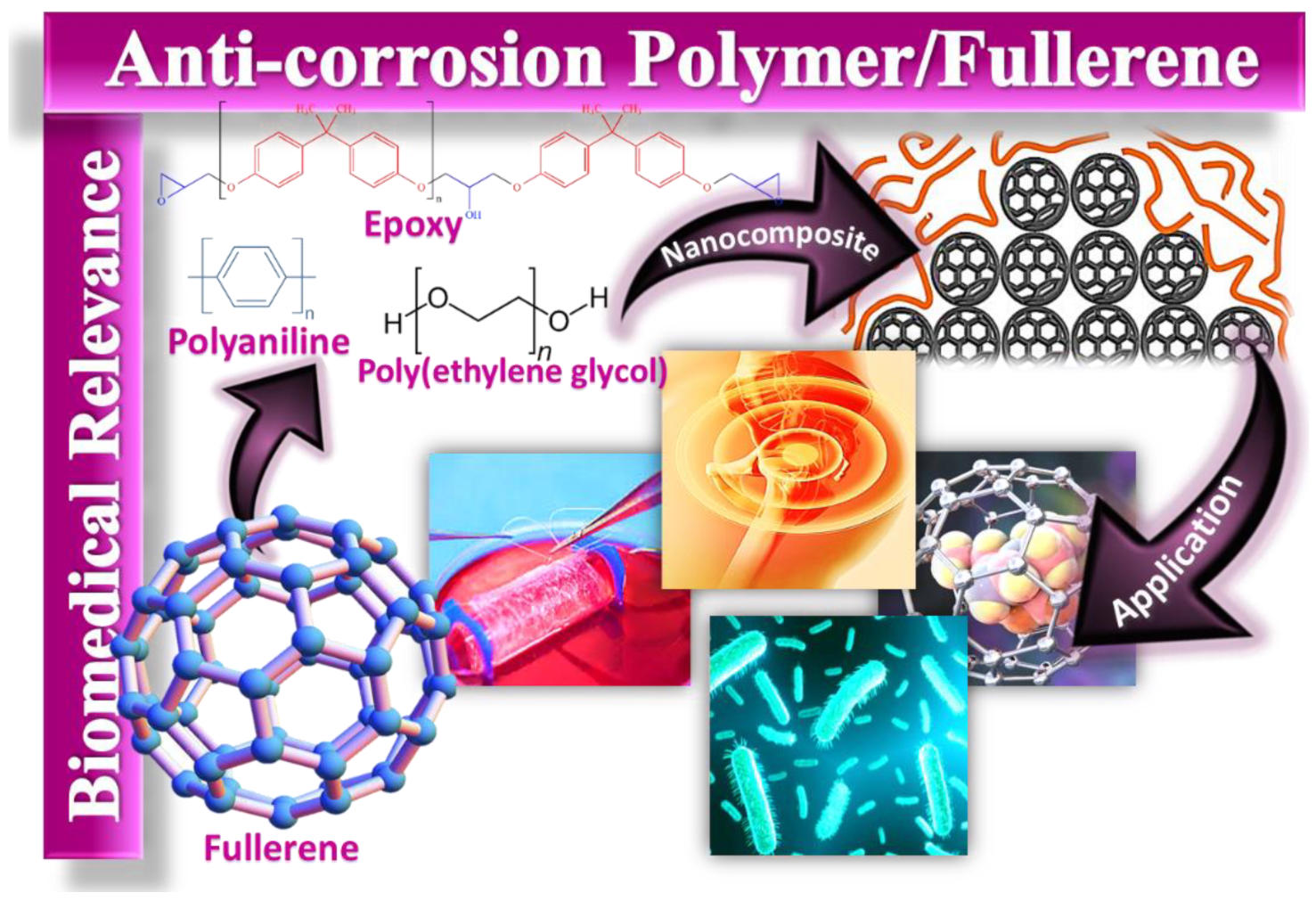
Potential of Polymer/Fullerene Nanocomposites for Anticorrosion Applications in the Biomedical Field
Abstract
1. Introduction
2. Corrosion-Resistant Polymeric Nanocomposites
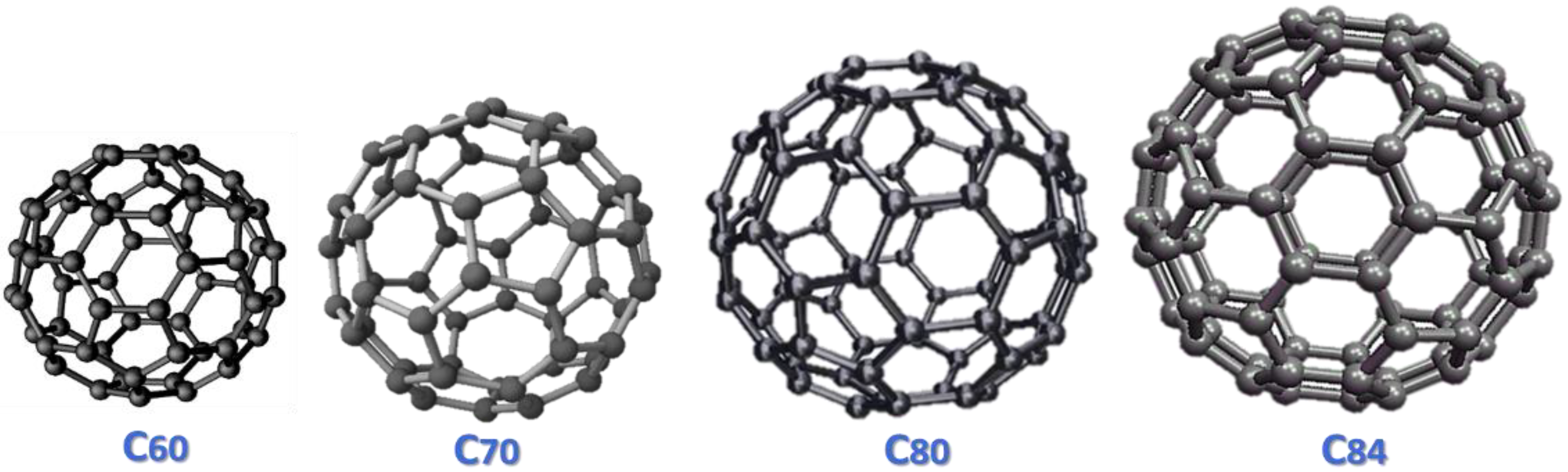
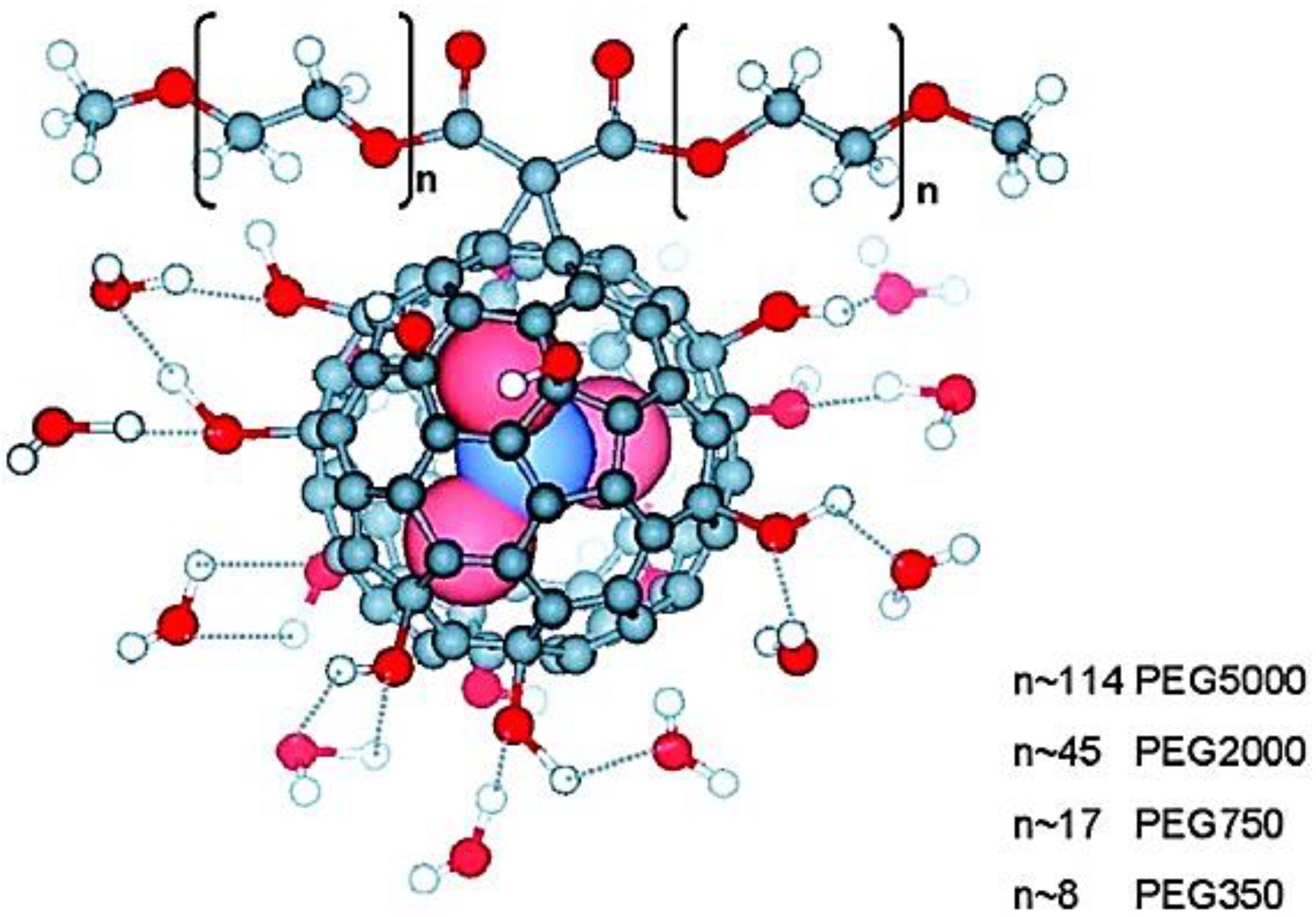
3. Fullerene
4. Polymer/Fullerene Nanocomposites for Corrosion Resistance Intended for Biomedical Applications
5. Future Challenges and Summary
Funding
Conflicts of Interest
References
- Ikubanni, P.; Oki, M.; Adeleke, A.; Adesina, O.; Omoniyi, P.; Akinlabi, E. Electrochemical Studies of the Corrosion Behavior of Al/SiC/PKSA Hybrid Composites in 3.5% NaCl Solution. J. Compos. Sci. 2022, 6, 286. [Google Scholar] [CrossRef]
- Zhong, S.; Li, J.; Cai, Y.; Yi, L. Novel surfactant-free waterborne acrylic-silicone modified alkyd hybrid resin coatings containing nano-silica for the corrosion protection of carbon steel. Polym. -Plast. Technol. Mater. 2019, 58, 866–878. [Google Scholar] [CrossRef]
- Omoniyi, P.; Abolusoro, O.; Olorunpomi, O.; Ajiboye, T.; Adewuyi, O.; Aransiola, O.; Akinlabi, E. Corrosion Properties of Alloy Reinforced with Wood Particles. J. Compos. Sci. 2022, 6, 189. [Google Scholar] [CrossRef]
- Abdalla, M.; Sims, A.; Mehanny, S.; Haghshenas, M.; Gupta, M.; Ibrahim, H. In Vitro Electrochemical Corrosion Assessment of Magnesium Nanocomposites Reinforced with Samarium (III) Oxide and Silicon Dioxide Nanoparticles. J. Compos. Sci. 2022, 6, 154. [Google Scholar] [CrossRef]
- Kausar, A. Performance of corrosion protective epoxy blend-based nanocomposite coatings: A review. Polym. -Plast. Technol. Mater. 2020, 59, 658–673. [Google Scholar] [CrossRef]
- Kausar, A. Green nanocomposites for energy storage. J. Compos. Sci. 2021, 5, 202. [Google Scholar] [CrossRef]
- Lopez, A.M.; Mateo-Alonso, A.; Prato, M. Materials chemistry of fullerene C 60 derivatives. J. Mater. Chem. 2011, 21, 1305–1318. [Google Scholar] [CrossRef]
- Jehoulet, C.; Obeng, Y.S.; Kim, Y.T.; Zhou, F.; Bard, A.J. Electrochemistry and Langmuir trough studies of fullerene C60 and C70 films. J. Am. Chem. Soc. 1992, 114, 4237–4247. [Google Scholar] [CrossRef]
- Gu, X.; Yan, H.; Kurosawa, T.; Schroeder, B.C.; Gu, K.L.; Zhou, Y.; To, J.W.; Oosterhout, S.D.; Savikhin, V.; Molina-Lopez, F.; et al. Comparison of the morphology development of polymer–fullerene and polymer–polymer solar cells during solution-shearing blade coating. Adv. Energy Mater. 2016, 6, 1601225. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Zhao, J.; Wang, X.; Xiong, C.; Song, L.; Ding, R.; Han, P.; Li, W. Self-healing performance and corrosion resistance of graphene oxide–mesoporous silicon layer–nanosphere structure coating under marine alternating hydrostatic pressure. Chem. Eng. J. 2019, 361, 792–804. [Google Scholar] [CrossRef]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Tang, S.; Deng, Y.; Zheng, X.; Bai, Y.; Fang, Y.; Dong, Q.; Wei, H.; Huang, J. Composition engineering in doctor-blading of perovskite solar cells. Adv. Energy Mater. 2017, 7, 1700302. [Google Scholar] [CrossRef]
- Razza, S.; Castro-Hermosa, S.; Di Carlo, A.; Brown, T.M. Research update: Large-area deposition, coating, printing, and processing techniques for the upscaling of perovskite solar cell technology. APL Mater. 2016, 4, 091508. [Google Scholar] [CrossRef]
- Goodarzi, S.; Da Ros, T.; Conde, J.; Sefat, F.; Mozafari, M. Fullerene: Biomedical engineers get to revisit an old friend. Mater. Today 2017, 20, 460–480. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Siva, T.; Ramadoss, A. Surface functionalized bioceramics coated on metallic implants for biomedical and anticorrosion performance–a review. J. Mater. Chem. B 2021, 9, 9433–9460. [Google Scholar] [CrossRef]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical applications of carbon nanomaterials: Fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Leygraf, C. Atmospheric Corrosion; John Wiley & Sons: New York, NY, USA, 2016. [Google Scholar]
- Selvi, S.T.; Raman, V.; Rajendran, N. Corrosion inhibition of mild steel by benzotriazole derivatives in acidic medium. J. Appl. Electrochem. 2003, 33, 1175–1182. [Google Scholar] [CrossRef]
- Armelin, E.; Ocampo, C.; Liesa, F.; Iribarren, J.I.; Ramis, X.; Alemán, C. Study of epoxy and alkyd coatings modified with emeraldine base form of polyaniline. Prog. Org. Coat. 2007, 58, 316–322. [Google Scholar] [CrossRef]
- Situ, Y.; Ji, W.; Liu, C.; Xu, J.; Huang, H. Synergistic effect of homogeneously dispersed PANI-TiN nanocomposites towards long-term anticorrosive performance of epoxy coatings. Prog. Org. Coat. 2019, 130, 158–167. [Google Scholar] [CrossRef]
- Tareq, S. Fabrication and Characterisation of Polymeric Nano-Composites; Western Sydney University: Penrith, Australia, 2019. [Google Scholar]
- Bhattacharya, M. Polymer nanocomposites—A comparison between carbon nanotubes, graphene, and clay as nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, P.; John, M.J.; Pothen, L.; Sreekala, M.S.; Thomas, S. Natural fibre and polymer matrix composites and their applications in aerospace engineering. In Advanced Composite Materials for Aerospace Engineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 365–383. [Google Scholar]
- Garces, J.M.; Moll, D.J.; Bicerano, J.; Fibiger, R.; McLeod, D.G. Polymeric nanocomposites for automotive applications. Adv. Mater. 2000, 12, 1835–1839. [Google Scholar] [CrossRef]
- Burris, D.L.; Boesl, B.; Bourne, G.R.; Sawyer, W.G. Polymeric nanocomposites for tribological applications. Macromol. Mater. Eng. 2007, 292, 387–402. [Google Scholar] [CrossRef]
- Kumar, S.A.; Meenakshi, K.S.; Sankaranarayanan, T.; Srikanth, S. Corrosion resistant behaviour of PANI–metal bilayer coatings. Prog. Org. Coat. 2008, 62, 285–292. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Siju, C.; Mahanta, D.; Patil, S.; Madras, G. Conducting polyaniline–nano-TiO2 composites for smart corrosion resistant coatings. Electrochim. Acta 2009, 54, 1249–1254. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.; Gupta, V.; Tomar, M. Fabrication and characterization of ZnO-TiO2-PANI (ZTP) micro/nanoballs for the detection of flammable and toxic gases. J. Hazard. Mater. 2019, 370, 126–137. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Wang, Y.; Lim, S.; Luo, J.; Xu, Z. Tribological and corrosion behaviors of Al2O3/polymer nanocomposite coatings. Wear 2006, 260, 976–983. [Google Scholar] [CrossRef]
- González, M.; Saidman, S. Electrodeposition of polypyrrole on 316L stainless steel for corrosion prevention. Corros. Sci. 2011, 53, 276–282. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.; Rhee, K.Y. Aqueous phase polymeric corrosion inhibitors: Recent advancements and future opportunities. J. Mol. Liq. 2021, 348, 118387. [Google Scholar] [CrossRef]
- Hien, N.; Garcia, B.; Pailleret, A.; Deslouis, C. Role of doping ions in the corrosion protection of iron by polypyrrole films. Electrochim. Acta 2005, 50, 1747–1755. [Google Scholar] [CrossRef]
- Kausar, A. Corrosion prevention prospects of polymeric nanocomposites: A review. J. Plast. Film. Sheeting 2019, 35, 181–202. [Google Scholar] [CrossRef]
- Chang, C.-W. Electrical and Thermal Transport Measurements on Nano-Structured Materials; University of California: Berkeley, CA, USA, 2006. [Google Scholar]
- Yan, Q.L.; Gozin, M.; Zhao, F.Q.; Cohen, A.; Pang, S.P. Highly energetic compositions based on functionalized carbon nanomaterials. Nanoscale 2016, 8, 4799–4851. [Google Scholar] [CrossRef]
- Giacalone, F.; Martin, N. Fullerene polymers: Synthesis and properties. Chem. Rev. 2006, 106, 5136–5190. [Google Scholar] [CrossRef]
- Akasaka, T.; Wakahara, T.; Nagase, S.; Kobayashi, K.; Waelchli, M.; Yamamoto, K.; Kondo, M.; Shirakura, S.; Maeda, Y.; Kato, T. Structural determination of the La@ C82 isomer. J. Phys. Chem. B 2001, 105, 2971–2974. [Google Scholar] [CrossRef]
- Mojica, M.; Alonso, J.A.; Méndez, F. Synthesis of fullerenes. J. Phys. Org. Chem. 2013, 26, 526–539. [Google Scholar] [CrossRef]
- Withers, J.C.; Loutfy, R.O.; Lowe, T.P. Fullerene commercial vision. Fuller. Nanotub. Carbon Nanostructures 1997, 5, 1–31. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C 60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Timerkaev, B.; Gevorgyan, R.; Zalyalieva, A.; Timerkaeva, D. Plasma-Chemical Synthesis of Nanodiamonds on the Surface of a Microarc Discharge Cathode. J. Eng. Phys. Thermophys. 2022, 95, 1–6. [Google Scholar] [CrossRef]
- Xie, S.Y.; Huang, R.B.; Yu, L.J.; Ding, J.; Zheng, L.S. Microwave synthesis of fullerenes from chloroform. Appl. Phys. Lett. 1999, 75, 2764–2766. [Google Scholar] [CrossRef]
- Chen, C.; Lou, Z. Formation of C60 by reduction of CO2. J. Supercrit. Fluids 2009, 50, 42–45. [Google Scholar] [CrossRef]
- Scott, L.T. Methods for the chemical synthesis of fullerenes. Angew. Chem. Int. Ed. 2004, 43, 4994–5007. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.-R.; Therezien, M.; Budarz, J.F.; Wessel, L.; Lin, S.; Xiao, Y.; Wiesner, M.R. Comparison of the photosensitivity and bacterial toxicity of spherical and tubular fullerenes of variable aggregate size. J. Nanoparticle Res. 2011, 13, 5121–5127. [Google Scholar] [CrossRef]
- Modi, A.; Koratkar, N.; Lass, E.; Wei, B.; Ajayan, P.M. Miniaturized gas ionization sensors using carbon nanotubes. Nature 2003, 424, 171–174. [Google Scholar] [CrossRef]
- Gallo, M.; Favila, A.; Glossman-Mitnik, D. DFT studies of functionalized carbon nanotubes and fullerenes as nanovectors for drug delivery of antitubercular compounds. Chem. Phys. Lett. 2007, 447, 105–109. [Google Scholar] [CrossRef]
- Nierengarten, J.-F. Chemical modification of C 60 for materials science applications. New J. Chem. 2004, 28, 1177–1191. [Google Scholar] [CrossRef]
- Dmitruk, N.; Borkovskaya, O.Y.; Havrylenko, T.; Naumenko, D.; Petrik, P.; Meza-Laguna, V.; Basiuk, E. Effect of chemical modification of thin C60 fullerene films on the fundamental absorption edge. Semicond. Phys. Quantum Electron. Optoelectron. 2010, 13, 180–185. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Strongly optical absorptive nanofluids and rheology in bonded fullerene C60 via poly (vinyl pyrrolidone) molecules in water. Fuller. Nanotub. Carbon Nanostructures 2017, 25, 143–150. [Google Scholar] [CrossRef]
- Behera, M.; Ram, S. Variation of optical properties, rheology, and microstructure in fullerene/poly (vinyl pyrrolidone) nanofluids with fullerene content in n-butanol. Fuller. Nanotub. Carbon Nanostructures 2016, 24, 154–161. [Google Scholar] [CrossRef]
- Wang, X.; Tang, F.; Qi, X.; Lin, Z.; Battocchi, D.; Chen, X. Enhanced protective coatings based on nanoparticle fullerene C60 for oil & gas pipeline corrosion mitigation. Nanomaterials 2019, 9, 1476. [Google Scholar]
- Liu, D.; Zhao, W.; Liu, S.; Cen, Q.; Xue, Q. Comparative tribological and corrosion resistance properties of epoxy composite coatings reinforced with functionalized fullerene C60 and graphene. Surf. Coat. Technol. 2016, 286, 354–364. [Google Scholar] [CrossRef]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.; Kasiriha, S. Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- Fadl, A.; Abdou, M.; Al-Elaa, S.A.; Hamza, M.; Sadeek, S. Evaluation the anti-corrosion behavior, impact resistance, acids and alkali immovability of nonylphenol ethoxylate/TiO2 hybrid epoxy nanocomposite coating applied on the carbon steel surface. Prog. Org. Coat. 2019, 136, 105263. [Google Scholar] [CrossRef]
- Gobara, M.; Baraka, A.; Akid, R.; Zorainy, M. Corrosion protection mechanism of Ce 4+/organic inhibitor for AA2024 in 3. 5% NaCl. RSC Adv. 2020, 10, 2227–2240. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Chen, J.; Zhang, J.; Fang, Q. Dopamine modified metal-organic frameworks on anti-corrosion properties of waterborne epoxy coatings. Prog. Org. Coat. 2017, 109, 126–134. [Google Scholar] [CrossRef]
- Qiang, Y.; Guo, L.; Li, H.; Lan, X. Fabrication of environmentally friendly Losartan potassium film for corrosion inhibition of mild steel in HCl medium. Chem. Eng. J. 2020, 406, 126863. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.J.; Weng, G.J.; Su, Y. The effects of temperature and alignment state of nanofillers on the thermal conductivity of both metal and nonmetal based graphene nanocomposites. Acta Mater. 2020, 185, 461–473. [Google Scholar] [CrossRef]
- Farooq, S.; Razzaq, H.; Razzaque, S.; Khan, B.A.; Qaisar, S. Structural and physical impacts of nanofillers in ionogels: A comprehensive overview. Polym. Compos. 2019, 40 (Suppl. S1), E11–E23. [Google Scholar] [CrossRef]
- Kausar, A. Applications of polymer/graphene nanocomposite membranes: A review. Mater. Res. Innov. 2019, 23, 276–287. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, J.; Sun, J.; He, W. Corrosion inhibition of methanol towards stainless steel bipolar plate for direct formic acid fuel cell. Int. J. Hydrogen Energy 2020, 45, 30924–30931. [Google Scholar] [CrossRef]
- Xavior, M.A.; Kumar, H.P. Processing and characterization techniques of graphene reinforced metal matrix composites (GRMMC); a review. Mater. Today: Proc. 2017, 4, 3334–3341. [Google Scholar]
- Boppana, S.B.; Dayanand, S.; Kumar, M.A.; Kumar, V.; Aravinda, T. Synthesis and characterization of nano graphene and ZrO2 reinforced Al 6061 metal matrix composites. J. Mater. Res. Technol. 2020, 9, 7354–7362. [Google Scholar] [CrossRef]
- Sittner, F.; Enders, B.; Jungclas, H.; Ensinger, W. Corrosion properties of ion beam modified fullerene thin films on iron substrates. Surf. Coat. Technol. 2002, 158, 368–372. [Google Scholar] [CrossRef]
- Kausar, A. Fullerene nanofiller reinforced epoxy nanocomposites—Developments, progress and challenges. Mater. Res. Innov. 2021, 25, 175–185. [Google Scholar] [CrossRef]
- Turan, M.E.; Sun, Y.; Aydin, F.; Zengin, H.; Turen, Y.; Ahlatci, H. Effects of carbonaceous reinforcements on microstructure and corrosion properties of magnesium matrix composites. Mater. Chem. Phys. 2018, 218, 182–188. [Google Scholar] [CrossRef]
- Tseluikin, V. Electrodeposition and properties of composite coatings modified by fullerene C60. Prot. Met. Phys. Chem. Surf. 2017, 53, 433–436. [Google Scholar] [CrossRef]
- Dwivedi, N.; Satyanarayana, N.; Yeo, R.J.; Xu, H.; Ping Loh, K.; Tripathy, S.; Bhatia, C.S. Ultrathin carbon with interspersed graphene/fullerene-like nanostructures: A durable protective overcoat for high density magnetic storage. Sci. Rep. 2015, 5, 1–16. [Google Scholar] [CrossRef]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A.; Jafari-Tarzanagh, Y. Oxidized fullerene/sol-gel nanocomposite for corrosion protection of AM60B magnesium alloy. Surf. Coat. Technol. 2020, 385, 125400. [Google Scholar] [CrossRef]
- Liu, W.; Speranza, G. Functionalization of Carbon Nanomaterials for Biomedical Applications. C J. Carbon Res. 2019, 5, 72. [Google Scholar] [CrossRef]
- Akbarzadeh, S.; Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M.; Naderi, R. Fabrication of highly effective polyaniline grafted carbon nanotubes to induce active protective functioning in a silane coating. Ind. Eng. Chem. Res. 2019, 58, 20309–20322. [Google Scholar] [CrossRef]
- Yang, N.; Yang, T.; Wang, W.; Chen, H.; Li, W. Polydopamine modified polyaniline-graphene oxide composite for enhancement of corrosion resistance. J. Hazard. Mater. 2019, 377, 142–151. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in conductive polyaniline-based nanocomposites for biomedical applications: A review. J. Med. Chem. 2019, 63, 1–22. [Google Scholar] [CrossRef]
- Gupta, A. Development & Characterization of Anti-corrosive & Anti-fouling Functional Surfaces; Indian Institute of Technology Delhi: Delhi, India, 2019. [Google Scholar]
- Wahby, M.H.; Atta, A.M.; Moustafa, Y.M.; Ezzat, A.O.; Hashem, A.I. Hydrophobic and Superhydrophobic Bio-Based Nano-Magnetic Epoxy Composites as Organic Coating of Steel. Coatings 2020, 10, 1201. [Google Scholar] [CrossRef]
- Nikafshar, S.; McCracken, J.; Dunne, K.; Nejad, M. Improving UV-Stability of epoxy coating using encapsulated halloysite nanotubes with organic UV-Stabilizers and lignin. Prog. Org. Coat. 2020, 151, 105843. [Google Scholar] [CrossRef]
- Satheesan, B.; Mohammed, A.S. Tribological characterization of epoxy hybrid nanocomposite coatings reinforced with graphene oxide and titania. Wear 2020, 466, 203560. [Google Scholar]
- Feng, Y.; Cui, Y.; Zhang, M.; Li, M.; Li, H. Preparation of Tung Oil-Loaded PU/PANI Microcapsules and Synergetic Anti-Corrosion Properties of Self-Healing Epoxy Coatings. Macromol. Mater. Eng. 2020, 306, 2000581. [Google Scholar] [CrossRef]
- Rajabi, M.; Rashed, G.; Zaarei, D. Assessment of graphene oxide/epoxy nanocomposite as corrosion resistance coating on carbon steel. Corros. Eng. Sci. Technol. 2015, 50, 509–516. [Google Scholar] [CrossRef]
- Kozlov, G.; Dolbin, I. Aggregation of Nanofiller in Polymer/Carbon Nanotube Composites. J. Appl. Mech. Tech. Phys. 2020, 61, 263–266. [Google Scholar] [CrossRef]
- Zare, Y. Study of nanoparticles aggregation/agglomeration in polymer particulate nanocomposites by mechanical properties. Compos. Part A: Appl. Sci. Manuf. 2016, 84, 158–164. [Google Scholar] [CrossRef]
- Zuev, V.V. Polymer Nanocomposites Containing Fullerene C60 Nanofillers. Macromol. Symp. 2011, 301, 157–161. [Google Scholar] [CrossRef]
- Badamshina, E.; Estrin, Y.; Gafurova, M. Nanocomposites based on polyurethanes and carbon nanoparticles: Preparation, properties and application. J. Mater. Chem. A 2013, 1, 6509–6529. [Google Scholar] [CrossRef]
- Zuev, V.V.; Shlikov, A.V. Polyamide 12/fullerene C60 composites: Investigation on their mechanical and dielectric properties. J. Polym. Res. 2012, 19, 1–6. [Google Scholar] [CrossRef]
- Geringer, J.; Macdonald, D.D. Modeling fretting-corrosion wear of 316L SS against poly (methyl methacrylate) with the Point Defect Model: Fundamental theory, assessment, and outlook. Electrochim. Acta 2012, 79, 17–30. [Google Scholar] [CrossRef]
- Qi, K.; Sun, Y.; Duan, H.; Guo, X. A corrosion-protective coating based on a solution-processable polymer-grafted graphene oxide nanocomposite. Corros. Sci. 2015, 98, 500–506. [Google Scholar] [CrossRef]
- Wang, D.; Wang, W.; Lu, H.; You, C.; Liang, L.; Liu, C.; Xiang, H.; Chen, Y. Charge transfer of ZnTPP/C60 cocrystal-hybridized bioimplants satisfies osteosarcoma eradication with antitumoral, antibacterial and osteogenic performances. Nano Today 2022, 46, 101562. [Google Scholar] [CrossRef]
- Braga, J.d.O.; dos Santos, D.M.; Cotting, F.; Lins, V.F.; Leão, N.M.; Soares, D.C.; Mazzer, E.M.; Houmard, M.; Figueiredo, R.B.; Nunes, E.H. Surface modification of magnesium with a novel composite coating for application in bone tissue engineering. Surf. Coat. Technol. 2022, 433, 128078. [Google Scholar] [CrossRef]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A. Sol-gel coating filled with SDS-stabilized fullerene nanoparticles for active corrosion protection of the magnesium alloy. Surf. Coat. Technol. 2021, 419, 127292. [Google Scholar] [CrossRef]
- Pourhashem, S.; Ghasemy, E.; Rashidi, A.; Vaezi, M.R. A review on application of carbon nanostructures as nanofiller in corrosion-resistant organic coatings. J. Coat. Technol. Res. 2020, 17, 19–55. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Chairman, C.A.; Balasundaram, R. Magnesium matrix composite for biomedical applications through powder metallurgy–Review. Mater. Today Proc. 2020, 27, 736–741. [Google Scholar] [CrossRef]
- Wang, J.; Qing, Y.; Xiao, L.; Wang, Y.; Bao, X.; Qin, Y.; Zhang, J.; Zhang, K. Design of new-type F-FLC artificial joint coatings via fluorine incorporation and fullerene-like structure construction. Surf. Coat. Technol. 2020, 385, 125419. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon nanomaterials against pathogens; the antimicrobial activity of carbon nanotubes, graphene/graphene oxide, fullerenes, and their nanocomposites. Adv. Colloid Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Lyon, D.Y.; Adams, L.K.; Falkner, J.C.; Alvarez, P.J. Antibacterial activity of fullerene water suspensions: Effects of preparation method and particle size. Environ. Sci. Technol. 2006, 40, 4360–4366. [Google Scholar] [CrossRef]
- Zhao, X.; Li, P.; Guo, B.; Ma, P.X. Antibacterial and conductive injectable hydrogels based on quaternized chitosan-graft-polyaniline/oxidized dextran for tissue engineering. Acta Biomater. 2015, 26, 236–248. [Google Scholar] [CrossRef]
- Wang, B.; Gao, X.; Piao, G. Preparation of polyaniline-doped fullerene whiskers. Int. J. Polym. Sci. 2013, 2013, 1–4. [Google Scholar] [CrossRef]
- Ansari, M.J.; Soltani, A.; Ramezanitaghartapeh, M.; Singla, P.; Aghaei, M.; Fadafan, H.K.; Khales, S.A.; Shariati, M.; Shirzad-Aski, H.; Balakheyli, H. Improved antibacterial activity of sulfasalazine loaded fullerene derivative: Computational and experimental studies. J. Mol. Liq. 2022, 348, 118083. [Google Scholar] [CrossRef]
- Keykhosravi, S.; Rietveld, I.B.; Couto, D.; Tamarit, J.L.; Barrio, M.; Céolin, R.; Moussa, F. [60] Fullerene for medicinal purposes, a purity criterion towards regulatory considerations. Materials 2019, 12, 2571. [Google Scholar] [CrossRef]
- Sukhodub, L.; Sukhodub, L.; Kumeda, M.; Prylutska, S.; Deineka, V.; Prylutskyy, Y.I.; Ritter, U. C60 fullerene loaded hydroxyapatite-chitosan beads as a promising system for prolonged drug release. Carbohydr. Polym. 2019, 223, 115067. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, H.; Wang, L.; Li, L.; Wang, H.; Wang, Z.; Li, Z.; Chen, C.; Hou, L.; Zhang, C. PEI-derivatized fullerene drug delivery using folate as a homing device targeting to tumor. Biomaterials 2013, 34, 251–261. [Google Scholar] [CrossRef]
- Kazemzadeh, H.; Mozafari, M. Fullerene-based delivery systems. Drug Discov. Today 2019, 24, 898–905. [Google Scholar] [CrossRef]
- Hsiao, C.-W.; Bai, M.-Y.; Chang, Y.; Chung, M.-F.; Lee, T.-Y.; Wu, C.-T.; Maiti, B.; Liao, Z.-X.; Li, R.-K.; Sung, H.-W. Electrical coupling of isolated cardiomyocyte clusters grown on aligned conductive nanofibrous meshes for their synchronized beating. Biomaterials 2013, 34, 1063–1072. [Google Scholar] [CrossRef]
- Thong, N.M.; Vo, Q.V.; Le Huyen, T.; Van Bay, M.; Dung, N.N.; Thao, P.T.T.; Nam, P.C. Functionalization and antioxidant activity of polyaniline–fullerene hybrid nanomaterials: A theoretical investigation. RSC Adv. 2020, 10, 14595–14605. [Google Scholar] [CrossRef]
- Heidari, A. Treatment of breast cancer brain metastases through a targeted nanomolecule drug delivery system based on dopamine functionalized multi–wall carbon nanotubes (mwcnts) coated with nano graphene oxide (GO) and protonated polyaniline (PANI) in situ during the polymerization of aniline autogenic nanoparticles for the delivery of anti–cancer nano drugs under synchrotron radiation. Br. J. Res. 2017, 4, 16. [Google Scholar]
- Abd Elkodous, M.; El-Sayyad, G.S.; Abdelrahman, I.Y.; El-Bastawisy, H.S.; Mosallam, F.M.; Nasser, H.A.; Gobara, M.; Baraka, A.; Elsayed, M.A.; El-Batal, A.I. Therapeutic and diagnostic potential of nanomaterials for enhanced biomedical applications. Colloids Surf. B: Biointerfaces 2019, 180, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K.; Mishra, A.K.; Rauta, P.R.; Al-Sehemi, A.G.; Pannipara, M.; Sett, A.; Bratovcic, A.; Avula, S.K.; Mohanta, T.K.; Saravanan, M. Exploring the Bioactive Potentials of C60-AgNPs Nano-Composites against Malignancies and Microbial Infections. Int. J. Mol. Sci. 2022, 23, 714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Fatouros, P.P.; Shu, C.Y.; Reid, J.; Owens, L.S.; Cai, T.; Gibson, H.W.; Long, G.L.; Corwin, F.D.; Chen, Z.J.; et al. High Relaxivity Trimetallic Nitride (Gd3N) Metallofullerene MRI Contrast Agents with Optimized Functionality. Bioconjugate Chem. 2010, 21, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Bakir, M. Design and Characterization of Aromatic Thermosetting Copolyester Resin for Polymer Matrix Nanocomposites; University of Illinois at Urbana-Champaign: Illinois, IL, USA, 2019. [Google Scholar]
- Kausar, A. Emerging research trends in polyurethane/graphene nanocomposite: A review. Polym. Plast. Technol. Eng. 2017, 56, 1468–1486. [Google Scholar] [CrossRef]
- Kausar, A. Graphene nanomesh and polymeric material at cutting edge. Polym. Plast. Technol. Mater. 2019, 58, 803–820. [Google Scholar] [CrossRef]
- Adamczyk, L.; Kulesza, P.J. Fabrication of composite coatings of 4-(pyrrole-1-yl) benzoate-modified poly-3, 4-ethylenedioxythiophene with phosphomolybdate and their application in corrosion protection. Electrochim. Acta 2011, 56, 3649–3655. [Google Scholar] [CrossRef]
- Pavase, T.R.; Lin, H.; Hussain, S.; Li, Z.; Ahmed, I.; Lv, L.; Sun, L.; Shah, S.B.H.; Kalhoro, M.T. Recent advances of conjugated polymer (CP) nanocomposite-based chemical sensors and their applications in food spoilage detection: A comprehensive review. Sens. Actuators B: Chem. 2018, 273, 1113–1138. [Google Scholar] [CrossRef]
- Gergely, A.; Pászti, Z.; Hakkel, O.; Drotár, E.; Mihály, J.; Kálmán, E. Corrosion protection of cold-rolled steel with alkyd paint coatings composited with submicron-structure types polypyrrole-modified nano-size alumina and carbon nanotubes. Mater. Sci. Eng. B 2012, 177, 1571–1582. [Google Scholar] [CrossRef]
- Selim, M.S.; Shenashen, M.; El-Safty, S.A.; Higazy, S.; Selim, M.M.; Isago, H.; Elmarakbi, A. Recent progress in marine foul-release polymeric nanocomposite coatings. Prog. Mater. Sci. 2017, 87, 1–32. [Google Scholar] [CrossRef]
- Vlasa, A.; Varvara, S.; Pop, A.; Bulea, C.; Muresan, L.M. Electrodeposited Zn–TiO 2 nanocomposite coatings and their corrosion behavior. J. Appl. Electrochem. 2010, 40, 1519–1527. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kausar, A. Potential of Polymer/Fullerene Nanocomposites for Anticorrosion Applications in the Biomedical Field. J. Compos. Sci. 2022, 6, 394. https://doi.org/10.3390/jcs6120394
Kausar A. Potential of Polymer/Fullerene Nanocomposites for Anticorrosion Applications in the Biomedical Field. Journal of Composites Science. 2022; 6(12):394. https://doi.org/10.3390/jcs6120394
Chicago/Turabian StyleKausar, Ayesha. 2022. "Potential of Polymer/Fullerene Nanocomposites for Anticorrosion Applications in the Biomedical Field" Journal of Composites Science 6, no. 12: 394. https://doi.org/10.3390/jcs6120394
APA StyleKausar, A. (2022). Potential of Polymer/Fullerene Nanocomposites for Anticorrosion Applications in the Biomedical Field. Journal of Composites Science, 6(12), 394. https://doi.org/10.3390/jcs6120394






