White Ginger Nanocellulose as Effective Reinforcement and Antimicrobial Polyvinyl Alcohol/ZnO Hybrid Biocomposite Films Additive for Food Packaging Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Nanocellulose and Hybrid Biocomposite Films
2.3. Sample’s Morphology Using SEM
2.4. Tensile Test
2.5. Fourier-Transform Infrared (FTIR) Spectroscopy
2.6. X-ray Diffraction (XRD)
2.7. Thermogravimetric Analysis (TGA)
2.8. Antimicrobial Activity
3. Results and Discussions
3.1. FESEM Images of the Fractured Surface
3.2. FTIR Spectra
3.3. X-ray Diffraction
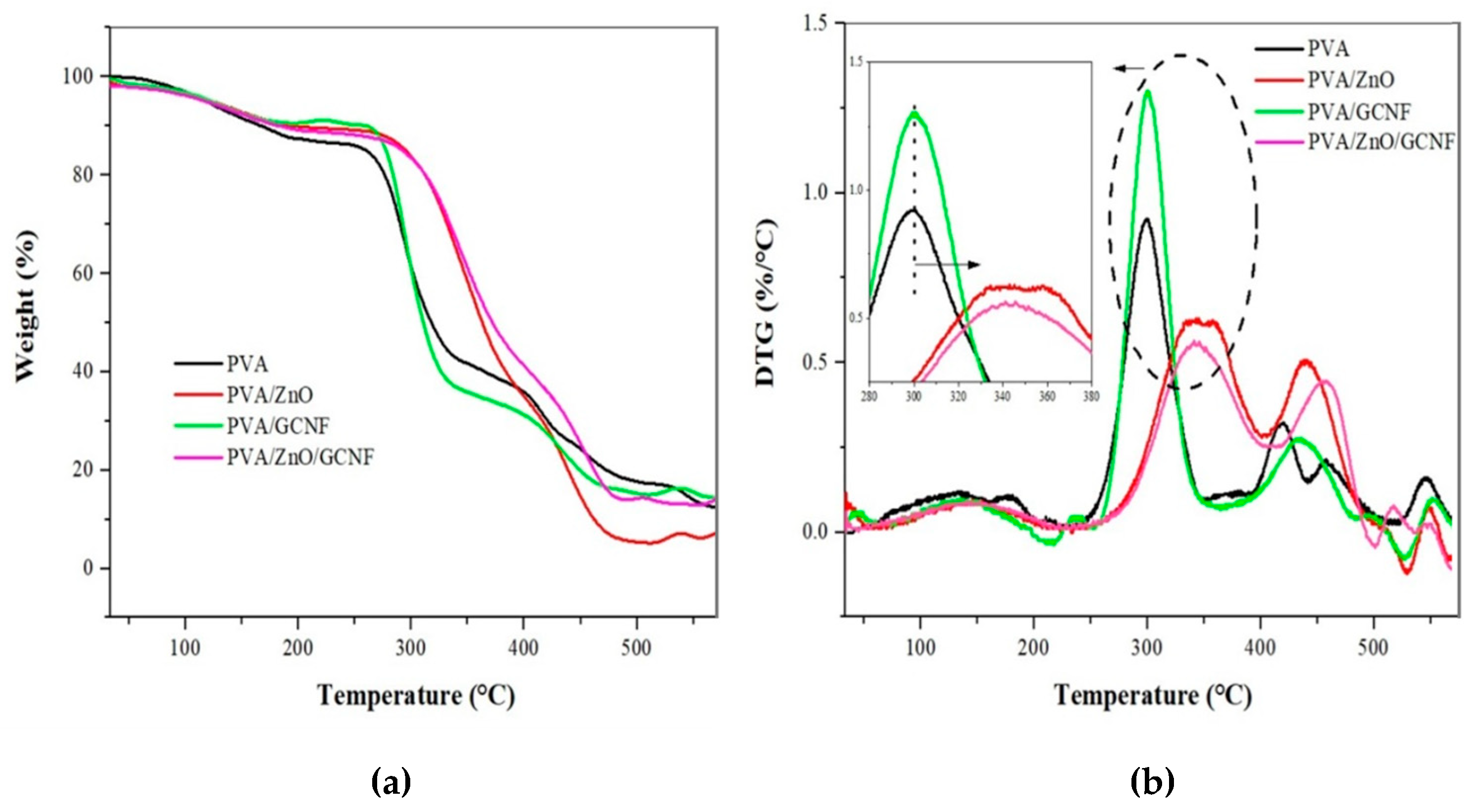
3.4. Thermal Analysis
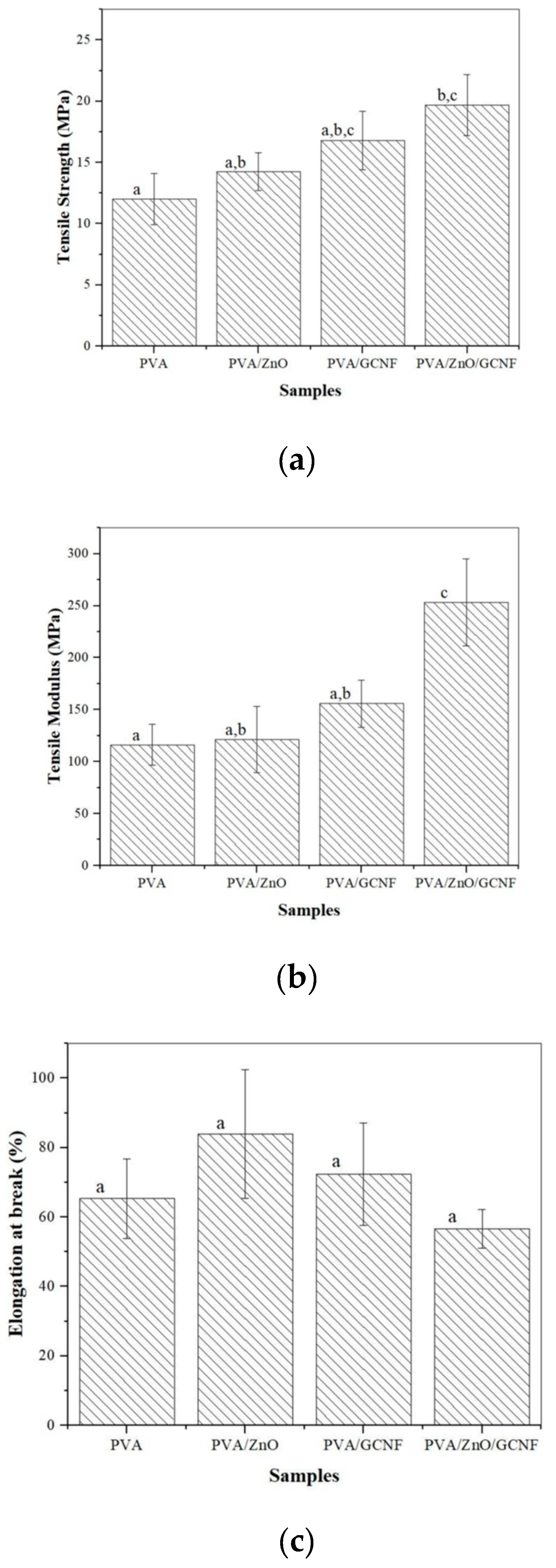
3.5. Tensile Properties
3.6. Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maurya, M.; Maurya, N.K.; Bajpai, V. Effect of SiC Reinforced Particle Parameters in the Development of Aluminium Based Metal Matrix Composite. Evergreen 2019, 6, 200–206. [Google Scholar] [CrossRef]
- Syahrial, A.Z.; Miqdad, M.; Syahrial, A.Z. Effect of Nano Al2O3 Addition and T6 Heat Treatment on Characteristics of AA7075 / Al2O 3 Composite Fabricated by Squeeze Casting Method for Ballistic Application Effect of Nano Al2O3 Addition and T6 Heat Treatment on Characteristics of AA. Evergreen 2022, 9, 531–537. [Google Scholar]
- Rahmadiawan, D.; Aslfattahi, N.; Nasruddin, N.; Saidur, R.; Arifutzzaman, A.; Mohammed, H.A. MXene Based Palm Oil Methyl Ester as an Effective Heat Transfer Fluid. J. Nano Res. 2021, 68, 17–34. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and Intelligent Biodegradable Packaging Films Using Food and Food Waste-Derived Bioactive Compounds: A Review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Mathew, S.; Mathew, J.; Radhakrishnan, E.K. Polyvinyl Alcohol/Silver Nanocomposite Films Fabricated under the Influence of Solar Radiation as Effective Antimicrobial Food Packaging Material. J. Polym. Res. 2019, 26, 1–10. [Google Scholar] [CrossRef]
- Agarwal, S.; Hoque, M.; Bandara, N.; Pal, K.; Sarkar, P. Synthesis and Characterization of Tamarind Kernel Powder-Based Antimicrobial Edible Films Loaded with Geraniol. Food Packag. Shelf Life 2020, 26, 100562. [Google Scholar] [CrossRef]
- Oner, B.; Meral, R.; Ceylan, Z. Determination of Some Quality Indices of Rainbow Trout Fillets Treated with Nisin-Loaded Polyvinylalcohol-Based Nanofiber and Packed with Polyethylene Package. Lwt 2021, 149, 111854. [Google Scholar] [CrossRef]
- Lee, H.; You, J.; Jin, H.J.; Kwak, H.W. Chemical and Physical Reinforcement Behavior of Dialdehyde Nanocellulose in PVA Composite Film: A Comparison of Nanofiber and Nanocrystal. Carbohydr. Polym. 2020, 232, 115771. [Google Scholar] [CrossRef] [PubMed]
- Abral, H.; Atmajaya, A.; Mahardika, M.; Hafizulhaq, F.; Kadriadi; Handayani, D.; Sapuan, S.M.; Ilyas, R.A. Effect of Ultrasonication Duration of Polyvinyl Alcohol (PVA) Gel on Characterizations of PVA Film. J. Mater. Res. Technol. 2020, 9, 2477–2486. [Google Scholar] [CrossRef]
- Abral, H.; Ikhsan, M.; Rahmadiawan, D.; Handayani, D.; Sandrawati, N.; Sugiarti, E.; Novi, A. Anti-UV, Antibacterial, Strong, and High Thermal Resistant Polyvinyl Alcohol/Uncaria Gambir Extract Biocomposite Film. J. Mater. Res. Technol. 2022, 17, 2193–2202. [Google Scholar] [CrossRef]
- Darbasizadeh, B.; Fatahi, Y.; Feyzi-barnaji, B.; Arabi, M.; Motasadizadeh, H.; Farhadnejad, H.; Moraffah, F.; Rabiee, N. Crosslinked-Polyvinyl Alcohol-Carboxymethyl Cellulose/ZnO Nanocomposite Fibrous Mats Containing Erythromycin (PVA-CMC/ZnO-EM): Fabrication, Characterization and in-Vitro Release and Anti-Bacterial Properties. Int. J. Biol. Macromol. 2019, 141, 1137–1146. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhao, Y.; Miao, B.; Abo-Dief, H.M.; Qu, M.; Pashameah, R.A.; Xu, B.B.; Huang, M.; Algadi, H.; Liu, X.; et al. Hydrothermally Synthesized ZnO-RGO-PPy for Water-Borne Epoxy Nanocomposite Coating with Anticorrosive Reinforcement. Prog. Org. Coatings 2022, 172, 107153. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Ma, D.; Ahmed, S.; Qin, W.; Liu, Y. Effects of Ultrasonication Duration and Graphene Oxide and Nano-Zinc Oxide Contents on the Properties of Polyvinyl Alcohol Nanocomposites. Ultrason. Sonochem. 2019, 59, 104731. [Google Scholar] [CrossRef] [PubMed]
- Dyartanti, E.R.; Widiasa, I.N.; Purwanto, A.; Susanto, H. Nanocomposite Polymer Electrolytes in PVDF/ZnO Membranes Modified with PVP for Use in LiFePo4 Batteries. Evergreen 2018, 5, 19–25. [Google Scholar] [CrossRef]
- Jacob, J.; Peter, G.; Thomas, S.; Haponiuk, J.T.; Gopi, S. Chitosan and Polyvinyl Alcohol Nanocomposites with Cellulose Nanofibers from Ginger Rhizomes and Its Antimicrobial Activities. Int. J. Biol. Macromol. 2019, 129, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B. Nepal Ginger Profile 2016: An Assessment of Commercial Ginger Cultivated in Nepal; Nepal Market Development Nepal & Ginger Producers and Traders: Kathmandu, Nepal, 2016. [Google Scholar]
- Thomas, S.; Gopi, S.; Jacob, J.; Haponiuk, J.; Peter, G. Use of Ginger Nanofibers for the Preparation of Cellulose Nanocomposites and Their Antimicrobial Activities. Fibers 2018, 6, 79. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Pratama, A.B.; Fajri, N.; Sapuan, S.M.; Ilyas, R.A. Transparent and Antimicrobial Cellulose FIlm from Ginger Nanofiber. Food Hydrocoll. 2020, 98, 105266. [Google Scholar] [CrossRef]
- Abral, H.; Ariksa, J.; Mahardika, M.; Handayani, D.; Aminah, I.; Sandrawati, N.; Sapuan, S.M.; Ilyas, R.A. Highly Transparent and Antimicrobial PVA Based Bionanocomposites Reinforced by Ginger Nanofiber. Polym. Test. 2019, 81, 106186. [Google Scholar] [CrossRef]
- Abral, H.; Kurniawan, A.; Rahmadiawan, D.; Handayani, D.; Sugiarti, E.; Muslimin, A.N. Highly Antimicrobial and Strong Cellulose-Based Biocomposite Film Prepared with Bacterial Cellulose Powders, Uncaria Gambir, and Ultrasonication Treatment. Int. J. Biol. Macromol. 2022, 208, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ariawan, D.; Raharjo, W.P.; Diharjo, K.; Raharjo, W.W.; Kusharjanta, B. Influence of Tropical Climate Exposure on the Mechanical Properties of RHDPE Composites Reinforced by Zalacca Midrib Fibers. Evergreen 2022, 09, 662–672. [Google Scholar]
- Sosiati, H.; Yuniar, N.D.M.; Saputra, D.; Hamdan, S. The Influence of Carbon Fiber Content on the Tensile, Flexural, and Thermal Properties of the Sisal/PMMA Composites. Evergreen 2022, 9, 32–40. [Google Scholar] [CrossRef]
- Rahmadiawan, D.; Abral, H.; Nasruddin, N.; Fuadi, Z. Stability, Viscosity, and Tribology Properties of Polyol Ester Oil-Based Biolubricant Filled with TEMPO-Oxidized Bacterial Cellulose Nanofiber. Int. J. Polym. Sci. 2021, 2021, 5536047. [Google Scholar] [CrossRef]
- Fuadi, Z.; Rahmadiawan, D.; Kurniawan, R.; Mulana, F.; Abral, H. Effect of Graphene Nanoplatelets on Tribological Properties of Bacterial Cellulose/Polyolester Oil Bio-Lubricant. Front. Mech. Eng. 2022, 8, 810847. [Google Scholar] [CrossRef]
- Abral, H.; Pratama, A.B.; Handayani, D.; Mahardika, M.; Aminah, I.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. Antimicrobial Edible Film Prepared from Bacterial Cellulose Nanofibers/Starch/Chitosan for a Food Packaging Alternative. Int. J. Polym. Sci. 2021, 2021, 6641284. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 36, 493–496. [Google Scholar] [CrossRef]
- Chen, C.; Ding, R.; Yang, S.; Wang, J.; Chen, W.; Zong, L.; Xie, J. Development of Thermal Insulation Packaging Film Based on Poly(Vinyl Alcohol) Incorporated with Silica Aerogel for Food Packaging Application. Lwt 2020, 129, 109568. [Google Scholar] [CrossRef]
- Abral, H.; Soni Satria, R.; Mahardika, M.; Hafizulhaq, F.; Affi, J.; Asrofi, M.; Handayani, D.; Sapuan, S.M.; Stephane, I.; Sugiarti, E.; et al. Comparative Study of the Physical and Tensile Properties of Jicama (Pachyrhizus Erosus) Starch Film Prepared Using Three Different Methods. Starch/Staerke 2019, 71, 1–9. [Google Scholar] [CrossRef]
- Hezma, A.M.; Rajeh, A.; Mannaa, M.A. An Insight into the Effect of Zinc Oxide Nanoparticles on the Structural, Thermal, Mechanical Properties and Antimicrobial Activity of Cs/PVA Composite. Colloids Surfaces A Physicochem. Eng. Asp. 2019, 581, 123821. [Google Scholar] [CrossRef]
- Mahardika, M.; Abral, H.; Kasim, A.; Arief, S.; Hafizulhaq, F.; Asrofi, M. Properties of Cellulose Nanofiber/Bengkoang Starch Bionanocomposites: Effect of Fiber Loading. LWT Food Sci. Technol. 2019, 116, 108554. [Google Scholar] [CrossRef]
- Abral, H.; Fajri, N.; Mahardika, M.; Handayani, D.; Sugiarti, E.; Kimd, H.J. A Simple Strategy in Enhancing Moisture and Thermal Resistance and Tensile Properties of Disintegrated Bacterial Cellulose Nanopaper. J. Mater. Res. Technol. 2020, 9, 8754–8765. [Google Scholar] [CrossRef]
- Channa, I.A.; Ashfaq, J.; Gilani, S.J.; Shah, A.A.; Chandio, A.D.; Jumah, M.N. Bin UV Blocking and Oxygen Barrier Coatings Based on Polyvinyl Alcohol and Zinc Oxide Nanoparticles for Packaging Applications. Coatings 2022, 12, 897. [Google Scholar] [CrossRef]
- Abral, H.; Hartono, A.; Hafizulhaq, F.; Handayani, D.; Sugiarti, E.; Pradipta, O. Characterization of PVA/Cassava Starch Biocomposites Fabricated with and without Sonication Using Bacterial Cellulose Fiber Loadings. Carbohydr. Polym. 2018, 206, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Khan, R.; Ul-Islam, M.; Khan, T.; Wahid, F. Bacterial Cellulose-Zinc Oxide Nanocomposites as a Novel Dressing System for Burn Wounds. Carbohydr. Polym. 2017, 164, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.L.; Pawar, S.G.; Chougule, M.A.; Raut, B.T.; Godse, P.R.; Sen, S.; Patil, V.B. Structural, Morphological, Optical, and Electrical Properties of PANi-ZnO Nanocomposites. Int. J. Polym. Mater. 2012, 61, 809–820. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khattak, W.A.; Ullah, M.W.; Khan, S.; Park, J.K. Synthesis of Regenerated Bacterial Cellulose-Zinc Oxide Nanocomposite Films for Biomedical Applications. Cellulose 2014, 21, 433–447. [Google Scholar] [CrossRef]
- Sarwar, M.S.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A. Preparation and Characterization of PVA/Nanocellulose/Ag Nanocomposite Films for Antimicrobial Food Packaging. Carbohydr. Polym. 2018, 184, 453–464. [Google Scholar] [CrossRef]
- Abral, H.; Dalimunthe, M.H.; Hartono, J.; Efendi, R.P.; Asrofi, M.; Sugiarti, E.; Sapuan, S.; Park, J.-W.; Kim, H.-J. Characterization of Tapioca Starch Biopolymer Composites Reinforced with Micro Scale Water Hyacinth Fibers. Starch Stärke 2018, 70, 1700287. [Google Scholar] [CrossRef]
- Asrofi, M.; Abral, H.; Kurnia, Y.; Sapuan, S.M.; Kim, H. Effect of Duration of Sonication during Gelatinization on Properties of Tapioca Starch Water Hyacinth Fiber Biocomposite. Int. J. Biol. Macromol. 2018, 108, 167–176. [Google Scholar] [CrossRef]
- Asrofi, M.; Abral, H.; Kasim, A.; Pratoto, A.; Mahardika, M.; Hafizulhaq, F. Characterization of the Sonicated Yam Bean Starch Bionanocomposites Reinforced By Nanocellulose Water Hyacinth Fiber (Whf): The Effect of Various Fiber Loading. J. Eng. Sci. Technol. 2018, 13, 2700–2715. [Google Scholar]
- Syafri, E.; Kasim, A.; Abral, H.; Asben, A. Cellulose Nanofibers Isolation and Characterization from Ramie Using a Chemical-Ultrasonic Treatment. J. Nat. Fibers 2019, 16, 1145–1155. [Google Scholar] [CrossRef]
- Jayakumar, A.; Heera, K.V.; Sumi, T.S.; Joseph, M.; Mathew, S.; Praveen, G.; Nair, I.C.; Radhakrishnan, E.K. Starch-PVA Composite Films with Zinc-Oxide Nanoparticles and Phytochemicals as Intelligent PH Sensing Wraps for Food Packaging Application. Int. J. Biol. Macromol. 2019, 136, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/Starch/Propolis/Anthocyanins Rosemary Extract Composite Films as Active and Intelligent Food Packaging Materials. J. Food Saf. 2020, 40, e12725. [Google Scholar] [CrossRef]
- Bazzi, M.; Shabani, I.; Mohandesi, J.A. Enhanced Mechanical Properties and Electrical Conductivity of Chitosan/Polyvinyl Alcohol Electrospun Nanofibers by Incorporation of Graphene Nanoplatelets. J. Mech. Behav. Biomed. Mater. 2022, 125, 104975. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ding, H.; Qi, G.; Li, C.; Xu, P.; Zheng, T.; Zhu, X.; Kenny, J.M.; Puglia, D.; Ma, P. Highly Transparent PVA/Nanolignin Composite Films with Excellent UV Shielding, Antibacterial and Antioxidant Performance. React. Funct. Polym. 2021, 162, 104873. [Google Scholar] [CrossRef]
- Amalraj, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Preparation, Characterization and Antimicrobial Activity of Polyvinyl Alcohol/Gum Arabic/Chitosan Composite Films Incorporated with Black Pepper Essential Oil and Ginger Essential Oil. Int. J. Biol. Macromol. 2020, 151, 366–375. [Google Scholar] [CrossRef]
- Cano, A.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Physical and Antimicrobial Properties of Starch-Pva Blend Films as Affected by the Incorporation of Natural Antimicrobial Agents. Foods 2016, 5, 3. [Google Scholar] [CrossRef]
- Francis, D.V.; Thaliyakattil, S.; Cherian, L.; Sood, N.; Gokhale, T. Metallic Nanoparticle Integrated Ternary Polymer Blend of PVA/Starch/Glycerol: A Promising Antimicrobial Food Packaging Material. Polymers 2022, 14, 1379. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent Bacterial Growth Inhibition and Mechanism of Antibacterial Activity of Zinc Oxide Nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.D.C.; Hernández-Carranza, P.; Cid-Pérez, T.S.; Ávila-Sosa, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Antimicrobial Activity of Ginger (Zingiber Officinale) and Its Application in Food Products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- Rinanda, T.; Isnanda, R.P. Zulfitri Chemical Analysis of Red Ginger (Zingiber Officinale Roscoe Var Rubrum) Essential Oil and Its Anti-Biofilm Activity against Candida Albicans. Nat. Prod. Commun. 2018, 13, 1587–1590. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Gutha, Y.; Zhang, W. Antibacterial Property and Biocompatibility of Chitosan/Poly(Vinyl Alcohol)/ZnO (CS/PVA/ZnO) Beads as an Efficient Adsorbent for Cu(II) Removal from Aqueous Solution. Colloids Surfaces B Biointerfaces 2017, 156, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, K.; Somashekarappa, H.M. In Vitro Biocompatibility and Antibacterial Activity of Gamma Ray Crosslinked ZnO/PVA Hydrogel Nanocomposites. Mater. Today Proc. 2018, 5, 21314–21321. [Google Scholar] [CrossRef]




| Samples | PVA (g) | Distilled Water (mL) | GCNF (g) | ZnO (g) |
|---|---|---|---|---|
| PVA | 10 | 90 | - | - |
| PVA/ZnO | 10 | 89.5 | - | 0.5 |
| PVA/GCNF | 10 | 65.7 | 24.3 or 0.1 | - |
| PVA/ZnO/GCNF | 10 | 65.2 | 24.3 or 0.1 | 0.5 |
| Samples | The Peak Position at 2θ around 19.6° | Tm (°C) | d-Spacing [Å] at 2θ (19.6°) | FWHM (°) of the Peak at 2θ (19.6°) |
|---|---|---|---|---|
| PVA | 19.686 | 300 | 4.510 | 0.614 |
| PVA/GCNF | 19.667 | 301 | 4.514 | 0.614 |
| PVA/ZnO | 19.344 | 341 | 4.589 | 0.512 |
| PVA/ZnO/GCNF | 19.236 | 345 | 4.614 | 0.409 |
| Author | Material | Tensile Strength (MPa) |
|---|---|---|
| Dieter et al. [This Work] | PVA/ZnO/GCNF | 19.7 |
| Abral et al. [33] | PVA/cassava starch | 17.2 |
| Sarwar et al. [37] | PVA/nanocellulose/Ag | 12.32 |
| Jayakumar et al. [42] | PVA/starch/nutmeg oil/ZnO/jamun extract | 26 |
| Mustafa et al. [43] | PVA/starch/propolis/anthocyanins rosemary extract | 6 |
| Bazzi et al. [44] | PVA/chitosan/graphene nanoplatelets | 11 |
| Yang et al. [45] | PVA/nanolignin | 24.3 |
| Amalraj et al. [46] | PVA/gum arabic/chitosan | 11.8 |
| Cano et al. [47] | PVA/neem oil | 21.5 |
| Francis et al. [48] | PVA/starch/glycerol | 18.05 |
| Samples | The Inhibition Zone Diameter with Standard Deviation (mm) | ||||
|---|---|---|---|---|---|
| SA | BS | EC | PA | CA | |
| PVA | 0 | 0 | 0 | 0 | 0 |
| PVA/ZnO | 5.5 ± 0.04 | 5.3 ± 0.2 | 5.1 ± 0.7 | 4.7 ± 0.1 | 9.6 ± 1.5 |
| PVA/GCNF | 8.8 ± 1.8 | 12.4 ± 0.5 | 11.9 ± 1.7 | 12.0 ± 1.6 | 10.1 ± 1.0 |
| PVA/ZnO/GCNF | 13.6 ± 0.3 | 13.0 ± 0.5 | 14.5 ± 0.3 | 12.5 ± 0.9 | 11.2 ± 1.0 |
| Positive Control | 26.9 ± 0.2 | 25.6 ± 0.5 | 30.7 ± 0.4 | 26.5 ± 0.2 | 23.5 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahmadiawan, D.; Abral, H.; Yesa, W.H.; Handayani, D.; Sandrawati, N.; Sugiarti, E.; Muslimin, A.N.; Sapuan, S.M.; Ilyas, R.A. White Ginger Nanocellulose as Effective Reinforcement and Antimicrobial Polyvinyl Alcohol/ZnO Hybrid Biocomposite Films Additive for Food Packaging Applications. J. Compos. Sci. 2022, 6, 316. https://doi.org/10.3390/jcs6100316
Rahmadiawan D, Abral H, Yesa WH, Handayani D, Sandrawati N, Sugiarti E, Muslimin AN, Sapuan SM, Ilyas RA. White Ginger Nanocellulose as Effective Reinforcement and Antimicrobial Polyvinyl Alcohol/ZnO Hybrid Biocomposite Films Additive for Food Packaging Applications. Journal of Composites Science. 2022; 6(10):316. https://doi.org/10.3390/jcs6100316
Chicago/Turabian StyleRahmadiawan, Dieter, Hairul Abral, Wahyu Hidayat Yesa, Dian Handayani, Neny Sandrawati, Eni Sugiarti, Ahmad Novi Muslimin, S. M. Sapuan, and R. A. Ilyas. 2022. "White Ginger Nanocellulose as Effective Reinforcement and Antimicrobial Polyvinyl Alcohol/ZnO Hybrid Biocomposite Films Additive for Food Packaging Applications" Journal of Composites Science 6, no. 10: 316. https://doi.org/10.3390/jcs6100316
APA StyleRahmadiawan, D., Abral, H., Yesa, W. H., Handayani, D., Sandrawati, N., Sugiarti, E., Muslimin, A. N., Sapuan, S. M., & Ilyas, R. A. (2022). White Ginger Nanocellulose as Effective Reinforcement and Antimicrobial Polyvinyl Alcohol/ZnO Hybrid Biocomposite Films Additive for Food Packaging Applications. Journal of Composites Science, 6(10), 316. https://doi.org/10.3390/jcs6100316









