | |
| LAGP20 | 16.48 | 65.93 | 17.58 | 0.20 | 3.75 |
| LAGP30 | 25.28 | 58.99 | 15.73 | 0.30 | 3.75 |
| LAGP40 | 34.48 | 51.72 | 13.79 | 0.40 | 3.75 |
| LAGP50 | 44.12 | 44.12 | 11.76 | 0.50 | 3.75 |
| LAGP60 | 54.22 | 36.14 | 9.63 | 0.60 | 3.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denney, J.; Huang, H. Thermal Decomposition Characteristics of PEO/LiBF4/LAGP Composite Electrolytes. J. Compos. Sci. 2022, 6, 117. https://doi.org/10.3390/jcs6040117
Denney J, Huang H. Thermal Decomposition Characteristics of PEO/LiBF4/LAGP Composite Electrolytes. Journal of Composites Science. 2022; 6(4):117. https://doi.org/10.3390/jcs6040117
Chicago/Turabian StyleDenney, Jacob, and Hong Huang. 2022. "Thermal Decomposition Characteristics of PEO/LiBF4/LAGP Composite Electrolytes" Journal of Composites Science 6, no. 4: 117. https://doi.org/10.3390/jcs6040117
APA StyleDenney, J., & Huang, H. (2022). Thermal Decomposition Characteristics of PEO/LiBF4/LAGP Composite Electrolytes. Journal of Composites Science, 6(4), 117. https://doi.org/10.3390/jcs6040117






