Thermal Qualification of the UHTCMCs Produced Using RF-CVI Technique with VMK Facility at DLR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Processing
2.2. Post Test Materials Characterisation
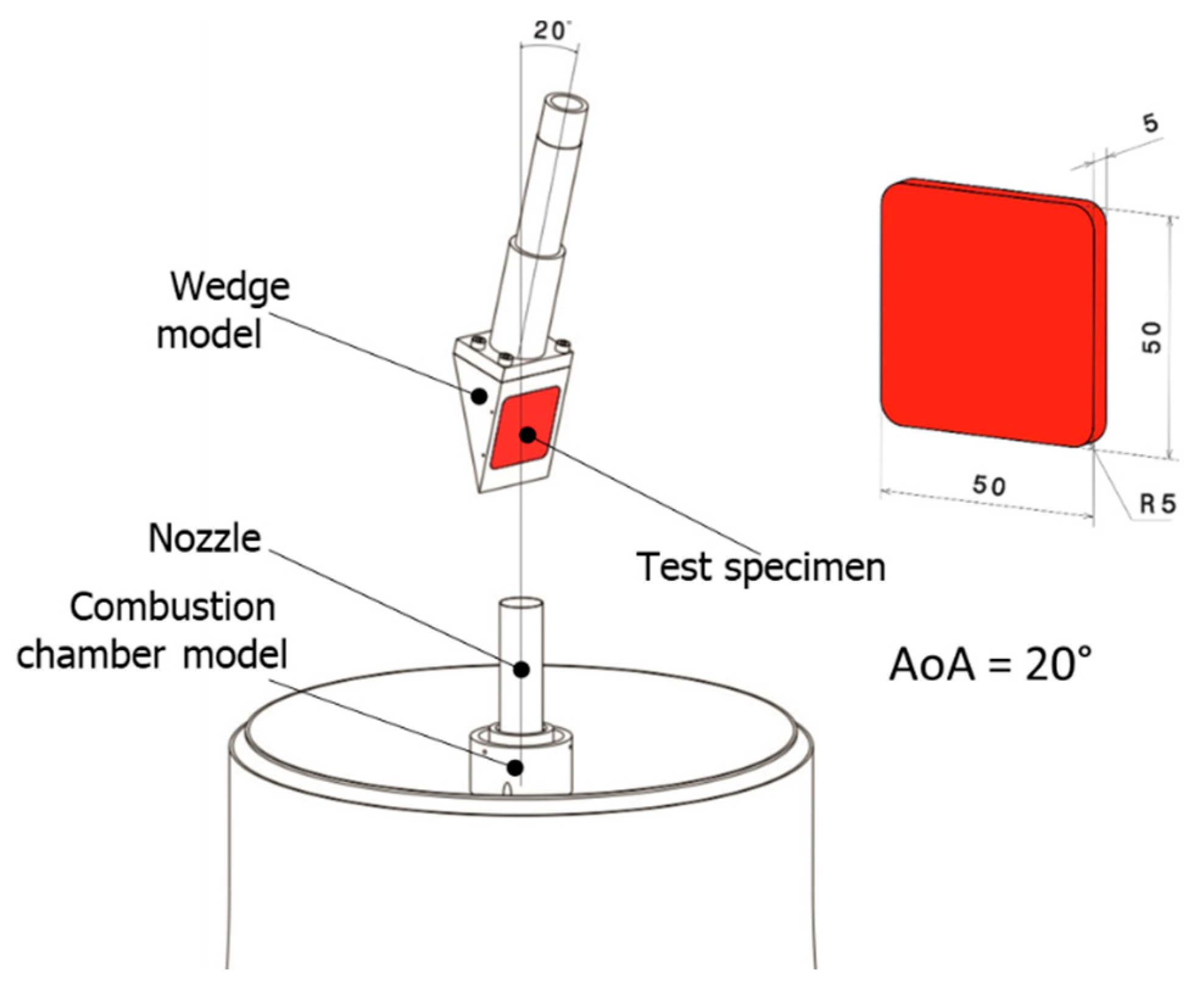
2.3. VMK Test Facilities
2.4. Specimen Preparation
2.5. Test Conditions
3. Results and Discussion
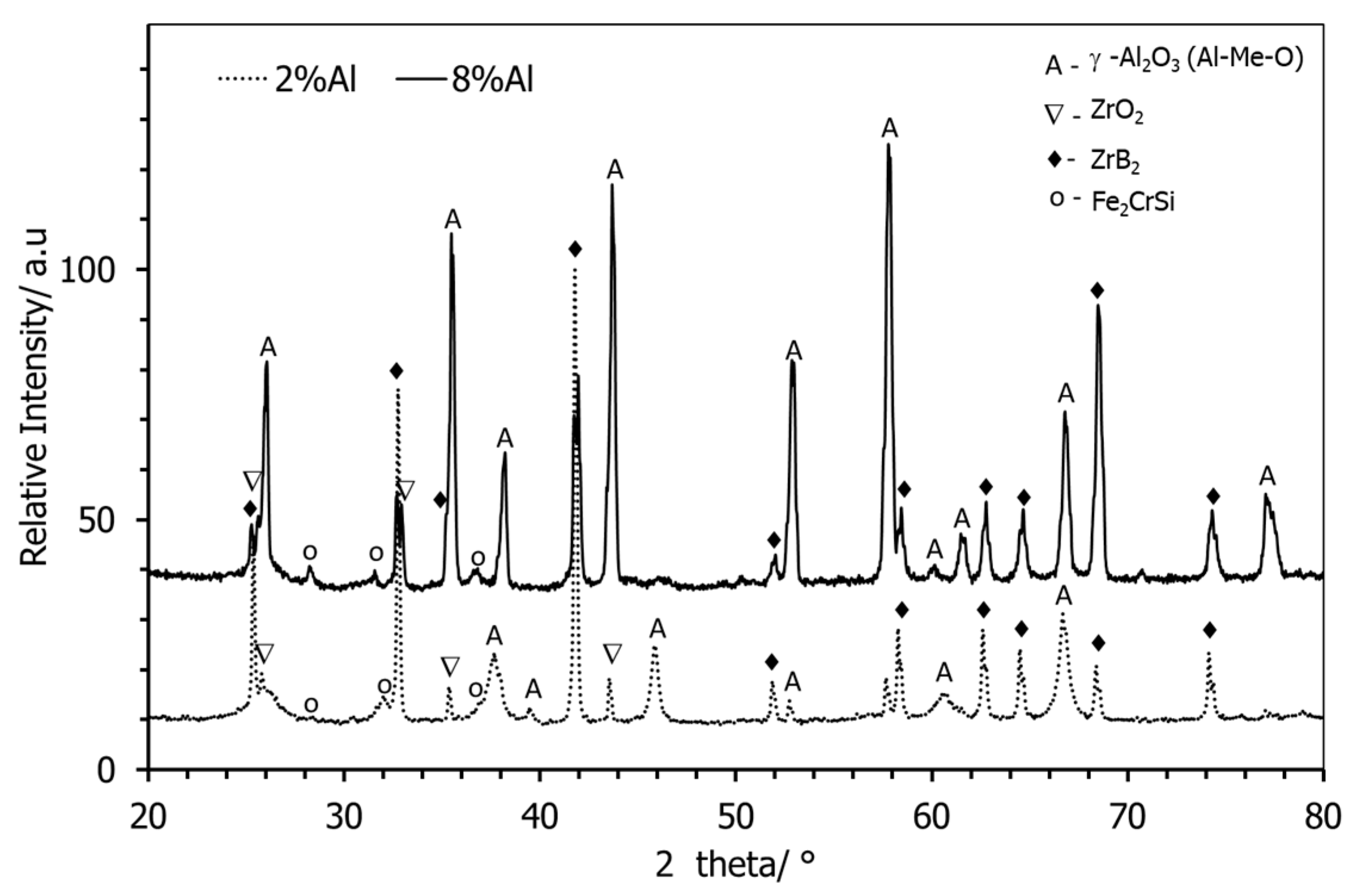
3.1. Starting Materials
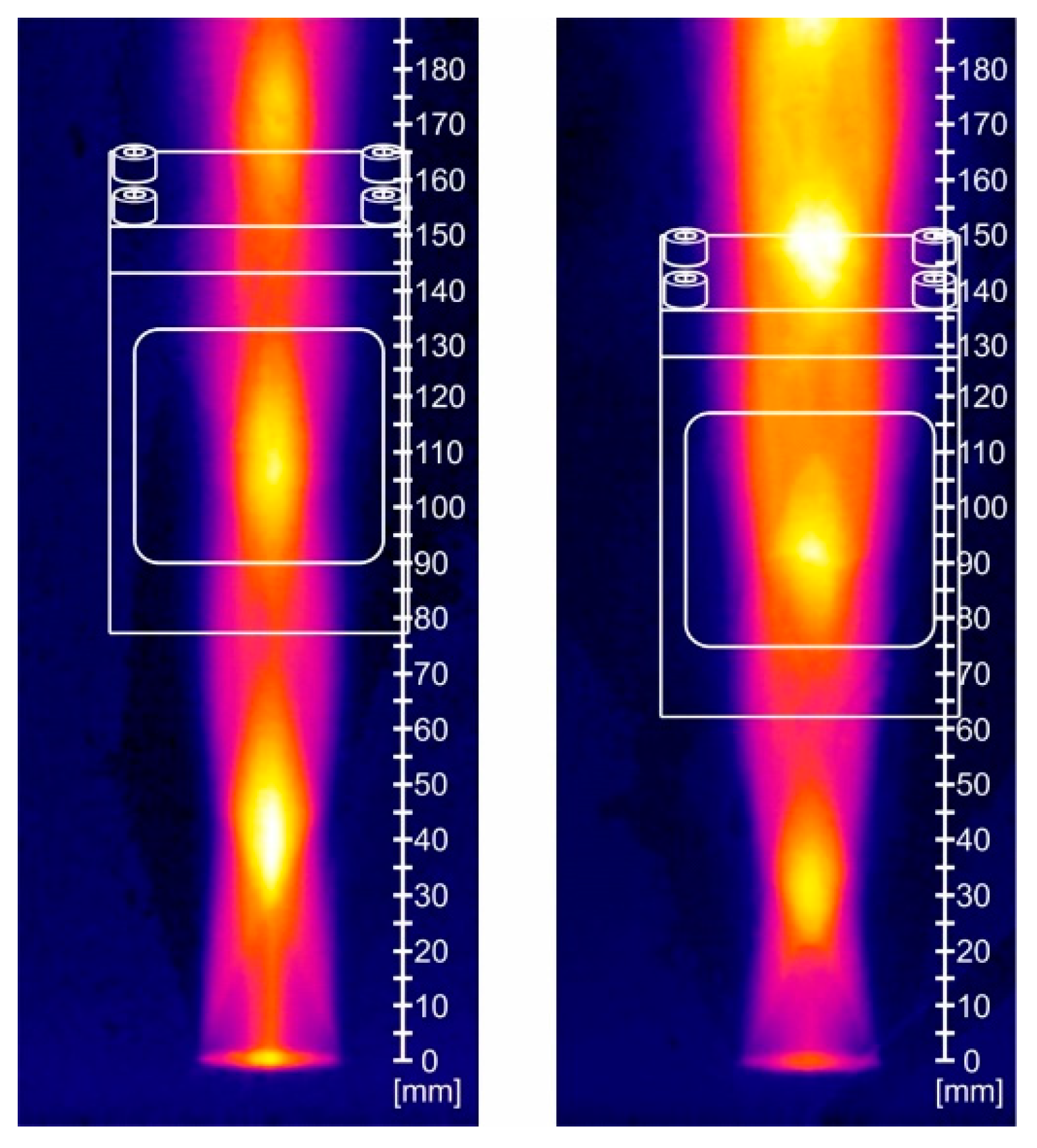
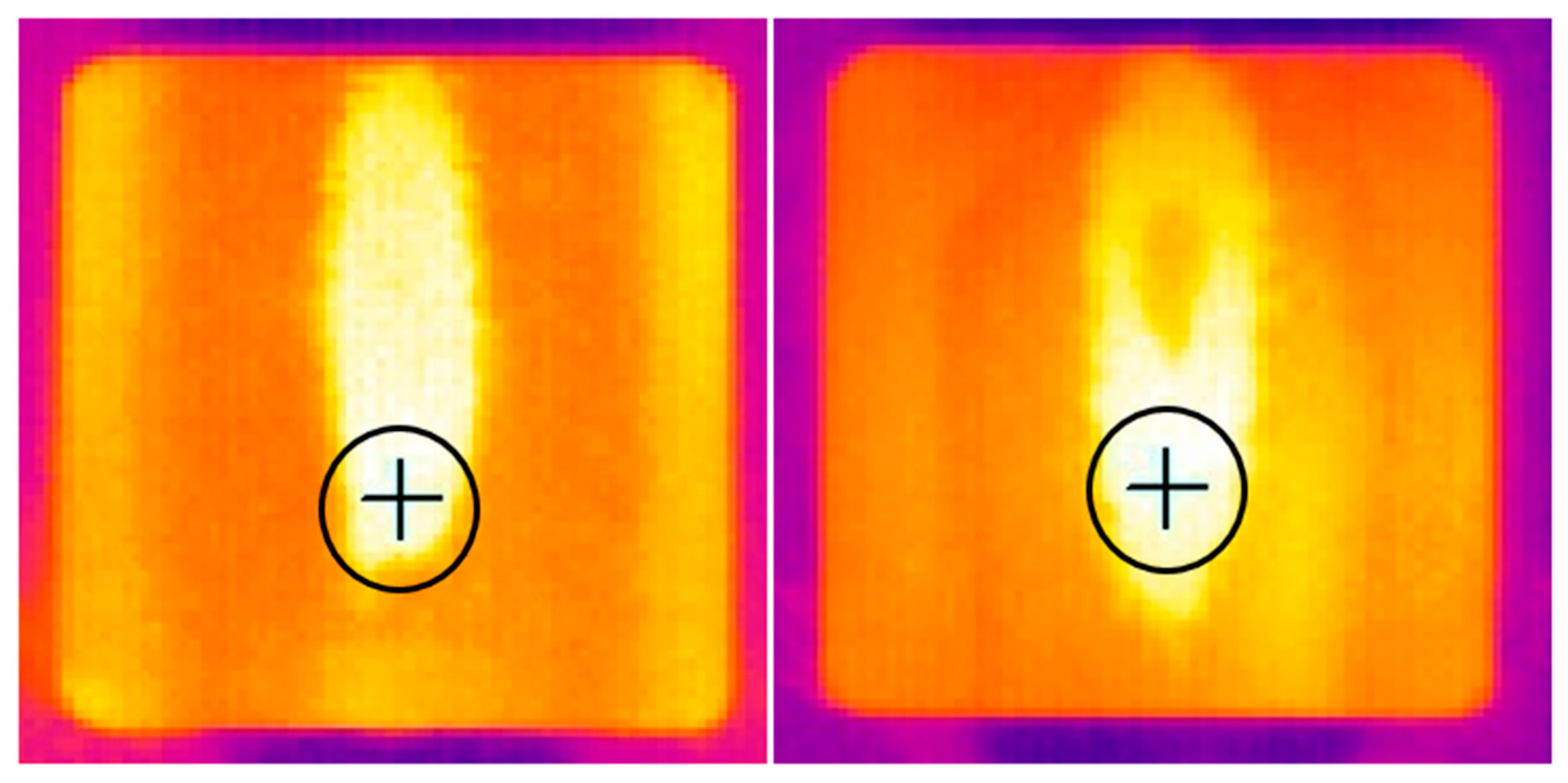
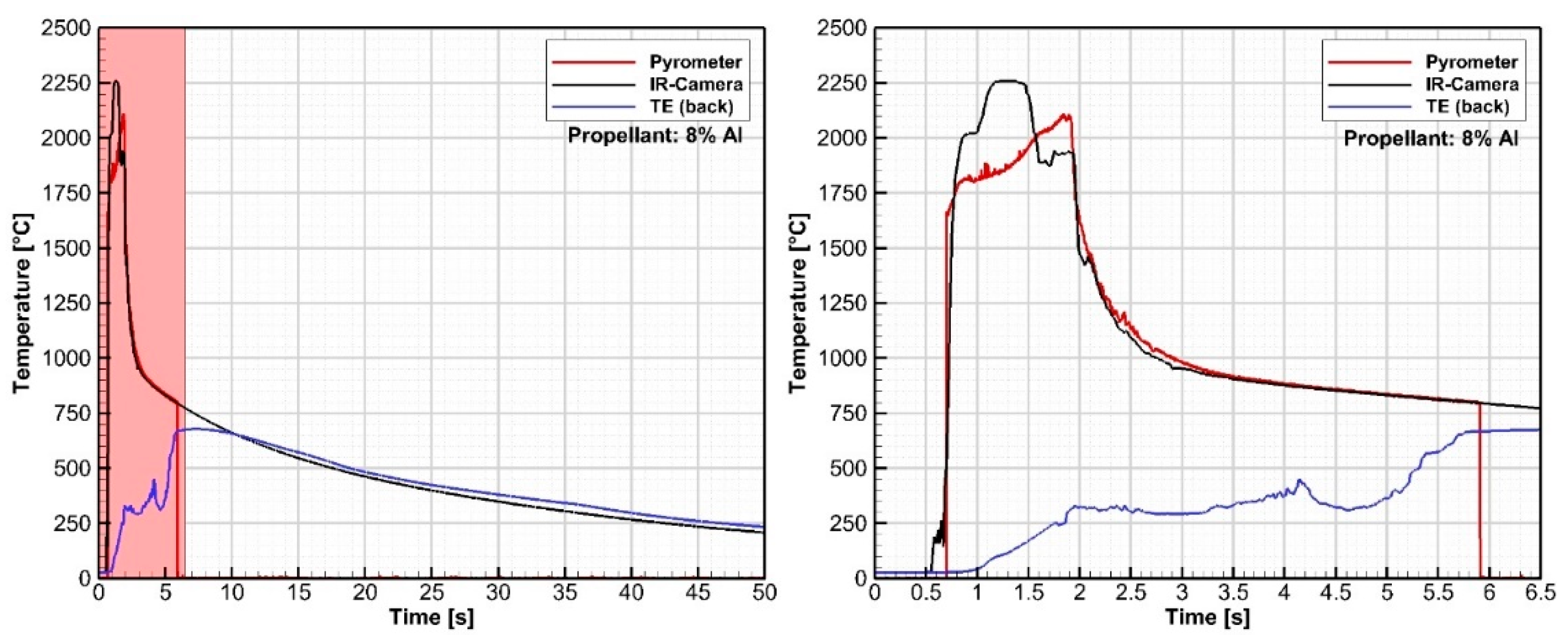
3.2. Propulsion Test Results (DLR)
3.2.1. Phase 1 Tests
3.2.2. Phase II Tests
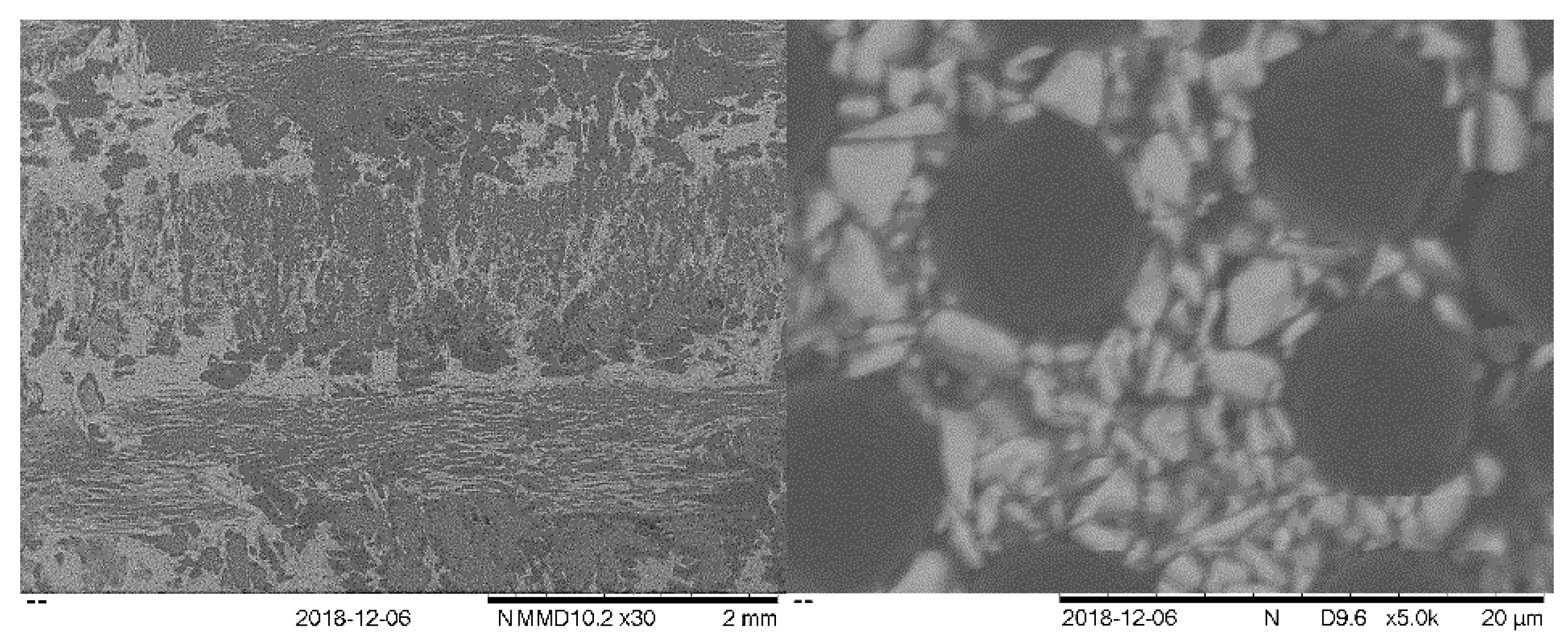
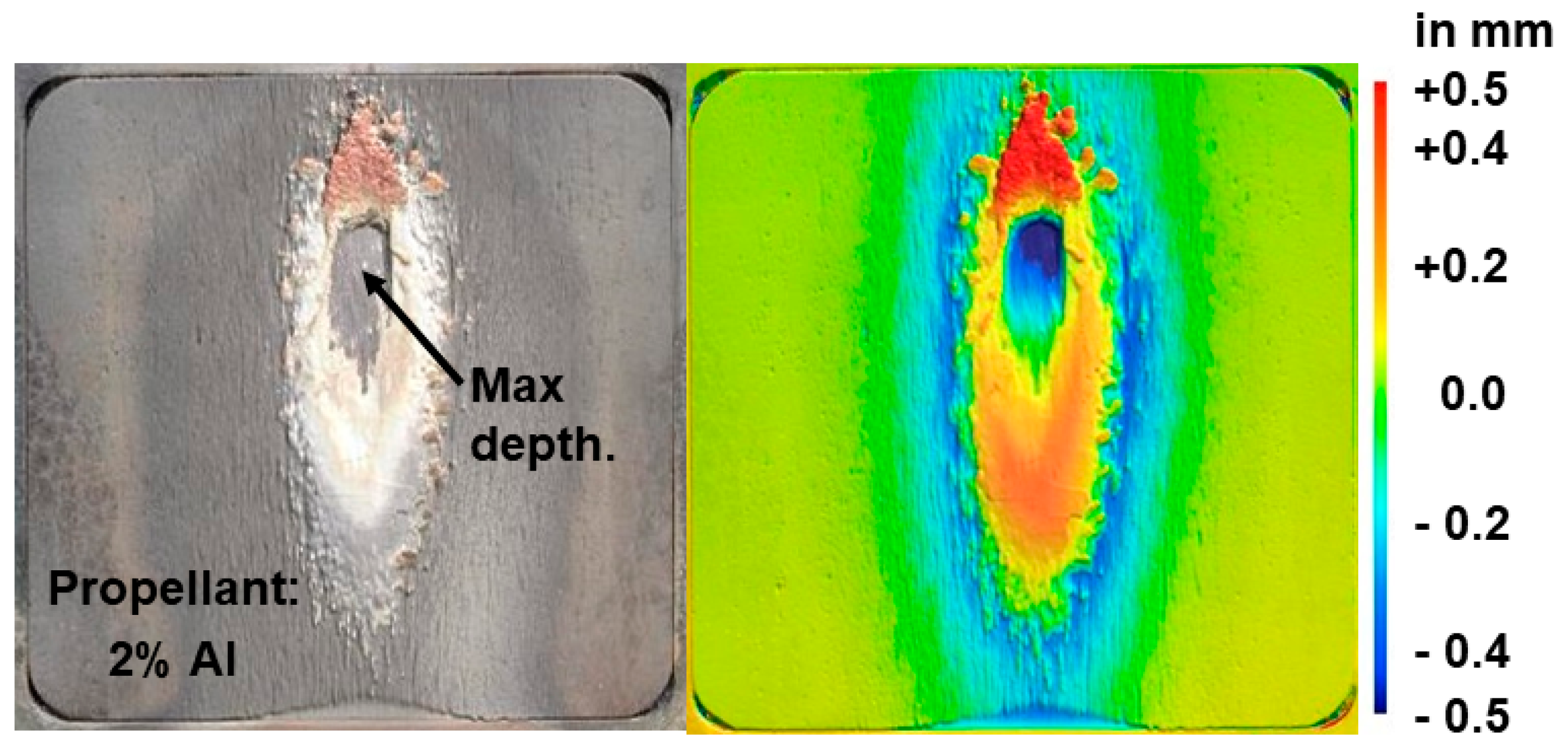
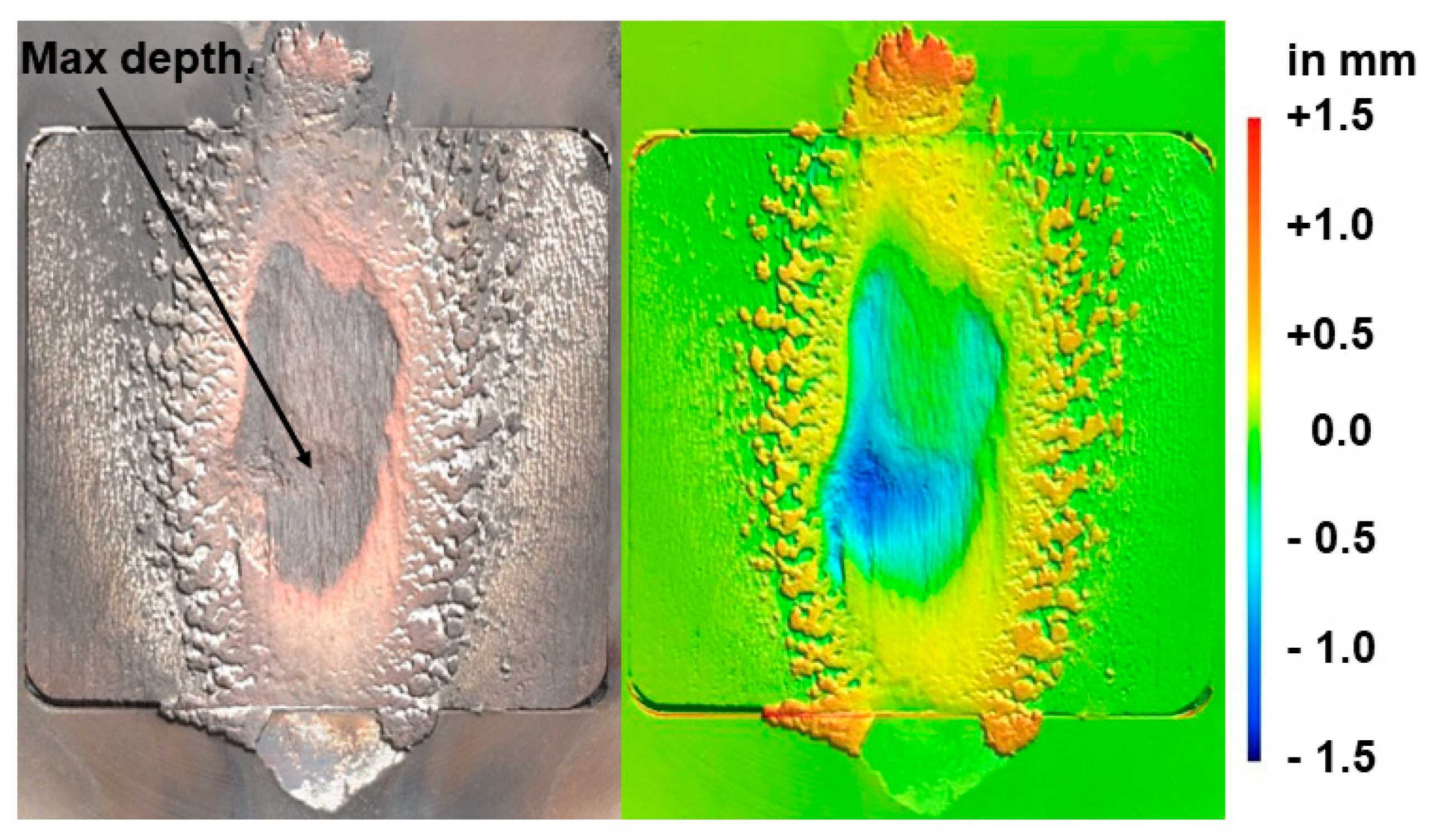
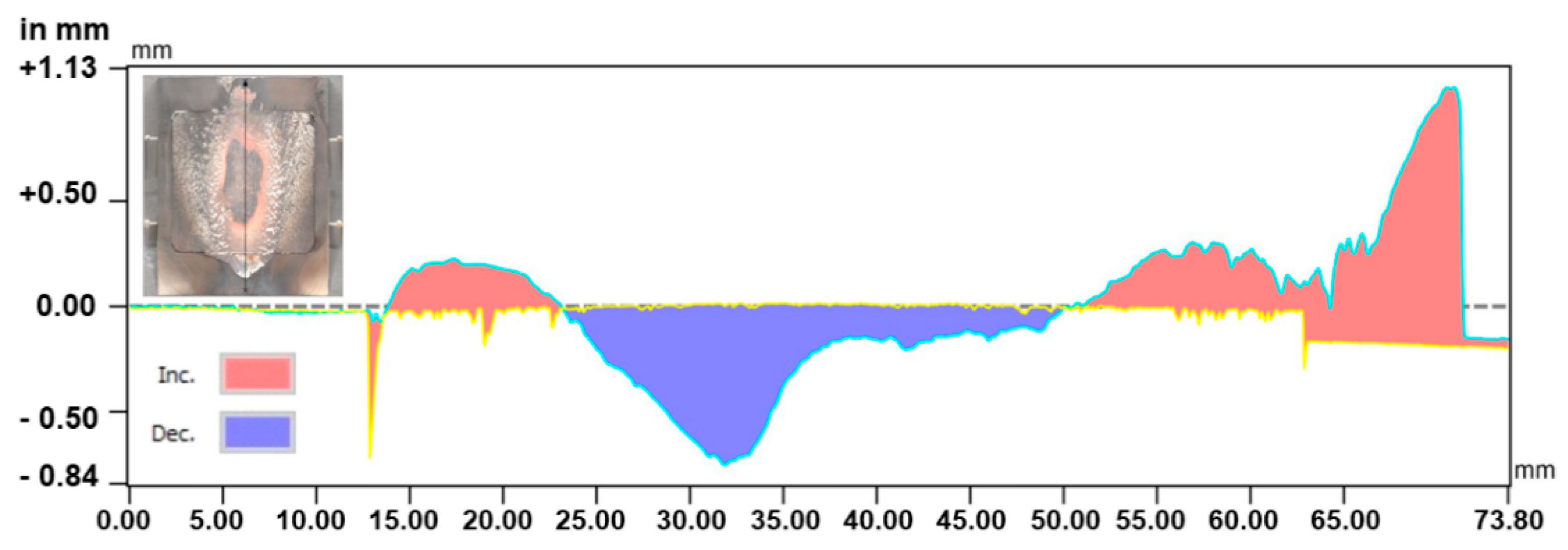
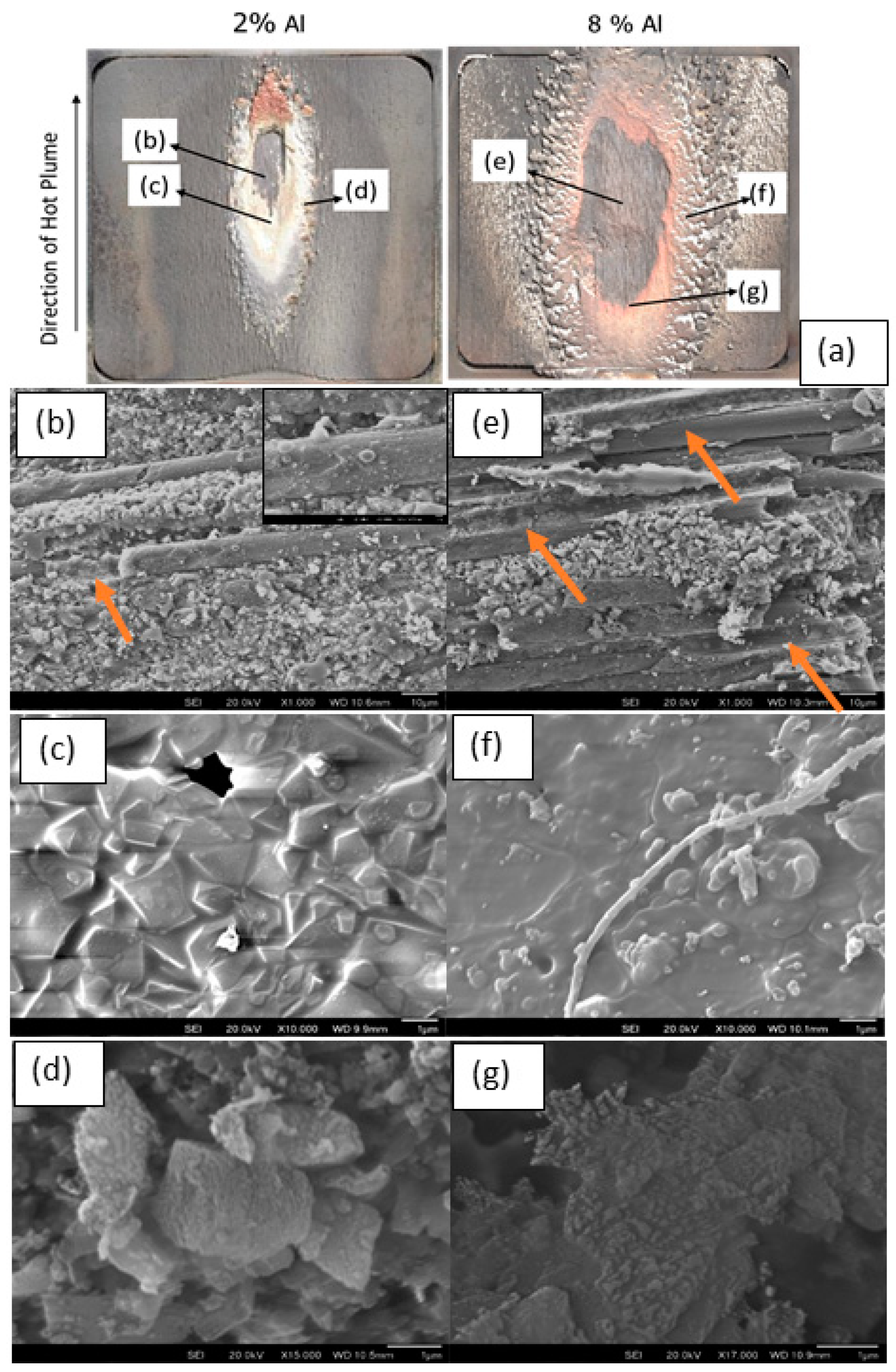
3.2.3. Post-Test Characterisation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silvestroni, L.; Kleebe, H.J.; Fahrenholtz, W.G.; Watts, J. Super-strong materials for temperatures exceeding 2000 °C. Sci. Rep. 2017, 7, 40730. [Google Scholar] [CrossRef] [Green Version]
- Mungiguerra, S.; Di Martino, G.D.; Cecere, A.; Savino, R.; Zoli, L.; Silvestroni, L.; Sciti, D. Ultra-high-temperature testing of sintered ZrB2-based ceramic composites in atmospheric re-entry environment. Int. J. Heat Mass Transf. 2020, 156, 119910. [Google Scholar] [CrossRef]
- Binner, J.; Porter, M.; Baker, B.; Zou, J.; Venkatachalam, V.; Diaz, V.R.; D’Angio, A.; Ramanujam, P.; Zhang, T.; Murthy, T.S.R.C. Selection, processing, properties and applications of ultra-high temperature ceramic matrix composites, UHTCMCs—A review. Int. Mater. Rev. 2019, 65, 389–444. [Google Scholar] [CrossRef]
- Sciti, D.; Silvestroni, L.; Monteverde, F.; Vinci, A.; Zoli, L. Introduction to H2020 project C3HARME–next generation ceramic composites for combustion harsh environment and space. Adv. Appl. Ceram. 2018, 117, s70–s75. [Google Scholar] [CrossRef] [Green Version]
- Zoli, L.; Sciti, D.; Vinci, A.; Galizia, P.; Monteverde, F.; Failla, S.; Silvestroni, L. Ultra-High Temperature Ceramic Matrix Composites. Encycl. Mater. Tech. Ceram. Glas. 2021, 2, 340–352. [Google Scholar]
- Jayaseelan, D.D.; Xin, Y.; Vandeperre, L.; Brown, P.; Lee, W.E. Development of multi-layered thermal protection system (TPS) for aerospace applications. Compos. Part B Eng. 2015, 79, 392–405. [Google Scholar] [CrossRef]
- Savino, R.; Fumo, M.D.; Paterna, D.; Serpico, M. Aerothermodynamic study of UHTC-based thermal protection systems. Aerosp. Sci. Technol. 2005, 9, 151–160. [Google Scholar] [CrossRef]
- Fahrenholtz, W.G.; Hilmas, G.E.; Li, R. Densification of ultra-refractory transition metal diboride ceramics. Sci. Sinter. 2020, 52, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wuchina, E.; Opila, E.; Opeka, M.; Fahrenholtz, W.; Talmy, I. UHTCs: Ultra-High Temperature Ceramic materials for extreme environment applications. Electrochem. Soc. Interface 2007, 16, 30–36. [Google Scholar] [CrossRef]
- Tang, S.; Hu, C. Design, Preparation and Properties of Carbon Fiber Reinforced Ultra-High Temperature Ceramic Composites for Aerospace Applications: A Review. J. Mater. Sci. Technol. 2017, 33, 117–130. [Google Scholar] [CrossRef]
- Vinci, A.; Zoli, L.; Galizia, P.; Kütemeyer, M.; Koch, D.; Sciti, D. Reactive melt in\filtration of carbon fibre reinforced ZrB2/B composites with Zr2Cu. Compos. Part A Appl. Sci. Manuf. 2020, 137, 105973. [Google Scholar] [CrossRef]
- Kütemeyer, M.; Koch, D. Development of Ultra High Temperature Matrix Composites Using a Reactive Melt Infiltration Process. Ph.D. Thesis, Karlsruhe Institute of Technology, Karlsruhe, Germany, 2021. [Google Scholar]
- Zoli, L.; Vinci, A.; Galizia, P.; Gutièrrez-Gonzalez, C.F.; Rivera, S.; Sciti, D. Is spark plasma sintering suitable for the densification of continuous carbon fibre—UHTCMCs? J. Eur. Ceram. Soc. 2019, 40, 2597–2603. [Google Scholar] [CrossRef]
- Naslain, R.; Langlais, F.; Fedou, R. The CVI processing of ceramic matrix composites. J. Phys. Colloq. 1989, 50, 191–207. [Google Scholar] [CrossRef]
- Vignoles, G.L. 8—Chemical vapor deposition/infiltration processes for ceramic composites. In Advances in Composites Manufacturing and Process; Woodhead Publishing Series in Composites Science and Engineering: Cambridge, UK, 2015; Volume 56, pp. 147–176. [Google Scholar]
- Galizia, P.; Vinci, A.; Zoli, L.; Monteverde, F.; Binner, J.; Venkatachalam, V.; Lagos, M.; Reimer, T.; Jain, N.; Sciti, D. Retained strength of UHTCMCs after oxidation at 2278 K. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106523. [Google Scholar] [CrossRef]
- Rubio, V.; Ramanujam, P.; Binner, J. Ultra high temperature ceramic composite materials. Adv. Appl. Ceram. 2018, 117, 56–61. [Google Scholar] [CrossRef]
- Triesch, K.; Krohn, E.-O. The Vertical Test Section (VMK) at DFVLR in Koeln-Porz (Status 1986); Technical Translation ESA-TT-1053; European Space Agency: Cologne, Germany, 1987. [Google Scholar]
- Baker, B.; Rubio, V.; Ramanujam, P.; Binner, J.; Hussain, A.; Ackerman, T.; Brown, P.; Dautremont, I. Development of a slurry injection technique for continuous fibre ultra-high temperature ceramic matrix composites. J. Eur. Ceram. Soc. 2019, 39, 3927–3937. [Google Scholar] [CrossRef]
- Baker, B.; Venkatachalam, V.; Zoli, L.; Vinci, A.; Failla, S.; Sciti, D.; Binner, J. Ablation behaviour of carbon fibre ultra-high temperature composites at oblique angles of attack. Mater. Des. 2021, 212, 110199. [Google Scholar] [CrossRef]
- Wang, T.; Li, H.; Shen, Q.; Li, K.; Li, W.; Song, Q.; Zhang, S. Dependence of mechanical properties on microstructure of high-textured pyrocarbon prepared via isothermal and thermal gradient chemical vapor infiltration. Compos. Part B Eng. 2020, 192, 107982. [Google Scholar] [CrossRef]
- William, G.F. The ZrB2 Volatility Diagram. J. Am. Ceram. Soc. 2005, 88, 3509–3512. [Google Scholar]
- Shi, C.; Alderman, O.; Berman, D.; Du, J.; Neuefeind, J.; Tamalonis, A.; Weber, J.K.R.; You, J.; Benmore, C.J. The structure of amorphous and deeply supercooled liquid alumina. Front. Mater. 2019, 6, 38. [Google Scholar] [CrossRef]
- Levin, I.; Brandon, D. Metastable alumina polymorphs: Crystal structures and transition sequences. J. Am. Ceram. Soc. 1998, 81, 1995–2012. [Google Scholar] [CrossRef]
- Tarasi, F.; Medraj, M.; Dolatabadi, A.; Oberste-Berghaus, J.; Moreau, C. Amorphous and crystalline phase formation during suspension plasma spraying of the alumina–zirconia composite. J. Eur. Ceram. Soc. 2011, 31, 2903–2913. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Liu, L.L.; Xiao, L.Y.; Wang, Z.X. Thermal decomposition of HTPB/AP and HTPB/HMX mixtures with low content of oxidizer. J. Therm. Anal. Calorim. 2014, 119, 1673–1678. [Google Scholar] [CrossRef]
- Wang, F.; Wu, Z.; Shangguan, X.; Sun, Y.; Feng, J.; Li, Z.; Chen, L.; Zuo, S.; Zhuo, R.; Yan, P. Preparation of mono-dispersed, high energy release, core/shell structure Al nanopowders and their application in HTPB propellant as combustion enhancers. Sci. Rep. 2017, 7, 5228. [Google Scholar] [CrossRef]
- Chen, Y.; Guildenbecher, D.R.; Hoffmeister, K.N.G.; Sojka, P.E. Digital imaging holography and pyrometry of aluminum drop combustion in solid propellant plumes. In Optical InfoBase Conference Paper; Optica Publishing Group: Heidelberg, Germany, 2016. [Google Scholar]
- Izumi, Y.; Shiba, T. Characterization of the Alumina-Boria Catalyst. Bull. Chem. Soc. Jpn. 1964, 37, 1797–1809. [Google Scholar] [CrossRef]
- Sato, S.; Kuroki, M.; Sodesawa, T.; Nozaki, F.; Maciel, G.E. Surface structure and acidity of alumina-boria catalysts. J. Mol. Catal. A Chem. 1995, 104, 171–177. [Google Scholar] [CrossRef]





| Propellant | Throat Diameter, ∅th (mm) | Pressure, p0 (bar) | Temperature, T0 (K) | Combustion Time (s) |
|---|---|---|---|---|
| 2% Al | 11.0 | 30 | ~2700 | ~0.9 |
| 8% Al | 7.0 | 60 | ~2900 | ~1.3 |
| UHTCMC Material | Sample #1 with 2% Al | Sample #2 with 8% Al |
|---|---|---|
| Rz vertical (avg)/µm | 49.6 | 49.2 |
| Rz horizontal (avg)/µm | 65.9 | 64.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, V.; Blem, S.; Gülhan, A.; Binner, J. Thermal Qualification of the UHTCMCs Produced Using RF-CVI Technique with VMK Facility at DLR. J. Compos. Sci. 2022, 6, 24. https://doi.org/10.3390/jcs6010024
Venkatachalam V, Blem S, Gülhan A, Binner J. Thermal Qualification of the UHTCMCs Produced Using RF-CVI Technique with VMK Facility at DLR. Journal of Composites Science. 2022; 6(1):24. https://doi.org/10.3390/jcs6010024
Chicago/Turabian StyleVenkatachalam, Vinothini, Sergej Blem, Ali Gülhan, and Jon Binner. 2022. "Thermal Qualification of the UHTCMCs Produced Using RF-CVI Technique with VMK Facility at DLR" Journal of Composites Science 6, no. 1: 24. https://doi.org/10.3390/jcs6010024
APA StyleVenkatachalam, V., Blem, S., Gülhan, A., & Binner, J. (2022). Thermal Qualification of the UHTCMCs Produced Using RF-CVI Technique with VMK Facility at DLR. Journal of Composites Science, 6(1), 24. https://doi.org/10.3390/jcs6010024






