Hydrogen Bonds in Blends of Poly(N-isopropylacrylamide), Poly(N-ethylacrylamide) Homopolymers, and Carboxymethyl Cellulose
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis and Mixtures of Homopolymers
2.3. Preparation of Blends Based on Thermoresponsive Polymers and CMC
2.4. Molecular Characterization
2.5. Properties
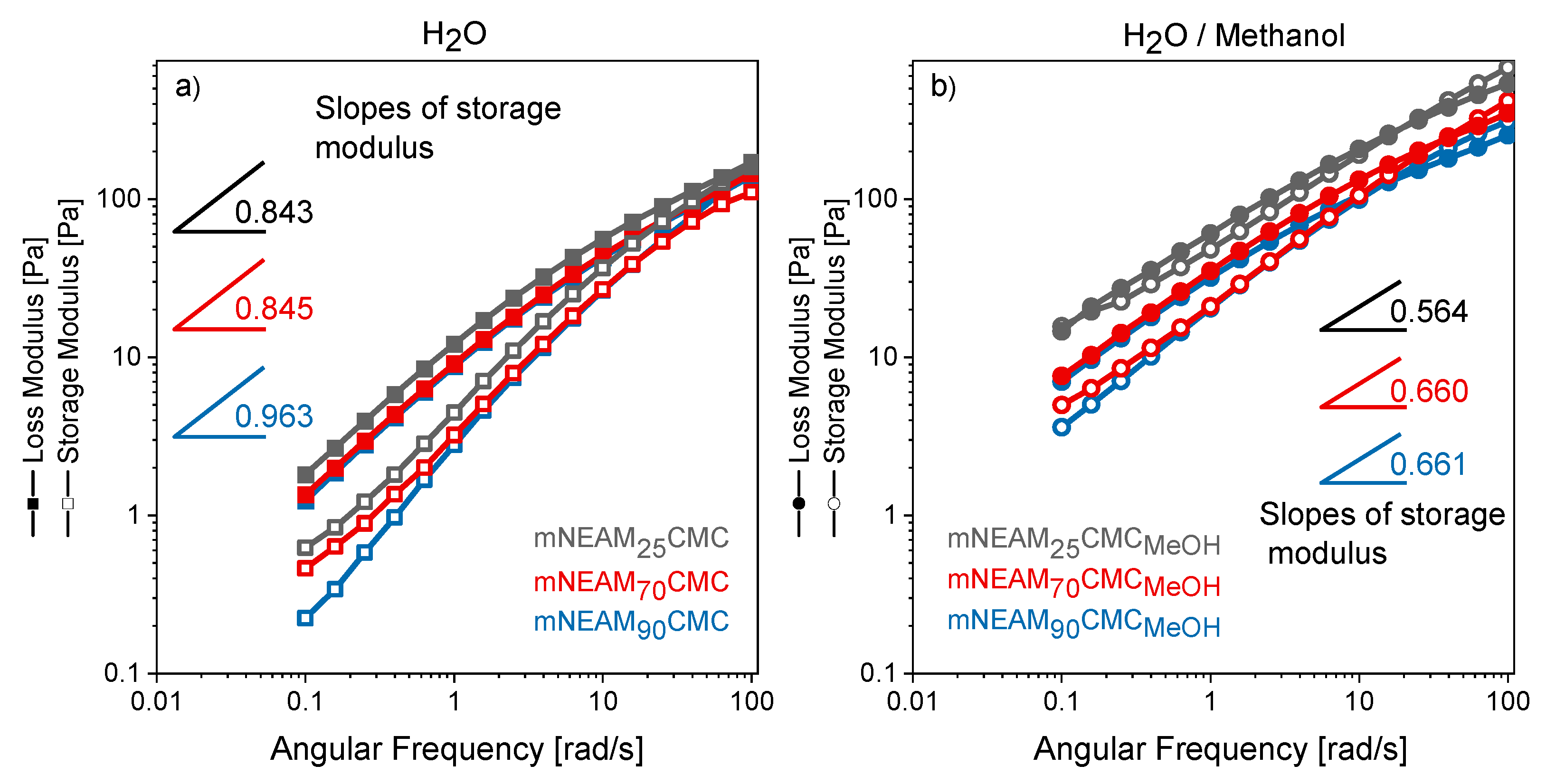
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Declarations
References
- Komorowska, P.; Różańska, S.; Różański, J. Effect of the degree of substitution on the rheology of sodium carboxymethylcellulose solutions in propylene glycol/water mixtures. Cellulose 2017, 24, 4151–4162. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and control able delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Kono, H.; Onishi, K.; Nakamura, T. Characterization and bisphenol A adsorption capacity of beta-cyclodextrin-carboxymethylcellulose-based hydrogels. Carbohydr. Polym. 2013, 98, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Alvarez-Lorenzo, C.; Concheiro, A. Cationic cellulose hydrogels: Kinetics of the cross-linking process and characterization as pH-/ion-sensitive drug delivery systems. J. Control. Release 2003, 86, 253–265. [Google Scholar] [CrossRef]
- Yang, X.H.; Zhu, W.L. Viscosity properties of sodium carboxymethylcellulose solutions. Cellulose 2007, 14, 409–417. [Google Scholar] [CrossRef]
- Vasile, C.; Bumbu, G.G.; Dumitriu, R.P.; Staikos, G. Comparative study of the behavior of carboxymethyl cellulose-g-poly(N-isopropylacrylamide) copolymers and their equivalent physical blends. Eur. Polym. J. 2004, 40, 1209–1215. [Google Scholar] [CrossRef]
- Gulrez, S.K.H.; Al-Assaf, S.; Phillips, G.O. Hydrogels: Methods of Preparation, Characterisation and Applications; IntechOpen LimitedPublisher: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Xiao, C.; Gao, Y. Preparation and properties of physically crosslinked sodium carboxymethylcellulose/poly(vinyl alcohol) complex hydrogels. J. Appl. Polym. Sci. 2008, 107, 1568–1572. [Google Scholar] [CrossRef]
- Chatterjee, A.; Das, B. Radii of gyration of sodium carboxymethylcellulose in aqueous and mixed solvent media from viscosity measurement. Carbohydr. Polym. 2013, 98, 1297–1303. [Google Scholar] [CrossRef]
- Sharma, R.; Das, B.; Nandi, P.; Das, C. Viscosity of sodium carboxymethylcellulose in ethylene glycol-water mixed solvent media: Separation of the influences of polyion conformation and electrostatic interactions on the reduced viscosity. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 1196–1202. [Google Scholar] [CrossRef]
- Marques, N.D.N.; Balaban, R.D.C.; Halila, S.; Borsali, R. Synthesis and characterization of carboxymethylcellulose grafted with thermoresponsive side chains of high LCST: The high temperature and high salinity self-assembly dependence. Carbohydr. Polym. 2018, 184, 108–117. [Google Scholar] [CrossRef]
- Aubry, T.; Bossard, F.; Staikos, G.; Bokias, G. Rheological study of semidilute aqueous solutions of a thermoassociative copolymer. J. Rheol. 2003, 47, 577–587. [Google Scholar] [CrossRef]
- Bokias, G.; Mylonas, Y.; Staikos, G.; Bumbu, G.G.; Vasile, C. Synthesis and Aqueous Solution Properties of Novel Thermoresponsive Graft Copolymers Based on a Carboxymethylcellulose Backbone. Macromolecules 2001, 34, 4958–4964. [Google Scholar] [CrossRef]
- Do Nascimento Marques, N.; de Lima, B.V.; Silveira, V.R.; Lima, B.L.B.; Maia, A.M.S.; Balaban, R.C. PNIPAM-based graft copolymers prepared using potassium persulfate as free-radical initiator: Synthesis reproducibility. Colloid Polym. Sci. 2016, 294, 981–991. [Google Scholar] [CrossRef]
- Vasile, C.; Marinescu, C.; Vornicu, R.; Staikos, G. Enzymatic degradation of thermoresponsive poly(N-sopropylacrylamide) grafted to carboxymethylcellulose copolymers. J. Appl. Polym. Sci. 2003, 87, 1383–1386. [Google Scholar] [CrossRef]
- Farag, R.K.; EL-Saeed, S.M.; Abdel-Raouf, M.E. Synthesis and investigation of hydrogel nanoparticles based on natural polymer for removal of lead and copper(II) ions. Desalination Water Treat. 2016, 57, 16150–16160. [Google Scholar] [CrossRef]
- Biswas, C.S.; Wang, Q.; Du, B.; Stadler, F.J. Testing of the effect of parameters on the cononsolvency of random copolymer gels of N-isopropylacrylamide and N-ethylacrylamide in methanol-water mixed solvents by simple gravimetric method. Polym. Test. 2017, 62, 177–188. [Google Scholar] [CrossRef]
- Pagonis, K.; Bokias, G. Upper critical solution temperature—Type cononsolvency of poly(N,N-dimethylacrylamide) in water—Organic solvent mixtures. Polymer 2004, 45, 2149–2153. [Google Scholar] [CrossRef]
- Sui, X.; Chen, Q.; Hempenius, M.A.; Vancso, G.J. Probing the Collapse Dynamics of Poly(N-isopropylacrylamide) Brushes by AFM: Effects of Co-nonsolvency and Grafting Densities. Small 2011, 7, 1440–1447. [Google Scholar] [CrossRef]
- Scherzinger, C.; Schwarz, A.; Bardow, A.; Leonhard, K.; Richtering, W. Cononsolvency of poly-N-isopropyl acrylamide (PNIPAM): Microgels versus linear chains and macrogels. Curr. Opin. Colloid Interface Sci. 2014, 19, 84–94. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ringsdorf, H.; Venzmer, J. Methanol-water as a co-nonsolvent system for poly(N-isopropylacrylamide). Macromolecules 1990, 23, 2415–2416. [Google Scholar] [CrossRef]
- Schild, H.G.; Muthukumar, M.; Tirrell, D.A. Cononsolvency in mixed aqueous solutions of poly(N-isopropylacrylamide). Macromolecules 1991, 24, 948–952. [Google Scholar] [CrossRef]
- Winnik, F.M.; Ottaviani, M.F.; Bossmann, S.H.; Garcia-Garibay, M.; Turro, N.J. Consolvency of poly(N-isopropylacrylamide) in mixed water-methanol solutions: A look at spin-labeled polymers. Macromolecules 1992, 25, 6007–6017. [Google Scholar] [CrossRef]
- Asano, M.; Winnik, F.M.; Yamashita, T.; Horie, K. Fluorescence Studies of Dansyl-Labeled Poly(N-isopropylacrylamide) Gels and Polymers in Mixed Water/Methanol Solutions. Macromolecules 1995, 28, 5861–5866. [Google Scholar] [CrossRef]
- Biswas, C.S.; Patel, V.K.; Vishwakarma, N.K.; Mishra, A.K.; Bhimireddi, R.; Rai, R.; Ray, B. Synthesis, characterization, and drug release properties of poly(N-isopropylacrylamide) gels prepared in methanol-water cononsolvent medium. J. Appl. Polym. Sci. 2012, 125, 2000–2009. [Google Scholar] [CrossRef]
- Biswas, C.S.; Patel, V.K.; Vishwakarma, N.K.; Mishra, A.K.; Saha, S.; Ray, B. Synthesis and characterization of stereocontrolled poly(N-isopropylacrylamide) hydrogel prepared in the presence of Y(OTf)3 Lewis acid. Langmuir 2010, 26, 6775–6782. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.S.; Vishwakarma, N.K.; Patel, V.K.; Mishra, A.K.; Saha, S.; Ray, B. Synthesis and Study of the Properties of Stereocontrolled Poly(N-isopropylacrylamide) Gel and Its Linear Homopolymer Prepared in the Presence of a Y(OTf)3 Lewis Acid: Effect of the Composition of Methanol–Water Mixtures as Synthesis Media. Langmuir 2012, 28, 7014–7022. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.W.; Napper, D.H. Volume phase transitions of poly(N-isopropylacrylamide) latex particles in mixed water-N,N-dimethylformamide solutions. Chem. Phys. Lett. 1996, 256, 51–56. [Google Scholar] [CrossRef]
- Biswas, C.S.; Patel, V.K.; Vishwakarma, N.K.; Mishra, A.K.; Ray, B. Synthesis and characterization of porous poly(N-isopropylacrylamide) hydrogels prepared in ethanol-water mixtures. J. Appl. Polym. Sci. 2011, 121, 2422–2429. [Google Scholar] [CrossRef]
- Yoshida, M.; Omichi, H.; Katakai, R. Relaxation Phenomena of Thermo-Shrinkage of Methacryloyl Polymer Gels with Pendant α-Amino Acid Groups. Polym. J. 1993, 25, 215–217. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.; García-Peñas, A.; Sharma, G.; Kumar, A.; Stadler, F.J. Competition between Physical Cross-Linking and Phase Transition Temperature in Blends Based on Poly(N-isopropylacrylamide-co-N-ethylacrylamide) Copolymers and Carboxymethyl Cellulose. Macromol. Chem. Phys. 2020, 221, 2000081. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C. 1H NMR Spectroscopy. In Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- García-Peñas, A.; Biswas, C.S.; Liang, W.; Wang, Y.; Stadler, F.J. Lower Critical Solution Temperature in Poly(N-Isopropylacrylamide): Comparison of Detection Methods and Molar Mass Distribution Influence. Macromol. Chem. Phys. 2019, 220, 1900129. [Google Scholar] [CrossRef]
- García-Peñas, A.; Wang, Y.; Muñoz-Bonilla, A.; Fernández-García, M.; Stadler, F.J. Lower critical solution temperature sensitivity to structural changes in poly(N -isopropyl acrylamide) homopolymers. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1386–1393. [Google Scholar] [CrossRef]
- Xue, W.; Huglin, M.B.; Jones, T.G.J. Parameters Affecting the Lower Critical Solution Temperature of Linear and Crosslinked Poly(N-ethylacrylamide) in Aqueous Media. Macromol. Chem. Phys. 2003, 204, 1956–1965. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, X.X. Lower critical solution temperatures of N-substituted acrylamide copolymers in aqueous solutions. Polymer 1999, 40, 6985–6990. [Google Scholar] [CrossRef]
- Biswas, C.S.; Wang, Q.; Galluzzi, M.; Wu, Y.; Navale, S.T.; Du, B.; Stadler, F.J. Versatile Mechanical and Thermoresponsive Properties of Macroporous Copolymer Gels. Macromol. Chem. Phys. 2017, 218, 1600554. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, L.; Zhang, X.; Dai, H. Synthesis and solution properties of temperature-sensitive copolymers based on NIPAM. J. Appl. Polym. Sci. 2010, 116, 1099–1105. [Google Scholar] [CrossRef]
- Cheng, R.-S.; Yang, H.; Yan, X.-H.; Wang, Z.-L.; Li, L. The Solvation and Desolvation Process in the Course of Coil-globule Transition of Aqueous Poly( N-isopropylacrylamide) Solution. Chem. J. Chin. Univ. 2001, 22, 1262–1264. [Google Scholar]
- Garcia-Penas, A.; Biswas, C.S.; Liang, W.; Wang, Y.; Yang, P.; Stadler, F.J. Effect of Hydrophobic Interactions on Lower Critical Solution Temperature for Poly(N-isopropylacrylamide-co-dopamine Methacrylamide) Copolymers. Polymers 2019, 11, 991. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, S.; Vatankhah-Varnoosfaderani, M.; GhavamiNejad, A.; Obiweluozor, F.O.; Du, B.; Stadler, F.J. Self-associations and temperature dependence of aqueous solutions of zwitterionically modified N-isopropylacrylamide copolymers. Rheol. Acta 2015, 54, 501–516. [Google Scholar] [CrossRef]
- Hofmeister, F. About the science of the effect of salts. Arch. Fuer Exp. Pathol. Pharmakol. 1888, 24, 247–260. [Google Scholar] [CrossRef] [Green Version]
- Paricaud, P.; Galindo, A.; Jackson, G. Understanding liquid-liquid immiscibility and LCST behaviour in polymer solutions with a Wertheim TPT1 description. Mol. Phys. 2003, 101, 2575–2600. [Google Scholar] [CrossRef]
- Zhang, Z. Switchable and Responsive Surfaces and Materials for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Wang, Q.; Biswas, C.S.; Galluzzi, M.; Wu, Y.; Du, B.; Stadler, F.J. Random copolymer gels of N-isopropylacrylamide and N-ethylacrylamide: Effect of synthesis solvent compositions on their properties. RSC Adv. 2017, 7, 9381–9392. [Google Scholar] [CrossRef] [Green Version]
- Biswas, C.S.; Wang, Q.; Du, B.; Stadler, F.J. Sorption and desorption properties of random copolymer hydrogels of N-isopropylacrylamide and N-ethylacrylamide: Effect of monomer composition. J. Appl. Polym. Sci. 2017, 134, 45176. [Google Scholar] [CrossRef]
- de Lima, B.V.; Vidal, R.R.L.; do N. Marques, N.; Maia, A.M.S.; de C. Balaban, R. Temperature-induced thickening of sodium carboxymethylcellulose and poly(N-isopropylacrylamide) physical blends in aqueous solution. Polym. Bull. 2012, 69, 1093–1101. [Google Scholar] [CrossRef]
- Dixit, S.; Crain, J.; Poon, W.C.; Finney, J.L.; Soper, A.K. Molecular segregation observed in a concentrated alcohol—Water solution. Nature 2002, 416, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Winter, H.H.; Chambon, F. Analysis of Linear Viscoelasticity of a Cross-Linking Polymer at the Gel Point. J. Rheol. 1986, 30, 367–382. [Google Scholar] [CrossRef]
- Hyun, K.; Wilhelm, M.; Klein, C.O.; Cho, K.S.; Nam, J.G.; Ahn, K.H.; Lee, S.J.; Ewoldt, R.H.; McKinley, G.H. A review of nonlinear oscillatory shear tests: Analysis and application of large amplitude oscillatory shear (LAOS). Prog. Polym. Sci. 2011, 36, 1697–1753. [Google Scholar] [CrossRef]
- Guillet, P.; Mugemana, C.; Stadler, F.J.; Schubert, U.S.; Fustin, C.-A.; Bailly, C.; Gohy, J.-F. Connecting micelles by metallo-supramolecular interactions: Towards stimuli responsive hierarchical materials. Soft Matter 2009, 5, 3409–3411. [Google Scholar] [CrossRef]
- Vatankhah-Varnoosfaderani, M.; Hashmi, S.; GhavamiNejad, A.; Stadler, F.J. Rapid self-healing and triple stimuli responsiveness of a supramolecular polymer gel based on boron–catechol interactions in a novel water-soluble mussel-inspired copolymer. Polym. Chem.-UK 2014, 5, 512–523. [Google Scholar] [CrossRef]
- Yan, Z.-C.; Stadler, F.-J.; Guillet, P.; Mugemana, C.; Fustin, C.-A.; Gohy, J.-F.; Bailly, C. Linear and Nonlinear Dynamic Behavior of Polymer Micellar Assemblies Connected by Metallo-Supramolecular Interactions. Polymers 2019, 11, 1532. [Google Scholar] [CrossRef] [Green Version]





| Name | NIPAM (mol) | NEAM (mol) | AIBN (mol) | DMF (mL) |
|---|---|---|---|---|
| pNIPAM | 0.022 | -- | 6 × 10−4 | 15 |
| pNEAM | -- | 0.028 | 6 × 10−4 | 14 |
| Name | pNIPAM (%) | pNEAM (%) |
|---|---|---|
| mNEAM25 | 75 | 25 |
| mNEAM70 | 30 | 70 |
| mNEAM90 | 10 | 90 |
| Name | Polymer Concentration (wt.%) | CMC Concentration (wt.%) | Water: MeOH (Ratio) |
|---|---|---|---|
| mNEAM25CMC | 10 | 2.5 | 100:0 |
| mNEAM25CMCMeOH | 10 | 2.5 | 50:50 |
| mNEAM70CMC | 10 | 2.5 | 100:0 |
| mNEAM70CMCMeOH | 10 | 2.5 | 50:50 |
| mNEAM90CMC | 10 | 2.5 | 100:0 |
| mNEAM90CMCMeOH | 10 | 2.5 | 50:50 |
| Name | NIPAM [mol.%] | NEAM [mol.%] |
|---|---|---|
| mNEAM90 | 8 | 92 |
| mNEAM70 | 21 | 69 |
| mNEAM25 | 75 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Peñas, A.; Liang, W.; Hashmi, S.; Sharma, G.; Saeb, M.R.; Stadler, F.J. Hydrogen Bonds in Blends of Poly(N-isopropylacrylamide), Poly(N-ethylacrylamide) Homopolymers, and Carboxymethyl Cellulose. J. Compos. Sci. 2021, 5, 240. https://doi.org/10.3390/jcs5090240
García-Peñas A, Liang W, Hashmi S, Sharma G, Saeb MR, Stadler FJ. Hydrogen Bonds in Blends of Poly(N-isopropylacrylamide), Poly(N-ethylacrylamide) Homopolymers, and Carboxymethyl Cellulose. Journal of Composites Science. 2021; 5(9):240. https://doi.org/10.3390/jcs5090240
Chicago/Turabian StyleGarcía-Peñas, Alberto, Weijun Liang, Saud Hashmi, Gaurav Sharma, Mohammad Reza Saeb, and Florian J. Stadler. 2021. "Hydrogen Bonds in Blends of Poly(N-isopropylacrylamide), Poly(N-ethylacrylamide) Homopolymers, and Carboxymethyl Cellulose" Journal of Composites Science 5, no. 9: 240. https://doi.org/10.3390/jcs5090240
APA StyleGarcía-Peñas, A., Liang, W., Hashmi, S., Sharma, G., Saeb, M. R., & Stadler, F. J. (2021). Hydrogen Bonds in Blends of Poly(N-isopropylacrylamide), Poly(N-ethylacrylamide) Homopolymers, and Carboxymethyl Cellulose. Journal of Composites Science, 5(9), 240. https://doi.org/10.3390/jcs5090240











