Abstract
Polymer blend or composite, which is a combination of two or more polymers and fillers such as semiconductors, metals, metal oxides, salts and ceramics, are a synthesized product facilitating improved, augmented or customized properties, and have widespread applications for the achievement of functional materials. Polymer materials with embedded inorganic fillers are significantly appealing for challenging and outstanding electric, dielectric, optical and mechanical applications involving magnetic features. In particular, a polymer matrix exhibiting large values of dielectric constant (ε′) with suitable thermal stability and low dielectric constant values of polymer blend, having lesser thermal stability, together offer significant advantages in electronic packaging and other such applications in different fields. In this review paper, we focused on the key factors affecting the dielectric properties and its strength in thin film of inorganic materials loaded poly methyl meth acrylate (PMMA) based polymer blend (single phase) or composites (multiple phase), and its consequences at low and high frequencies are explored. A wide range of different types of PMMA based polymer blends or composites, which are doped with different fillers, have been synthesized with specific tailoring of their dielectric behavior and properties. A few of them are discussed in this manuscript, with their different preparation techniques, and exploring new ideas for modified materials.
1. Introduction
In the last few decades, researchers are showing their interest and attention towards materials which have challenging dielectric properties with suitable structural and mechanical strength. They are focused on modern technologies on the new materials and blends, having distinct ‘amalgamation properties’. Dielectric materials with high dielectric constants have wide applications in capacitors, energy storage electronic devices, microelectronic devices and devices working at high operating frequencies [1,2,3]. The advance technology is to make electronic devices smaller, i.e., miniaturization demands low dielectric constant materials for packaging and insulation. Therefore, the synthesis of two types of materials has currently created a lot of attention among scholars and researchers. For its desired application, materials with high dielectric constant should have high dielectric strength, low chemical resistance, low dielectric losses, a frequency independent response and good mechanical properties [4,5,6]. It is difficult to obtain the unique combination of all properties in one material (component). Usually, ferroelectric materials show a high dielectric constant and low losses, but due to the brittle nature, it has limitations in its fabrications and shape [7,8]. The current review paper focuses on PMMA based polymer blends or composites with filler materials for challenging results in dielectric properties.
The selection of suitable doping material is a major clause. Consequently, due to the excessive existence of different polymer materials, a focused and properly defined categorization is needed. The first class of materials are the materials having high dielectric constant (ε′) values containing low dielectric loses and highly loaded fillers, which depends on varying frequencies, the concentration of doping and fine dispersion. Several such polymer composites (PC) have wide applications in electronic devices for frequency ranges from 20 Hz to 1 MHz, and thus different characteristics and behavior in a wide window from audio frequency (AF) to radio frequency (RF) ranges have been observed [6,9,10]. Thus, the first types of materials were found to be suitable for their applications in capacitors, dielectric elastomer or energy store electronic devices, which can also withstand varying thermal conditions. The second types of materials are the class II materials, which have low dielectric constant values with lesser thermal stability. Due to the inherent characteristics of such II class materials; these have great advantages in electronic packaging and similar applications [6,7,8,9,10].
The preparation of a material blend (single phase) or composite (multiphase) [11,12] from two constituent polymer with different physio-chemical properties is a strategic way to tune the properties, since few of the materials in their pristine form do not meet with the necessary requirements for most of the technological needs and applications. So, the selection of the materials and the use of other components as fillers to achieve the tuned values, and as well as their synthesis route along with their quantitative analysis and dispersion rate, all plays a significant role in ensuring the desired functional properties of the finally synthesized material.
In particular, the choice of the host material is usually critical for many applications. The performance and final properties of the synthesized material are primarily affected by its structure and morphology [12], secondarily by the mutual arrangement of the constituent particles, but mostly on the interaction between host and guest polymers. The polymer materials represent an appealing behavior as they can be controlled, designed and tailored to a tuned wide range of dielectric, thermal and optical properties [10,11,12,13].
As the polymer has stretching quality, and due to its regular arrangement pattern, it can be used in several architectural designs. Large numbers of polymer composites have been developed as matrix incorporation with different fillers, and their effects on size and shapes [14,15] were reported.
Among the wide variety of most occurring polymer, poly methyl methacrylate (PMMA) has been one of the distinguished and broadly studied polymers, due to its challenging and promising features. Its suitability and compatibility with other polymer and materials is due to the chemical inertness property of the polymer. PMMA is an amorphous, low cost, chemically stable, strongly hydrophilic and highly transparent polymer [6,14,15]. Its good gelatinizing and high solvent properties, good outdoor weather ability and excellent environmental stability make it popular for researchers working in the broad field of material science. It has good resistance towards acid and has high surface resistance. Although it has sound features, there are still some limitations with some of its properties, such as thermal stability and ionic conductivity. Pristine PMMA possess electrical conductivity 10−19 S/cm, which is improved to 10−5 S/cm for the PMMA based lithium ion conducting nano solid polymer electrolytes (NSPEs) by varying the temperature from 27–55 °C [16,17,18,19].
Their excellent optical property due to transparency in the visible region is another feature which makes this polymer popular in many technological and productive fields. PMMA embedded with inorganic or organic materials, cast into films, have been broadly used to yield satisfying properties such as photoconductivity, nonlinear optical properties and photoluminescence, etc., [20,21,22].
The present manuscript highlights the dielectric properties and electrical behavior of PMMA doped with different inorganic fillers in different weight ratios, to obtain suitable results of dielectric properties. Their final consequences revealed the dependence of dielectric behavior on the dispersion factor of the polymer matrix and fillers, the content of the fillers, the shape and size of the fillers and porosity [20,21].
This article is divided in three main parts; the first part focuses on the synthesis of the materials by mixing both filler and host materials, mainly in solid forms such as powder, palette or film. The second part is with the aim of providing a comprehensive or detailed description of an up-to-date survey on the state of the art PMMA based composites or blends, with a focus on the parameters affecting their characteristics. In the final section, the case studies are concerned with a few examples, and their comparative response is sketched and classified according to their applications. Collectively, these three frames can be pointed as: (i) The composite films are usually prepared by different synthesis techniques like sol-gel methods, solution casting and the use of ultrasound and microwaves for perfect miscibility [23,24,25], which are discussed one by one. (ii) Different cases are taken with PMMA as the host material to study the dielectric properties of the synthesized material for frequencies varied from low (few hertz) to high (1 Megahertz). (iii) The loading of different inorganic fillers viz.: metal oxides (such as ZnO, Al2O3, SnO2, SiO2, TiO2), salts and ceramics. All of these frames show different results when interacted with matrix polymers [20,25,26].
2. Scientific Approach to Design Polymer Composites
It has already been discussed that large numbers of experiments have been performed for the preparation and synthesis of polymer blends/composites [19,20,21,22,23,24,25,26]. In this framework, several synthetic and processing issues have been taken into consideration. The mixing of organic polymer and inorganic filler particles may lead to particle agglomeration in distinct or indistinct phases, resulting in different optical, electrical, dielectric, thermal and mechanical properties. The undefined particles’ grow, which can be seen in a variety of composite systems in which bunch and undefined particles are involved. These unmodified inorganic particles with fillers in most of the polymer matrices, whatever their size, nature and structure, tend to add their involvement and affect the aggregate results. Such problems can be controlled on the basis of the selection of each polymer, which can tune with added fillers, and their interaction can be suitably modified and supported with the surrounding environment [26,27,28]. In the next section of this review, the key concerned factors are the suitable preparation techniques, which can control the technical difficulties related to their uniform dispersion, tuned interfacial support and congeniality between matrix and fillers. Among the different planning and techniques employed to produce polymer based composite materials, three main techniques are described here. The first method involves the advanced technique of simply mixing the polymers. In this technique, the dispersion of the guest particles in a monomer solution by the chemical mixing of two components, results in a perfectly homogenous dispersion of guest particle and host polymer matrix to get an evenly distributed polymer composite (PC). The second technique helps to form a perfectly blended uniform thickness film, in which some solvent is taken for the miscibility of the polymer materials and fillers and subjected to continuous stirring on a magnetic stirrer with 250 to 500 rpm (revolutions per minute) at an ambient temperature from +4 °C to +40 °C in a closed laboratory room [11,20]. The synthesized material in the form of a film, by using solution casting or ultrasonicator, is compatible and suitable for further investigation and applications [26,27,28,29,30,31].
The third technique involves a microwave radiation technique for proper dispersion. The solution of polymer composites and solvent are exposed to ultrasound and microwaves [32].
3. Experimental Techniques for the Synthesis of Composites
Experiments and techniques carried out for the synthesis of composite films may affect its dielectric properties to some extent, due to the different mixing mechanism. The direct mixing or in situ polymerization techniques can be used, but with these two methods, the filler particles did not dispersed uniformly. This forced the scientific community to develop the following methods to disperse one kind of material into another, so that proper mixing can be ensured [24,32].
3.1. Sol Gel Method
The sol gel method is an accurate way to produce solid materials from tiny molecules in materials science. This method is especially used for the fabrication of metal oxides (MO) such as the oxides of silicon (Si) or titanium (Ti). Because of its simplicity and versatility (it can be cast in various forms), this technique is used for the development of sensors (viz.: bio and optical) [26,28,32]. As compared to the organic polymers, sol-gel material is chemically, thermally and mechanically stable; therefore, their applications are convenient in harsh environments [33], so this technique is used for the synthesis of polymer blends/composite films. In this technique, the monomers are converted into a colloidal solution (sol), which acts as the precursor for an integrated network or gel for either discrete particles or network polymers. Sol-gel can be caste in monoliths, thin films, fibers and powders. This permits various configurations of sensing elements or materials for different applications.
3.2. Solution Casting Technique
The principle of the solution casting method is based on ‘Stokes law’ [20]. The polymer has the matrix phase easily dissolved in the solution, whereas the NPs are dispersed in the same solution or a different solution and, finally, both of them become intermixed. The solution is dispersed on clay after the process of swelling within the solvent. A decrease in entropy occurs due to the distortion of solvent molecules from the polymer/clay composites. In this manner, the entropy decreases to establish an intercalated chain. Due to the evaporation of solvent molecules, a nano composite structure is established and to do this, either a magnetic stirrer or an ultra-sonicator is required, with temperature control [27,28]. In this method, different polymers (host and guest polymers) are equally merged to form the suitable solution. The polymer (being the matrix phase) dissolved easily in solution I (with proper solvent) whereas, the NPs dispersed either in solution I (same solution) or in solution II (different solution). These are then allowed to be mixed by putting them on a stirrer for the definite/specific time for proper miscibility [32,34]. Generally, the films of perfect miscibility and uniform thickness are synthesized by this method, which is the most popular and easy, and can be performed in normal laboratories with normal precautionary measures. The whole process is summarized in Figure 1.
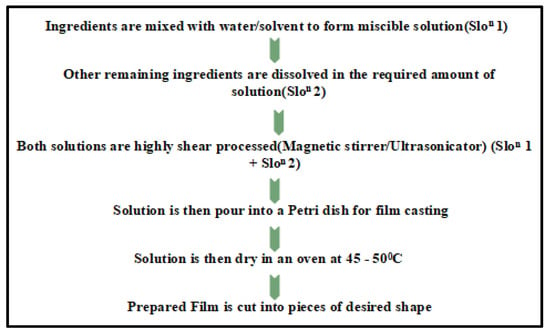
Figure 1.
Solution casting technique.
3.3. Radiation Technique
There are many other techniques used by the researchers to prepare solid polymer nano electrolyte films, using an ultra sonicator or microwave radiation. It was found that for the preparation of MMT, dispersed PMMA-PEO-LiClO4 PNC film, such as radiation techniques, are implemented. In this, Microwave Radiation (MW), Ultra Sonicated (US), US-MW irradiated, and Solution Cast (SC) are used in the manner shown in Scheme 1, below; the polymer composite films are then further characterized by an impedance analyzer for their dielectric properties [32,33,34].
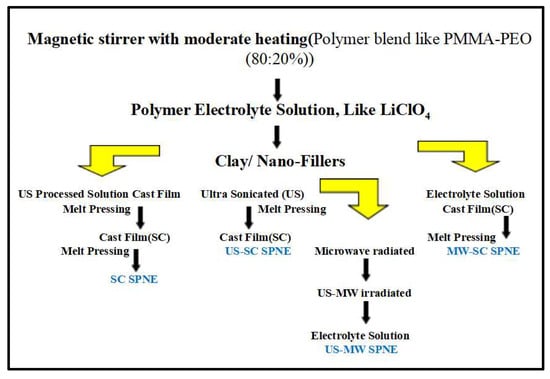
Scheme 1.
Steps for the preparation of nano composite films by different methods: SC, US, MW and US-MW by melt pressing. Reprinted from [32] with the permission of Springer Nature.
Different schemes and practices used for obtaining perfectly miscible PCs films:
There are various techniques and methods to obtain perfectly dissolved and miscible solutions using different materials, organic or inorganic, by using some solvents inert in nature. The first technique is the most commonly used method, a. Solution Cast (SC) In this method, firstly, polymer blends are dissolved using acetonitrile and THF (tetra hydro furan), then, PEO is homogeneously mixed in ionic salt using a magnetic stirrer [20]. Further, MMT and PMMA are stirred, and these solutions are further stirred for a few more hours and finally poured into a Teflon Petri dish and dried to form a film of the required thickness. A portion is taken from it and subjected to another treatment. Mentioned here as the Ultrasonic processed SC (US-SC) method, b. The second portion of the polymeric electrolyte solutions with different wt% of MMT was sonicated by an ultra sonicator (250 W power, 25 kHz frequency), as shown in the above chart 1, for up to 10 min duration with 15 s pause after every 15 s. In this process, the stainless-steel rod (i.e., sonotrode) was directly immersed into the electrolyte solution and we set it to ultrasound (strong dose). These ‘ultra-sonicated solutions’ were cast in Petri dishes and set to evaporate solvent, and the US processed electrolyte films were then obtained [32,33]. The third technique is c. Ultra Sonication followed by microwave irradiation (US-MW). The third part of each of the electrolyte solutions were initially ultra-sonicated and subsequently irradiated by microwave energy, i.e., em-energy used for the domestic purpose microwave oven (600 W power, 2.45 GHz frequency) for up to 2 min duration and with a 10–15 s irradiation step for intermediate cooling. The SC method was used to obtain free standing ‘US-MW-processed prepared electrolyte films’ (SPNE) [32,34]. The last technique is microwave irradiation on solution casting solution. d. Microwave-irradiated SC (MW-SC) method. In this forth part, each of the electrolyte solutions are irradiated by MW, em-energy used for a domestic purpose microwave oven (600 W power, 2.45 GHz frequency) for 2 min duration with 10–15 s irradiation steps for intermediate cooling. The solution was then cast onto Teflon Petri dishes and floated on a mercury bath to achieve the films of equal thickness. MW-irradiated SC films were achieved by the evaporation of solvent [32,33,34,35]. Films or materials synthesized using ultrasound and microwaves did not show much difference in dielectric values with those values obtained from simple solution casting techniques, but a slight accuracy in the parameters of physical quantities was observed.
4. Factors Affecting Dielectric Properties of Polymer Composites
4.1. Dispersion Mechanism in Composites
Another important factor which affects the dielectric properties to some extent is dispersion, or the mixing behavior of both filler materials and polymer matrix [36,37]. The effect of the homogeneous dispersion is a pivotal factor because, as the content of fillers in PMMA increases, the bundling tendency increases and thus affects the uniform distribution, which increased some order of the crystallinity of PMMA, as observed in Figure 2, for the case of nanotube and PMMA [37].
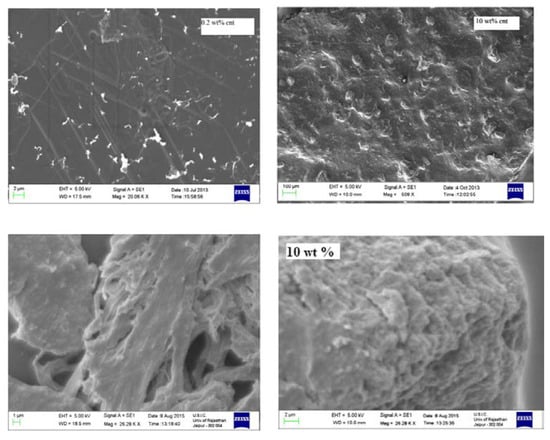
Figure 2.
SEM micrographs representing miscibility of MWNT/PMMA with the increasing content of MWNT. Reprinted from [37] with the permission of Materials Research Express.
According to M. Supova et al. [13], a properly dispersed method generally yields more desirable composite properties. After perfect blending, the synthesized material can be in blend or composite form, thus, the dielectric materials are classified with their multi-phase or single phase: single phase materials are known as blends, which do not always provide all the essential features and therefore seeks great attention from researchers to discuss multi-phase materials or composites [13,15,24]. In multi-phase materials the ‘inter spatial relationships’ are explained on the basis of connectivity between fillers and polymer composites. This also affects the dielectric and other physical properties of the blend composites [10,11,12].
A new concept, which emerged out for the PNCs, is the substantial increase in the interface formed between the nano fillers and the polymers. It happened when one of the characteristic sizes of the fillers is reduced to a nm scale, as shown in Figure 3. For spherically shaped fillers, the interface area increases by 106, when its radius is lowered from micrometer to nanometer. This considerable increase in the interface area thus amplifies the interaction between polymer blend matrix and nano fillers, and this interaction makes a profound contribution to the ‘macroscopic material properties’ of the PNCs; their properties are different to those of the bulk polymers [13,36,37].
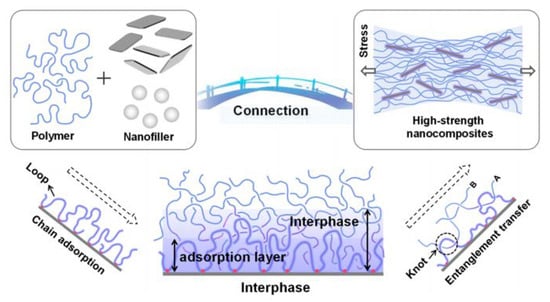
Figure 3.
Formation and transfer mechanism of the interfacial phase in polymer nano composites from [24] under creative common license.
4.2. Filler Size and Shape
As Huang, J. et al. [24] commented, and as shown above, for the filler size and its effect on the consequences of dielectric properties, the dielectric constant (ε″) of a synthesized material depends on the inter facial (IF) polarization. When the size of the filler particles decreases, the ‘effective surface area’ of a filler in connection with the matrix polymer increases, which leads to the increment in IFP (inter facial polarization), which further improves the dielectric constant value, but loses the strength of dielectric properties [13,14,15,24]. One of the research studies revealed that the needle-shaped TiO2 fillers increased the dielectric constant more than the spherically shaped TiO2 fillers, when composed with polymeric liquid crystals at a lower volume than spherical ones. Another investigation has also reported that, as the filler size taken in nano scale, there would be an effective increase in dielectric losses; this is due to agglomerate tendency, which exhibit in NPs, or it can state that PNCs materials have higher dielectric losses than materials of micro scale composites [15,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34].
4.3. Porosity
Another key factor affecting dielectric properties in polymer based nano composites with fillers is the porosity. Usually, the porosity is not desirable in ceramics. The porosity can be reduced, and the permittivity and dielectric losses of dense ceramics can be estimated by the porosity. The porosity decreases the VBD (Breakdown Voltage) resulting in the degradation of the ‘electrical reliability performance’ of the material, but it does not affect the leakage current. It is concluded from earlier consequences that using the fillers with a small dielectric constant value, or high porosity of the material, reduces the values of dielectric properties of the composite polymer materials [6,12,13,19].
4.4. Loading of Fillers
On increasing the weight of filler content in polymer nano composites, dielectric constant values greatly increased. However, in the case of ceramic fillers, a large content of ceramic makes the synthesized composite heavy and bulky, due to this, an increase in the loss of flexibility is observed resulting in the poor dispersion of fillers [6,12,19,20,24,26]. The loading of filler also affects the dielectric loss and the strength of a composite. B.K. Sharma et al. reported in their work that the dielectric loss factor decreased with the addition of ZnO in the PANI matrix at room temperature [38]. The authors also stated that the dielectric loss increased with the loading of semiconductors [31,32]. At a low filler loading, the composites exhibited high dielectric values with high dielectric strength. In this article we also included the effect on conductivity of the polymer composites with increasing loading fillers, at both low and high frequencies and the applications of the polymer nano composites doped with different inorganic fillers.
5. Dielectric Properties and Relaxation Behavior of PMMA and PMMA Based Composite Films
PMMA is a linear and amorphous thermo plastic polymer with high transparency [6]. PMMA transmits light in the range 360–1000 nm with almost nil loss. It is stable when exposed in sunlight. It has an extraordinary characteristic to resist against “oxidative photo degradation” [39,40]. Because of its perfect stability in different weathering situations, it is classified as hard and rigid, but brittle in nature. Its tensile strength and compressive property are satisfactorily sufficient for its wide applications and popularity in industries and research [40,41].
Its inherent resistance is also good and can be improved by special coatings or suitable loadings. It has the property to withstand both acidic and alkaline media due to its large hydrolytic resistance. It is non-reactive with many other inorganic materials, non-polar solvents, acids and alkaline, etc., [41]. Further, the benefit of PMMA is its easy availability and low cost. Some of its properties match to other occurring polymers but few among them can be produced from its liquid, nonvolatile and low-cost monomers [39,41]. It has been reported that the conformational characteristics of the polymer chain is due to the micro structure of the polymer network, and this property helps to define its physical properties such as chain, flexibility, miscibility and glass transition behavior. The dielectric constant of PMMA, measured at 1 kHz and 1 MHz, are found to be 3.0 and 2.6, respectively, at 25 °C [42,43].
Although the dielectric properties of PMMA and PMMA based polymer or composites/blends have been studied thoroughly by many researchers, its optical, thermal and electrical capabilities were also explored. From the latest research, it was found that PMMA with different fillers behaves differently, especially for the dielectric properties of PMMA [41].
As the next sections describe the tuning of dielectric properties of PMMA by forming composites with different inorganic materials (fillers), it is essential to review the structure of PMMA. In this context, PMMA is polymerized from the ‘monomer methyl meth acrylate by initiators (free radical), such as ‘peroxides and azo-compounds via free radical’ vinyl polymerization and are exemplified as below. It has excellent retention ability and compatibility with liquid electrolytes and exhibits good interfacial stability towards lithium electrodes. Moreover, few studies showed that the dielectric properties of such composites are also changed and affected due to the polymer chain tacticity, such as the relaxation time Tg [30,31,32,41]. The α-relaxation were studied for iso-tactic PMMA thin films supported on aluminum [44] where the dielectric properties are changed due to the change in the thickness of the film [41,44], which is a concept of stereo chemistry having relative arrangement within the polymer chains adjacent to chiral centers. It is studied that there are 3 ‘stereo regular arrangements’ obtained from PMMA viz.: (a) Iso-tactic; (b) Syndi-tactic; and (c) A-tactic, as shown in Figure 4.
Figure 4.
Poly methyl-meth-acrylate (PMMA).
In iso-tactic PMMA (iso-PMMA), the chiral centers have the same configuration, whereas in syndicate PMMA alternate chiral centers have the same configuration and, in atactic PMMA there is a random distribution of the substituent group [6,24,25,41]. For atactic PMMA (at-PMMA), dielectric measurement was performed under high pressure CO2 at different pressures (P) and temperatures (T). The dielectric loss of at-PMMA in the transparent glassy state is asymmetric because of the disturbance in density for the amorphous structure. The peak shifts to higher frequencies, additionally, the relaxation strength was found to increase with the increasing CO2 pressure, and in the glassy state the dielectric constant structure became more symmetric with increasing content of CO2 pressure. The study showed that the “apparent activation energy” has decreased with CO2 at high pressure [41,45]. Apart from these examples, glassy featured PMMA material has a broad application in medical sciences. It has several merits including high bio compatibility, reliability, along with some interesting physical qualities i.e., it is tasteless, odorless, it persists tissue irritation and toxicity [46,47]. Moreover, PMMA as modified PMMA or PMMA based polymer blend materials, also possess several properties with interesting change in behavior and variant applications, preferred in numerous biomedical fields such as ‘contact and intraocular lenses’ [48], ‘bone cement in orthopedic surgery’, and ‘removable dentures’ [49,50]. Further, it also has vast applications in interior design, and transparent glass substitutes by transparent dielectric films of PMMA [6]. PMMA as a host polymer is blended with other polymer such as PEO as PMMA/PEO—Salt-MMT (Mont Morillonite), PEG-MMT as PMMA-PEG-MMT, and PEO-SiO2 as PEO–PMMA-SiO2 [51,52], etc., and are synthesized with simple, low cost green chemistry techniques either by the sol-gel method, solution casting techniques for the accrual of electrical, and dielectric properties with high thermal stability. Several researchers highlighted such polymer blends/composite and their multi-functional applications in electronics or microelectronic devices [53,54]. PMMA has advantages in the automotive industry and domestic appliances. It is used for glazing in air crafts and boats and is also used as rear lamp light fixtures [6,51,52,53,54,55].
The dielectric properties of PMMA based on different polymer blends/composites with fillers, with incorporation such as semiconductors, metals, metal oxides (ZnO, SiO2, SnO2, TiO2 & Al2O3) or salts such as PMMA/PEO-Salt-MMT (Mont Morillonite), PMMA-PEG-MMT, PEO–PMMA-SiO2, Al-PMMA-TiO2–Al (sandwich structure), PMMA-PVC-LiTFSI-BmImTFSI-SiO2, PMMA-(CCTO)CaCu3TiO12, and PMMA-STMO(Sr2TiMnO6) [20,25,28,31,32,34,56,57,58,59,60,61], etc., were investigated by several authors, taken into consideration at temperature ranges from 28 °C to 55 °C. Recent studies and reviews revealed that the reduced values of dielectric losses and enhanced values of dc conductivity for different frequency ranges can be achieved by loading different concentrations of nano particles such as ZnO, SiO2, SnO2, Al2O3, etc., and highlighted the material’s applications in electromagnetic shielding, optoelectronic, microelectronic devices and automobile industries, such as glazing in air crafts or boats. Some of the metal oxides have good absorbing quality for electromagnetic radiations when doped in polymer, and can be used for electromagnetic shielding [6,20,52,53].
It is also analyzed from previous theories that solid polymer electrolytes (SPEs) composites have lower conductivity due to high restriction in the motion of the ‘polymer molecules’, affecting the performance efficiency of the devices that use SPEs. SPEs promoted the development of ‘gel’ or ‘plasticized’ polymer electrolytes, whose conductivity is comparable to that of the ‘liquid electrolytes’. However, these materials again suffer from weak mechanical strength and stability due to the presence of ‘volatile solvents’ [47,55,56,57,58,59,60,61,62]. To reduce such a gap, numerous modified chemical and physical techniques have been adopted by researchers, including polymer blending and doping.
Polymer blends, or composites with improved dielectric properties and functionality, can be tuned with significant advantages in new technological electric and medical fields. So, such polymer blends or composites with nano-fillers represent an underutilized and valuable resource, accounting for a diverse range of applications and energy storage challenges [63,64]. Special attention has been given towards new materials, along with these new conducting materials, having fast ion-conducting properties (conductivity ranges from of 10−5–10−1 S cm−1) at 27 °C. Such materials are called ‘Super Ionic Solids’ or ‘Fast Ion Conductors’, depending on their structures, compositions, phases and their physical properties [6,24,41,42,43,44,45,65,66,67,68,69,70,71]. Thus, different factors are considered while designing and synthesizing the materials, such as synthesis methods, fillers used, size, shape, surface treatment of filler, etc., [6,15,33,41]. Here, we emphasis, mainly on the effect on dielectric properties of composite polymer (PMMA) loaded with varying concentration of inorganic fillers, salts or ceramics (Figure 5) along with their synthesis and fabrication techniques at ambient temperature (30 °C), with the comparative studies of differently loaded PNCs.
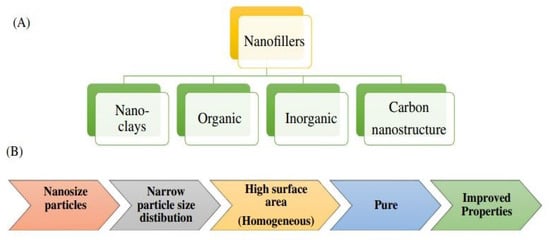
Figure 5.
(A) Classification of nano fillers on the basis of chemical nature. (B) Process for incorporation of nano fillers in polymer nano composites from [33] with the permission of Polymer Composites, WILEY.
Filler Materials: Nano Additives Reinforcement
Inorganic fillers like metal oxides such as ZnO, Al2O3, SnO2, SiO2, TiO2, ceramics (MMT), and salts such as LiClO4, etc., are used as fillers, especially in nano-forms. These NPs have different absorbing, thermal, miscibility and conductivity behavior, which on mixing (by x-wt%) with different polymer blends affect their dielectric properties at different frequency ranges (20 Hz to 1 MHz); a few of the PNCs are also reported with varying temperature. Studies reflect that the dielectric properties of PNCs also depend on the techniques discussed above for their synthesis, including the SC technique, US, MW radiation or the US-MW process [20,24,31,32,33,34,35,36,37]. Molecular bonds between the ‘inorganic filler’ and the ‘polymer blend matrix’ plays an important role for their structure and surface morphology, which affects its electrical band gap and alters its dielectric properties. This ‘interfacial connection’ (IC) is essential for transmitting the applied load from the ‘blend matrix’ to the ‘type of fillers’, where structural and thermal specifications could be affected with the molecular bonding. Inorganic additives can have crystalline or amorphous structures with their peak intensities and size, which is characterized with ‘X-Ray Diffraction (XRD)’ and ‘Scanning Electron Microscope (SEM)’ [20,26,28]. A few of the filler NPs are reported here, with their dramatic change in dielectric properties.
5.1. Alumina (Al2O3) Nanoparticles
Sengwa and Shobhna reported that the conductivity of PEO-PMMA increases with filler Al2O3 nano-powder, and is the leading contender among the ‘ceramic nano materials’, which is cost-effective and bears promising thermal abilities [33]. It is a semi crystalline material with a relative dielectric constant εr (at 1 MHz and 30 °C) as 9.7. It is a ‘transition ceramic material’ with an ultra-variety of meta-stable structural phase each having a different ‘degree of crystallinity’ and ‘thermo mechanical properties’. Because of these factual clarities, Al2O3 NPs (as inorganic fillers) are largely consumed for the polymer nano composite film preparations and also referred to as the ‘novel NSPEs’ [26]. It has been suggested that using Al2O3 in the polymer blend (PEO-PMMA (50–50 wt%)), the blend matrix significantly reduces its crystallinity and dielectric permittivity and it too restricts the ‘cooperative chain segmental dynamics’ of the polymer blend, which unevenly changes with the further addition of an increased concentration of Al2O3. Using the solution cast method, the film of Al2O3 doped polymer blend PMMA/PVA (50:50 wt%) was prepared and then subjected to impedance analyzer at temperature 31 °C to find the values of ε′ and ε″ for the frequencies, ranging from 20 Hz–1 MHz. A slight decrease in the net values of ε′, ε″ and loss tangent (tan δ) was observed, whereas the electric modulus M′ was increased for the prepared film. The observed conductivity was less (ranges 1 × 10−13 to 7 × 10−9 S/cm) for the audio frequency range (20 Hz–20 KHz) to lower the radio frequency range (20 KHz–1 MHz) with very high impedance, which reflects its use in electric insulators or polymeric dielectric substrate. Studies on the addition of Al2O3 in polymer blend PEO-PMMA-LiTFSI suggest that the reduction in the ‘degree of crystallinity’ of the polymer matrix, and the increase in the ‘mobility of the polymer chains’ and thus strengthens the Li+ ion transport capacities in the film, and improves the ‘ionic conductivity’. It is observed that PEO-PMMA-LiTFSI has conductivity 6.71 × 10−7 S/cm but when Al2O3 is added to this (i.e., PEO-PMMA-LiTFSI-Al2O3), the conductivity value is raised to 9.39 × 10−7. It is also noted that raising the content of LiTFSI in the film, the ratio of Eo and Li+ (Eo/Li+) reduces from 20 to 10 or the ionic conductivity is increased from 3.01 × 10−7 to 9.0 × 10−7 S/cm. Such films are used in the lithium batteries of mobile phones, laptop and digital devices. The incorporation of Al2O3 in PEO-PMMA-LiClO4 gives the high values of the ionic conductivity. At a low frequency range, its values are around 2 × 10−8 S/cm and for high frequencies these raise to 7 × 10−6 S/cm. P. Sharma noticed its behavior with the addition of Al2O3 used as inorganic nano fillers (INF) in PEO-PMMA-AgNO3 with 4 wt%, significantly increases conductivity than its un-doped form (~10−9 S/cm). Thus, such PMMA based polymers nano composite on doping with Al2O3 due to its low loss tangent, dielectric constant and high conductivity, influenced the PNC films [26,27,31,32,33,34].
5.2. Titanium Dioxide (TiO2) Nanoparticles
TiO2 is another gem in this family, which can tune the dielectric properties significantly. It is noticed that a decrease in ε′ values is small when the frequency of the applied field increased from 20 Hz to 1 MHz. This finding confirmed the suitability of these nano composites as good quality nano dielectrics for developing the operative ac electric field for numerous types of ‘microelectronic devices’ [35,36]. Additionally, the ε′ spectra and the curve obtained with the frequency variation are different when compared with different nano fillers in different concentrations to the PMMA matrix. The addition of TiO2 NP in the PMMA matrix appreciably lowered the ε′ values of the resulted composite films, as compared to that of the pristine (pure) PMMA film. According to the literature, the dielectric permittivity (ε′) values of the TiO2 nano materials (At 1 kHz) at the ambient temperature (27 °C) is nearly one hundred (ie ε′ ≃ 100) [24,60,61,62,63]. The TiO2 (up to the 3 wt%) filler loaded films exhibited a lowering in ε′ values initially, and then a little rise in values for up to 5 wt% loading. These spectral changes confirm that the interaction of TiO2 with the PMMA structure is distinctive in regard to the alteration done in the ‘dipolar ordering’ of the PMMA-chains. Structural dynamics is slightly complex with the change in filler concentration or x wt% of TiO2 nano filler containing PNC films. An irregular variation in AC electrical conductivity was observed for the TiO2 loaded PNC films with the increase of these fillers’ concentrations. The comparative results of these broad band gap metal oxide semiconductors (viz. TiO2 nano crystallites) filled PMMA based PNCs films could be suitably promising with regard to their effectiveness for tailoring and developing the better energy storage and high-power electronic devices with impressive efficiency. Additionally, Adebahr [59,72,73,74,75], in his work, used amorphous Al-PMMA-TiO2-Al, a sandwich structured film prepared by the dip coating method, which showed high dielectric constant values (ε′ = 26.8). An increase in the values of ε′ from 14.3 to 26.8 and tan δ from 0.1 to 0.79 with temperature is reasonably good, which is used as high dielectric layer in thin film transistors. A prominent and significant increase in the values of ε′ & ε″ was observed due to incorporation of increasing wt% of nano filler TiO2, and a frequency change from lower to higher in PEO-PMMA-LiClO4. The real and imaginary values of permittivity (ε′ and ε″) at a low frequency range, and at the interface between the polymer film and electrodes, attributes to the free charge build up. An enhancement in conductivity is also shown due to the rapid dissolution of salt because of ion filler interaction, resulting in a higher number of free charge carriers [62,63,76,77,78]. The theoretical studies also revealed the irregular variation in electrical conductivity for TiO2 loaded PMMA polymer with different wt% and different frequency ranges from 20 Hz to 1 MHz.
Another Most Demanding Metal Oxide Nano Particles (MO NPs)
5.3. Zinc Oxide (ZnO) Nanoparticles
According to Noto et al., ZnO is highly crystalline and the particle size tp and relative dielectric constant εr (at 1 MHz and 30 °C) of ZnO are tp < 100 nm and εr = 10.26, respectively [24,34,64,65,66,67]. When the frequency of the applied field increased from 20 Hz to 1 MHz at 30 °C, a gradual lowering in ε′ values is observed in a film containing ZnO nano particles dispersed in PMMA matrix. This confirms the suitability of these nano composites as high-quality nano dielectrics for developing the operative ac electric field in various types of microelectronic devices. ZnO dispersed nanoparticles in PMMA-matrix appreciably increased the values of ε′ of the developed composite films, as compared to that of the pure (pristine) PMMA film. In another work of Wu W. [65], the dielectric permittivity (ε′) values of the ZnO nano materials at 1 kHz frequency and at the ambient temperature reported is ε′ ≃ 40 [61,63], which is much higher as compared to that of the pure PMMA, and therefore their dispersion in this polymer matrix should have increased ε′ values with the increase of ZnO concentrations by the simple mixing of the constituents of a dielectric composition. ε″ and tan δ spectra of the different wt% of ZnO doped PNC films exhibited a broader peak in the lower frequency region, which can be ascribed to the ‘ester group rotations’ of the repeat units of the PMMA-chain (i.e., β-relaxation process) [24,46]. The incorporation of ZnO (1–5 wt%) in the PNC films, a relaxation peak for -COOCH3 groups rotational dynamics is noted at lower frequencies. Findings also show a non-linear rise in electrical ac conductivity for the ZnO containing PNC films. The PNC film of ZnO (1–5 wt%) doped in PMMA-PVA blend with 50–50 wt% is prepared by the solution casting technique and its dielectric properties is investigated using impedance spectroscopy for frequency 20 Hz to 1 MHz; results revealed lower electrical conductivity and high impedance, thus such films are utilized in electric insulators. When ZnO is incorporated in PEO-PMMA-LiClO4 by the SC technique [63], using acetonitrile as solvent, and the electrical properties viz: dielectric constant (ε′) and complex permittivity ε″ values of the prepared films fall very steeply from a lower frequency range to a higher frequency range. Spectra of tan δ increases slightly thus the lowering of ionic conductivity is small for ZnO loaded polymer blended films. In our earlier work [34], we reported the increase in conductivity with filler percentage of ZnO incorporated in PVDF/PMMA and it is suitable for microelectronic applications because of the direct dependence between the dielectric parameter and the mean relaxation time in the polymer chain SD (segmental dynamics). Similarly, B.K. Sharma et al. [38] reported an increase in electrical conductivity (1 S/cm) when compared with its intact form to the polymer composite containing ZnO content with increasing weight % in poly aniline (PANI) at temperature (30 °C) [38].
5.4. Tin Oxide (SnO2) Nanoparticles
Initially, SnO2 is taken as a nano filler owing to its n-type direct energy band gap (Egd = 3.6 eV) with broad functionalities and large potential applications [24,51]. The particle size ‘tp’ and ‘relative dielectric constant’ εr (at 1 MHz and 30 °C) of SnO2 are tp < 100 nm and εr = 34.5, respectively. PMMA/x-wt% SnO2 films at 30 °C reveals the gradual decrease in ε′ values for the frequency ranges from 20 Hz to 1 MHz of the applied field. Further, the shapes of the ε′ spectra for all these nano dielectrics are almost similar with the variation in frequencies, but it is substantially different when compared with differently loaded nano fillers, i.e., SnO2 in the PMMA-matrix. The dielectric permittivity (ε′) value of SnO2 nano materials (1 kHz) at ambient temperature is observed as ε′ ≃ 250 [20,47,51]. Again, the higher values of ε′ were noted as compared to pure PMMA, thus their dispersion in this polymer matrix should have increased ε′ values, which is due to increased concentration ratios of SnO2 in the respective PNC films. The ε″ and tan δ spectra in a few SnO2 concentrated PNC films exhibited the ‘relaxation process peak’ closer to the lower end frequencies, and for the remaining films, it is expected to have this process peak for frequencies less than 20 Hz. The consequences reveal that the ‘rotational dynamics of the -COOCH3 group’ occur relatively faster in ZnO nanoparticles fillers as compared to the nano fillers, such as SnO2 and TiO2 doped PMMA-matrix [20]. Structural dynamics are slightly slow for the SnO2 nano filler-based PNC films, whereas it is relatively complex for the TiO2 nano filler containing PNC films, with the dispersion of different variation of filler concentration in these PNCs films [20,51]. Additionally, a relaxation peak for ‘-COOCH3 groups rotational dynamics’ was noted at lower frequencies. An anomalous increase in electrical ac conductivity was confirmed for the increasing x wt% of SnO2 dispersed films [20]. The comparative consequences of the broad band gap metal oxide semiconductors viz. Zinc Oxide, Tin Oxide, and Titanium Oxide nano crystallites/nano particle loaded PMMA-matrix based PNCs films, could be strongly satisfying and promising in favor to their effectiveness for generating new ‘power consumption and storage devices’. Thus, PMMA loaded with ZnO, SnO2 and TiO2 films have shown wide applications in opto-electronics, organo-electronics and microelectronic devices [20,26,34].
5.5. Silica (SiO2) Nanoparticles
Silica (SiO2) has attracted considerable academic, industrial and technological interest, which is taken as dopant in the polymer blend PMMA-PEO PNCs films. SiO2 is amorphous and the particle size tp and relative dielectric constant εr (at 1 MHz and 30 °C) of SiO2 are tp < 15 nm and εr = 3.8, respectively [25]. Fumed silica, an inorganic filler, was produced from a ‘Continuous Flame Hydrolysis Technique (CFHT’)’ of silicon tetrachloride in a hydrogen–oxygen flame [52]. There are two techniques involved in the production of fumed silica: firstly, ‘silicon tetrachloride (SiCl4)’ is converted to the gas phase; then, it is allowed to react with water to yield SiO2 and ‘hydrochloric acid (HCl)’. It can be seen that the RN value is relatively high for the ‘SiO2 dispersed NSPE film’ and low for the ‘ZnO dispersed NSPE film’ [34]. RN is inversely related with εr and particle size, thus a high value of RN for the SiO2 dispersed NSPE film may be due to the ‘relatively low dielectric constant’ and small ‘particle size of the SiO2’ [25]. The satisfying results confirmed the particle size of the dispersed nano fillers, which also influences the RN values of the NSPE films, i.e., the smaller particle sized nano fillers cause high RN values of the NSPE film [33,67]. Silica, when added with PMMA-PVA by 50/50 wt% ratio with increasing wt%, gives a lesser value of ε′ (<2.5), significantly low ε″ and tan δ reduces gradually with a frequency range (lower to higher), whereas conductivity increases linearly, and impedance decreases linearly, which results in ohmic behavior. Through the dispersion of x-wt% of SiO2 in different ratios of PEO/PMMA (75/25%, 50/50%, 25/75%), PNC film were generated by solution cast technique using di-chloro methane as a solvent. The dielectric relaxation process of the prepared films confirms that the ‘polymers cooperative chain segmental dynamics’ becomes significantly slow when merely 1% of SiO2 NPs were dispersed in polymer blend matrix. Around 1 MHz/30 °C the ε′ value of film is around 2 to 2.5 with very low dielectric loss ε″, which suggests the suitability of these films as a low permittivity nano dielectric substrate [33,52,78,79,80,81].
SiO2 loaded PEO-PMMA-LiClO4 shows an increased value of conductivity from 2 × 10−8 to 2.1 × 10−5 S/cm [67]. The synthesis of P(VDF–HFP)–PMMA–LiCF3SO3–(PC+DEC)–SiO2 composite polymer electrolytes and the maximum ionic conductivity of this polymer electrolyte system is found to be 1 × 10−3 S cm−1 at 303 K. Mechanical and thermal stabilities at temperatures above and below their melting points were examined for the CPEs, and enhanced values are observed by developing a 3D network via H-bonding among the aggregates. In accordance with Ahmad [52], who outlined the effects of fumed silica nano particles on the ionic conductivity of polymer electrolytes, it is expected to be decreased at temperatures above melting point, whereas it increased below the melting point [33,49].
5.6. Lithium Triflate (LiCF3SO3) as Ionic Salt and Mont Morillonite (MMT) Clay
Sengwa, Choudhary and Dhatarwal published the influences of UV and microwave preparation methods on structural and dielectric properties on the polymer electrolytes (PEs). As such, PEs are attracting much attention due to its potential applications in ‘electrochemical devices’ such as ‘rechargeable batteries’ and ‘fuel cells’, etc. Electrical conductivity is the main property for such [32] electrolyte films and several researchers have made great endeavors to raise the values of electrical conductivities [52,53,54,55,56,57]. The authors reported that when MMT (× wt%) is dispersed in PEO-PMMA-LiCF3SO3, PNC film is prepared by different methods and is characterized by an impedance analyzer for its dielectric properties. It is noted that the values of ‘dielectric constant’ and ‘dielectric loss’ changes with its preparation methods, and also with the amount dissolved in it. MW, US, US-MW irradiated and SC methods were used to prepare these PNC films and the value of σdc is obtained by using , where ‘ts is thickness’ and ‘A is surface area’ of the film. The σdc value for 5 wt% by different technique of preparation which revealed that:
σdc (MW) > σdc (US-MW) > σdc (US) > σdc (SC)
Here, it is noticed that the dominance of intercalated structures by using different techniques reduces the ‘crystalline morphology’ of the electrolyte materials, which favors the enhancement of ‘ionic conductivity’, which is the major cause for greater σdc due to the ‘MR-technique’ than the σdc obtained for the films prepared by the ‘SC method’. It is also found that the value of σdc for 10% MMT is greater than σdc value for 5% MMT, but the difference is not remarkable.
5.7. Lithium Chlorate Ionic Salt and MMT-Clay
At room temperature, these PNC films containing low salt ratios have significant ionic conductivity values, which reveals their potential applications in ‘electro chromic devices’ and also as ‘electrolyte material for lithium-ion batteries’. The intercalated amorphous structures of PMMA-PEO-LiClO4+ × wt% of MMT clay is superior and novel than the electrolyte blend of PMMA-LiClO4+ × wt% of MMT and the intercalated PEO-LiClO4+ × wt% of MMT electrolyte [68,69,70,71,72]. It is also recorded that the same methods for the sample preparation are not always effective for getting high ionic conductivity of electrolyte films containing different salts concentration. The dc conductivity “σdc” values of these PNCEs films increases linearly from 10−8 to 10−5 S cm−1 with the increasing percentage of salts concentration at Troom [71,72].
5.8. Sr2TiMnO6 (STMO)/CaCu3Ti4O12 (CCTO) Ceramics
According to P. Thomas, PMMA and STMO were fabricated via melt mixing followed by the hot pressing technique. An impedance analyzer is used for the characterization of dielectric properties. STMO content in PMMA is up to 50 wt% where the glass transition (Tg) temperature of the PMMA polymer and their composites shows no variation or difference, whereas the permittivity (real and complex) found were very high, i.e., 30.9 at 100 Hz for the PMMA-STMO-50 wt% composites, indicating the possibility of using these materials for ‘capacitor applications’ [73]. The thermal stability of such polymer blends was enhanced by the incorporation of STMO fillers. As the content of STMO is increased in polymer blend there is an improvement in polymer density and hardness [73]. The composite PMMA-CaCu3Ti4O12 fabrication process was outlined by Thomas and Dakshayini by ‘melt mixing’ followed by hot pressing, and the permittivity obtained in this case is found to be 4.9 at 100 Hz, which is raised to 15.7 at 100 Hz for 40 wt%. PMMA-CCTO has low dielectric loss, due to which PMMA-CCTO can be exploited for the high frequency ‘capacitor applications’ [64]. We can see that PMMA with STMO ceramic has high values as compared to PMMA with CCTO, and so it reflects the usage in different fields [74,75,76,77,78].
6. Some Reported Experimental Results (Case Studies)
In this last section, the case study for the dielectric properties of 25 polymer composites are studied at low and high frequencies with constant filler quantities, to observe the dependence of dielectric constant (ε′), dielectric permittivity (ε″), loss tangent (tan δ), conductivity and electric modulus (M′) and the results are categorized.
It is known from studies by several researches that polymer and inorganic fillers have their own physical properties, but when mixed with each other, depending on its structural and dielectric properties, either it takes the formation of a nano composite polymer electrolytea micro composite polymer electrolyte or a (NSPE material with a significant band gap and significant properties. Some of the polymer mixtures with different preparation techniques are focused upon here, showing their augmented data and values. Extensive work has been carried out so far and is still in practice by scientists to discuss the morphological, structural, thermal and dielectric properties of numerous composite polymer materials for the authenticity of their multi functionality as novel composite polymer materials. In this article, along with enlightening the knowledge on the synthesis of various materials with fillers their dielectric properties viz. dielectric constant and dielectric loss, conductivity is recorded at 20 Hz and 1 MHz to differentiate the composite polymers materials used as conductors or insulators.
Here, mainly polar polymers, namely, PEO, PVA, PVP, PMMA and their blends, take PMMA as a base polymer which is then further loaded with different inorganic nanofillers in different weight ratios, viz. MMT clay, ‘salts LiTFSI’, ‘LiClO4’, and ceramics such as: ‘SCTO and CCTO’. Metal Oxides such as Al2O3, ZnO, TiO2, SiO2 and SnO2 are discussed with their dielectric properties tabulated in Table 1 [20,24,25,26,27,31,32,33,34,35,38,41,42,43,44,45,46,47,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79].

Table 1.
Values of dielectric parameters (ε′, ε″, tan δ, M′ and σ′) of different sample materials with loading fillers.
After a thorough study of these modified material composites and their structure, transition time, and the dielectric parameters of their values are collected and framed in Table 1. The dielectric constant, dielectric loss, conductivity, electric modulus and impedance values at 20 Hz and 1 MHz are noticed and further plotted, shown in Figure 6, Figure 7A,B and Figure 8A,B, and a comparative analysis have been conducted. Characteristic graphs represent these dielectric parameters for low frequencies; it is independent to materials, but for higher frequencies, peaks are shown.
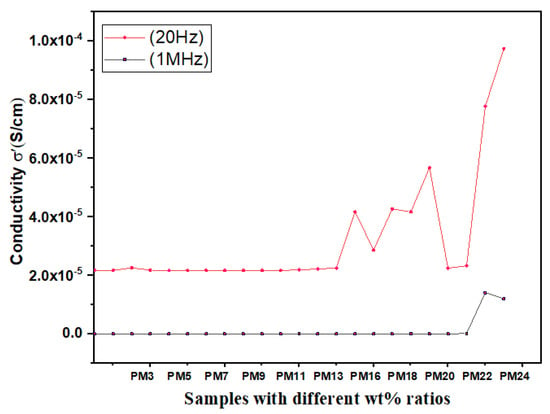
Figure 6.
Conductivity “σ” dependence on different polymer nano composite films at frequencies 20 Hz and 1 MHz.
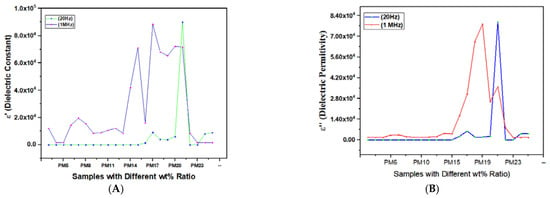
Figure 7.
Variation in dielectric constant ε′ (A) and dielectric permittivity ε″ (B) of different polymer nano composite films at frequencies 20 Hz and 1 MHz.
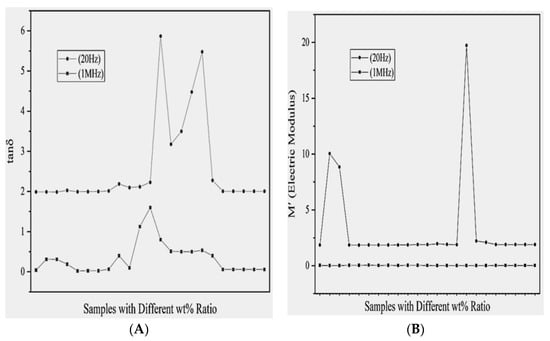
Figure 8.
Variation in (A) dielectric loss ‘tanδ’ and (B) electric modulus ‘M’ of different polymer composite samples at low (20 Hz) and high (1 MHz) frequencies.
This theory reveals the classification of dielectric properties of the material blends and composites having versatile applications in electronics and sensing materials.
The following graphs and table are reported here for comparative study and analysis.
The spectra of σ’ represents that the values are relatively high at frequency 20 Hz, whereas the values reduce for higher frequencies (up to 1 MHz frequency) due to fast ion oscillation. It is also observed that ac conductivity is increased due to high conduction for frequency raised from 20 Hz to 1 MHz.
From the graphs in Figure 7A,B, it is said that the dielectric properties of TiO2 doped multifunctional polymer NCs has nonlinear behavior. However, ZnO dispersed matrix composites named as high-performance flexible nano dielectrics with high value in dielectrics constant and improved conductivity at low frequencies (20 Hz), which are used in multifunctional microelectronic devices [75,76,77,78,79,80]. Significantly enhanced dielectric properties and ‘chain segmental dynamics’ were observed in SnO2 doped NCs [20]. Material permittivity is a static property, whereas conductivity is purely dependent on its dynamic property, thus to connect relation between these physical quantities a perfect time scale selection is required. Thus, from above graph, it is clear that the magnitudes of the dielectric constant for higher frequencies are low for both the values of the real and imaginary part of dielectric permittivity. The dielectric constant of the given or selected samples shows the amount of electrical energy that can be stored on applying voltage [69,70,71,72,73,74,75]. Thus, a dielectric material can become polarized when subjected to an electric field; on polarization of such materials, the electric field is thus reduced. Additionally, to correlate conductivity to dielectric constant we need to find the loss factor of our materials, which is the ratio of (ε″/ε′). All the samples containing PMMA have less values of loss factor, which for a higher frequency range (1 MHz) is less, as compared to a lower frequency range (20 Hz).
The graph in Figure 8A shows that the loss is relatively high for the materials containing PEO/PMMA (50/50 wt%)/LiClO4 (3 wt%), PEO/PMMA (50/50 wt%)/LiClO4 (3 wt%)/Al2O3 (3 wt%) and PEO/PMMA (50/50 wt%)/LiClO4 (3wt%)/SiO2 (3 wt%), but overall the loss is not very high. Its dielectric constant value is increased with the loading filler and their increasing content, comparing the data from Table 1 it is clear that the polymer blend PMMA/PVC has ε′ is equal to 0.17, the polymer blend with filler metal oxides such as PMMA/PVA/ZnO or SnO2 has nearly 1.8 dielectric constant. However, for the polymer blend with filler salts or salt + metal oxides such as PEO/PMMA + LiClO4 + ZnO, ε′ is 90,000, an extremely good result of the dielectric constant and properties. Further, it is clear for some of the samples that the measurement of loss factor at very high frequency shows no change, but for low frequencies it has high values in the case of metal oxide doped PMMA, than for the salt filler PMMA. Electric modulus is the important dielectric property of the polymer blends and physically corresponds to the relaxation of the electric field in the materials, electric modulus for low frequency variation shows changes for the differently doped polymer blends, while for higher frequencies (1 MHz depicted) it becomes independent to the dopants and their concentrations in the PMMA based polymer blends depicted in Table 1 [18,19,20,42,43,44,45,46,47,67,68,69,70,71,72,73,74,75]. Additionally, the complex permittivity is the inverse of permittivity of the material. This helps in finding relaxation phenomenon in the ceramic materials, as well as in ionic conductors.
7. Future Development and Perspectives
PMMA is an amorphous, cheap, chemically inert, extremely hydrophilic and glassy transparent polymer. It has good outdoor weather ability and excellent environmental stability; along with this, it also has good resistance towards acid and has high surface resistance. Although it has sound features, there are still some limitations as it is not ideal in all cases, especially when meeting with mechanical requirements. The addition of fillers to PMMA matrix may enhance mechanical properties, along with outstanding performance in electrical or optical applications. These metal oxides modify and increase the efficiency of material composites and enhance their durability. As in the case of resins, the recycled or modified PMMA may change the chemical or physical properties of acrylic resins [36]. It is worth noting that these fillers, along with remarkable results in dielectric constant and conductivity, etc., will improve mechanical strength. Some materials may have excellent results when immersed in water using modified PMMA, rather than using pristine PMMA. This is due to PMMA with fillers or additives having the lowest value of solubility, which increases the lifetime under water medium. Such materials are in demand and that is the reason why research in this field is growing faster and is appreciated in every field of science.
8. Conclusions
This review paper provides an overview of studies on the synthesis of PMMA based PNCs loaded with different wt% of fillers at ambient temperature, and its application on the basis of the results of dielectric constant, published in articles on PNCs, reflects that reinforcing the polymer with a rising amount of nano fillers prominently improves the polymer’s material properties. The amorphous nature of pristine PMMA is changed to crystalline form to some extent when doped with MO NPs. In this manuscript, we analyzed the improved and enhanced dielectric strength with high values of dielectric properties of PNCs doped with different type of fillers, with their increasing concentration among these dielectric properties; the dielectric constant plays a major role and is a big challenge to study. Furthermore, the wide-scale production of nano dielectrics with improved dielectric properties is a huge issue.
Inorganic fillers viz. ceramics fillers improve the dielectric constant and its electrical conductivity from 10−13 S cm−1 to 10−6 S cm−1 of polymer composites; however, when compared with metal oxides fillers they reduce the dielectric strength and mechanical properties. Additionally, in the case of salts, we obtained satisfying results as compared to non-doped polymer composites. Table 2 reflects that the synthesized materials have low values of conductivity (10−14–10−8 S cm−1) and the materials are used as insulators or dielectric substrate. It is also noted that the uniform dispersion of nano filler with polymer matrix results in a substantial increase in its dielectric properties and the ‘low percolation threshold’ plays a prime role in improving the dielectric properties. From the above analysis, PMMA associated polymer blend with inorganic fillers/modified PMMA, with the increasing concentrations in the polymer matrix, increases the dielectric parameters; that is, the values for ε′, ε″, tan δ, M’ and σ’ values for MO NPs with 3 wt% is less as compared to the 10 wt% of MOs doped materials. For the MWNT doped PMMA material films the perfect miscibility for 10 wt% is compared to 2 wt%, which influences the enhancement of ε′ value, resulting in a significant increase in conductivity. This too influences its mechanical and optical characteristics. These affected values changed their electrical behavior and their uses in distinguished fields. It is clear from the graphs in Figure 6 that the conductivity of the materials is improved for blends in which the doping of salt like LiTFSI, LiClO4, etc., sample no PM 25 {PVC/LiTFSI/BmImTFSI (92 wt%) + SiO2 (8 wt%)} and shows maximum conductivity values 8 × 10−5 S/cm. Such high values of conductivity are due to the hopping mechanism and have wide applications in good conductivity electronic devices. With the enhanced value of electrical conductivity, i.e., high charge carrier mobilities, lithium-based polymer blends or composites have wide applications in electronic devices such as light emitting diodes (LED) and liquid crystal devices (LCD), as located in Table 2. It is also concluded that metal oxides such as SiO2 doped polymer blends have very low conductivity values ~10−13 S cm−1; such materials are widely used as insulators or dielectric substrates. From Table 2 and from the spectra of ε′, ε″, tan δ, M’, and σ’ in Figure 6, Figure 7 and Figure 8, it can be seen that the values are relatively high at lower frequencies that are around 20 Hz, whereas the values fall at higher frequencies (up to 1 MHz frequency). At higher frequencies, the conductivity reduces, which is due to the fast oscillations and thus the net ionic motion becomes less along a definite direction than that in the presence of a static condition i.e., at low frequencies (20 Hz). Materials with high dielectric constants can store more energy as compared to those materials with low dielectric constants; good dielectric materials have high electric susceptibility. The dielectric values of polymer composite less than 10, i.e., ε′ < 10 are used as insulator devices whereas 50 > ε′ >10 are used in transistor or electronic devices, but for ε′ above thousands, the polymer composite materials are used as Ion conducting devices (Table 2). Additionally, these polymer composites with fillers will have high durability due to an increase in mechanical strength by using such a polymer with fillers.

Table 2.
Polymer composite films with conductivity values and applications.
Author Contributions
Writing—original draft, F.D.; Writing—review & editing, M.B., F.D. and K.S.; Supervision, M.B. and A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Arbatti, M.; Shan, X.; Cheng, Z.-Y. Ceramic–Polymer Composites with High Dielectric Constant. Adv. Mater. 2007, 19, 1369–1372. [Google Scholar] [CrossRef]
- Popielarz, R.; Chiang, C.K.; Nozaki, R.; Obrzut, J. Dielectric properties of polymer/ferroelectric ceramic composites from 100 Hz to 10 GHz. Macromolecules 2001, 34, 5910. [Google Scholar] [CrossRef]
- Billah, S.M. Dielectric Polymers in Functional Polymers. Polymers and Polymeric Composites: A Reference Series, 2nd ed.; Mazumder, M.J., Sheardown, H., Al Ahmed, A., Eds.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-92067-2. [Google Scholar]
- Huang, X.; Jiang, P.; Tanaka, T. A review of dielectric polymer composites with high thermal conductivity. IEEE Electr. Insul. Mag. 2011, 27, 8–16. [Google Scholar] [CrossRef]
- Al-Bataineh, Q.M.; Ahmad, A.; Alsaad, A.M.; Ahmad, D. Optical characterization of PMMA/metal oxide nanoparticles thin films: Bandgap engineering using a novel derived model. Heliyon 2021, 7, e05952. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.; Liew, C.-W.; Ramesh, K. Evaluation and investigation on the effect of ionic liquid onto PMMA-PVC gel polymer blend electrolytes. J. Non-Cryst. Solids 2011, 357, 2132–2138. [Google Scholar] [CrossRef]
- Sharp, K.G. Advance Materials. Wiley Online Libr. 1998, 10, 1243–1248. [Google Scholar] [CrossRef]
- Schubert, U.; Hu¨sing, N. Synthesis of Inorganic Materials, 2nd ed.; Wiley VCH: Weinheim, German, 2005; ISBN 978-3-527-31037-1. [Google Scholar]
- Schubert, U. Polymers Reinforced by Covalently Bonded Inorganic Clusters. Chem. Mater. 2001, 13, 3487–3494. [Google Scholar] [CrossRef]
- Work, W.J.; Horie, K.; Hess, M.; Stepto, R.F.T. Definitions of terms related to polymer blends, composites, and multiphase polymeric materials. Pure Appl. Chem. 2004, 76, 1985–2007. [Google Scholar] [CrossRef]
- Deeba, F.; Gupta, A.K.; Kulshrestha, V.; Bafna, M.; Jain, A. Investigations on dielectric properties of PVDF/PMMA blends. Mater. Today Proc. Sci. Direct 2022, 66, 3547–3552. [Google Scholar] [CrossRef]
- Shobhna, C.; Sengwa, R.J. Effect of different inorganic nanoparticles on the structural, dielectric and ion transportation properties of polymers blend based nanocomposite solid polymer electrolytes. Electrochem. Acta 2017, 247, 924–941. [Google Scholar] [CrossRef]
- Suporva, M.; Martynkova, G.S.; Barabaszova, K. Effect of Nano fillers Dispersion in Polymer Matrices: A Review. Sci. Adv. Mater. 2011, 3, 1–25. [Google Scholar] [CrossRef]
- Ghule, B.; Laad, M. Polymer composites with improved dielectric properties: A review. Ukr. J. Phys. 2021, 66, 166–177. [Google Scholar] [CrossRef]
- Guo, N.; Di-Benedetto, S.A.; Tewari, P.; Lanagan, M.T.; Ratner, M.A.; Marks, T.J. Nanoparticle, size, shape, and interfacial effects on leakage current density, permittivity, and breakdown strength of metal oxide-polyolefin nanocomposites: Experiment and theory. Chem. Mater 2010, 22, 1567. [Google Scholar] [CrossRef]
- Sebastian, M.T.; Jantunen, H. Polymer-Ceramic Composites of 0-3 Connectivity for Circuits in Electronics: A Review. Int. J. Appl. Ceram. Technol. 2010, 7, 415–434. [Google Scholar] [CrossRef]
- Bafna, M.; Sain, N.; Khandelwal, A.; Deeba, F.; Gupta, A.K. Study of refractive index and dispersion behavior of KMnO4 doped poly-methyl-methacrylate(PMMA)composites. Mater. Today Proc. Sci. Direct. 2022, 66, 3481–3486. [Google Scholar] [CrossRef]
- Fenton, D.; Parker, J.; Wright, P. Complexes of alkali metal ions with poly (ethylene oxide). Polymer 1973, 14, 589. [Google Scholar] [CrossRef]
- Ramesh, S.; Liew, C.W.; Morris, E.; Durairaj, R. Effect of PVC on ionic conductivity, crystallographic structural, morphological and thermal characterizations in PMMA–PVC blend-based polymer electrolytes. Thermochim. Acta 2010, 511, 140–146. [Google Scholar] [CrossRef]
- Sengwa, R.; Dhatarwal, P. Polymer nanocomposites comprising PMMA matrix and ZnO, SnO2, and TiO2 nanofillers: A comparative study of structural, optical, and dielectric properties for multifunctional technological applications. Opt. Mater. 2021, 113, 110837. [Google Scholar] [CrossRef]
- Lee, I.-Y.S.; Niidome, Y.; Matsuo, T.; Yamada, S. Temperature Effects on Molecular Alignments at the Surface of Ultrathin Films Studied by SHG and Fluorescence Techniques. Anal. Sci. 1997, 13, 343–346. [Google Scholar] [CrossRef]
- Rawlins, K.; Lees, A.; Fuerniss, S.; Papathomas, K. Brightly Phosphorescent Trinuclear Copper(I) Complexes of Pyrazolates: Substituent Effects on the Supramolecular Structure and Photophysics. Chem. Mater. 1996, 8, 1540–1544. [Google Scholar] [CrossRef]
- Farmer, S.C.; Patten, T.E. Photoluminescent Polymer/Quantum Dot Composite Nanoparticles. Chem. Mater. 2001, 13, 3920–3926. [Google Scholar] [CrossRef]
- Huang, J.; Zhou, J.; Liu, M. Interphase in Polymer Nanocomposites. JACS Au 2022, 2, 280–291. [Google Scholar] [CrossRef]
- Ramesh, S.; Wen, L.C. Investigation on the effects of addition of SiO2 nanoparticles on ionic conductivity, FTIR, and thermal properties of nanocomposite PMMA–LiCF3SO3–SiO2. Ionics 2010, 16, 255–262. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Choudhary, S.; Dhatarwal, P. Investigation of alumina nanofiller impact on the structural and dielectric properties of PEO/PMMA blend matrix-based polymer nanocomposites. Adv. Compos. Hybrid Mater. 2019, 2, 162–175. [Google Scholar] [CrossRef]
- Deshmukh, K.; Ahamed, M.B.; Deshmukh, R.R.; Sada Sivuni, K.K.; Ponama, D.; Pasha, S.K.K.; Almaded, M.A.A.; Polu, A.R.; Chidambaram, K. The Minerals, Metals & Materials Society. J. Electron. Mater. 2017, 46, 2406–2418. [Google Scholar] [CrossRef]
- Sharma, P.; Kanchan, D.K.; Gondaliya, N.; Jayswal, M.; Joge, P. Influence of nano filler on conductivity in PEO-PMMA-AgNO3 polymer blends. Indian J. Pure Appl. Sci. 2013, 51, 346–349. [Google Scholar]
- Zhang, H.Q.; Jin, Y.; Qiu, Y. The optical and electrical characteristics of PMMA film prepared by spin coating method, IOP conference series. Mater. Sci. Eng. 2015, 87, 012032. [Google Scholar] [CrossRef]
- O’Brien, S.B.G.; Hayes, M. A model for dip-coating of a two liquid mixture. Int. J. Math. Math. Sci. 2002, 29, 313–324. [Google Scholar] [CrossRef]
- Ullah, N.; Cui, J.; Ren, X.; Mei, H.; Xu, K.; Idrees, M.; Mei, X. Structural, optical, and electrical characterizations of silver nanowire/single-layer graphene oxide composite film. Appl. Surf. Sci. 2022, 602, 154343. [Google Scholar] [CrossRef]
- Sengwa, R.J.; Choudhary, S.; Dhatarwal, P. Influences of ultrasonic- and microwave-irradiated preparation methods on the structural and dielectric properties of (PEO–PMMA)–LiCF3SO3–x wt% MMT nanocomposite electrolytes. Ionics 2014, 21, 95–109. [Google Scholar] [CrossRef]
- Das, P.P.; Chaudhary, V.; Ahmad, F.; Manral, A. Effect of nanotoxicity and enhancement in performance of polymer compo-sites using nanofillers: A state-of-the-art review. Polym. Compos. 2021, 42, 2152–21270. [Google Scholar] [CrossRef]
- Deeba, F.; Gupta, A.K.; Kulshrestha, V.; Bafna, M.; Jain, A. Analysing the dielectric properties of ZnO doped PVDF/PMMA blend composite. J. Mater. Sci. Mater. Electron. 2022, 33, 23703–23713. [Google Scholar] [CrossRef]
- Sengwa, R.; Dhatarwal, P.; Choudhary, S. Role of preparation methods on the structural and dielectric properties of plasticized polymer blend electrolytes: Correlation between ionic conductivity and dielectric parameters. Electrochim. Acta 2014, 142, 359–370. [Google Scholar] [CrossRef]
- Al-Jmmal, A.Y.; Nada, Z.; Taqa, A.A. The effect of recycled PMMA on some physical and chemical properties of acrylic resin denture base. Int. J. Enhanc. Res. Sci. Technol. Eng. 2018, 7, 2319–7463. [Google Scholar]
- Verma, M.; Patidar, D.; Sharma, K.B.; Saxena, N.S. An approach to correlate experimental and theoretical thermal conductivity of MWNT/PMMA polymer composites. Mater. Res. Express 2015, 2, 095302. [Google Scholar] [CrossRef]
- Sharma, B.K.; Gupta, A.K.; Khare, N.; Dhawan, S.K.; Gupta, H.C. Synthesis and characterization of polyaniline ZnO composite and its dielectric behavior. Synth. Met. 2009, 159, 391. [Google Scholar] [CrossRef]
- Hawkins, W.L. Polymer Stabilization; Wiley Inter Science: New York, NY, USA, 1972; p. 188. [Google Scholar]
- Bamford, C.H.; Tipper, C.F.H. Degradation of Polymers; Compton, R.G., Ed.; Elsevier: Amsterdam, The Netherlands, 1975; Volume 14, p. 367. ISBN 9780080868080. [Google Scholar]
- Silvia, G.; Daniele, C.; Vito, D.N.; Lidia, A.; Eugenia, T. PMMA: A key macromolecular component for dielectric low-k hybrid inorganic-organic polymer films. Eur. Polym. J. Sci. Direct. 2007, 43, 673–696. [Google Scholar]
- Schreyer, G. Kunstoffe. Polym. A Prop. Database 1972, 55, 737. [Google Scholar]
- Shindo, H.; Murakami, I.; Yamamura, H. Contemporary topics in polymer science. J. Polym. Sci. 1969, 7, 297. [Google Scholar] [CrossRef]
- Sharp, J.S.; Forrest, J.A. Thickness dependence of the dynamics in thin films of isotactic poly (methylmethacrylate). Eur. Phys. J. E Soft Mater. 2003, 12, S97. [Google Scholar] [CrossRef]
- Hirota, S.; Tominaga, Y.; Asai, S.; Sumita, M.J. Dielectric relaxation behavior of poly(methyl methacrylate) under high-pressure carbon dioxide. Polym. Sci. Part B Polym. Phys. 2005, 43, 295. [Google Scholar] [CrossRef]
- Bruce, D.W. Inorganic Materials Series: Energy Materials, 1st ed.; Bruce, D.W., O’Hare, D., Walton, R.I., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; p. 305. [Google Scholar] [CrossRef]
- Kumar, D.; Hashmi, S.A. Ionic liquid based sodium ion conducting gel polymer electrolytes. Solid State Ion. 2010, 181, 416–423. [Google Scholar] [CrossRef]
- Shtilman, M.I. New Concepts in Polymer Science, 1st ed.; Polymeric Biomaterials; VSP B.V.: Oud-Beijerland, The Netherlands, 2003; Volume 15, Available online: https://openlibrary.org/books/OL22567643M (accessed on 13 August 2022)ISBN 9067643890/9789067643894.
- Vaishya, R.; Chauhan, M.; Vaish, A. Bone cement: Review article. J. Clin. Ortho Paedics Trauma 2013, 4, 157–163. [Google Scholar] [CrossRef]
- Sabri, B.A.; Satgunam, M.; Abreeza, N.M.; Abed, A.N. A review on enhancements of PMMA denture base material with different nano-fillers. Cogent Eng. 2021, 8, 1875968. [Google Scholar] [CrossRef]
- Choudhary, S. Structural and dielectric properties of (PEO–PMMA)–SnO2 nanocomposites. Compos. Commun. 2017, 5, 54–63. [Google Scholar] [CrossRef]
- Ahmad, S.; Agnihotry, S.A. Nanocomposite electrolytes with fumed silica in poly(methyl methacrylate): Thermal, rheological and conductivity studies. J. Power Sources 2005, 140, 151–156. [Google Scholar] [CrossRef]
- Orlandi, M.O. Tin Oxide Materials: Synthesis, Properties, and Applications, 1st ed.; Orlandi, M.O., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-815924-8. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric Nanocomposites with Insulating properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Aashis, S.; Roy, S.; Gupta, S.; Sindhu, A.; Parveen, C.; Ramamurthy, P. Dielectric properties of novel PEO/ZnO hybrid nanocomposites films. Compos. Part B Eng. 2013, 47, 314–319. [Google Scholar]
- Bafna, M.; Gupta, A.K.; Khanna, R.K. Dielectric properties of potassium permangnate PMMA composite films. J. Emerg. Technol. Innov. Res. 2018, 5, 433. [Google Scholar]
- Ismail, L.N.; Farahiyah, S.A.; Habibah, Z.; Herman, S.H.; Rusop, M. Dielectric and physical properties of PMMA: TiO2 thin films by varying TiO2 concentration. In Proceedings of the IEEE Symposium on Humanities, Science and Engineering Research, Kuala Lumpur, Malaysia, 24–27 June 2012; pp. 259–262, ISBN 978-1-4673-1311-7. [Google Scholar]
- Dhatarwal, P.; Sengwa, R.J. Dielectric polarisation and relaxation process of the lithium ion conducting polymer blend matrix based electrolyte; effect of TiO2 nano filler. SN Appl. Sci. 2020, 2, 833. [Google Scholar] [CrossRef]
- Adebahr, J.; Byrne, N.; Forsyth, M.; MacFarlane, D.R.; Jacobson, P. Enhancement of ion dynamics in PMMA–based gels with addition of TiO2 nano–particles. Electrochim. Acta 2003, 48, 2099–2103. [Google Scholar] [CrossRef]
- Wypych, A.; Bobowska, I.; Tracz, M.; Opasinska, A.; Kadlubowski, S.; Kaliszewska, A.K.; Grobelny, J.; Wojciechowski, P. Dielectric properties and characterization of titanium dioxide obtained by different chemistry methods. J. Nanomater. 2014, 2014, 124814. [Google Scholar] [CrossRef]
- Rekik, H.; Ghallabi, Z.; Royaud, I.; Arous, M.; Seytre, G.; Boiteux, G.; Kallel, A. Dielectric relaxation behavior in semicrystalline polymer PVDF/TiO2 nanocomposites. Compos. Part B Eng. 2013, 45, 1119–1206. [Google Scholar] [CrossRef]
- Landau, L.D.; Levich, B.G. Dragging of a liquid by a moving plate. Acta Physiochim. 1942, 17, 42–54. [Google Scholar]
- Farheen, S.; Mathad, R.D. Effect of Nano Filler(TiO2) on conductivity in PEO-PMMA-LiClO4 polymer electrolyte. Int. J. Adv. Sci. Technol. 2015, 81, 49–52. [Google Scholar] [CrossRef]
- Lanje, A.S.; Sharma, S.J.; Ningthoujam, R.S.; Ahn, J.S.; Pode, R.B. Low temperature dielectric studies of zinc oxide (ZnO) nanoparticles prepared by precipitation method. Adv. Powder Technol. 2013, 24, 331–335. [Google Scholar] [CrossRef]
- Wu, W.; Uang, X.; Li, S.; Jiang, P.; Toshikatsu, T. Novel three-dimensional zinc oxide super structures for high dielectric constant polymer composites capable of withstanding high electric field. J. Phys. Chem. C. 2012, 116, 24887. [Google Scholar] [CrossRef]
- Alsaad, A.M.; AlDairy, A.R.; Ahmad, A.A.; Al Anbar, S.A.; Al B’ataineh, M.Q. Synthesis and characterization of as-grown doped polymerized (PMMA/PVA)/ZnO NPs hybrid thin films. Artic. Polym. Bull. Res. Gate 2022, 79, 2019–2040. [Google Scholar] [CrossRef]
- Noto, V.D.; Lavina, S.; Negro, E.; Pace, G.; Gross, S.; Depaoli, G.; Vidali, M. Dielectric low-k composite films based on PMMA, PVC and methylsiloxane-silica: Synthesis. Elsevier J. Non-Cryst. Solids 2007, 353, 2878–2888. [Google Scholar]
- Sengwa, R.J.; Dhatarwal, P. Predominantly chain segmental relaxation dependent ionic conductivity of multi-phase semicrystalline PVDF/PEO/LiClO4 solid polymer elctrolytes. Electrochim. Acta 2020, 338, 135890. [Google Scholar] [CrossRef]
- Dhatarwala, P.; Choudharya, S.; Sengwaa, R.J. Electrochemical performance of Li+-ion conducting solid polymer electrolytes based on PEO–PMMA blend matrix incorporated with various inorganic nano particles for the lithium ion batteries. Compos. Commun. 2018, 10, 11–17. [Google Scholar] [CrossRef]
- Shobhna, C.; Sengwa, R.J.; Choudhary, S. Dielectric properties and structural dynamics of melt compounded hot-pressed poly(ethylene oxide)–organophilic montmorillonite clay nanocomposite films. Indian Academy of Sciences. Bull. Mater. Sci. 2012, 35, 19–25. [Google Scholar]
- Achari, V.B.; Reddy, T.J.R.; Sharma, A.K.; Rao, V.V.R.N. Electrical, optical, and structural characterization of polymer blend (PVC/PMMA) electrolyte films. Ionics 2007, 13, 349–354. [Google Scholar] [CrossRef]
- Rajendran, S.; Bama, V.S.; Prabhu, M.R. Effect of lithium salt concentration in PVA-PMMA based gel polymer electrolytes. Ionics 2010, 16, 27–32. [Google Scholar] [CrossRef]
- Thomas, P.; Dakshayini, B.S.; Kushwaha, H.S.; Vaish, R. Effect of Sr2TiMnO6 fillers on mechanical, dielectric and thermal behaviour of PMMA polymer. J. Adv. Dielectr. 2015, 5, 155001. [Google Scholar] [CrossRef]
- Thomas, P.; Ravindran, R.S.E.; Varma, K.B.R. Dielectric properties of Poly(methyl methacrylate) (PMMA)/CaCu3Ti4O12 composites. In Proceedings of the 2012 IEEE 10th International Conference on Properties and Applications of Dielectric Materials, Banglore, India, 24–28 July 2012. [Google Scholar]
- Joseph, J.; Deshmuk, K.; Chidambaram, K.K.; Faisal, M.; Selvarajan, E.; Sadasivuni, K.K.; Ahamed, M.B.; Pasha, S.K.K. Dielectric and electromagnetic interference shielding properties of germanium dioxide nanoparticle reinforced poly(vinyl chloride) and poly(methylmethacrylate) blend nanocomposites. J. Mater. Sci. Mater. Electron. 2018, 29, 20172–20188. [Google Scholar] [CrossRef]
- Idrees, M.; Batool, S.; Javed, M.S.; Ahmad, M.; Khan, Q.U.; Imran, M.; Rasaki, S.A.; Mwizerwa, J.P.; Chen, Z. Adsorption and electrochemical facet of polymer precursor to yield meso porous carbon ceramic. Sep. Purif. Technol. 2021, 275, 119199. [Google Scholar] [CrossRef]
- Zha, J.W.; Zheng, M.S.; Fan, B.; Dang, Z.M. Polymer-based dielectrics with high permittivity for electric energy storage: A review. Nano Energy 2021, 89, 106438. [Google Scholar] [CrossRef]
- Bafna, M.; Gupta, A.K.; Khanna, R.K. Effect of potassium chromate nanoparticles on the optical properties of poly (methyl methacrylate) (PMMA) films. Mater. Today Proc. 2019, 10, 38. [Google Scholar] [CrossRef]
- Deeba, F.; Bafna, M.; Jain, A. Tuning of electrical properties of polymer blends or composites by doping of salts and inorganic fillers: A review. SGVU Int. J. Environ. Sci. Technol. 2021, 8, 46. [Google Scholar]
- Chitra, S.; Mahalakshmi, P.; Radha, K.P. Vibrational and Impedance analysis of polymer electrolyte based on PMMA complexed with adipic acid. Int. J. Multidiscip. Educ. Res. 2016, 1, 15–18. [Google Scholar]
- Sharma, P.; Kanchan, D.K.; Gondaliya, N. Effect of nano-filler on structural and Ionic transport properties of plasticized polymer electrolyte. Sci. Res. 2012, 2, 38–44. [Google Scholar] [CrossRef]
- Sun, Q.J.; Zhao, X.H. Polymer Nanocomp, Polymer Nanocomposites for Pressure Sensors, 1st ed.; Zhou, Y., Ding, G., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Bouknaitir, I.; Panniello, A.; Teixeira, S.S.; Kreit, L.; Corricelli, M.; Striccoli, M.; Costa, L.C.; Achour, M.E. Optical and dielectric properties of PMMA (poly(methyl methacrylate))/carbon dots composites. Polym. Compos. 2019, 40, 312–319. [Google Scholar] [CrossRef]
- Liew, C.W.; Ramesh, S.; Durairaj, R. Impact of low viscosity ionic liquid on PMMA-PVC-LiTFSI polymer electrolytes based on AC impedance, dielectric behavior and HATR-FTIR characteristics. J. Mater. Res. 2012, 27, 2996–3004. [Google Scholar] [CrossRef]
- Nagao, D.; Kinoshita, T.; Watanabe, A.; Konno, M. Fabrication of highly refractive, transparent BaTiO3/poly (methyl methacrylate) composite films with high permittivities. Polym. Int. 2011, 60, 1180–1184. [Google Scholar] [CrossRef]
- Rathesh, R.; Sebastian, M.T. Polymer Ceramic Composites for Microwave Applications. In Microwave Materials and Applications; Sebastian, M.T., Jantunen, H., Ubic, R., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2017; Chapter 11; p. 481. ISBN 978111920854. [Google Scholar]
- Strawbridge, I.; James, P.F. Study of polymer films. J. Non-Cryst. Solids 1986, 82, 366–372. [Google Scholar] [CrossRef]
- Choudhary, S.; Sengwa, R.J. Structural and dielectric studies of amorphous and semi crystalline polymers blend-based polymer nanocomposites electrolytes. J. Appl. Polym. Sci. 2015, 132, 41311. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).