Green Reduction of Graphene Oxide Involving Extracts of Plants from Different Taxonomy Groups
Abstract
:1. Introduction
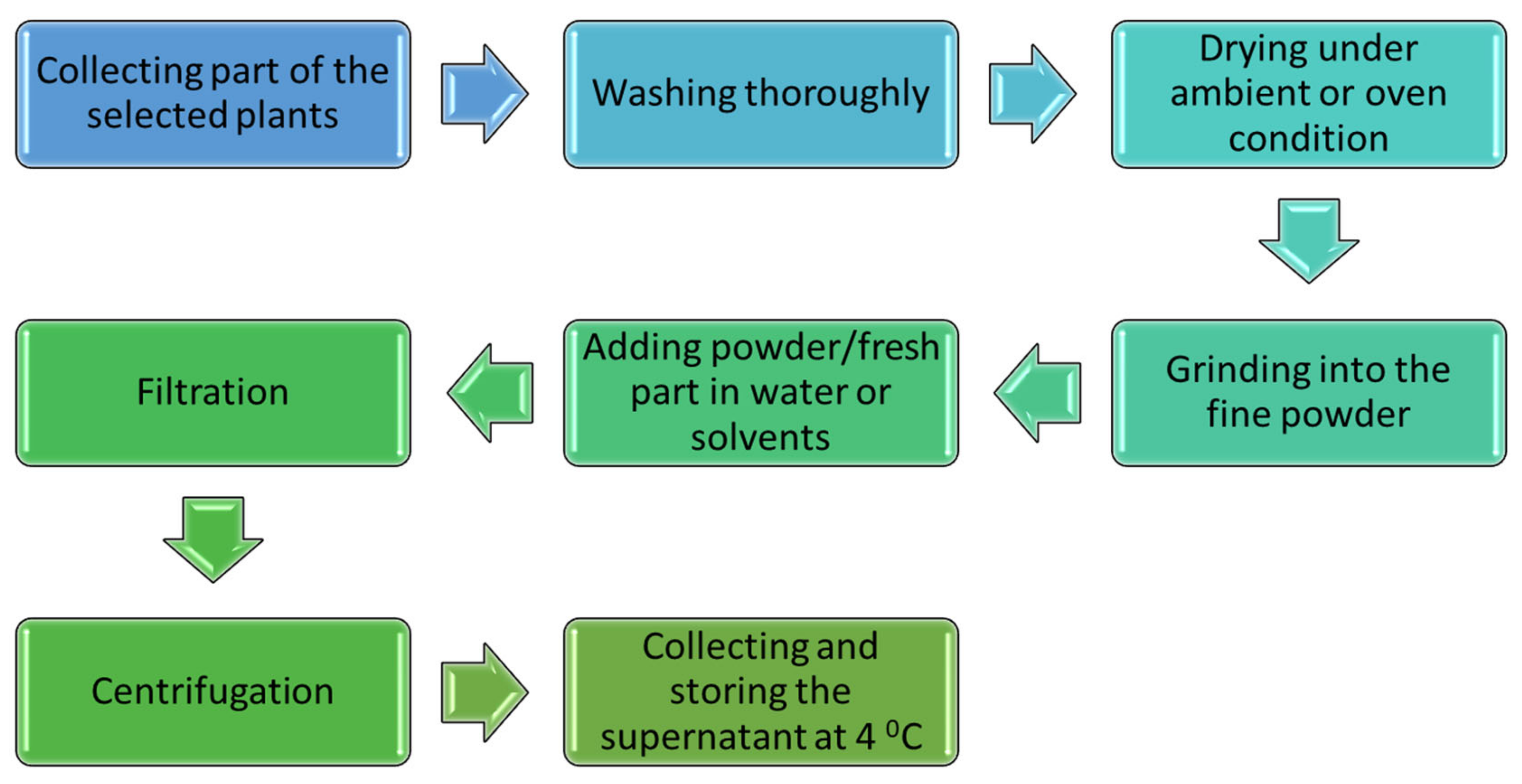
2. Preparation of Aqueous Extracts of Plants
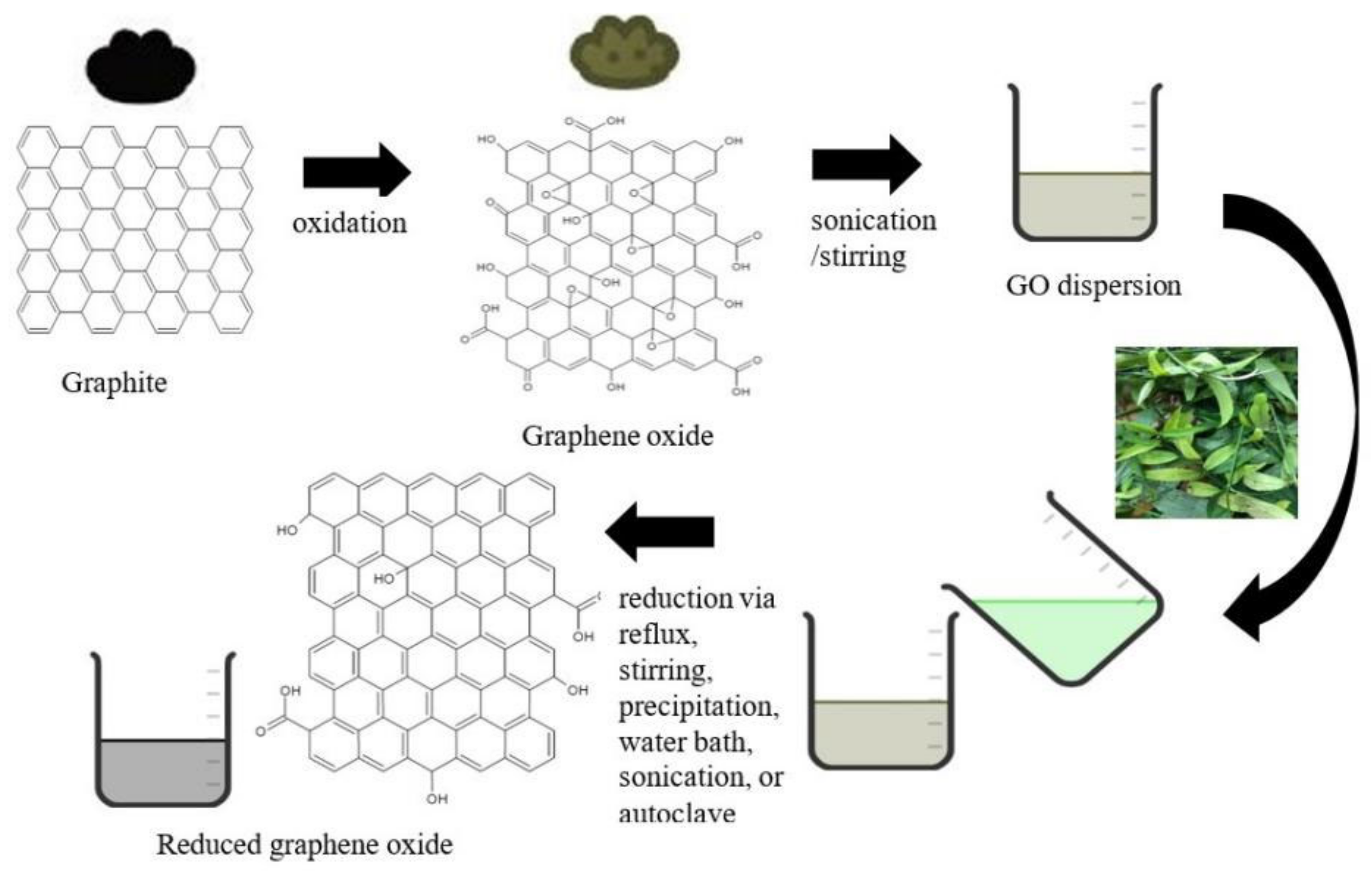
3. The Synthesis of rGO
4. Applications
4.1. Biomedical Applications
4.2. Supercapacitors
4.3. Photocatalytic Degradation
4.4. Antioxidant
4.5. Sensors
4.6. Nanocomposite
5. Characterizations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paton, K.R.; Varrla, E.; Backes, C.; Smith, R.J.; Khan, U.; O’Neill, A.; Boland, C.; Lotya, M.; Istrate, O.M.; King, P.; et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014, 13, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Yu, D.; Zeng, C.; Miao, Z.; Dai, L. Biocompatible graphene oxide-based glucose biosensors. Langmuir 2010, 26, 6158–6160. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.X.; Wang, M.; Chen, T.; Lou, X.W.; Li, C.M. A hierarchically nanostructured composite of MnO2/conjugated polymer/graphene for high-performance lithium ion batteries. Adv. Energy Mater. 2011, 1, 736–741. [Google Scholar] [CrossRef]
- Jo, G.; Choe, M.; Lee, S.; Park, W. The application of graphene as electrodes in electrical and optical devices. Nanotechnology 2012, 23, 112001. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-Based Antibacterial Paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K.; Patrice, D.; Yadav, B.S.; Materny, A. One step in-situ synthesis of CeO2 nanoparticles grown on reduced graphene oxide as an excellent fluorescent and photocatalyst material under sunlight irradiation. Phys. Chem. Chem. Phys. 2016, 18, 11157–11167. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.K.; Gope, S.; Rana, D.; Roy, I.; Sarkar, G.; Sadhukhan, S.; Bhattacharya, A.; Pramanik, K.; Chattopadhyay, S.; Chakraborty, M.; et al. Physical and electrical characterization of reduced graphene oxide synthesized adopting green route. Bull. Mater. Sci. 2016, 39, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Romero, A.; Lavin-Lopez, M.P.; Sanchez-Silva, L.; Valverde, J.L.; Paton-Carrero, A. Comparative study of different scalable routes to synthesize graphene oxide and reduced graphene oxide. Mater. Chem. Phys. 2018, 203, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- Brodie, B.C. XIII. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar]
- Staudenmaier, L. Method for the preparation of the graphite acid. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Agarwal, V.; Zetterlund, P.B. Strategies for reduction of graphene oxide—A comprehensive review. Chem. Eng. J. 2021, 405, 127018. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Jain, Y.; Kumari, M.; Gupta, R.; Sharma, S.K.; Sachdev, K. Synthesis and Characterization of Graphene Oxide (GO) and Reduced Graphene Oxide (rGO) for Gas Sensing Application. Macromol. Symp. 2017, 376, 1700006. [Google Scholar] [CrossRef]
- Longo, A.; Verucchi, R.; Aversa, L.; Tatti, R.; Ambrosio, A.; Orabona, E.; Coscia, U.; Carotenuto, G.; Maddalena, P. Graphene oxide prepared by graphene nanoplatelets and reduced by laser treatment. Nanotechnology 2017, 28, 224002. [Google Scholar] [CrossRef]
- Tran, D.N.H.; Kabiri, S.; Losic, D. A green approach for the reduction of graphene oxide nanosheets using non-aromatic amino acids. Carbon N. Y. 2014, 76, 193–202. [Google Scholar] [CrossRef]
- Azizighannad, S.; Mitra, S. Stepwise reduction of Graphene Oxide (GO) and its effects on chemical and colloidal properties. Sci. Rep. 2018, 8, 10083. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon N. Y. 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile Synthesis and characterization of graphene nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Si, Y.; Samulski, E.T. Synthesis of Water Soluble Graphene 2008. Nano Lett. 2008, 8, 1679–1682. [Google Scholar] [CrossRef] [PubMed]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Mei, X.; Ouyang, J. Ultrasonication-assisted ultrafast reduction of graphene oxide by zinc powder at room temperature. Carbon N. Y. 2011, 49, 5389–5397. [Google Scholar] [CrossRef]
- Agharkar, M.; Kochrekar, S.; Hidouri, S.; Azeez, M.A. Trends in green reduction of graphene oxides, issues and challenges: A review. Mater. Res. Bull. 2014, 59, 323–328. [Google Scholar] [CrossRef]
- Medha, G.; Sharmila, C.; Anil, G. Green Synthesis and Characterization of Nanocrystalline Graphene Oxide. Int. Res. J. Sci. Eng. 2017, 1, 29–34. [Google Scholar]
- Shubha, P.; Namratha, K.; Aparna, H.S.; Ashok, N.R.; Mustak, M.S.; Chatterjee, J.; Byrappa, K. Facile green reduction of graphene oxide using Ocimum sanctum hydroalcoholic extract and evaluation of its cellular toxicity. Mater. Chem. Phys. 2017, 198, 66–72. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Green reduction of graphene oxide by aqueous phytoextracts. Carbon N. Y. 2012, 50, 5331–5339. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Eppakayala, V.; Dayem, A.A.; Kwon, D.; Kim, J. Biocompatibility effects of biologically synthesized graphene in primary mouse embryonic fibroblast cells. Nanoscale Res. Lett. 2013, 8, 393. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Han, J.W.; Park, J.H.; Eppakayala, V.; Kim, J.H. Ginkgo biloba: A natural reducing agent for the synthesis of cytocompatible graphene. Int. J. Nanomed. 2014, 9, 363–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lalitha, M.J.F.P. Phyto-reduction of graphene oxide using the aqueous extract of Eichhornia crassipes (Mart.) Solms. Int. Nano Lett. 2014, 4, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Al-marri, A.H.; Khan, M.; Mohri, N.; Adil, S.F.; Al-Warthan, A.; Siddiqui, M.R.H.; Alkhathlan, H.Z.; Berger, R.; Tremel, W.; et al. Pulicaria glutinosa plant extract: A green and eco- friendly reducing agent for the preparation of highly reduced graphene oxide. RSC Adv. 2014, 4, 24119–24125. [Google Scholar] [CrossRef]
- Lee, G.; Kim, B.S. Biological reduction of graphene oxide using plant leaf extracts. Biotechnol. Prog. 2014, 30, 463–469. [Google Scholar] [CrossRef]
- Kumar, G.G.; Babu, K.J.; Nahm, K.S.; Hwang, Y.J. A facile one-pot green synthesis of reduced graphene oxide and its composites for non- enzymatic hydrogen peroxide sensor applications. RSC Adv. 2014, 4, 7944–7951. [Google Scholar] [CrossRef]
- Atarod, M.; Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis of a Cu/reduced graphene oxide/ Fe3O4 nanocomposite using Euphorbia wallichii leaf extract and its application as a recyclable and heterogeneous catalyst for the reduction of 4- nitrophenol and rhodamine B. RSC Adv. 2015, 5, 91532–91543. [Google Scholar] [CrossRef]
- Jana, M.; Saha, S.; Samanta, P.; Chandra, N.; Hee, J.; Kuila, T. Investigation of the capacitive performance of tobacco solution reduced graphene oxide. Mater. Chem. Phys. 2015, 151, 72–80. [Google Scholar] [CrossRef]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Nagabhushana, H.; Sharma, S.C. Spinach assisted green reduction of graphene oxide and its antioxidant and dye absorption properties. Ceram. Int. 2015, 41, 4810–4813. [Google Scholar] [CrossRef]
- Chamoli, P.; Sharma, R.; Das, K.; Kar, K.K. Mangifera indica, Ficus religiosa and Polyalthia longifolia leaf extract-assisted green synthesis of graphene for transparent highly conductive film. RSC Adv. 2016, 6, 96355–96366. [Google Scholar] [CrossRef] [Green Version]
- Chettri, P.; Vendamani, V.S.; Tripathi, A.; Pathak, A.P.; Tiwari, A. Self assembly of functionalised graphene nanostructures by one step reduction of graphene oxide using aqueous extract of Artemisia vulgaris. Appl. Surf. Sci. 2016, 362, 221–229. [Google Scholar] [CrossRef]
- Sadhukhan, S.; Kumar, T.; Rana, D.; Roy, I. Studies on synthesis of reduced graphene oxide (RGO) via green route and its electrical property. Mater. Res. Bull. 2016, 79, 41–51. [Google Scholar] [CrossRef]
- Xing, F.Y.; Guan, L.L.; Li, Y.L.; Jia, C.J. Biosynthesis of reduced graphene oxide nanosheets and their in vitro cytotoxicity against cardiac cell lines of Catla catla. Environ. Toxicol. Pharmacol. 2016, 48, 110–115. [Google Scholar] [CrossRef]
- Baioun, A.; Kellawi, H.; Falah, A. A modified electrode by a facile green preparation of reduced graphene oxide utilizing olive leaves extract. Carbon Lett. 2017, 24, 47–54. [Google Scholar] [CrossRef]
- Elif, Ö.; Belma, Ö.; Ilkay, Ş. Production of biologically safe and mechanically improved reduced graphene oxide/hydroxyapatite composites. Mater. Res. Express 2017, 4, 015601. [Google Scholar] [CrossRef]
- Chandu, B.; Sai, V.; Mosali, S.; Mullamuri, B.; Bollikolla, H.B. A facile green reduction of graphene oxide using Annona squamosa leaf extract. Carbon Lett. 2017, 21, 74–80. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhuang, Z.; Jin, X.; Chen, Z. A facile and green preparation of reduced graphene oxide using Eucalyptus leaf extract. Appl. Surf. Sci. 2017, 422, 469–474. [Google Scholar] [CrossRef]
- Moosa, A.A.; Jaafar, J.N. Green reduction of graphene oxide using tea leaves extract with applications to lead ions removal from water. Nanosci. Nanotechnol. 2017, 7, 38–47. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, X.; Liu, F.; Jin, J.; Liu, L.; Zhi, Y.; Chen, Z.; Zhou, Z.; Yu, J. Green synthesis of graphene nanosheets and their in vitro cytotoxicity against human prostate cancer (DU 145) cell lines. Nanomater. Nanotechnol. 2017, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, G.; Sas, S.; Wadhwa, S.; Mathur, A.; McLaughlin, J.; Roy, S.S. Aloe vera assisted facile green synthesis of reduced graphene oxide for electrochemical and dye removal applications. RSC Adv. 2017, 7, 26680–26688. [Google Scholar] [CrossRef] [Green Version]
- Ramanathan, S.; Elanthamilan, E.; Obadiah, A.; Durairaj, A.; Merlin, J.P.; Ramasundaram, S.; Vasanthkumar, S. Aloe vera (L.) Burm f. extract reduced graphene oxide for supercapacitor application. J. Mater. Sci. Mater. Electron. 2017, 28, 16648–16657. [Google Scholar] [CrossRef]
- Mahata, S.; Sahu, A.; Shukla, P.; Rai, A.; Singh, M.; Rai, V.K. Bio-inspired unprecedented synthesis of reduced graphene oxide: Catalytic probe for electro-/chemical reduction of nitro group in aqueous medium. New J. Chem. 2018, 42, 2067–2073. [Google Scholar] [CrossRef]
- Jin, X.; Li, N.; Weng, X.; Li, C.; Chen, Z. Green reduction of graphene oxide using Eucalyptus leaf extract and its application to remove dye. Chemosphere 2018, 208, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Mahata, S.; Sahu, A.; Shukla, P.; Rai, A.; Singh, M.; Rai, V.K. The novel and efficient reduction of graphene oxide using Ocimum sanctum L. leaf extract as an alternative renewable bio-resource. New J. Chem. 2018, 42, 19945–19952. [Google Scholar] [CrossRef]
- Elemike, E.E.; Onwudiwe, D.C.; Wei, L.; Lou, C.; Zhao, Z. Synthesis of nanostructured ZnO, AgZnO and the composites with reduced graphene oxide (rGO-AgZnO) using leaf extract of Stigmaphyllon ovatum. J. Environ. Chem. Eng. 2019, 7, 103190. [Google Scholar] [CrossRef]
- Fahiminia, M.; Shamabadi, N.S.; Nasrollahzadeh, M.; Mohammad Sajadi, S. Phytosynthesis of Cu/rGO using Euphorbia cheiradenia Boiss extract and study of its ability in the reduction of organic dyes and 4-nitrophenol in aqueous medium. IET Nanobiotechnol. 2019, 13, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.K.; Sadhukhan, S.; Rana, D.; Bhattacharyya, A.; Chattopadhyay, D.; Chakraborty, M. Green approaches to synthesize reduced graphene oxide and assessment of its electrical properties. Nano Struct. Nano Objects 2019, 19, 100362. [Google Scholar] [CrossRef]
- Khojasteh, H.; Safajou, H.; Mortazavi-Derazkola, S.; Salavati-Niasari, M.; Heydaryan, K.; Yazdani, M. Economic procedure for facile and eco-friendly reduction of graphene oxide by plant extracts; a comparison and property investigation. J. Clean. Prod. 2019, 229, 1139–1147. [Google Scholar] [CrossRef]
- Lin, Z.; Weng, X.; Ma, L.; Sarkar, B.; Chen, Z. Mechanistic insights into Pb(II) removal from aqueous solution by green reduced graphene oxide. J. Colloid Interface Sci. 2019, 550, 1–9. [Google Scholar] [CrossRef]
- Mahmudzadeh, M.; Yari, H.; Ramezanzadeh, B.; Mahdavian, M. Highly potent radical scavenging-anti-oxidant activity of biologically reduced graphene oxide using Nettle extract as a green bio-genic amines- based reductants source instead of hazardous hydrazine hydrate. J. Hazard. Mater. 2019, 371, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Ruan, J.; Wang, S. Biosynthesized of reduced graphene oxide nanosheets and its loading with paclitaxel for their anti cancer effect for treatment of lung cancer. J. Photochem. Photobiol. B Biol. 2019, 191, 13–17. [Google Scholar] [CrossRef]
- Veisi, H.; Tamoradi, T.; Karmakar, B.; Mohammadi, P.; Hemmati, S. In situ biogenic synthesis of Pd nanoparticles over reduced graphene oxide by using a plant extract (Thymbra spicata) and its catalytic evaluation towards cyanation of aryl halides. Mater. Sci. Eng. C 2019, 104, 109919. [Google Scholar] [CrossRef] [PubMed]
- Lingaraju, K.; Raja Naika, H.; Nagaraju, G.; Nagabhushana, H. Biocompatible synthesis of reduced graphene oxide from Euphorbia heterophylla (L.) and their in-vitro cytotoxicity against human cancer cell lines. Biotechnol. Rep. 2019, 24, e00376. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Chen, Y.; Fang, Z. In-vitro photothermal therapy using plant extract polyphenols functionalized graphene sheets for treatment of lung cancer. J. Photochem. Photobiol. B Biol. 2020, 204, 111587. [Google Scholar] [CrossRef]
- Faiz, M.S.A.; Azurahanim, C.A.C.; Raba, S.A.; Ruzniza, M.Z. Low Cost and Green Approach in The Reduction of Graphene Oxide (GO) Using Palm Oil Leaves Extract for Potential in Industrial Applications. Results Phys. 2020, 16, 102954. [Google Scholar] [CrossRef]
- Sabayan, B.; Goudarzian, N.; Moslemin, M.H.; Mohebat, R. Green synthesis and high efficacy method for reduced graphene oxide by Zataria multiflora extract. J. Environ. Treat. Tech. 2020, 8, 488–496. [Google Scholar]
- Olorunkosebi, A.A.; Eleruja, M.A.; Adedeji, A.V.; Olofinjana, B.; Fasakin, O.; Omotoso, E.; Oyedotun, K.O.; Ajayi, E.O.B.; Manyala, N. Optimization of graphene oxide through various Hummers’ methods and comparative reduction using green approach. Diam. Relat. Mater. 2021, 117, 108456. [Google Scholar] [CrossRef]
- Parthipan, P.; Al-Dosary, M.A.; Al-Ghamdi, A.A.; Subramania, A. Eco-friendly synthesis of reduced graphene oxide as sustainable photocatalyst for removal of hazardous organic dyes. J. King Saud Univ. Sci. 2021, 33, 101438. [Google Scholar] [CrossRef]
- Andrianiaina, H.; Razanamahandry, L.C.; Sackey, J.; Ndimba, R.; Khamlich, S.; Maaza, M. Synthesis of graphene sheets from graphite flake mediated with extracts of various indigenous plants from Madagascar. Mater. Today Proc. 2021, 36, 553–558. [Google Scholar] [CrossRef]
- Punniyakotti, P.; Aruliah, R.; Angaiah, S. Facile synthesis of reduced graphene oxide using Acalypha indica and Raphanus sativus extracts and their in vitro cytotoxicity activity against human breast (MCF-7) and lung (A549) cancer cell lines. 3 Biotech 2021, 11, 157. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Zhang, S.; Xie, C.; Liu, Q.; Yang, S. Fabrication of Erythrina senegalensis leaf extract mediated reduced graphene oxide for cardiac repair applications in the nursing care. Inorg. Nano Metal Chem. 2021, 51, 143–149. [Google Scholar] [CrossRef]
- Jha, P.K.; Khongnakorn, W.; Chawenjkigwanich, C.; Chowdhury, M.S.; Techato, K. Eco-friendly reduced graphene oxide nanofilter preparation and application for iron removal. Separations 2021, 8, 68. [Google Scholar] [CrossRef]
- Kartick, B.; Srivastava, S.K.; Srivastava, I. Green Synthesis of Graphene. J. Nanosci. Nanotechnol. 2013, 13, 4320–4324. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, F.; Salavati-Niasari, M.; Badiei, A.; Mohandes, F. Green synthesis and characterization of graphene nanosheets. Mater. Res. Bull. 2015, 63, 51–57. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Saha, S.; Barman, A.; Roy, S.S. Grape extract assisted green synthesis of reduced graphene oxide for water treatment application. Mater. Lett. 2015, 160, 355–358. [Google Scholar] [CrossRef]
- Maddinedi, S.B.; Mandal, B.K. Biofabrication of Reduced Graphene Oxide Nanosheets Using Terminalia bellirica Fruit Extract. Curr. Nanosci. 2016, 12, 94–102. [Google Scholar] [CrossRef]
- Hou, D.; Liu, Q.; Cheng, H.; Li, K. Graphene Synthesis via Chemical Reduction of Graphene Oxide Using Lemon Extract. J. Nanosci. Nanotechnol. 2017, 17, 6518–6523. [Google Scholar] [CrossRef]
- Hou, D.; Liu, Q.; Cheng, H.; Zhang, H.; Wang, S. Green reduction of graphene oxide via Lycium barbarum extract. J. Solid State Chem. 2017, 246, 351–356. [Google Scholar] [CrossRef]
- Ansari, M.Z.; Siddiqui, W.A. Deoxygenation of graphene oxide using biocompatible reducing agent Ficus carica (dried ripe fig). J. Nanostructure Chem. 2018, 8, 431–440. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.Z.; Lone, M.N.; Sajid, S.; Siddiqui, W.A. Novel Green Synthesis of Graphene Layers using Zante Currants and Graphene Oxide. Orient. J. Chem. 2018, 34, 2832–2837. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.Z.; Rahul, J.; Siddiqui, W.A. Novel and green synthesis of chemically reduced graphene sheets using Phyllanthus emblica (Indian Gooseberry) and its photovoltaic activity. Mater. Res. Express 2019, 6, 055027. [Google Scholar] [CrossRef]
- Raja, A.; Rajasekaran, P.; Selvakumar, K.; Arivanandhan, M.; Asath Bahadur, S.; Swaminathan, M. Green approach to the preparation of reduced graphene oxide for photocatalytic and supercapacitor application. Optik 2019, 190, 21–27. [Google Scholar] [CrossRef]
- Parthipan, P.; Cheng, L.; Rajasekar, A.; Govarthanan, M.; Subramania, A. Biologically reduced graphene oxide as a green and easily available photocatalyst for degradation of organic dyes. Environ. Res. 2021, 196, 110983. [Google Scholar] [CrossRef] [PubMed]
- Panicker, N.J.; Sahu, P.P. Green reduction of graphene oxide using phytochemicals extracted from Pomelo grandis and Tamarindus indica and its supercapacitor applications. J. Mater. Sci. Mater. Electron. 2021, 32, 15265–15278. [Google Scholar] [CrossRef]
- Smina, C.S.; Lalitha, P.; Sharma, S.C.; Nagabhushana, H. Screening of anti-cancer activity of reduced graphene oxide biogenically synthesized against human breast cancer MCF-7 cell lines. Appl. Nanosci. 2021, 11, 1093–1105. [Google Scholar] [CrossRef]
- Haghighi, B.; Tabrizi, M.A. Green-synthesis of reduced graphene oxide nanosheets using rose water and a survey on their characteristics and applications. RSC Adv. 2013, 3, 13365–13371. [Google Scholar] [CrossRef]
- Chu, H.; Lee, C.; Tai, N. Green reduction of graphene oxide by Hibiscus sabdariffa L. to fabricate flexible graphene electrode. Carbon N. Y. 2014, 80, 725–733. [Google Scholar] [CrossRef]
- Suresh, D.; Udayabhanu; Nagabhushana, H.; Sharma, S.C. Clove extract mediated facile green reduction of graphene oxide, its dye elimination and antioxidant properties. Mater. Lett. 2015, 142, 4–6. [Google Scholar] [CrossRef]
- Hou, D.; Liu, Q.; Cheng, H.; Li, K.; Wang, D.; Zhang, H. Chrysanthemum extract assisted green reduction of graphene oxide. Mater. Chem. Phys. 2016, 183, 76–82. [Google Scholar] [CrossRef]
- Shahane, S.; Sidhaye, D. Facile biosynthesis of reduced graphene oxide nanostructures via reduction by tagetes erecta (marigold flower) plant extract. Int. J. Mod. Phys. B 2018, 32, 1840068. [Google Scholar] [CrossRef]
- Li, B.; Jin, X.; Lin, J.; Chen, Z. Green reduction of graphene oxide by sugarcane bagasse extract and its application for the removal of cadmium in aqueous solution. J. Clean. Prod. 2018, 189, 128–134. [Google Scholar] [CrossRef]
- Wijaya, R.; Andersan, G.; Santoso, S.P.; Irawaty, W. Green Reduction of Graphene Oxide using Kaffir Lime Peel Extract (Citrus hystrix) and Its Application as Adsorbent for Methylene Blue. Sci. Rep. 2020, 10, 667. [Google Scholar] [CrossRef]
- Suresh, D.; Udayabhanu; Kumar, P.M.; Nagabhushana, H.; Sharma, S.C. Cinnamon supported facile green reduction of graphene oxide, its dye elimination and antioxidant activities. Mater. Lett. 2015, 151, 93–95. [Google Scholar] [CrossRef]
- Han, W.; Niu, W.; Sun, B.; Shi, G.; Cui, X. Biofabrication of polyphenols stabilized reduced graphene oxide and its anti-tuberculosis activity. J. Photochem. Photobiol. B Biol. 2016, 165, 305–309. [Google Scholar] [CrossRef]
- Singh, A.; Ahmed, B.; Singh, A.; Ojha, A.K. Photodegradation of phenanthrene catalyzed by rGO sheets and disk like structures synthesized using sugar cane juice as a reducing agent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 204, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, P.; Baskey, M. Plant extract assisted synthesis of reduced graphene oxide sheet and the photocatalytic performances on cationic and anionic dyes to decontaminate wastewater. Adv. Nat. Sci. Nanosci. Nanotechnol. 2021, 12, 015008. [Google Scholar] [CrossRef]
- Jana, M.; Saha, S.; Khanra, P.; Chandra, N.; Kumar, S.; Kuila, T.; Hee, J. Bio-reduction of graphene oxide using drained water from soaked mung beans (Phaseolus aureus L.) and its application as energy storage electrode material. Mater. Sci. Eng. B 2014, 186, 33–40. [Google Scholar] [CrossRef]
- Maddinedi, S.B.; Mandal, B.K.; Vankayala, R.; Kalluru, P.; Pamanji, S.R. Bioinspired reduced graphene oxide nanosheets using Terminalia chebula seeds extract. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 117–124. [Google Scholar] [CrossRef]
- Chu, H.; Lee, C.; Tai, N. Green preparation using black soybeans extract for graphene-based porous electrodes and their applications in supercapacitors. J. Power Sources 2016, 322, 31–39. [Google Scholar] [CrossRef]
- Yaragalla, S.; Rajendran, R.; Jose, J.; Almaadeed, M.A.; Kalarikkal, N.; Thomas, S. Preparation and characterization of green graphene using grape seed extract for bioapplications. Mater. Sci. Eng. C 2016, 65, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Tayade, U.S.; Borse, A.U.; Meshram, J.S. Green reduction of graphene oxide and its applications in band gap calculation and antioxidant activity. Green Mater. 2019, 7, 143–155. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Khanra, P.; Mishra, A.K.; Kim, N.H.; Lee, J.H. A green approach for the reduction of graphene oxide by wild carrot root. Carbon N. Y. 2012, 50, 914–921. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Abouei, E.; Hatamie, S.; Ghasemi, E. Accelerated differentiation of neural stem cells into neurons on ginseng-reduced graphene oxide sheets. Carbon N. Y. 2013, 66, 395–406. [Google Scholar] [CrossRef]
- Vusa, C.S.R.; Berchmans, S.; Alwarappan, S. Facile and green synthesis of graphene. RSC Adv. 2014, 4, 22470–22475. [Google Scholar] [CrossRef]
- Khan, M.; Al-marri, A.H.; Khan, M.; Shaik, M.R.; Mohri, N.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-warthan, A.; Tremel, W.; et al. Green Approach for the Effective Reduction of Graphene Oxide Using Salvadora persica L. Root (Miswak) Extract. Nanoscale Res. Lett. 2015, 10, 281. [Google Scholar] [CrossRef] [Green Version]
- Thongpool, V.; Phunpheok, A.; Piriyawong, V.; Limsuwan, S. Green Approach for the Reduction of Graphene Oxide by Thai Shallot. Key Eng. Mater. 2016, 675–676, 696–699. [Google Scholar] [CrossRef]
- Khanam, P.N.; Hasan, A. Biosynthesis and characterization of graphene by using non-toxic reducing agent from Allium cepa extract: Anti-bacterial properties. Int. J. Biol. Macromol. 2019, 126, 151–158. [Google Scholar] [CrossRef]
- Rai, S.; Bhujel, R.; Biswas, J.; Swain, B.P. Biocompatible synthesis of rGO from ginger extract as a green reducing agent and its supercapacitor application. Bull. Mater. Sci. 2021, 44, 40. [Google Scholar] [CrossRef]
- Rahman, O.S.A.; Chellasamy, V.; Ponpandian, N.; Amirthapandian, S.; Panigrahi, B.K.; Thangadurai, P. A facile green synthesis of reduced graphene oxide by using pollen grains of Peltophorum pterocarpum and study of its electrochemical behavior. RSC Adv. 2014, 4, 56910–56917. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.Y.; Zhang, D. Aqueous colloids of graphene oxide nanosheets by exfoliation of graphite oxide without ultrasonication. Bull. Mater. Sci. 2011, 34, 25–28. [Google Scholar] [CrossRef]
- Aunkor, M.T.; Mahbubul, I.; Saidur, R.; Metselaar, H.S. The green reduction of graphene oxide. RSC 2016, 6, 27807–27828. [Google Scholar] [CrossRef]
- Bosch-navarro, C.; Coronado, E.; Mart-Gastaldo, C.; Sanchez-Royo, J.F.; Gomez, M.G. Influence of the pH on the synthesis of reduced graphene oxide under hydrothermal conditions. Nanoscale 2012, 4, 3977–3982. [Google Scholar] [CrossRef]
- Gotoh, Y.; Hiraiwa, K.; Nagayama, M. In vitro mineralization of osteoblastic cells derived from human bone. Bone Miner. 1990, 8, 239–250. [Google Scholar] [CrossRef]

| Methods | Oxidants | Reaction Time (h) | Temperature (°C) | Advantages | Drawbacks | Ref. |
|---|---|---|---|---|---|---|
| Brodie method | KClO3 HNO3 | 72–96 | 60 |
|
| [12] |
| Staudenmaier method | HNO3 H2SO4 KClO3 | 96 | 90 |
|
| [13] |
| Hummer’s method | NaNO3 H2SO4 KMnO4 | ~2 | 35,98 |
|
| [14] |
| Improved Hummer’s method | KMnO4 H3PO4 H2SO4 | 12 | 50 |
|
| [15] |
| No | Scientific Name | Reduction Method | Reduction Temperature (°C) | Reduction Time | Ref. |
|---|---|---|---|---|---|
| Leaf Extract | |||||
| 1 | Colocasia esculenta |
| RT 100 | 8 h 5 h | [31] |
| 2 | Mesua ferrea L. |
| RT 100 | 10 h 8 h | |
| 3 | Spinacia oleracea |
| 30 | 24 h | [32] |
| 4 | Ginkgo biloba |
| 37 | 24 h | [33] |
| 5 | Eichhornia crassipes |
| 100 | 10 h | [34] |
| 6 | Pulicaria glutinosa |
| 98 | 24 h | [35] |
| 7 | Prunus serrulate Magnolia Kobus Platanus orientalis Diopyros kaki Pinus desiflora Acer palmatum Ginkgo biloba |
| 95 | 12 h | [36] |
| 8 | Azadirachta indica |
| RT | 48 h | [37] |
| 100 | 24 h | |||
| 9 | Euphorbia wallichii |
| 100 | 6 h | [38] |
| 10 | Nicotiana tabacum L. |
| RT 100 | 24 h 24 h | [39] |
| 11 | Spinacia oleracea |
| 100 | 30 min | [40] |
| 12 | Ficus religiosa Mangifera indica Polyalthia longifolia |
| 50 | 24 h | [41] |
| 13 | Artemisia vulgaris |
| 90 | 6 h, 12 h | [42] |
| 14 | Paederia foetide L. |
| 50 | 12 h | [9] |
| 15 | Mangifera indica L. |
| 60 60–70 | 12 h 8 h | [43] |
| 16 | Platanus orientalis |
| 100 | 10 h | [44] |
| 17 | Olea europaea |
| 100 | 10 h | [45] |
| 18 | Melissa officinalis L. |
| RT | 12 h | [46] |
| 19 | Annona squamosa |
| 100 | 12 h | [47] |
| 20 | Eucalyptus |
| 80 | 8 h | [48] |
| 21 | Lantana camara |
| 50 | 6 h | [28] |
| 22 | Camellia sinensis |
| 90 | 1 h | [49] |
| 23 | Citrullus colocynthis |
| 100 | 14 h | [50] |
| 24 | Aloe vera |
| 95 | 24 h | [51] |
| 25 | Aloe vera (L.) Burm.f. |
| 80 | 5 h | [52] |
| 26 | Ocimum sanctum |
| 100 | 10 h | [29] |
| 27 | Anacardium occidentale Linn |
| 68 | 3 h | [53] |
| 28 | Eucalyptus |
| 80 | 8 h | [54] |
| 29 | Ocimum sanctum L. |
| 70 | 4 h | [55] |
| 30 | Stigmaphyllon ovatum |
| 60–70 | 24 h | [56] |
| 31 | Euphorbia cheiradenia Boiss |
| 80 | 7 h | [57] |
| 32 | Mentha arvensis |
| 80–95 | 3 h | [58] |
| 33 | Tribulus terrestris Mentha piperita |
| 180 | 12 h | [59] |
| 34 | Camellia sinensis |
| 80 | 8 h | [60] |
| 35 | Urtica dioica L. |
| 90 | 1 h | [61] |
| 36 | Euphorbia milli |
| RT | 48 h | [62] |
| 37 | Thymbra spicata |
| 100 | 12 h | [63] |
| 38 | Euphorbia heterophylla (L.) |
| 95 | 12 h | [64] |
| 39 | Memecylon edule |
| 60 | 12 h | [65] |
| 40 | Elaeis guineensis |
| 100 | 3 h | [66] |
| 41 | Zataria multiflora |
| 98 | 24 h | [67] |
| 42 | Memecylon edule |
| 60 | 12 h | [65] |
| 43 | Azadirachta indica |
| 30 | 24 h | [68] |
| 44 | Telfairia occidentalis | ||||
| 45 | Murraya koenigii |
| 100 | 12 h | [69] |
| 46 | Cinnamomum camphora cineoliferum |
| RT | 24 h | [70] |
| 47 | Phyllarthrom madagascariese K. Schum | ||||
| 48 | Acalypha indica |
| 100 | 12 h | [71] |
| 49 | Erythrina senegalensis |
| 95 | 24 h | [72] |
| 50 | Callistemon viminalis |
| 60 | 2 h | [73] |
| Fruit Extract | |||||
| 1 | Cocos nucifera L. |
| 80, 100 | 12 h, 24 h, 36 h | [74] |
| 2 | Punica granatum |
| RT | 12 h, 18 h, 24 h | [75] |
| 3 | Vitis vinifera |
| 95 | 1 h, 3 h, 6 h | [76] |
| 4 | Terminalia bellirica |
| 90 | 24 h | [77] |
| 5 | Citrus limon |
| 95 | 24 h | [78] |
| 6 | Lycium barbarum |
| 95 | 24 h | [79] |
| 7 | Ficus carica |
| 95 | 12 h | [80] |
| 8 | Zante currants |
| 95 | 48 h | [81] |
| 9 | Phyllanthus emblica |
| 95 | 3 h | [82] |
| 10 | Fragaria ananassa |
| 95 | 12 h | [83] |
| 11 | Phyllanthus emblica |
| 100 | 12 h | [84] |
| 12 | Citrus grandis |
| 95 | 12 h | [85] |
| 13 | Tamarindus indica | ||||
| 14 | Terminalia bellirica |
| 40 | 2 h | [86] |
| 15 | Helicteres isora | ||||
| 16 | Quercus infectoria | ||||
| Flower Extract | |||||
| 1 | Rosa damascena |
| 95 | 5 h | [87] |
| 2 | Hibiscus sabdariffa L. |
| 100 | 1 h | [88] |
| 3 | Syzygium aromaticum |
| 100 | 30 min | [89] |
| 4 | Chrysanthemum morifolium |
| 95 | 24 h | [90] |
| 5 | Tagetes erecta |
| 95 | 3 h | [91] |
| Peel Extract | |||||
| 1 | Citrus sinensis |
| RT | 10 h | [31] |
| 100 | 8 h | |||
| 2 | Citrus limeta |
| 50 | 6 h | [28] |
| 3 | Sugarcane bagasse |
| 95 | 12 h | [92] |
| 4 | Citrus hystrix |
| RT | 8 h | [93] |
| Bark/Stem Extract | |||||
| 1 | Cinnamomum zeylanicum |
| 100 | 45 min | [94] |
| 2 | Cinnamomum verum |
| 100 | 12 h | [95] |
| 3 | Saccharum officinarum |
| 50 150 | 3 h 12 h | [96] |
| 4 | Cedrelopsis grevei Baill |
| RT | 24 h | [70] |
| 5 | Alstonia scholaris |
| 90 | 1 h, 3 h | [97] |
| Seed Extract | |||||
| 1 | Phaseolus aureus L. |
| 30 | 24 h | [98] |
| 2 | Terminalia chebula |
| 90 | 24 h | [99] |
| 3 | Glycine max (L.) Merr. |
| 75,85,95 | 1 h | [100] |
| 4 | Vitis vinifera |
| RT | 10 h | [101] |
| 5 | Punica grantum |
| 98 | 8 h | [102] |
| Root Extract | |||||
| 1 | Daucus carota |
| RT | 48 h | [103] |
| 100 | 24 h | |||
| 2 | Asian red ginseng |
| 80 | 10 min | [104] |
| 3 | Daucus carota subsp. sativus |
| 90 | 1 h | [105] |
| 4 | Salvadora persica L. (miswak) |
| 98 | 24 h | [106] |
| 5 | Solanum tuberosum L. (potato) |
| 60 70–80 | 12 h 8 h | [43] |
| 6 | Allium ascalonicum (shallot) |
| RT | 72 h | [107] |
| 7 | Allium cepa (onion) |
| RT | 6 h | [108] |
| 8 | Catharanthus roseus |
| RT | 24 h | [70] |
| 9 | Raphanus sativus |
| 100 | 12 h | [71] |
| 10 | Zingiber officinale Roscoe |
| 90 | 4 h, 6 h, 8 h, 10 h,12 h | [109] |
| 11 | Acorus calamus |
| 40 | 2 h | [86] |
| Pollen Grain Extract | |||||
| 1 | Peltophorum pterocarpum |
| RT 450 | 24 h 90 min | [110] |
| 120 550 | 30 h 2 h | |||
| Scientific Name | Activity | Cell Lines | Strain | MIC | Concentration | Cell Viability (%) | IC50 | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| Spinacia oleracea | Cytotoxicity |
| NA | NA |
|
| NA | [32] | |
| Citrullus colocynthis | Cytotoxicity |
| NA | NA |
|
| NA | [50] | |
| Ocimum sanctum | Cytotoxicity |
| NA | NA |
|
| NA | [29] | |
| Euphorbia milli | Anticancer effect of rGO loaded paclitaxel |
| NA | NA |
|
| NA | [62] | |
| Euphorbia heterophylla (L.) | Cytotoxicity |
| NA | NA |
|
|
| [64] | |
| NA | NA |
|
|
| ||||
| Memecylon edule | Cytotoxicity |
| NA | NA |
|
| NA | [65] | |
|
|
| |||||||
|
|
| |||||||
|
|
| |||||||
| Acalypha indica | Cytotoxicity |
| NA | NA |
|
|
| [71] | |
|
|
|
| ||||||
| Raphanus sativus | Cytotoxicity |
|
|
|
| ||||
|
|
|
| ||||||
| Acorus calamus | Cytotoxicity |
| NA | NA |
|
|
| [86] | |
| Terminalia bellirica |
|
|
| ||||||
| Helicteres isora |
|
|
| ||||||
| Quercus infectoria |
|
|
| ||||||
| Cinnamomum verum | Anti-tuberculosis | NA |
| 200 µg/mL | NA | NA | NA | [95] | |
| Vitis vinifera (grape) | Anti-microbial | NA |
| 4 & 5 µg/mL | NA | NA | NA | [101] | |
| Anti-proliferative |
| NA | NA |
|
| ||||
|
|
| |||||||
| Allium cepa | Anti-bacterial | NA |
| NA |
|
|
| NA | [108] |
|
|
| |||||||
|
|
| |||||||
|
|
| |||||||
| Scientific Name | Resistance | Specific Capacitance | Charge-Discharge Cyclic Stability | Ref. |
|---|---|---|---|---|
| Hibiscus sabdariffa L. |
|
| NA | [88] |
|
| |||
| Phaseolus aureus L. |
|
| 98% after 1000 cycles | [98] |
| Nicotiana tabacum |
|
| ~112% after 1000 cycles | [39] |
| Peltophorum pterocarpum | NA |
| NA | [110] |
| Black soybean |
|
| 90% after 1000 cycles | [100] |
|
| |||
| Aloe vera (L.) Burm. f. mediated rGO | NA |
| NA | [52] |
| Fragaria ananassa | NA |
| 81% at 500 cycles | [83] |
| Citrus grandis |
|
| NA | [85] |
| Tamarindus indica |
|
| ||
| Ginger |
|
| 98% after 1000 cycles | [109] |
| Scientific Name | Dye Used | Metal Ions | Concentration | Amount of Photo-Catalyst | Time Required for Degradation (min) | Feature | % Removal | Adsorption Capacity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Spinacia oleracea | Methylene blue (MB) Malachite green (MG) | NA |
|
|
|
| NA | NA | [40] |
| Syzygium aromaticum | Methylene blue (MB) Malachite green (MG) | NA |
|
|
|
| NA | NA | [89] |
| Cinnamomum zeylanicum | Methylene blue (MB) Malachite green (MG) | NA |
|
|
|
| NA | NA | [94] |
| Eucalyptus | Methylene blue (MB) | NA |
|
|
| NA | NA | ~45 mg/g | [54] |
| Camellia sinensis | NA | Lead |
| NA | NA | NA |
| [49] | |
| Sugarcane bagasse | NA | Cadmium |
|
| NA | NA | NA | 24.47 mg/g | [92] |
| Aloe vera | Methylene blue (MB) | NA |
|
| NA | NA |
| NA | [51] |
| Camellia sinensis | NA | Lead (Pb (II)) |
|
| NA | NA |
| [60] | |
| Citrus hystrix | Methylene blue | NA |
|
| NA | NA | NA | 276.06 | [93] |
| Callistemon viminalis | NA | Iron | NA | NA | NA | NA |
| NA | [73] |
| Alstonia scholaris | Methylene blue (MB) Methyl orange (MO) | NA |
|
| NA | NA |
| NA | [97] |
| Phyllanthus emblica | Mixed dye (MB + MO) | NA |
|
|
|
|
| NA | [84] |
| Mixed dye (MB + MO) |
|
| |||||||
| Murraya koenigii | Methylene blue | NA |
|
|
|
|
| NA | [69] |
| Methyl orange |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perumal, D.; Albert, E.L.; Abdullah, C.A.C. Green Reduction of Graphene Oxide Involving Extracts of Plants from Different Taxonomy Groups. J. Compos. Sci. 2022, 6, 58. https://doi.org/10.3390/jcs6020058
Perumal D, Albert EL, Abdullah CAC. Green Reduction of Graphene Oxide Involving Extracts of Plants from Different Taxonomy Groups. Journal of Composites Science. 2022; 6(2):58. https://doi.org/10.3390/jcs6020058
Chicago/Turabian StylePerumal, Dharshini, Emmellie Laura Albert, and Che Azurahanim Che Abdullah. 2022. "Green Reduction of Graphene Oxide Involving Extracts of Plants from Different Taxonomy Groups" Journal of Composites Science 6, no. 2: 58. https://doi.org/10.3390/jcs6020058
APA StylePerumal, D., Albert, E. L., & Abdullah, C. A. C. (2022). Green Reduction of Graphene Oxide Involving Extracts of Plants from Different Taxonomy Groups. Journal of Composites Science, 6(2), 58. https://doi.org/10.3390/jcs6020058






