Abstract
Although in 1977 the first ceramic composite material had been introduced into the market, it was a long time before composite materials were qualified for medical applications. For a long period high purity alumina ceramics have been used as ball-heads and cups. Because of their brittleness, in 1986 yttria stabilized zirconia has been introduced into this application, because of higher strength and fracture toughness. However, due to its hydrothermal instability this material disappeared in orthopaedic applications in 2000. Meanwhile a composite materials based on an alumina matrix with dispersed metastable tetragonal zirconia particles and in-situ formed hexagonal platelets became the standard material for ceramic ball-heads, because of their excellent mechanical strength, hardness and improved fracture toughness. Especially fracture toughness can be improved further by special material formulations and tailored microstructure. It has been shown that a mixed stabilisation of zirconia by yttria and ceria with dispersed alumina and hexagonal platelets overcomes the hydrothermal instability and excellent materials properties can be achieved. Such materials do have big potential to be used in dental applications. Furthermore, these materials also can be seen as a new generation for ball-heads, because of their enhanced fracture toughness. All materials are described within these articles. In order to achieve the required properties of the materials, special raw materials are required. Therefore, it is quite important to understand and know the raw material manufacturing procedures.
1. Introduction
Ceramic materials have played an important role for many years. Their main use has been in bricks and in pottery. Because of its inertness and corrosion resistance, table wear is a quite popular, centuries-old application of ceramics. All goods named are made out of natural raw materials. About 90 years ago, the first publications showed alumina based ceramic materials based on synthetic alumina (Al2O3) powders [1].
In the early 50s of the last century, alumina ceramics became popular for some wear applications. Especially in the textile industry, it is still used as the most wear resistant material against yarns. Additional developments related to several applications, like cutting tools, lead to further improvements of the materials [2].
Due to its reversible phase transformation, zirconia (ZrO2) didn’t play a significant role for 80 years, because of the need of stabilizing oxides in order to keep a stable cubic crystal structure. In refractories, such zirconia became popular, because of their low thermal conductivity. However, mechanical strength properties of such kinds are quite limited. In mid 70s of the last century, it has been found that by a partial stabilization of the zirconia, special properties by microstructural design have been achieved [3]. Finally, in the early 80s it has been found that by stabilization of zirconia by yttria (Y2O3), the tetragonal modification can be stabilized. Such materials show a very high mechanical strength, and since that time, many new developments have been made.
Mixtures of alumina and zirconia were introduced in 1977 for ceramic cutting tools [4]. By incorporation of very fine grained zirconia particles into an alumina matrix, mechanical strength properties and fracture toughness can be enhanced. Finally, in 1991 it was published that reinforcement of alumina with zirconia and hexagonal platelets led to a further improvement of the materials.
One of the major uses of yttria stabilized zirconia started in 1986 when ball-heads for total hip prosthesis have been introduced into the market [5]. Today ball-heads on the bases of yttria stabilized zirconia do not play a role in this field. They have been substituted by composite ceramics. However, in the dental industry yttria stabilized zirconia ceramics are quite popular in prosthetics and as implants [6].
In the following, alumina, zirconia and its composites will be described more in detail.
2. High Purity Alumina
The most popular processing technology for making alumina powder is a disintegration of bauxite with caustic soda. By this process, called the Bayer-process, the alumina is dissolved at temperatures of about 120–140 °C and a pressure of about 2–3 bar. The Al(OH)4−-ions are then precipitated by the addition of seeds. These Al(OH)3 (aluminium hydroxide) precipitates normally contain impurities like magnesium, calcium, silica, iron and sodium ions. After a thermal treatment, the hydroxide changes to several intermediate oxides, before finally the thermodynamically stable α-phase of alumina is reached. Usually, this powder then is used for making synthetic ceramic materials [7].
One of the first applications for an alumina ceramic material has been the isolating component of spark plugs. Since densification during the sintering process of such alumina powders is very difficult, additional components are added. Therefore, most of the alumina ceramic materials contain silica (SiO2), magnesia (MgO) and/or calcium oxide (CaO) [8,9]. By addition of these components, glassy phases are formed and such glassy phases support the densification behaviour significantly. Besides spark plugs, which are usually based on 97 wt-% of alumina and additional silica and calcium oxide or magnesia, seal-discs and substrates for electronic applications are based on similar formulations. Unfortunately, these glassy-phase containing alumina ceramics tend to corrode under long term treatment in humid atmospheres.
Alumina ceramics with a purity of 99.7% based on Bayer-alumina still contain a certain small amount of calcium and silica impurities and do not have a very high sintering activity. Therefore, in order to achieve a density of at least 97.5% it is mandatory to apply sintering temperatures up to 1700 °C. By application of such high sintering temperatures a significant grain-growth occurs. Furthermore, grain-growth control is very difficult. Even the addition of magnesium oxide, which is well known as grain-growth inhibitor, does not help any more to control the growth of the grains at these high temperatures [10]. As a result, discontinuous grain growth occurs. Grain-size of such a kind varies significantly, and single grains with a size of 20 µm or more are quite often found in the microstructures. The typical mechanical strength of these materials is about 250–300 MPa. Figure 1 shows the microstructure of such a ceramic.

Figure 1.
Microstructure of alumina with a purity of 99.7% sintered at 1650 °C.
Looking back to the 1960s, only alumina powders based on the Bayer process have been available on the market. In parallel, already electro corundum has been applied for grinding applications. Electro corundum are alumina single crystals with a size of about 15–300 µm. Due to the melting process, which occurs in order to achieve the single crystals, the purity of these grains is much higher compared to alumina powders derived from the Bayer process. The only remaining impurities are small amounts of silica (SiO2) and sodium oxide (Na2O).
Erhard Dörre, a pioneer in the development of high purity alumina ceramics, recognized the advantage of the higher purity of electro corundum, milled the single crystals down to a size of about 0.6 µm, cleaned the milled powder with hydrofluoric acid in order to get rid of the silica impurities, followed by a second cleaning step with hydrochloric acid in order to get rid of sodium oxide. By this approach, he realized a high purity powder for making high purity ceramics. In addition, this ceramic material could be sintered at temperatures of less than 1600 °C. By addition of magnesia as grain-growth inhibitor a very uniform microstructure with a mean grain-size of about 5 µm has been realized (Figure 2) [11]. Furthermore, the density achieved has been at 99%. As a result of this, the mechanical strength could be increased to 420 MPa.
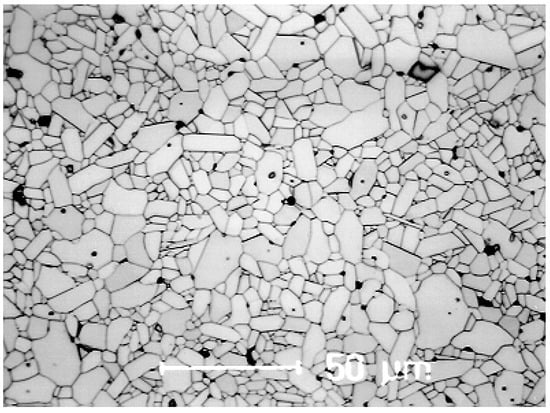
Figure 2.
Microstructure of 99.7% alumina based on purified and milled electro corundum.
The pioneering work of Dörre has been underestimated for a long period. Furthermore, the costs of Dörre’s material have been significantly higher. Therefore, managing people fought against this material, because they only have seen the higher costs and didn’t want to see the unique performance of this material.
Dörre’s approach shows the importance of the raw material in relation to the mechanical properties. Furthermore, by his approach he could avoid impurities, which are sensitive to corrosion. While in the Bayer alumina raw materials always small amounts of calcium oxide and silica are present, the high purity alumina ceramics made by Dörre no longer had any impurities. As a consequence of this, besides the improved mechanical properties, this material also shows a significant higher corrosion resistance.
Finally, Dörre achieved the break-through with this material in a publicly funded project, which has been related to bioceramic ball-heads. Mechanical strength and corrosion resistance are mandatory for a long-term stability. Since Dörre’s material fulfilled these requirements, finally his material has been qualified for bioceramic applications as ball-heads in total HIP replacement systems, while approaches on the basis of Bayer alumina only had limited success.
In 1970s, new powder processing routes for high purity alumina have been developed. All of these processes start from defined chemicals, which are isolated and afterwards transferred to high purity alumina. Typical precursor salts are Ammoniumaluminiumsulfate (Alaun) (NH4)Al(SO4)2·12H2O, Aluminiumchloride [Al(H2O)6]Cl3, Ammoniumaluminiumcarbonate (NH4)Al(CO3)2 or Aluminiumalkoxide Al(OR)3. These salts are easily dissolved and precipitation can be controlled, which means that the primary crystallites formed are influenced by the precipitation method. Especially for the Alkoxides by precipitation either hexagonal or ball-like precipitates can be tailored.
At this point it has to be stated that chemically derived alumina powders are significantly higher in costs compared to Bayer alumina. However, these powders can be sintered already below 1500 °C to a final density of 99.7% of the theoretical density with a very homogeneous fine grained microstructure of about 2.5–3 µm in oxidizing atmosphere (Figure 3, left) or between 1–2 µm after HIPing (Figure 3, right) in mean grain-size. In case these ceramics are only pre-fired to a density of about 97–98% and afterwards are hot isostatic pressed, the theorectical density of 3.98 g/cm3 is achieved. By this approach the homogeneous microstructure with a mean grain-size of about 1.5 µm or even less can be realized, and mechanical properties can be enhanced to about 620–650 MPa [12].
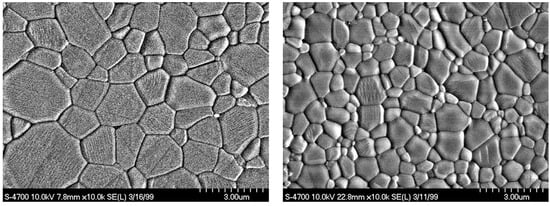
Figure 3.
Microstructure of alumina ceramics; left: starting powder with a mean grain-size of 0.33 µm; right: starting powder with a mean grain-size of 0.22 µm.
Because of the higher mechanical strength properties of high purity alumina ceramics, chemically derived powders have substituted Dörre’s alumina in 1987, followed by introduction of the HIP process in 1994. However, all of these materials with improved mechanical strength properties are extremely brittle and very stiff. It is well known that a brittle material with failures on the surface has a catastrophic breakage. This means that a failed part, i.e., a fractured ball-head, generates many fine particles. These have to be removed before a new ball-head can be replaced in the hip.
Taking into account that within a period of 20 years the mechanical strength properties have been increased by about 50% compared to the original material, it can be concluded that a continuous process improvement including new alumina powders, has been quite successful in order to enhance the mechanical strength properties and therefore the safety of ball-heads made out of alumina ceramics [13].
3. Zirconia
Zirconia did not play a significant role in engineering ceramic applications for a long period because it has a reversible phase transformation. While at room temperature the monoclinic phase is stable, it transfers at 1174 °C diffusion-less into a tetragonal modification. By cooling down, re-transformation into the monoclinic phase takes place. Figure 4 shows the hysteresis, which occurs during phase transformation in pure zirconia in comparison to doped zirconia with a different amount of stabilizing calcium oxide (CaO) [14]. Only after the addition of 19.5 Mol-% of CaO the expansion behaviour becomes reversible, while at lower concentrations, i.e., 5 Mol-%, does the hysteresis effect still occur. The phase transformation from monoclinic to tetragonal is combined with a re-orientation of the ions within the lattice. Due to the higher symmetry of the tetragonal modification, the density of it is 6.1 g/cm3, while in the monoclinic phase it is only 5.85 g/cm3. This means that for a non-stabilized sample, sintering takes place in the tetragonal modification and by cooling down, it re-transforms to monoclinic combined with a volume increase of about 4%. As a consequence of this, cracks are induced within the ceramic body and it is not stable. Figure 5 shows the symmetries in both modifications of the [111]-direction of the cationic lattice.
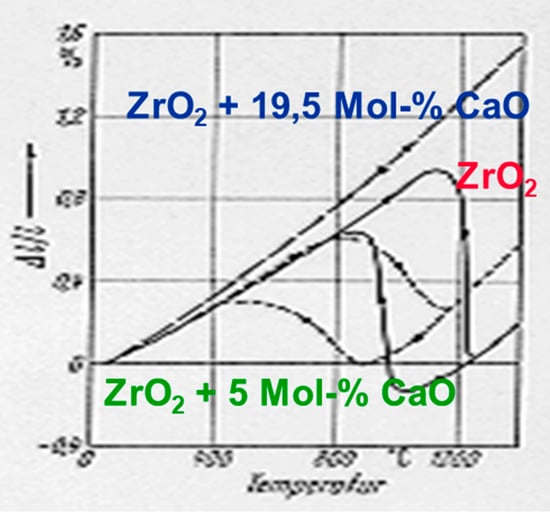
Figure 4.
Hysteresis curve of the reversible phase transformation in zirconia.
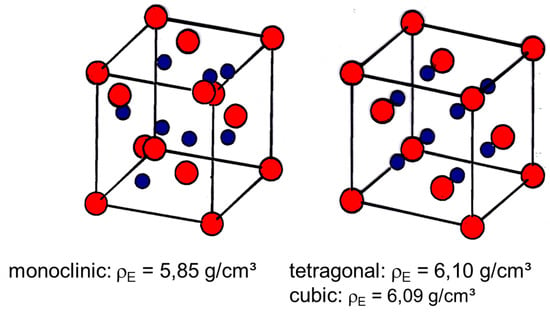
Figure 5.
Orientation of the Zr-ions in the monoclinic and tetragonal lattice.
3.1. PSZ Ceramics
In order to overcome the re-transformation from the higher symmetric modification to its monoclinic phase, MgO and/or CaO are added in a relatively high amount. As it can be seen in the zirconia-rich side of the phase diagram of the system ZrO2-MgO (Figure 6), the concentration of 12–13 Mol-% the cubic zirconia phase remains stable because of formation of a solid solution. Within this solid solution Mg2+-ions replace Zr4+-ions within the cationic lattice. In order to achieve neutrality, oxygen vacancies remain in the anionic lattice [15].
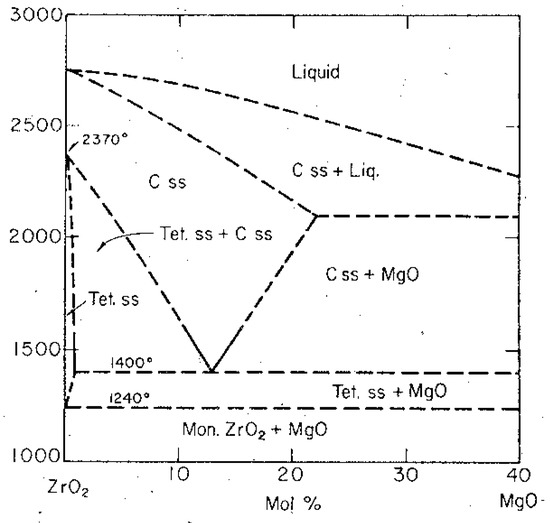
Figure 6.
Phase diagram of the system ZrO2-MgO.
Cubic fully stabilized zirconia doesn’t play an important role in high performance applications. Its major application has been in refractories as isolating material, because zirconia itself has a very low thermal conductivity. Besides, the reversible phase transformation zirconia powders are much more expensive compared to many alumina powders. From an economic point of view, this has been an additional argument against a broad application of cubic zirconia ceramics.
The raw material sources itself are mainly zircon sand, a zirconiumsilicate (ZrSiO4), which contains rare earth impurities, including uranium oxide (UO2). In South Africa the mineral Baddeleyite, a monoclinic zirconia material is found. Again, this mineral is usually accompanied by rare earth oxides and actinides [16].
For making the zirconia powders, there are different processing methods available. The simplest method is the thermal decomposition of zircon sand, ZrSiO4. As can be seen in the phase diagram (Figure 7), it decomposes at temperatures of 1685 °C into a solid solution of ZrO2 and SiO2 [16]. However, by this approach for making zirconia, a relatively high amount of impurities remains within the zirconia. The major impurity is remaining silica. Furthermore, all rare earth and actinides remain; i.e., the accompanying uranium- and thorium oxide remains within the zirconia grains as solid solution. Due to these impurities, it is obvious that such a raw material cannot be used in biomedical applications.
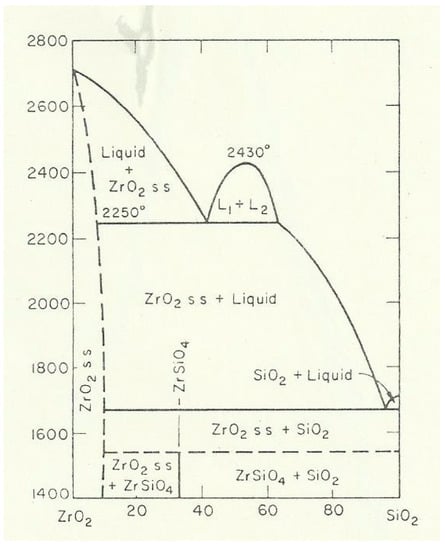
Figure 7.
Phase diagram of ZrSiO4.
A more suitable approach is the alkaline disintegration. By this approach, zircon sand is dissolved in caustic soda and the soluble sodiumzirconate Na2ZrO3 then is transformed into a defined chemical composition, i.e., with sulfuric acid into Zr5O8(SO4)2·H2O or with hydrochloric acid into zirconylchloride (ZrOCl2). The first salt can be thermally decomposed into ZrO2. ZrOCl2 can be cleaned and finally by shifting of the pH with ammonia into alkaline region, zirconiumoxyhydroxide (ZrO(OH)2) is precipitated. By calcination of the hydroxide finally a pure zirconia is achieved. It has to be remarked that in this processing route radioactive impurities can be reduced to a very low level, even below the detection limit [17].
It has to be remarked that neither by thermal separation nor by alkaline disintegration the hafnia (HfO2) can be separated from zirconia. This means that normally zirconia always contains an amount of about 1.5–2.0 wt-% of hafnia.
A third alternative approach for making zirconia powder is the carbo-thermic disintegration. By this method, zircon sand is mixed with carbon and chlorine and heat-treated at 900–1300 °C:
ZrSiO4:Hf,U,Th + C + Cl2 → ZrCl4 + HfCl4 + SiCl4 + UCl4 + ThCl4 + CO2
The formation of the chlorides is the only processing route in order to separate Zr4+- and Hf4+-ions. Furthermore, it is a very effective method to get rid of the radioactive impurities [18,19]. The separation of the Chlorides is made in a condensation column. Finally, the zirconium tetrachloride is directly transferred at about 250 °C in water steam into zirconia:
ZrCl4 + 2H2O → ZrO2 + 4HCl
By this method an extremely high purity zirconia powder with a nano-scaled particle size can be made. This powder behaves completely differently from all other known high purity zirconia powders. It is the most promising raw material in order to achieve excellent yttria stabilized zirconia ceramics. Furthermore, it is the best raw material to be composed with alumina in order to make composite materials. However, before being used in the ceramic body, it is mandatory to make a chemical pre-treatment of it. Figure 8 shows an image of the particles in comparison with a standard grade zirconia material. Table 1 summarizes the chemical impurity levels of the zirconia powders derived by different processing routes [17].
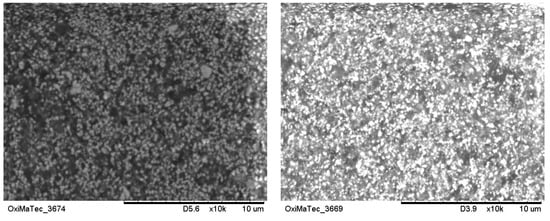
Figure 8.
Powder morphology of standard grade zirconia powder (left) and the nano-scaled powder (right) made by synthesis without calcination.

Table 1.
Overview table of zirconia powders derived by different processing routes.
As already mentioned, in order to have stable zirconia ceramics, additional oxides have to be added in order to retain either the cubic phase, or in the case of a partial stabilisation either to directly keep the tetragonal phase metastable until room temperature, or to have cubic matrix grains and within these grains tetragonal precipitates. The last named ceramics are well known under the name “PSZ” (partially stabilized zirconia). The most convenient stabilizing oxide for these kinds of ceramic materials is MgO [15,20].
Figure 6 shows the zirconia riche side of the phase diagram ZrO2-MgO. As can be seen in the phase diagram, the region, where the tetragonal phase is existing, is quite limited. It is only exisiting at high temperatures up to a concentration of about 0.5 Mol-% and disappears already below 1200 °C. Such a low concentration cannot stabilize the tetragonal zirconia modification.
Garvie et al. have shown a very interesting approach by a partial stabilization of zirconia with MgO. They reduced the amount of stabilizing MgO to about 9.2 Mol-% (3.2 wt-%), sintered at a temperature of 1750 °C and cooled the system quickly until about 800 °C [21]. Sintering at 1750 °C means that this process takes place in the region, where only a cubic solid solution is existing. During cooling, the material has to pass a region where tetragonal and cubic solid solution exists in parallel, i.e., during cooling tetragonal precipitates are formed within the cubic matrix grains. Either by optimizing the cooling rate or by heat treatment after sintering, these tetragonal precipitates can be developed within the cubic matrix grains. These cubic grains are in the size of 30–70 µm, while the tetragonal particles within the cubic grains are limited up to 0.2 µm in order to retain their tetragonal modification. A bigger size of these tetragonal precipitates lead to an immediate phase transformation to the monoclinic phase at room-temperature. So, the closer the tetragonal precipitates come to the critical coherence length, the higher the mechanical strength and fracture toughness. Figure 9 shows the typical microstructure of Mg-PSZ and its tetragonal precipitates.
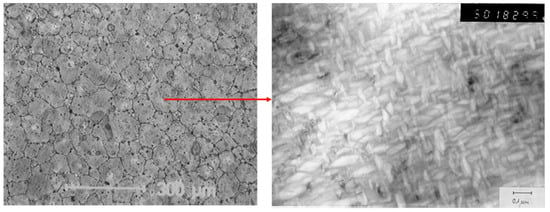
Figure 9.
Microstructure of Mg-PSZ (left) and its tetragonal precipitates within one grain (right).
Compared to cubic stabilized zirconia, which only has a mechanical strength of about 200–250 MPa and is very brittle, the Mg-PSZ grades show strength levels of 500–750 MPa. However, in the case of tetragonal precipitates being close to the critical coherence size, the material can only be used at room temperatures. In the event elevated temperatures are required in the application, the size of the precipitates has to be reduced and therefore the mechanical strength goes down to about 500 MPa. However, in this case the materials can be used up to about 800 °C. Due to its unique microstructure with its coarse grain-size, it fractures trans-granular at a high Weibull modulus of up to 25 (see Figure 10) [22].

Figure 10.
Fractured surface of Mg-PSZ.
Usually, Mg-PSZ ceramics show a hardness of HV10 ≈ 1150–1200. Its fracture toughness is about 5 MPa√m. While pure Mg-PSZ is quite critical in its behaviour related to phase transformation, a system based on co-stabilisation of MgO and Y2O3 is thermodynamically much more stable. Figure 11 shows the strength decrease of Mg-PSZ and Y-Mg-PSZ after treatment at 1100 °C. It is evident that mixed stabilisation causes significant benefits compared to stabilisation with only one single oxide [22,23].
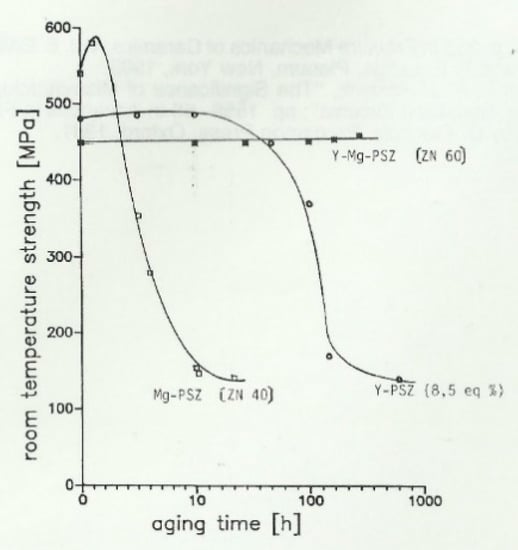
Figure 11.
Thermal stability of Y-Mg-PSZ vs. Mg-PSZ.
For making PSZ ceramics, raw materials are used, which come from the thermal decomposition process of zircon sand. Their chemical purity is limited (see Table 1) and most of these materials have a yellow colour, which goes back to the trace impurities of radioactive elements. Small amounts of silica, up to about 0.2 wt-% are not very critical for these kind of ceramic materials. Silica remains in the grain-boundaries and forms forsterite (MgSiO4). As a consequence of this, the zirconia matrix is destabilized. In order to avoid the formation of forsterite, it has been proposed to add small amounts of strontium oxide in order to make SrSiO4 and therefore retain the MgO within the cubic matrix grains [24].
In the past there have been attempts to qualify Mg-PSZ–based on high purity zirconia powders–ceramics for total hip replacement systems. Compared to alumina, Mg-PSZ has a higher fracture toughness and lower hardness. Mechanical strength properties are comparable. However, there was no breakthrough with these materials, although these kinds of materials do not undergo a hydrothermal decomposition reaction like Y-TZP ceramics.
3.2. TZP Ceramics
Yttria stabilized zirconia (Y-TZP) materials are quite popular, because within the system ZrO2-Y2O3 there is a broader range of tetragonal solid solution (see Figure 12). The tetragonal solid solution region exists up to an yttria content of 3 Mol-%. In practice, this means that by having powders with a high sintering activity, the tetragonal phase can be retained stable as long as the sintering temperature does not exceed 1450 °C.
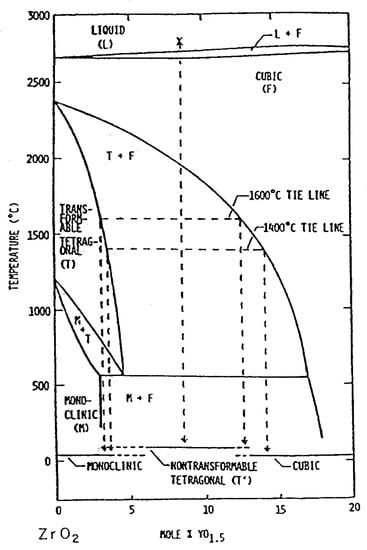
Figure 12.
Phase diagram of the system ZrO2-Y2O3.
Normally, high sintering activity of a powder is given when they are fine grained and have a high specific surface area. However, handling of these powders is much more difficult in pressing compared to more coarse powders, which are used for PSZ ceramics. Suitable zirconia powders are derived from purified ZrOCl2- and Zr5O8(SO4)2-precursor salts. During precipitation the formation of the crystallites is controlled severely, and very fine-grained particles of ZrO(OH)2 are precipitated. These particles can be calcined at different temperatures. Finally, the most popular powders—relatively easy to handle for making ceramics—have a specific surface area of about 8 m2/g. For high-end materials with a very high sintering activity, the specific surface area is about 15 m2/g.
For many years the distribution of yttria within the zirconia grains has already been made in the precursor salt solution through addition of a solution of YCl3. In the following precipitation process, the yttria is extremely homogeneously distributed within the zirconia grains. During the following calcination step a solid solution of yttria dissolved in the zirconia matrix takes place. Its particle size is about 0.2–0.3 µm. This approach is well known as the so-called “coprecipitation” process.
As it has been already described in detail, the carbo-clorination process also leads the very fine-grained zirconia powder (see Figure 8 right image). Although in principle yttria could be dispersed in the precursor salt, in practice this has never been done. In the late 1980s it was found that the surface properties of this zirconia raw material can be tailored by chemical treatment in order to adopt yttria onto the surface of the nano-scaled zirconia powder. This approach is the so-called “coating” process. Figure 13 shows the principle differences between these two different approaches for stabilisation of the tetragonal phase in the system ZrO2-Y2O3.
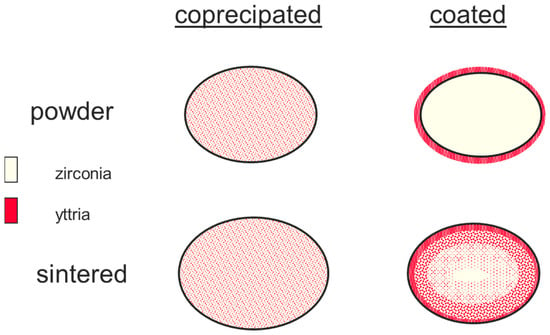
Figure 13.
Comparison of “coprecipitation” and “coating” technology (principle!).
Figure 13 illustrates the difference between “coprecipiatated” and “coated” powder. While in the starting grains of the coprecipitated material, there is a very homogeneous distribution of yttria, in the coated material the stabilizing yttria is on the surface of the grains. During sintering there is diffusion of yttria into zirconia with the formation of the solid solution. This diffusion reaction prevents and limits grain-growth. However, it has to be taken into account that the sintering temperature is kept at reasonably low conditions in order to realize a gradient of yttria within the zirconia grains. In case of a high sintering temperature, the gradient disappears. Since in the coprecipitated powder almost 75% of the particles are in its tetragonal modification, during sintering slight grain-growth occurs.
A quite interesting investigation was conducted in the early 1990s through application of high temperature X-ray investigations on the two different powders: while the coprecipitated powder already has about 75% of tetragonal phase, the remaining monoclinic phase transforms already at a temperature of about 1000 °C into tetragonal. By cooling to room temperature, the tetragonal phase remains stable [25]. Opposite to this behaviour, at the transition temperature of 1174 °C a first phase transformation from monoclinic is observed. Only by a higher temperature and time is there the transformation to the tetragonal phase. Both X-ray diffraction patterns can be seen in Figure 14. In the DTA-analysis there is also observed a small endothermal effect in the yttria coated powder at the transition temperature of 1174 °C (Figure 15). This grants proof of a slightly diffusion controlled phase transformation by yttria.
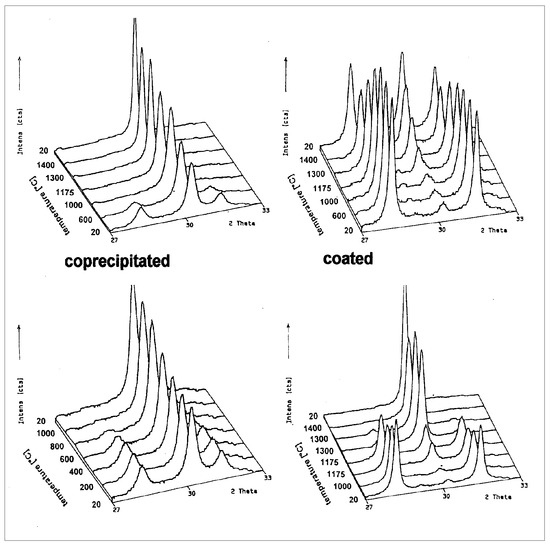
Figure 14.
High temperature X-ray diffraction pattern of coprecipitated and coated Y2O3-ZrO2 composition.
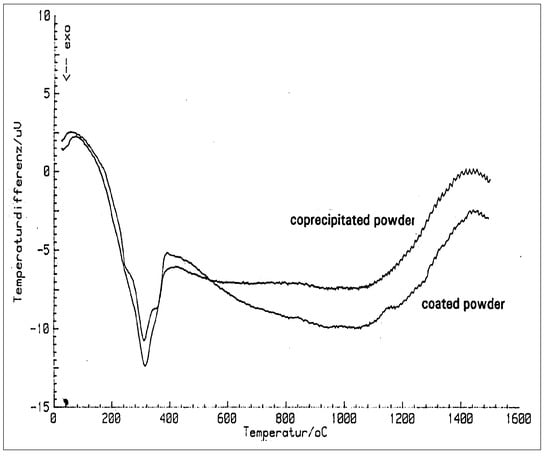
Figure 15.
DTA-analysis of coprecipitated and coated yttria-zirconia composites.
The sintering characteristics of the two Y-TZP materials are different: while the coprecipitated material starts earlier in the shrinkage, it requires higher sintering temperatures in order to reach the final density. Opposite to this, densification of coated powder starts later, but the shrinkage rate is higher and lower sintering temperatures are required. Dilatation experiments of the shrinkage behaviour are shown in Figure 16 [26]. For the coated material only 1390 °C are required in order to reach a density of 99.7%; the coprecipitated powder requires 1450 °C. Figure 17 shows the microstructure of the two materials. As it can be taken out of the picture, the microstructure of the coated material is finer.
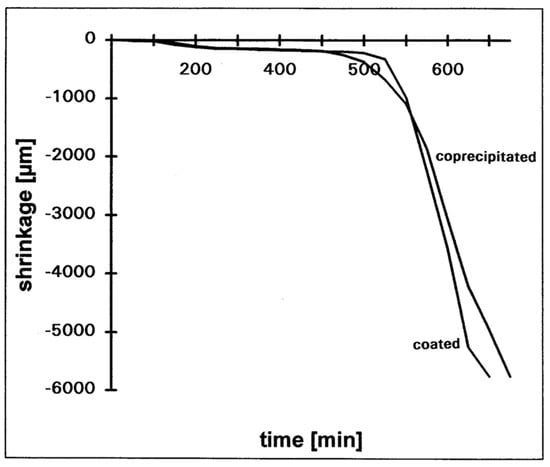
Figure 16.
Shrinkage behaviour of different Y-TZP materials.
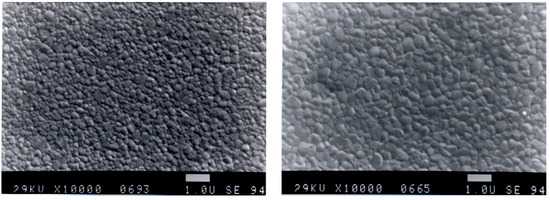
Figure 17.
Microstructure of Y-TZP derived by coating (left) and coprecipitation (right) method (magnification 10,000×).
Mechanical strength properties of the materials are comparable; its mechanical strength after sintering in oxidizing atmosphere is about σ ≈ 1000–1100 MPa. Due to the yttria gradient in the coated material, its fracture toughness is higher. The real difference between the two materials is in their hydrothermal stability. Aging experiments at 135 °C with a vapor pressure of 2 bar have been made in comparison with bio-grade alumina [27]. In both cases a decrease of strength is measured; however, it is more drastic for the coprecipitated material [28,29]. The relative strength decrease is shown in Figure 18; Figure 19 contains the development of the monoclinic phase with increasing aging time, and Figure 20 shows the thickness of the corroded layer. In Figure 21 the corroded layer of the two materials is shown after 48 h treatment.
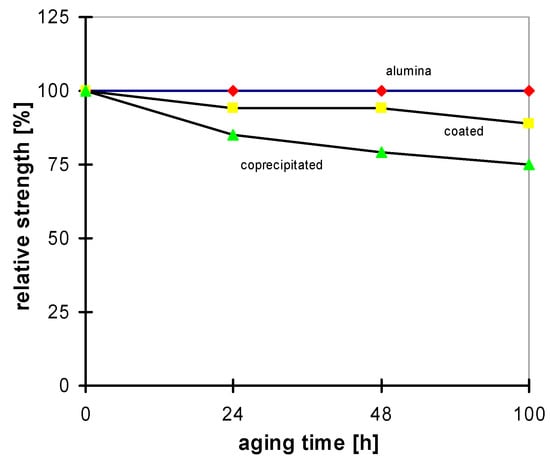
Figure 18.
Strength decrease of Y-TZP after hydrothermal treatment.
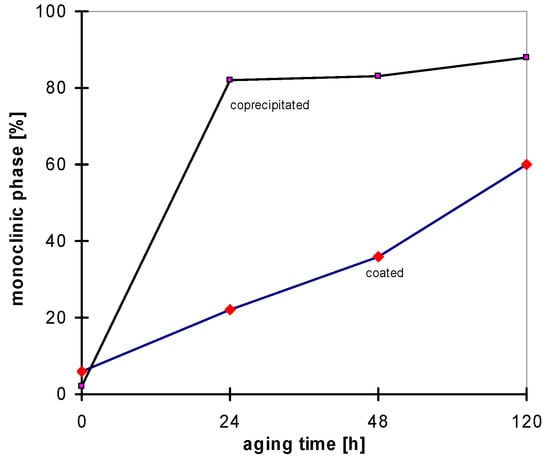
Figure 19.
Monoclinic phase of Y-TZP after hydrothermal ageing.
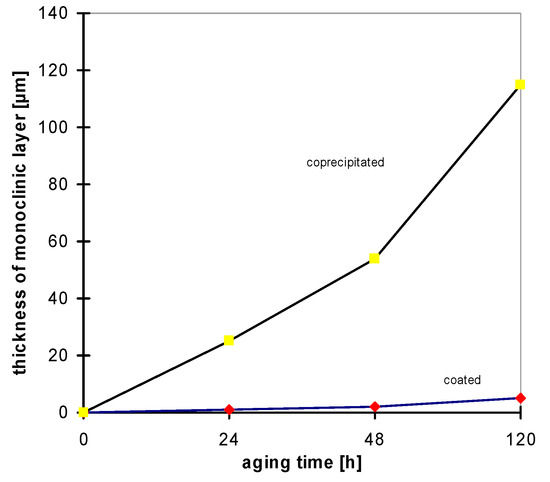
Figure 20.
Thickness of the corroded layer after hydrothermal treatment.
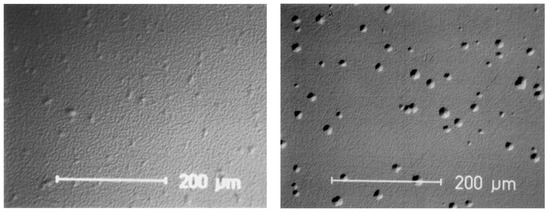
Figure 21.
Cross-sectioned polished surface after hydrothermal treatment for 48 h (left: coating technology, right: coprecipitation).
As it is shown in the above mentioned experiments, yttria stabilized zirconia based on the coating process has an enhanced resistance against hydrothermal decomposition compared to coprecipitated materials. Y-TZP ceramics have been very popular for being used in orthopaedic applications as ball-heads mated against UHMWPE (ultra high molecular weight polyethylene). Due to a production problem, many ball-heads fractured and therefore Y-TZP ceramics disappeared for this application.
Although Y-TZP based on coated zirconia has a higher stability, when the coprecipitated materials are made in a proper way, they also fulfill the requirements for being used as dental implants [30]. Because of the higher fracture toughness and its improved hydrothermal stability, coated ceramics are preferred. They are already on the market in the premium segment applications. Recent experiments related to the hydrothermal stability (treatment at 134 °C up to 100 h in an autoclave system) have shown its superior behaviour related to aging (Figure 22). Aging according to the linear drawing shows a parabolic behaviour (Figure 23). By Mehl–Avrami–Johnson drawing the linearity is demonstrated (Figure 24). The gradient is n = 0.273 ± 0.016 and lnk = −1.39 ± 0.045, which corresponds to a velocity constant of k = 0.25 h−1 [31]. Compared to Chevalier, aging of ZY is significantly slower than in coprecipitated Y-TZP [32].
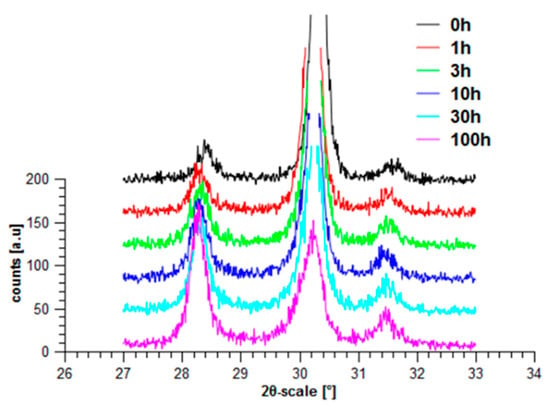
Figure 22.
Hydrothermal treatment of ZY (Y-TZP based on coating technology).
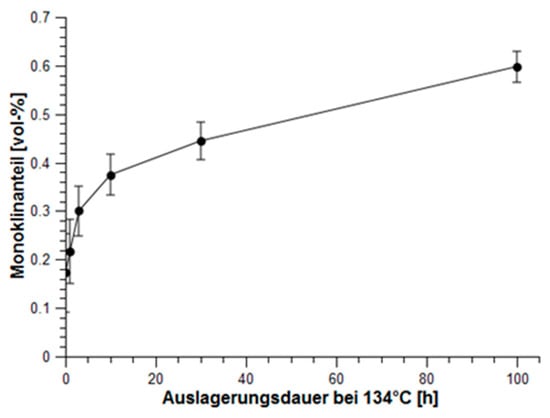
Figure 23.
Increase of monoclinic phase–linearly.
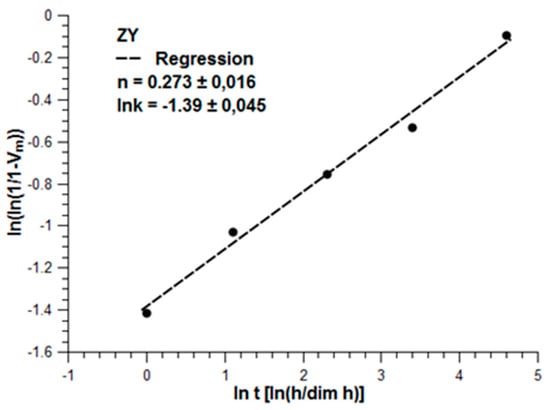
Figure 24.
Increase of monoclinic phase according to Mehl-Avrami-Johnson-kinetics.
Due to their biocompatibility and aesthetics, Y-TZP ceramics are of great interest in dental applications. It sounds that even in coprecipitated materials the longterm stability is good enough for these materials to be used in this application. For sure, the higher hydrothermal stability of Y-TZP made by the coating method is higher, but the more important argument is their higher fracture toughness.
4. Alumina Matrix Composites
In a certain sense, alumina with an amount of 92–96 wt-% contain additional oxides. But usually, only alumina, spinel and/or mullite can be detected in X-ray diffraction. In many cases X-ray diffraction is reduced to alumina, because the ingredients form a glassy phase, which reduces sintering temperatures. Opposite to these materials, composites are based on different materials. One of the oldest composites is the dispersion of Titaniumcarbide in an alumina matrix. Such materials have been used as ceramic cutting tools and are made by hot-pressing [33].
Dispersing of zirconia in an alumina matrix was discovered in the early 1970s. From the phase diagram it is well known that alumina and zirconia do not have a chemical reaction. The only interesting thing is that at a temperature of 1660 °C and a composition of 42.6 wt-% (47.2 Mol-%) alumina and 57.4 wt-% (52.8 Mol-%) zirconia there is a eutectic point (Figure 25) [34]. Due to the fact that there is no chemical reaction between alumina and zirconia, such a composite material has become of great interest. While first attempts at dispersing monoclinic zirconia particles within the alumina matrix increased the fracture toughness by inducing microcracks, no breakthrough was achieved because of its limited mechanical strength properties [35].
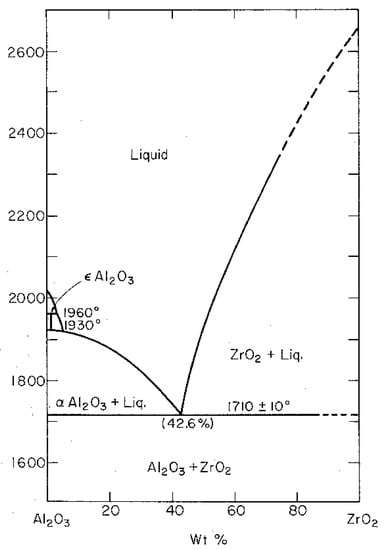
Figure 25.
Phase diagram of the system alumina-zirconia.
In 1977 it was recognized by Dworak and Olapinski that by dispersion of nanoscaled zirconia grains within the alumina matrix, they can be retained as metastable in its tetragonal modification at room temperature; i.e., alumina works as a stabilizing matrix. The zirconia particles, which have been based on the carbothermal disintergration process, may not be agglomerated, but rather homogeneously distributed within the alumina matrix [2]. In theory, each zirconia particle should be surrounded by alumina grains. Such a distribution guarantees a constraint of the tetragonal zirconia grains and keeps the tetragonal modification metastable until room-temperature. Later, it was found that the optimal size of the zirconia grains should be between 0.2–0.6 µm [36]. In case the particles are coarser, there is a spontaneous phase transformation to monoclinic, while the finer grains do not have a tendency to re-transform into their monoclinic phase. Without any additional stabilizing oxide, a zirconia concentration of 5–10 wt-% is relatively easy to handle [37]. It becomes very difficult at a concentration of up to 15-wt-% (10 Vol-%). Higher amounts of zirconia dispersed within the alumina matrix cannot be retained in its tetragonal modification. Figure 26 shows a typical microstructure of the system 90 wt-% Al2O3 and 10 wt-% (6.9 Vol-%) ZrO2.
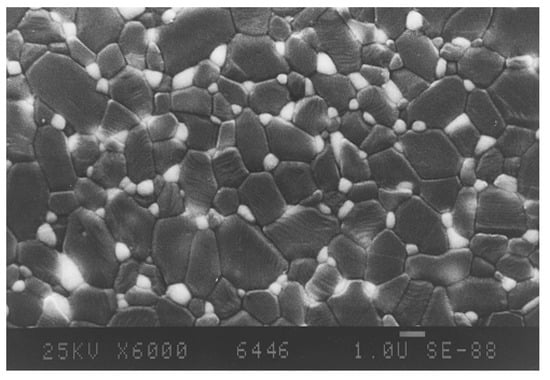
Figure 26.
Microstructure of ZTA-ceramics containing 10 wt-% of ZrO2.
Within a dispersion of 75 wt-% Al2O3 and 25 wt-% (18 Vol-%) ZrO2 it is impossible to retain the tetragonal modification of zirconia without any additional stabilizing oxide. The most common stabilizing oxide for zirconia is yttria. The very conventional approach is to use a coprecipitated yttria containing zirconia powder and disperse it in alumina. Normally stabilisation of zirconia is made with 3 Mol-% of yttria. However, due to the fact that we still have the stabilizing effect of alumina, such a stabilisation is too high. In order to reduce the stabilizing oxide, attempts have been made by using a mixture of 3 Mol-% yttria containing zirconia with a non-stabilized zirconia. Unfortunately, this approach has had only limited success.
Experimental work has shown that working with a 3 Mol-% yttria containing zirconia powder increases the mechanical strength, but decreases the fracture toughness compared to the approach with zirconia coming from the carbo-chlorination process in addition with the coating technology. However, because of the solubility of yttria in water suspension, it has been very difficult to retain the slurry stable during the milling process. During milling the solubility of yttria is enhanced further and it has been quite difficult to control the rheology. In laboratory experiments it has been shown that the higher amount of zirconia within the alumina matrix leads to an enhanced fracture toughness [38,39]. Corresponding to the higher fracture toughness, the hardness decreased. This is shown in Figure 27.
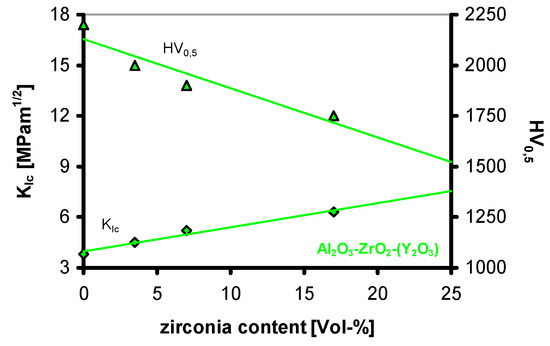
Figure 27.
Fracture toughness and hardness in the system Al2O3-ZrO2(Y).
While in the two-phase system based on alumina and zirconia, the rheology of the slurry is still not difficult, the situation immediately changes through the addition of yttria as mentioned above. This makes it extremely difficult to have a stable suspension during the body preparation process. During milling the formation of hydroxides influences the stability of the slurry significantly.
In the late 80th of the last century, the know-how related to rheology with defined chemicals has not been established. It has been quite popular to use commercially available dispersing agents. Unfortunately, the suppliers of these dispersing agents were not prepared to disclose any functional groups within their systems. So, it has been very difficult to understand the colloidal chemistry within a slurry. Since at this time the focus has been dedicated to new inorganic material formulations, processing technology, especially understanding colloidal chemistry, has been very limited. As a consequence of this, finally, in order to have a stable suspension, the following idea has been created: synthesis of a stable ternary chemical composition with yttria.
Due to the fact that the solubility of chromia in alumina is well known and in the literature has been described that only amounts of chromia of at least 1 wt-% shall lead to any influence in the alumina, it has been decided to synthesise the ternary material yttriumchromite, YCrO3. Furthermore, it has been assumed that during the sintering process the following chemical reaction may take place:
Al2O3 + ZrO2 + YCrO3 → Al2O3:Cr + ZrO2:Y
In detail this means that during the sintering process the ternary component is destroyed and the formation of solid solutions takes place.
Body preparation of the system containing alumina, zirconia and yttriumchromite (YCrO3) worked pretty well and the rheology of the slurry remained completely stable. Pressed and sintered parts are pink-coloured. In X-ray diffraction analysis, besides alumina, only tetragonal zirconia has been detected. At this time, it has been very surprising that the addition of only a small amount of chromia already caused a significant hardness increase combined with brittleness of the ceramic material. Even at very high amounts of zirconia, the material still stayed at high hardness and remained very brittle compared to the chromia-free system (Figure 28).
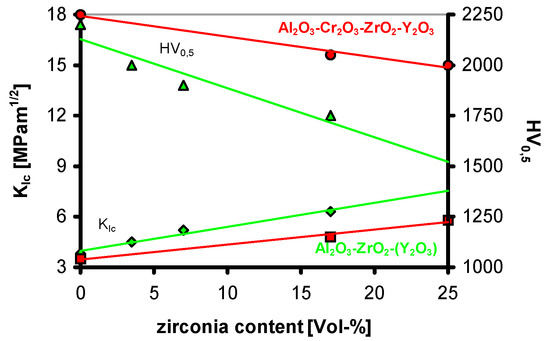
Figure 28.
Fracture toughness and hardness within the system Al2O3-ZrO2:Y-Cr2O3.
During the period that this development work was made, a group in Ceramatec (Salt Lake City, UT, USA), worked in the system CeO2-ZrO2-Al2O3-SrO. They have found, besides formation of tetragonal zirconia, hexagonal platelets with the chemical composition SrAl12O19 in the sintered bodies. At a certain concentration of strontium aluminate, these platelets enhanced fracture toughness significantly [40].
Following the approach of Cutler et al.,the addition of small amounts of strontium oxide within the system Al2O3-Cr2O3-ZrO2-Y2O3 finally showed a significantly higher fracture toughness at a high hardness compared to the four component system (Figure 29). In addition, this composition also had a very high mechanical strength of about 1000 MPa after sintering in oxidizing atmosphere. Application of HIPing leads to a strength of about 1200 MPa.
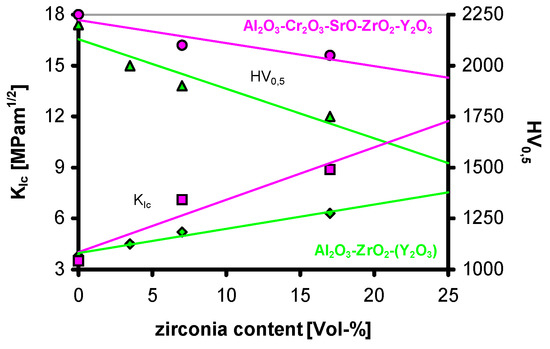
Figure 29.
Fracture toughness and hardness in the system Al2O3-Cr2O3-ZrO2-Y2O3-SrO.
Since strontium oxide is also not very stable in water suspension, again the approach of making a ternary oxide has been made: synthesis of strontiumzirconate (SrZrO3). Commercially available SrZrO3-powders could not be used because of their radioactive impurities. Therefore, the ternary oxide was made by a solid state chemical reaction using strontium carbonate and high purity zirconia.
The material system of the composite material has therefore been based on Al2O3-ZrO2-YCrO3-SrZrO3. During the sintering process therefore the following solid state chemical reactions take place:
Al2O3 + ZrO2 + YCrO3 → Al2O3:Cr + ZrO2:Y
Al2O3:Cr + ZrO2:Y + SrZrO3 → Al2O3:Cr + SrAl12−xCrxO19 + ZrO2:Y
In order to really have a reproducible product, the sintering schedule has to be kept under controlled conditions. During the thermal treatment, the above-mentioned solid state chemical reactions take place. At this point it has to be clearly stated that it is very important to use a pre-treated zirconia powder coming from the carbo-chlorine process, because of the formation of the gradient of yttria within the zirconia grains.
When only the mechanical strength is taken into account, a different zirconia powder can be used. However, only zirconia coming from the carbo-chlorination process finally forms a gradient with yttria, while conventional calcined zirconia powders don’t show this effect; they behave like coprecipitated zirconia powders. Certainly, there is no negative effect on the mechanical strength, but its fracture toughness decreases compared to the materials based on the yttria gradient in zirconia of the system. Furthermore, its behaviour related to aging can be compared to Y-TZP based on coprecipitated and coated zirconia powders.
As already described above, at higher zirconia concentrations it is mandatory to stabilize the zirconia grains at least in parts, due to the fact that even at higher zirconia additions, there is a constraint of the metastable tetragonal zirconia particles. However, in this case they have to be partly stabilized by addition of a stabilizing oxide, i.e., yttria. The amount of required yttria for stabilization depends on the amount and also on the size of the particles. For the commercially available material “Biolox delta”, which is based on the above described details, the yttria concentration required is 1.5 Mol-% related to zirconia—optimized for zirconia from carbo-clorination process—and its nanoscaled powder size. Figure 30 shows the typical microstructure of the material with zirconia coming from carbo-chlorine disintegration. In Figure 31 a fractured surface is shown. Table 2 summarizes the typical mechanical properties of this material.
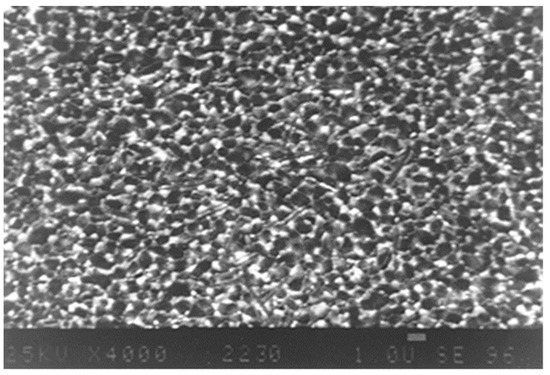
Figure 30.
Microstructure of the original “Biolox delta”, containing zirconia from carbo-chlorine processing route.
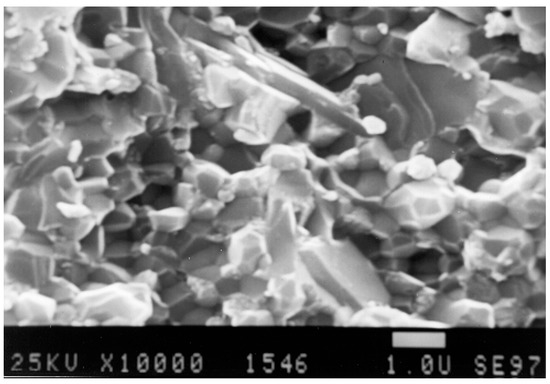
Figure 31.
Fractured surface of the original “Biolox delta”.

Table 2.
Material properties of original “Biolox delta”.
The above-mentioned composite material are three phase ceramics: alumina, metastable tetragonal zirconia and hexagonal platelets. Without platelets, the system is brittle. During the development work made for “Biolox delta”, we have also investigated the system Al2O3-Cr2O3-ZrO2-CeO2-SrO [38,39]. Within this system fracture toughness increases significantly, while the hardness remains reasonably high (Figure 32). However, in its mechanical strength properties it remains limited, because it is only about 550 MPa. Sintering only can take place in oxidizing atmosphere. By HIP treatment ceria is reduced from Ce4+ to Ce3+, which causes a complete de-stabilisation of the zirconia to its monoclinic modification.
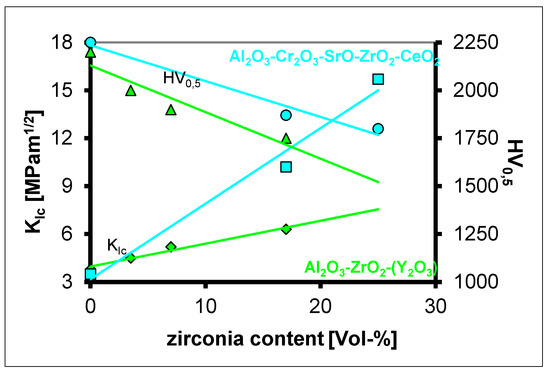
Figure 32.
Hardness and fracture toughness of the system Al2O3-Cr2O3-ZrO2-CeO2-SrO.
Although the mechanical strength level is limited, the material has a very high fracture toughness at a reasonable hardness. The mechanical results obtained are summarized in Table 3. Figure 33 shows the corresponding microstructure.

Table 3.
Material properties of an experimental grade composite material, using cerium oxide as stabilizing oxide for zirconia.
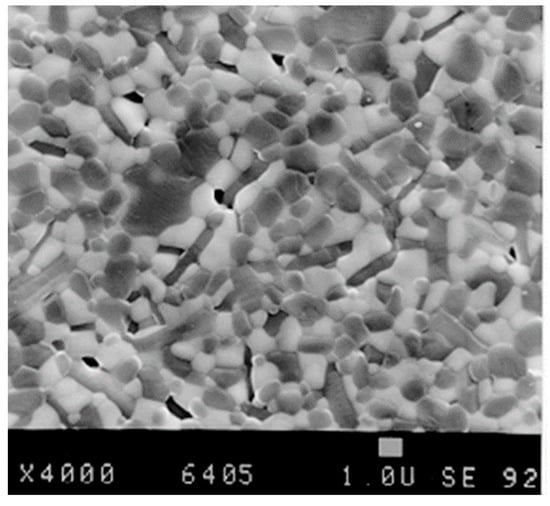
Figure 33.
Microstructure of a ceria stabilized alumina matrix composite.
In principle, the combination of strength properties coming from stabilisation of zirconia by yttria and fracture toughness coming from stabilisation of zirconia by ceria in combination with the platelet reinforcement might be an optimum material. So, taking into account the results obtained for the described system, as a logical consequence zirconia might be stabilised by yttria and ceria.
Another aspect which has to be taken into account are the results obtained in the system Al2O3-La2O3. Yasuoka et al. published a significant increase of strength and fracture toughness in an alumina matrix composite containing 20 Vol-% of LaAl11O18 [41]. Own experiments within the system Al2O3-Cr2O3-ZrO2-Y2O3-La2O3 have shown that platelet formation of LaAl11O18 is more extended compared to SrO. Furthermore, it is obvious that a higher amount of partially stabilized zirconia gives an additional benefit with respect to fracture toughness [42].
It is well known that ceria stabilized zirconia leads to ceramic materials with high fracture toughness, but limited strength. Dispersing of zirconia in an alumina matrix with yttria and ceria as stabilizing agents for zirconia has been studied intensively in a publicly funded European project (GRD1-199-10585). Within these investigations the following chemical composition has been found to be very promising:
55.3 wt-% Al2O3
0.7 wt-% Cr2O3
4.0 wt-% La2O3
36.45 wt-% ZrO2
1.05 wt-% Y2O3
2.5 wt-% CeO2
This chemical composition combines relatively high hardness, high mechanical strength and high fracture toughness in the sintered body. Its typical properties after HIPing are summarized in Table 4. Detailed stress analysis investigations have shown high compressive stresses at very low monoclinic content. This material also has been investigated very detailed with respect to its biocompatibility. Cytotoxicity and in-vitro cancerogeniticity tests have been made. No cytotoxic effect has been found. Finally, in-vivo experiments (implantation of ceramic parts into the bones of NewZealand white rabbits) confirmed the good biocompatibility of the material [43].

Table 4.
Mechanical properties of a composition based on an alumina matrix, containing platelets and about 40 wt-% of zirconia, partially stabilized by yttria and ceria.
Although these promising results already had been established until 2005, such a material formulation has not been regarded, when the ISO standard 6474/partII has been established. Its maximum amount of zirconia dispersed within the alumina matrix in this standard is limited to 30 wt-%. Since the results described above have been ignored for the new ISO-standard 6474/partII, such a promising material cannot be exploited in the bioceramic field. In order to realize a material with higher fracture toughness, it is mandatory to reduce the zirconia content to less than 30 wt-% and therefore, results obtained in the former development project GRD1-199-10585 cannot be exploited.
Meanwhile, the concept of zirconia stabilisation with a mixture of yttria and ceria in an alumina matrix was transferred to a material system with only about 25 wt-% of zirconia. During the development phase the following chemical composition sorted out, which combines high hardness, fracture toughness and mechanical strength:
72.5 wt-% Al2O3
2.5 wt-% La2O3
23.4 wt-% ZrO2
0.4 wt-% Y2O3
1.0 wt-% CeO2
0.2 wt-% Pr6O11
The typical material properties of this chemical composition are summarized in Table 5. The chemical material formulation contains in addition a small amount of praseodymium oxide. This oxide works as a “bridging” component between alumina and zirconia [44].

Table 5.
Material properties of an alumina matrix composite containing plateles and about 25 wt-% of zirconia, partially stabilized by yttria and ceria.
Again, it is confirmed that the stabilisation of zirconia by yttria and ceria in addition with platelets based on lanthanum aluminium oxide leads to good materials, especially taking into account the fracture toughness. So, the higher the fracture toughness, the better the reliability of the material; it becomes more resistant to slow crack growth behaviour.
Unfortunately, none of the new approaches in order to enhance the quality of these composite materials further was exploited to-date. For 20 years, there have been no material innovations for ceramic ball-heads. Meanwhile, the zirconia within “Biolox delta” has been substituted by a conventional zirconia. It appears as though nobody wants to take the risk to introduce a new, more innovative material into the field of orthopaedic applications because of long-term qualification procedures.
The above described material innovations show that it is possible to enhance the material properties further, and as a consequence of this, make the materials even more safe than they are today. It sounds like the hurdles, coming from regulatory affairs, have limited the amount of zirconia addition due to the existing ISO-standard and the high costs for qualification for bioceramic applications compared to the state-of-the-art, are too high and therefore the limiting factors.
While alumina ceramics have been improved step by step, it sounds like the existing composite material qualified for bioceramics does not have such a steady state improvement, but is optimized related to cost savings because of the use of a cheaper zirconia material.
5. Zirconia Matrix Composites
Alumina matrix composites, especially when doped with chromia, became quite famous in bioceramic applications. As it has been described above, there are made different developments in order to further enhance the fracture toughness of such kind of materials in order to further enhance the safety aspects related to potential fractures of ball-heads, which are quite popular in THR surgeries.
For a couple of years, it has been quite popular to use hard-hard pairings in hip surgeries. Meanwhile, mating of ceramic ball-heads with UHMWPE cups again achieved a higher priority, because of more or less no wear of the cup. Such a system also was popular in the late 80th and early 90th by using zirconia ball-heads in combination with UHMWPE. Unfortunately, by the limited hydrothermal stability, finally this solution disappeared, although Y-TZP based on the coating technology didn’t show such a big disadvantage compared to ceramics based on co-precipitated powders.
On the other hand, yttria stabilized zirconia has become quite popular in the dental industry. Nowadays, crowns based on this ceramic material are quite popular. Abutments and implants have also started to become interesting in dental restorations. Especially Y-TZP based on yttria coated zirconia powders have extremely good success in dental implantology [44]. Its major benefit is the better hydrothermal stability compared to the corresponding ceramics based on co-precipitated powders. As an alternative material for dental implants, yttria stabilized zirconia containing 20 wt-% of alumina is under discussion because of its high strength, but limited fracture toughness.
Since dental implants are embedded into natural bone, mechanical strength is less important. It is much more important to have a material available which is resistant against shear forces. Resistance against shear forces can be realized in ceramic materials with a high fracture toughness. Pure Ce-TZP ceramics have a very high fracture toughness of up to 20 MPa√m, but their mechanical strength of about 400–500 MPa is limited [45]. By addition of about 10 wt-% of alumina, the mechanical strength can be enhanced, but fracture toughness decreases to 8.62 MPa√m [46]. Based on these results and taking into account the experience of the alumina matrix composites, it makes sense to combine the yttria/ceria stabilisation and platelet reinforcement in a zirconia matrix.
In a very first approach, yttria stabilized zirconia derived by the coating method has been combined with platelets on the basis of lanthanum aluminium oxide. In practice, a mixture of 90 wt-% of yttria coated zirconia and 10 wt-% of Al2O3/La2O3 has been made (ZYA10P) [47]. The ratio of alumina and lanthanum oxide has been made in order to form about 60% of hexagonal platelets within the zirconia matrix during sintering. The sintering process itself is combined with a solid state chemical reaction and therefore has to be made in a very accurate manner; i.e., within the process, nuclides for platelet formation have to be made, before a homogeneous distribution of platelets within the microstructure can be obtained. Its principle mechanical properties are summarized in Table 6. Figure 34 shows the typical microstructure.

Table 6.
Typical mechanical properties of ZYA10P.

Figure 34.
Microstructure of ZYA10P, a material based on Y-TZP and LaAl11O18.
As can be seen within the table, mechanical strength properties correspond to Y-TZP. This material shows a higher fracture toughness compared to the pure Y-TZP material after HIP treatment. A small remaining porosity in the only sintered material shows a very high fracture toughness. This effect has to be related to the remaining micro-porosity within the microstructure. Its aging behaviour under hydrothermal conditions corresponds to Y-TZP made by coating technology.
In order to enhance the fracture toughness further, we have analysed the stress-strain behaviour of Y-TZP and Ce-TZP (both based on coating technology). Figure 35 shows the results obtained. While in Y-TZP we have linear-elastic behaviour, in Ce-TZP there is a plastic deformation.
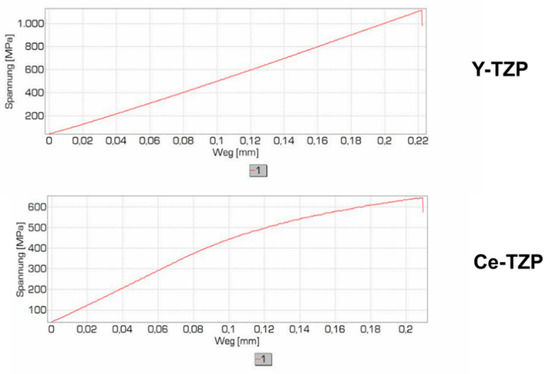
Figure 35.
Stress-strain behaviour of Y-TZP and Ce-TZP.
First investigations in the system Ce-TZP/SrO/Al2O3 have been made by Cutler et al. [37]. They have found a significant increase in mechanical strength in composites based on a Ce-TZP matrix and containing 15 Vol-% and 30 Vol-% of alumina, where about 30% of this addition contained hexagonal platelets of SrAl12O19. Normally, Ce-TZP has as mechanical strength of about 400 MPa, while the materials made by Cutler et al. showed 725 MPa. Hardness increased as well from 950 to 1350, while fracture toughness decreased slightly from 12.5 MPa√m to 11 MPa√m. Figure 36 shows the influence of the SrO amount, which is relevant for the platelet formation with respect to fracture toughness for two different amounts of alumina addition to the Ce-TZP matrix.
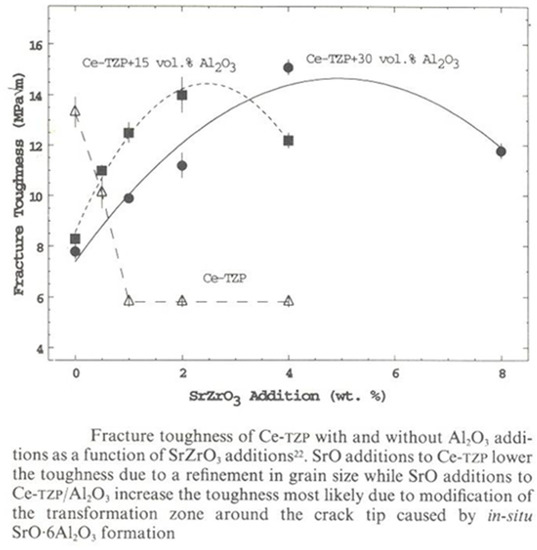
Figure 36.
Fracture toughness related to the addition of SrO and final platelet formation.
As already mentioned, mechanical strength properties of Ce-TZP are quite limited, but fracture toughness is quite high. Therefore, the approach has been made to again combine 90 wt-% of Ce-TZP with Alumina/Lanthanum oxide (ZCA10P) in order to realize an amount of about 90% of platelet formation during sintering. The materials properties achieved in this first approach are summarized in Table 7 and the corresponding microstructure is shown in Figure 37. From Table 7 it becomes obvious that also by this approach the mechanical strength increases significantly compared to pure Ce-TZP. Further optimisation of the amount of stabilizing ceria finally lead to a fracture toughness of 15 MPa√m at a reasonable mechanical strength of 760 MPa in the 4-point bending test [48].

Table 7.
Mechanical properties of Ce-TZP/LaAl11O18 composite.

Figure 37.
Microstructure of Ce-TZP/LaAl11O18 composite (ZCA10P).
Finally, we have investigated mixed stabilisation of zirconia by yttria and ceria in a system, which contains about 5 wt-% of alumina/lanthanum oxide. By this approach, the high mechanical strength properties can be retained and fracture toughness can be increased compared to Y-TZP. Initial results obtained are summarized in Table 8. Figure 38 shows the corresponding microstructure.

Table 8.
Mechanical properties of Y-Ce-TZP/Al2O3/LaAl11O18.
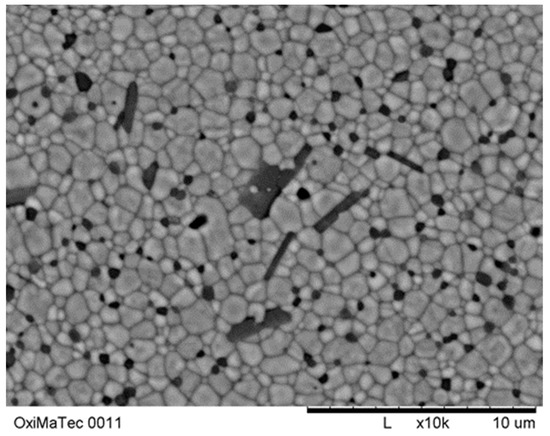
Figure 38.
Microstructure of preliminary Y-Ce-TZP/Al2O3/LaAl11O18.
Meanwhile, the material composition has been optimized further. In addition, the raw material has been substituted by a nanoscaled zirconia powder, derived from direct synthesis of zircon-tetra-chloride with water steam. This optimisation work finally leads to a material with excellent mechanical strength of σ = 1100–1200 MPa measured with 4-point bending test, a fracture toughness kIc = 12–14 MPa√m and a hardness of HV10 = 1250. The microstructure of this optimized material is shown in Figure 39. Within the zirconia matrix there are globular alumina particles and platelets homogeneously distributed.

Figure 39.
Microstructure of final Y-Ce-TZP/Al2O3/LaAl11O18 (ZA05P).
Both materials, ZCA10P and ZA05P, have finally been treated under hydrothermal conditions in the same way, just as ZY has been treated. Figure 40 shows the result obtained for ZCA10P (Ce-TZP/LaAl11O18) and Figure 41 the results obtained for ZA05P (Y-Ce-TZP/Al2O3/LaAl11O18). Opposite to ZY, which already has a significantly higher hydrothermal stability compared to conventional Y-TZP based on co-precipitated powders, there is no phase transformation.
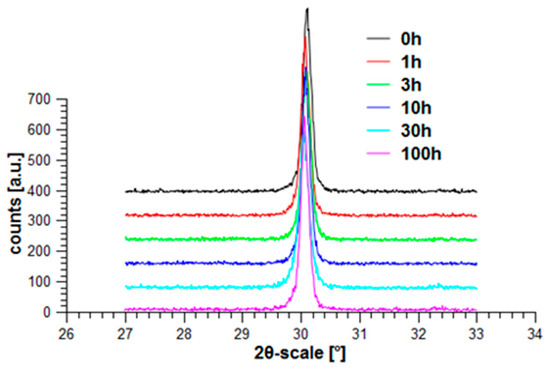
Figure 40.
Hydrothermal treatment of ZCA10P (Ce-TZP/LaAl11O18).
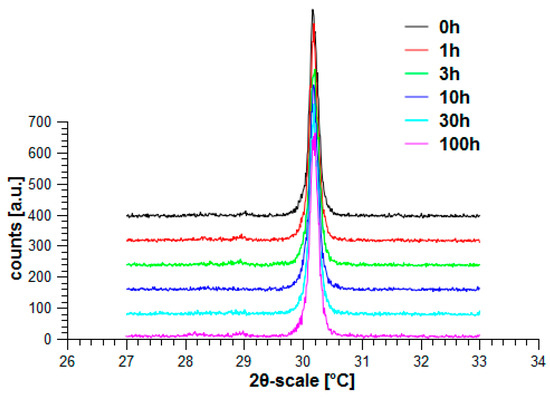
Figure 41.
Hydrothermal treatment of ZA05P (Y-Ce-TZP/Al2O3/LaAl11O18).
So, it can be concluded that both materials don’t show any hydrothermal decomposition reaction and therefore are preferred materials for bioceramic applications. Both materials have to be regarded as interesting alternative ceramic materials in total hip replacement systems, especially when mated against UHMWPE. Compared to the standard ZPTA (zirconia and platelet reinforced alumina) ceramics of today, these materials are less hard and have a significantly higher fracture toughness, which makes them even safer than the existing solution.
We expect that the excellent material properties of these platelet reinforced zirconia ceramics may play a very important role in dental industry in future. Because of their relatively low hardness (ZCA10P), they might become of interest for prosthetic applications, as well as for implants.
6. Conclusions
Two different classes of composite ceramic materials have been described in more detail. One of them is based on an alumina matrix and the other on a zirconia matrix. In case a very high hardness in combination with high strength is required, the alumina matrix composites are preferred. In case a combination of high mechanical strength and fracture toughness is required, a zirconia matrix material is preferred.
The description of the two different composite systems are only a few representative materials which were developed over the past 15 years. However, one can assume that through intelligent additional dopants these two material classes can be improved further and finally tailored to the required properties.
For sure, it is much more difficult to handle these kinds of systems during body preparation compared to the use of commercially available yttria stabilized zirconia. However, in order to really have progress in development of new products, it is mandatory to develop useful technologies for body preparation. The deep understanding of the colloidal processes, which occur in a suspension with the different oxides is mandatory in order to finally come to a good and homogeneous product. Since there are no spectroscopic methods available, the approach for understanding the processes more in detail requires experience in chemistry and a deep understanding of the raw material powders, as well as their behaviour in a suspension. Finally, it has to be pointed out that commercially available dispersing agents are not useful in order to understand the processes. Only the use of defined chemicals help to understand the colloidal chemistry.
Optimisation of the inorganic composition in the alumina/zirconia system requires well educated solid state chemists, who understand crystallography and the chemical solid state reactions, which take place during sintering. Taking into account the amount of different ceramic compositions compared to metallic materials, in ceramics there are only a few different materials compared to the many different materials based on iron. Even in stainless steel there are more materials with different properties available than all ceramic compositions available on the market. To transfer the philosophy of steel into ceramics, a high flexibility within the different companies, who still manufacture their own ready-to-press powders, is required.
Finally, it is state-of-the-art that a good ceramic material requires excellent and completely reproducible raw material powders. Such kinds of powders nowadays are available on the market; i.e., alumina based on the alkoxide process or zirconia based on the chloro-carbonation process. But these raw materials cannot be handled like cheap raw materials such as alumina coming from the Bayer-process or zirconia coming from the thermal decomposition of zircon sand. Chemically derived powders are much more difficult in handling and also in the forming steps, like the pressing of green parts. Finally, sintering is not only a densification process; it is applied to solid state chemistry, by which the densification behaviour can be improved further.
Author Contributions
The work related to platelet reinforced alumina- and zirconia matrix composites has been made together by the authors. Recent results reported for Y-TZP are also based on a close collaboration among the authors. All authors have read and agreed to the published version of the manuscript.
Funding
Research work related to Y-TZP has been funded within the BriteEuram project BE5172 (BIOHPZ) and the most recent results by OxiMaTec GmbH. Research work for reinforced alumina matrix composites has been funded by European Project GRD1-199-10585 (HYPERCER) and also by OxiMaTec GmbH. Finally, most of the work related to zirconia matrix materials has been financed by OxiMaTec GmbH and a part of it by AiF-project ZU4433101KI7.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Dörre, E.; Hübner, H. Alumina: Processing, Properties, and Applications; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1984; p. 5. [Google Scholar]
- Dworak, U.; Olapinski, H.; Thamerus, G. Festigkeitssteigerung von mehrphasigen keramischen Werkstoffen am Beispiel der Systeme ZrO2-ZrO2/Al2O3-ZrO2/Al2O3-TiC. Ber. DKG 1978, 55, 98. [Google Scholar]
- Garvie, R.C.; Hannink, R.H.; Pascoe, R.T. Ceramic steel? Nature 1975, 258, 703. [Google Scholar] [CrossRef]
- Dworak, U.; Olapinski, H. Sinterwerkstoff. Deutsches Patent DE27 44 700, 27 May 1987. [Google Scholar]
- Christel, P. Zirconia: The second generation of ceramics for total hip replacement. Bull. Hosp. Jt. Dis. Orthop. Inst. 1989, 49, 170. [Google Scholar]
- Piconi, C.; Rimondini, L.; Cerroni, L. La Zirconia in Odontoiatria; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Cockayne, B.; Jones, D.W. Modern Oxide Materials; Academic Press: London, UK, 1972. [Google Scholar]
- Glizen, W.H. Alumina as a Ceramic Material; American Ceramic Society: Columbus, OH, USA, 1970. [Google Scholar]
- Schüller, K.-H. Aluminiumoxid. In Handbuch der Keramik; Schmid: Freiburg, Germany, 1970. [Google Scholar]
- Johnson, W.C.; Coble, R.I. A Test of the Second-Phase and Impurity-Segregation Models for MgO-Enhanced Densification of Sintered Alumina. J. Am. Ceram. Soc. 1978, 61, 110. [Google Scholar] [CrossRef]
- Dörre, E.; (Feldmühle AG Plochingen, Düsseldorf, Deutschland). Personal communication, 1986.
- Burger, W. Maßgeschneiderte Hochleistungskeramiken für hochfeste und komplex Bauteile; Innovation und Technik: Altena, Germany, 2007; Volume 8. [Google Scholar]
- Willmann, G. Development in medical-grade alumina during the past two decades. J. Mater. Process. Technol. 1996, 56, 168. [Google Scholar] [CrossRef]
- Curtis, C.E. Development of zirconia resistant to thermal shock. J. Am. Ceram. Soc. 1974, 30, 180. [Google Scholar] [CrossRef]
- Grain, C.F. Phase Relations in the ZrO2-MgO System. J. Am. Ceram. Soc. 1967, 50, 288. [Google Scholar] [CrossRef]
- Geller, R.F.; Lang, S.M. Decomposition of ZrSiO4; NIST: Gaithersburg, MD, USA, 1959. [Google Scholar]
- Burger, W. Zirkonoxid in der medizintechnik. In Technische Keramische Werkstoffe; Kriegesmann, J., Ed.; Verlagsgrussp Deutscher Wirtschaftsdienst: Köln, Germany, 1996. [Google Scholar]
- Dransfield, G.P.; Fothergill, K.A.; Egerton, T.A. Euro Ceramics; De, G., Terpstra, R., Metselaar, R., Eds.; Elsevier: London, UK, 1989; Volume 1, p. 275. [Google Scholar]
- Blackburn, S.R.; Egerton, T.A.; Jones, A.G. Vapour phase synthesis of nitride ceramic powders using a DC plasma. Br. Ceram. Prod. 1991, 47, 87. [Google Scholar]
- Garvie, R.C.; Hannink, R.H. Sub-eutectoid aged Mg-PSZ alloy with enhanced thermal up-shock resistance. J. Mater. Sci. 1982, 17, 2637. [Google Scholar]
- Garvie, R.C.; Hannink, R.H.J.; Mekinnon, N.A. Partially Stabilized Zirconia Ceramics; Method of Making Said Ceramics, Dies Constructed of Said Ceramics Cutting Tools with Cutting Surface and Tappet Facings Formed of Said Ceramics. European Patent EP 0 013 599, 9 May 1984. [Google Scholar]
- Olapinski, H.; Dworak, U.; Burger, W. Ceramic Materials and Components for Engines; Bunk, W., Hausner, H., Eds.; Wiley: Hoboken, NJ, USA, 1987; p. 537. [Google Scholar]
- Dworak, U.; Olapinski, H.; Burger, W. Science and technology of zirconia III. In Advances in Ceramics; Heuer, A.H., Hobbs, L.W., Eds.; The American Ceramic Society: Columbus, OH, USA, 1988; Volume 24, p. 545. [Google Scholar]
- Drennan, J.; Hannink, R.H.J. Effect of SrO Additions on the Grain-Boundary Microstructure and Mechanical Properties of Magnesia-Partially-Stabilized Zirconia. J. Am. Ceram. Soc. 1986, 69, 541. [Google Scholar] [CrossRef]
- Burger, W.; Richter, H.G.; Piconi, C.; Vatteroni, R.; Cittadini, A.; Boccalari, M. Materials in medicine. J. Mater. Sci. 1997, 8, 113. [Google Scholar]
- Burger, W.; Richter, H.G.; Slawik, G.; Willmann, G. Sintering Behaviour of Medical Grade Zirconia (Y-TZP) Powders. Ceram. Trans. 1995, 48, 43. [Google Scholar]
- Richter, H.G.; Burger, W.; Willmann, G. Ceramic hip joint heads made from alumina and zirconia—A comparison. Ceram. Trans. 1995, 48, 73. [Google Scholar]
- Chevalier, J.; Cales, B.; Drouin, M. Low-temperature aging of Y-TZP ceramics. J. Am. Ceram. Soc. 1999, 82, 2150. [Google Scholar] [CrossRef]
- Zhang, F.; Venmeeusel, K.; Inokoshi, M.; Batuk, M.; Hadermann, J.; van Meerbeck, B.; Naert, I.; Vleugels, J. 3Y-TZP ceramics with improved hydrothermal degradation resistance and fracture toughness. J. Eur. Ceram. Soc. 2014, 34, 2453. [Google Scholar] [CrossRef]
- Nakamura, K.; Kauno, T.; Milleding, P.; Ortengren, U. Zirconia as a dental abutment material: A systematic review. Int. J. Prosthodont. 2010, 299. [Google Scholar]
- Kern, F. Non-Published Investigations on Several Zirconia Matrix Materials; Stuttgart University: Stuttgart, Germany, 2020. [Google Scholar]
- Chevalier, J.; Gremillard, L.; Deville, S. Low temperature degradation of zirconia and implication for biomedical implants. Annu. Rev. Mater. Res. 2007, 37, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Dörre, E.; (Feldmühle AG Plochingen, Düsseldorf, Deutschland). Personal communication, 1989.
- Cevales, G. Das Zustandsdiagramm Al2O3-ZrO2 und die Bestimmung einer neuen Hochtemperaturphase. Ber. Dtsch. Keram. Ges. 1968, 45, 217. [Google Scholar]
- Claussen, N. Fracture toughness of Al2O3 with an unstabilized ZrO2 dispersed phase. J. Am. Ceram. Soc. 1976, 59, 49. [Google Scholar] [CrossRef]
- Lange, F.F. Transformation toughening; part 3: Experimental observations in the ZrO2-Y2O3-system. J. Mater. Sci. 1982, 17, 247. [Google Scholar] [CrossRef]
- Lange, F.F.; Hirlinger, M.M. Hinderance of grain growth in Al2O3 by ZrO2 inclusions. J. Am. Ceram. Soc. 1984, 67, 164. [Google Scholar] [CrossRef]
- Burger, W. Umwandlungs-und plateletverstärkte Aluminiumoxidmatrixwerkstoffe. Keram. Z. 1997, 49, 1067. [Google Scholar]
- Burger, W. Umwandlungs-und plateletverstärkte Aluminiumoxidmatrixwerkstoffe. (Teil 2). Keram. Z. 1998, 50, 18. [Google Scholar]
- Cutler, R.A.; Mayhow, R.J.; Rettyxmean, K.M.; Virkar, A.V. High-Toughness Ce-TZP/Al2O3 Ceramics with Improved Hardness and Strength. J. Am. Ceram. Soc. 1991, 74, 179. [Google Scholar] [CrossRef]
- Yasuoka, M.; Hirao, K.; Brito, M.G.; Kanzaki, S. High-Strength and High-Fracture-Toughness Ceramics in the Al2O3/LaAl11O18 Systems. J. Am. Ceram. Soc. 1995, 78, 1853. [Google Scholar] [CrossRef]
- Burger, W.; Kiefer, G.; Bellido, E.; Andersch, H. Sintered Shaped Body Reinforced with Platelets. U.S. Patent 6,452,957, 17 September 2002. [Google Scholar]
- Burger, W. (coordinator) Results summarized in the final report of Brite Euram Project GRD1-199-10585, 2005.
- Ceramic Dental Implants: Where Do We Stand? Available online: https://www.straumann.com/en/discover/youtooth/article/esthetics/2020/ceramic-dental-implants-where-do-we-stand.html (accessed on 22 June 2020).
- EI Attaoui, H.; Saädaoui, M.; Chevalier, J.; Fantozzi, G. Static and cyclic crack propagation in Ce-TZP ceramics with different amounts of transformation toughening. J. Eur. Ceram. Soc. 2007, 27, 483. [Google Scholar] [CrossRef]
- Brochure “Nanozyr”; Panasonic Electric Works Yokkaichi Co., Ltd.: Tokyo, Japan, 2011.
- Burger, W. Oxidkeramik—Neue werkstoffe für die medizintechnik und industrielle anwendungen. In Keramische Werkstoffe; Hennicke, D., Ed.; Springer: Köln, Germany, 2012. [Google Scholar]
- Non-published results, based on European Patent EP 2 086 908 (2006) and EP 2 086 909 (2006).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).