Automated Identification of Defect Morphology and Spatial Distribution in Woven Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. X-ray Microtomography
2.3. Mesh Reconstruction
3. Characterization of Defects
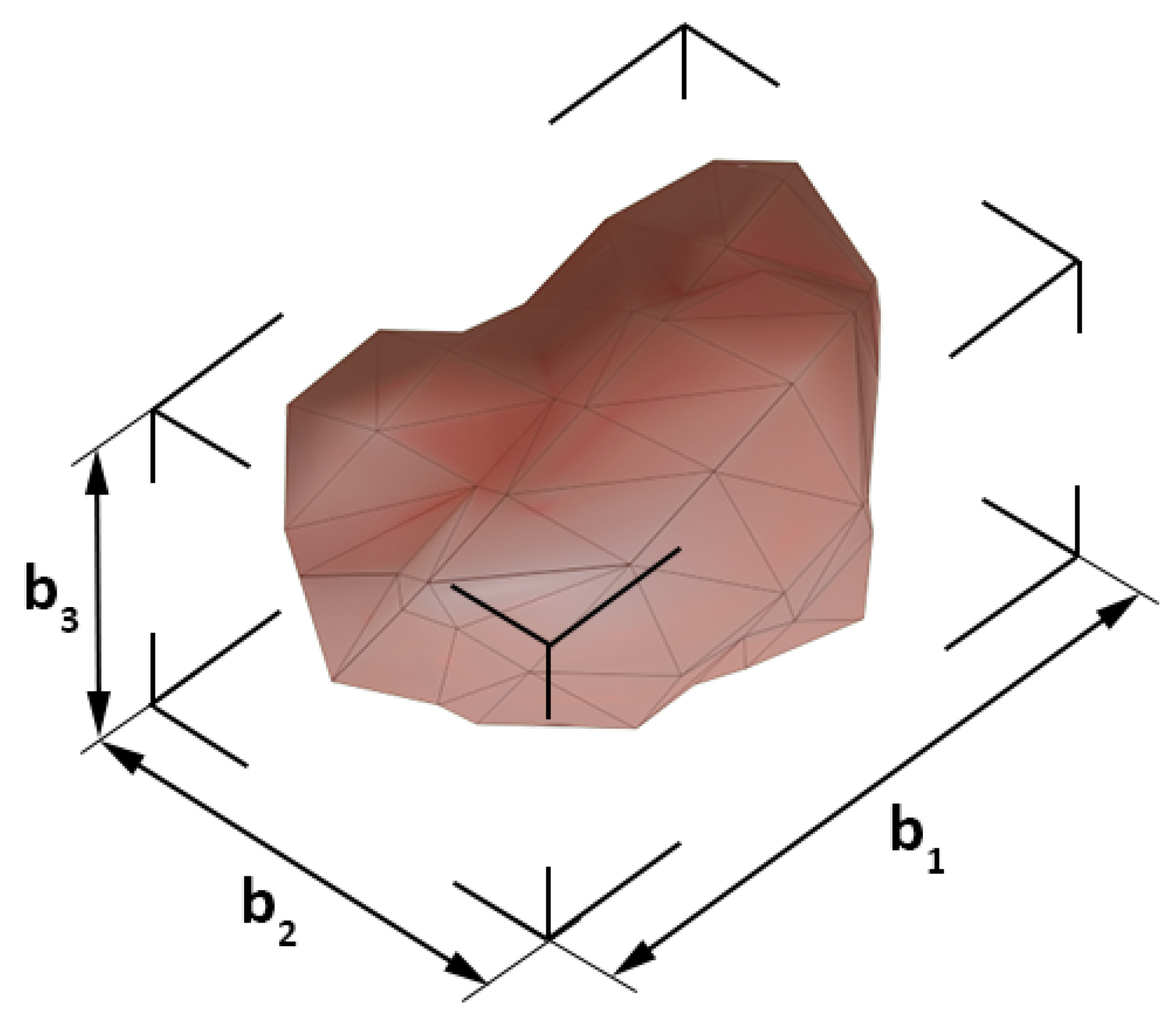
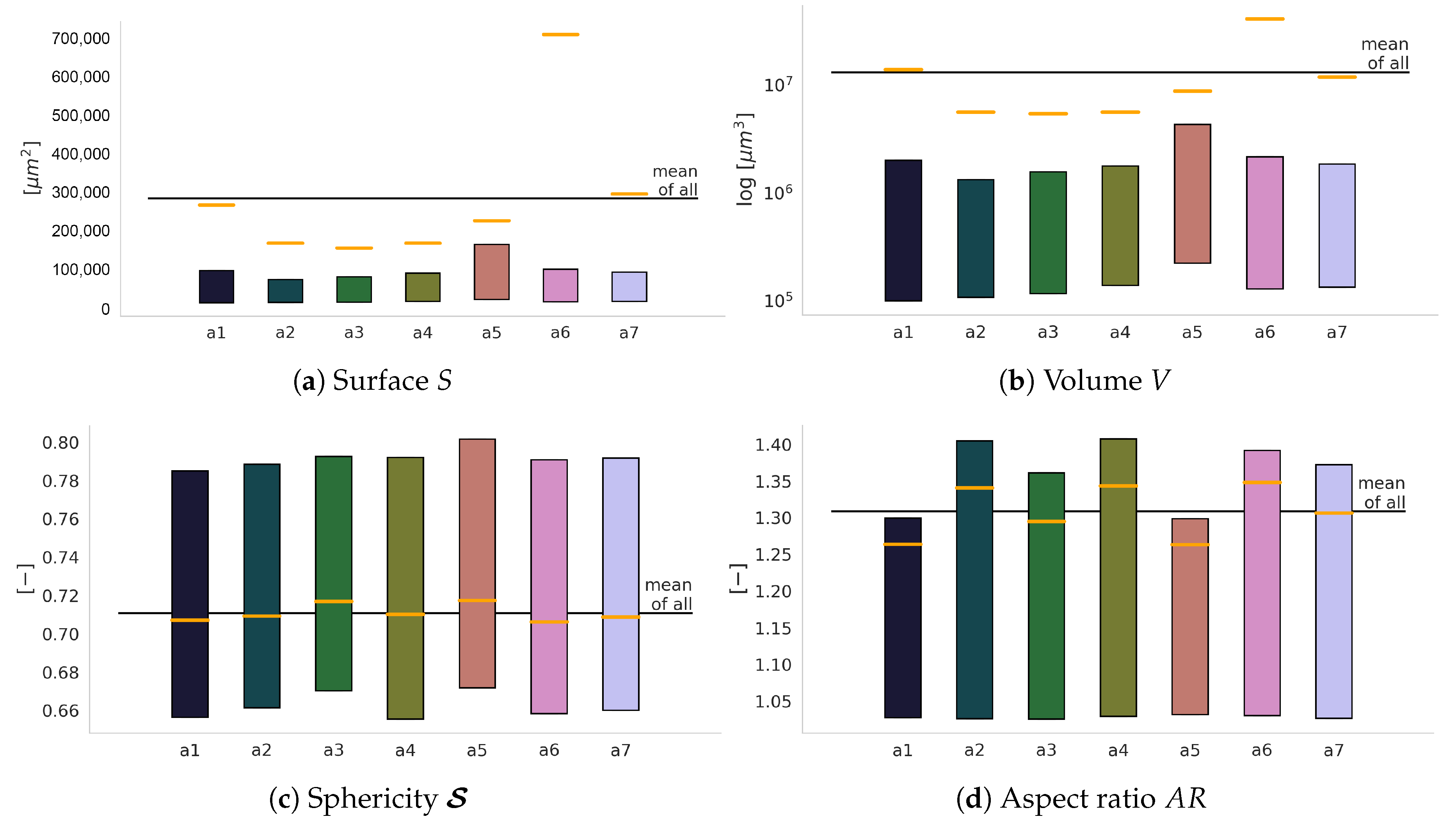
3.1. Geometric Features
- surface S,
- volume V,
- sphericity S,
- box dimensions , , ,
- aspect ratio .
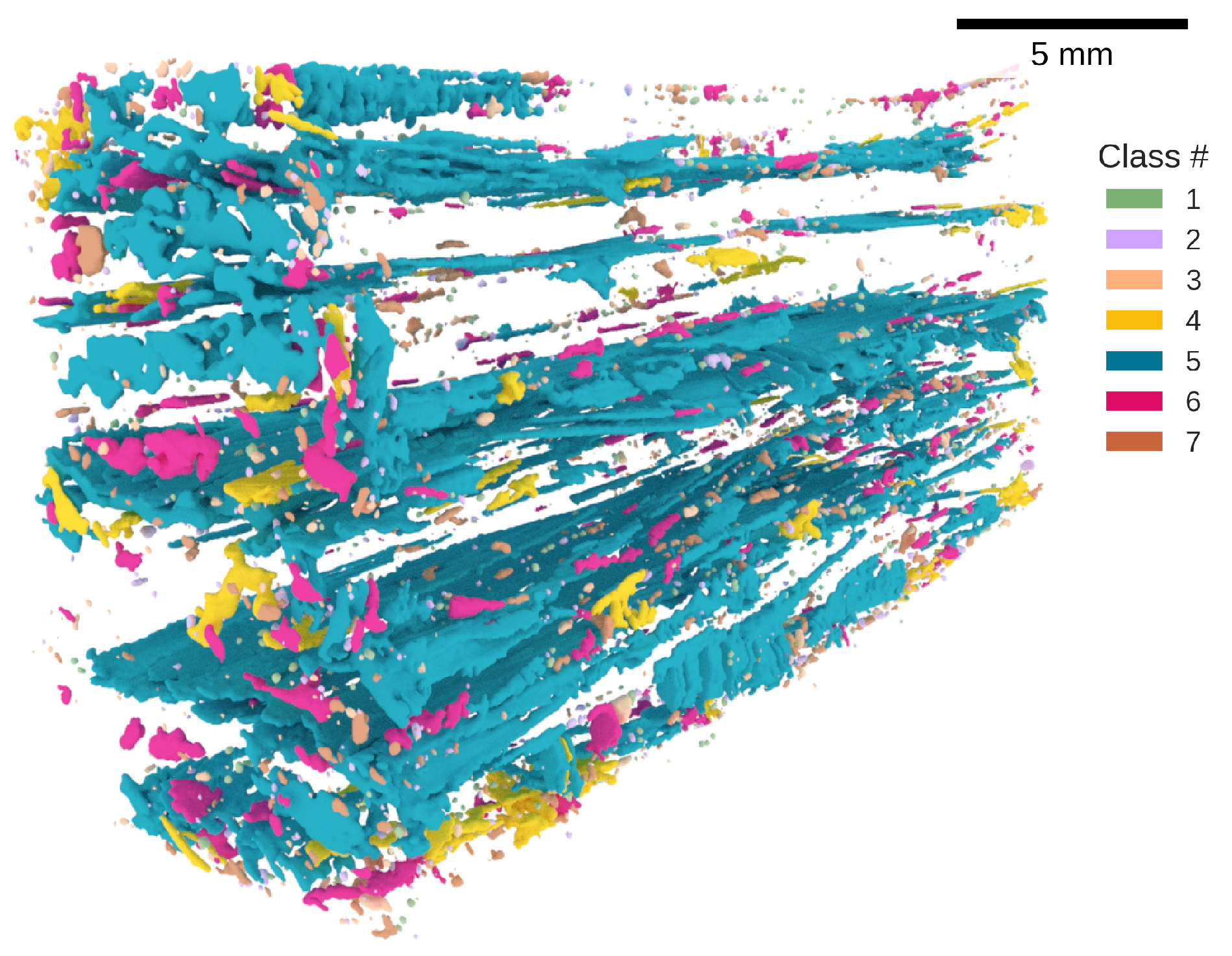
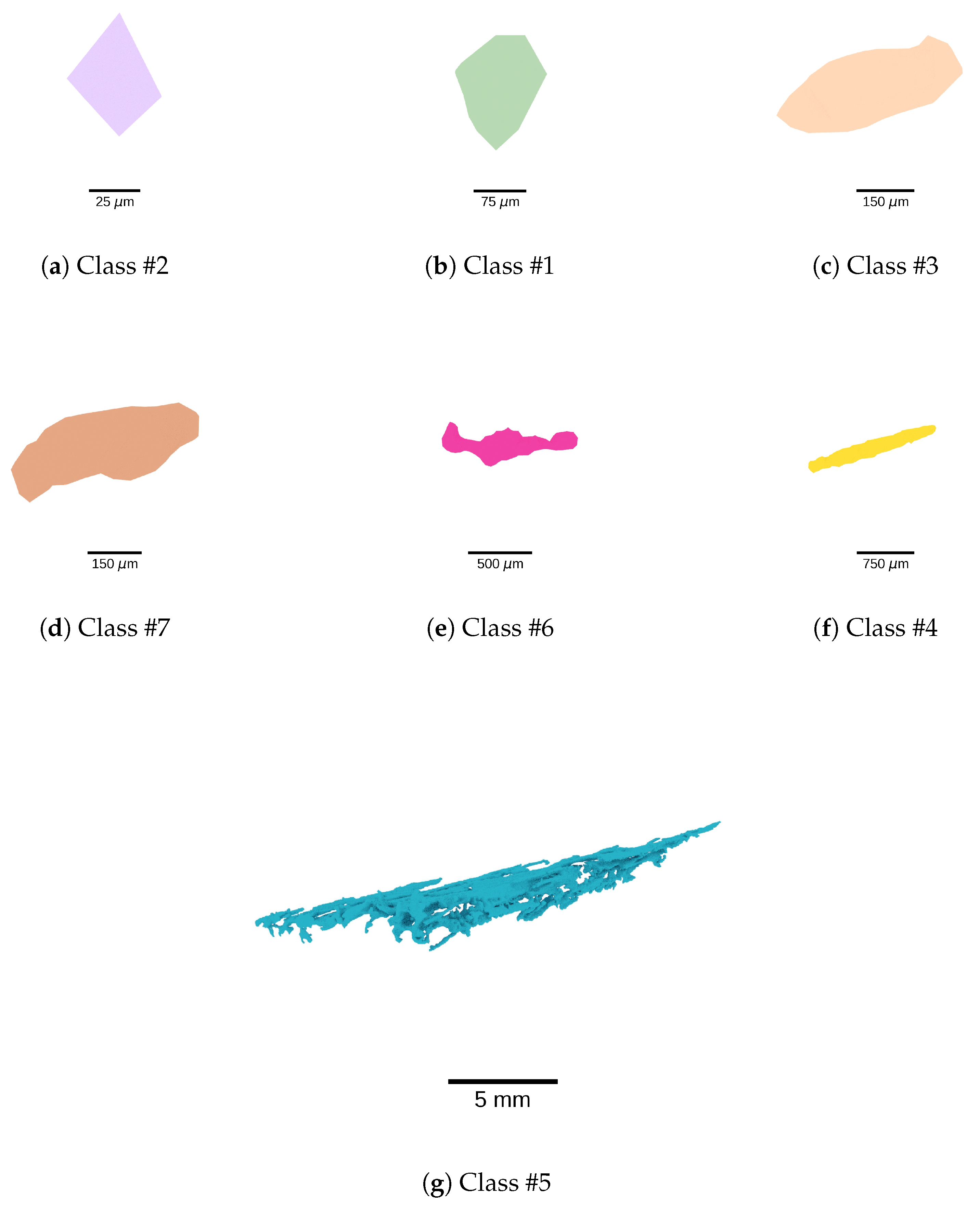
3.2. Morphological Classes
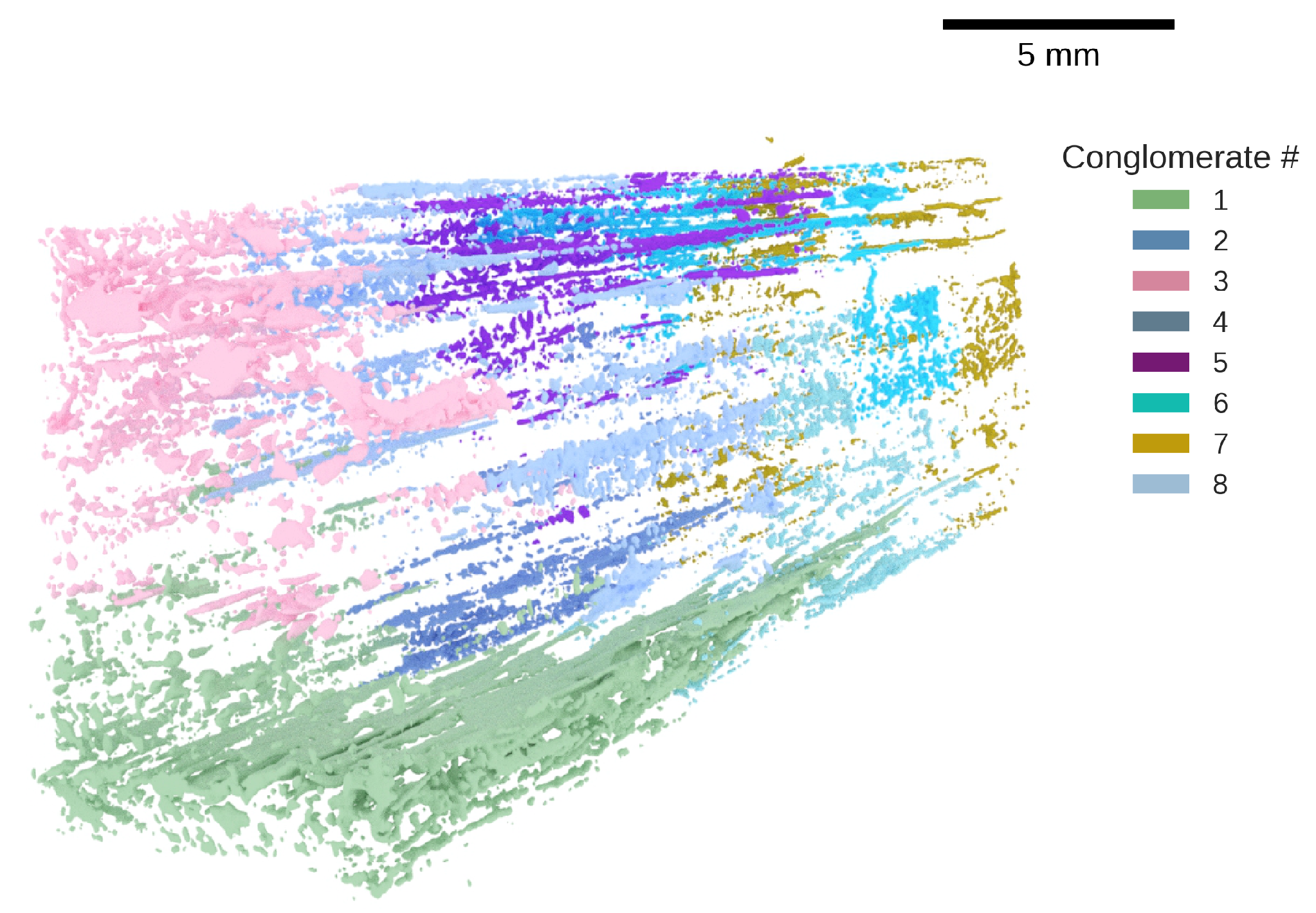
3.3. Spatial Features and Conglomerates
3.4. Defect Genome
4. Results and Discussion
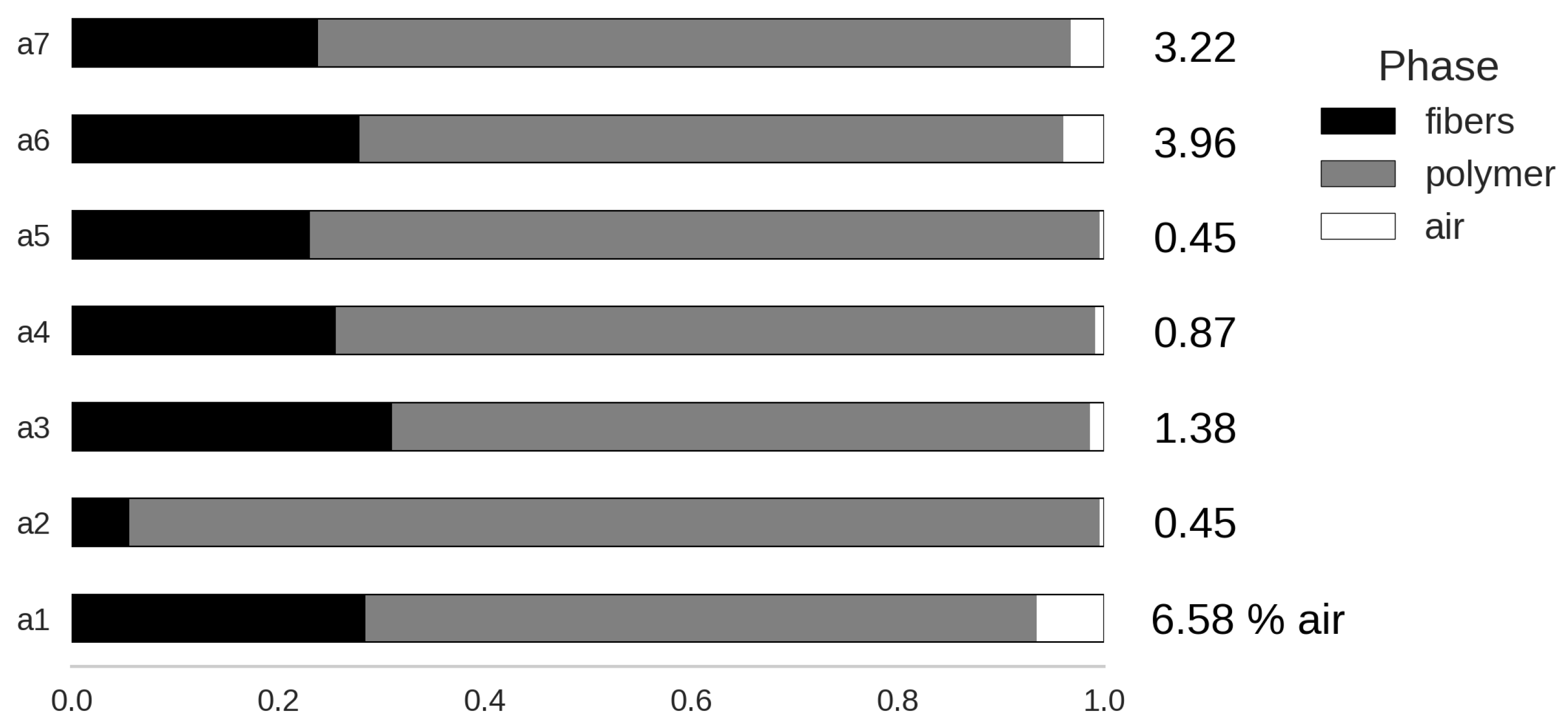
4.1. Phase Composition
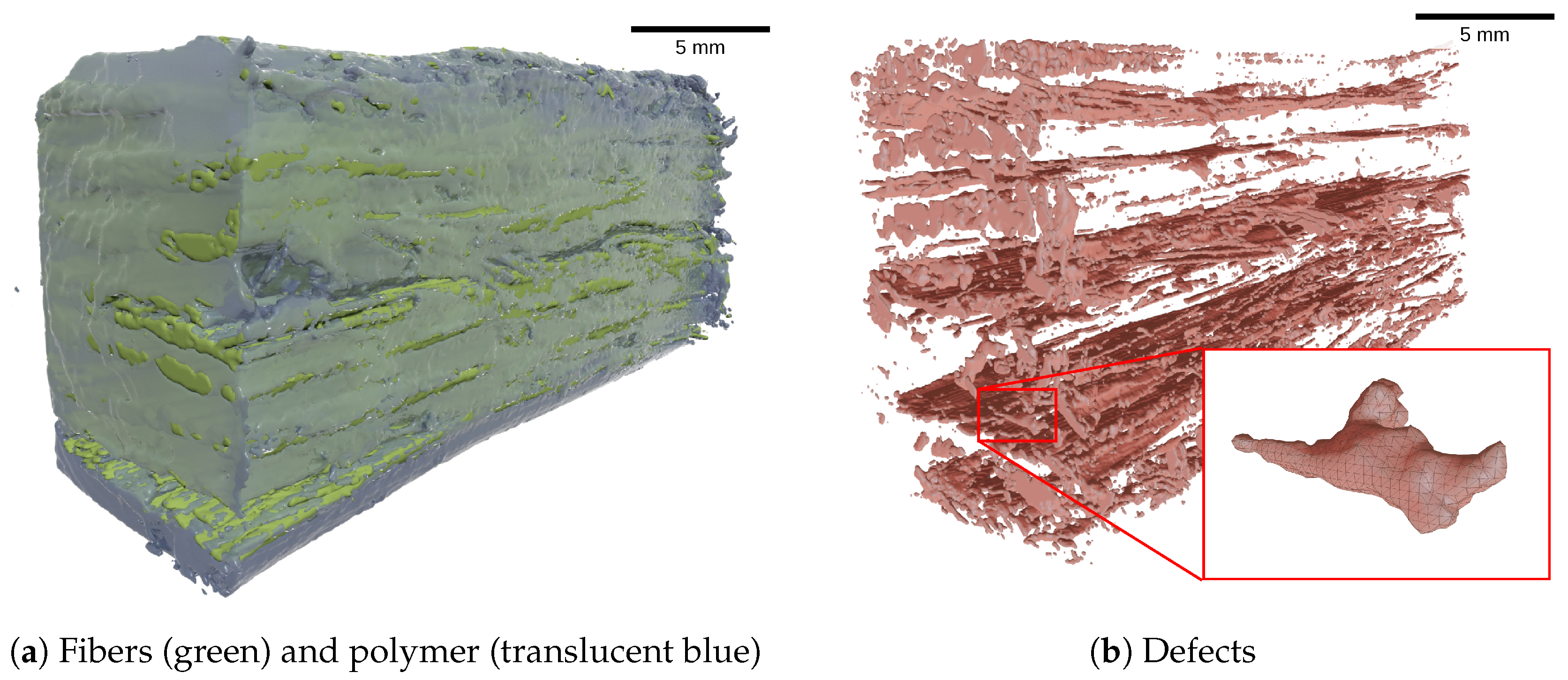
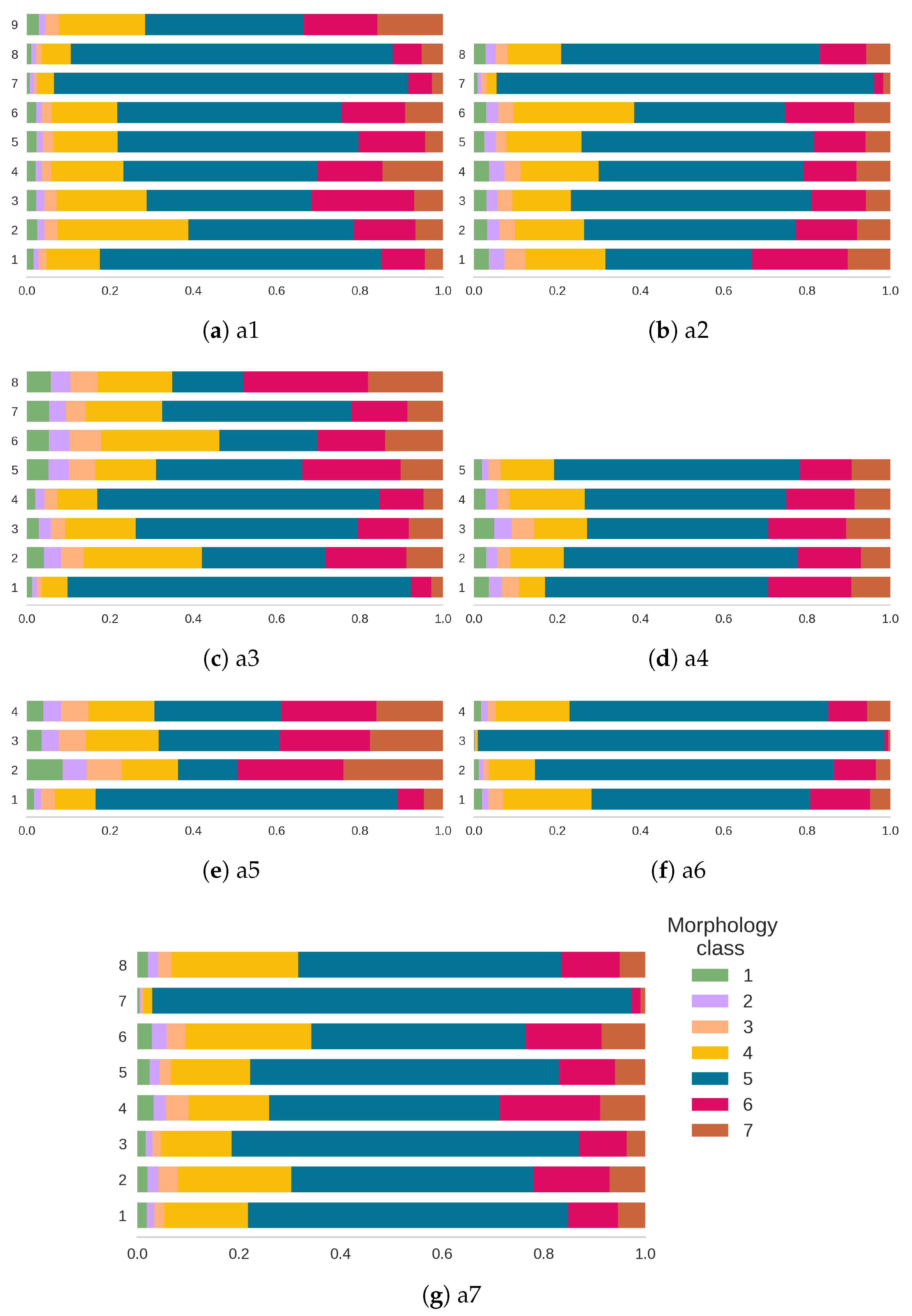
4.2. Defect Morphology
4.3. Defect Conglomerates
4.4. Defect Genomes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Algorithm A1: Processing of segmented tomograms to separate defects from the air around the specimen. |
| Input: Segmented tomogram (air phase only) Output: Two-dimensional image with defects and eliminated outside air copy as ; fill holes in ; dilate ; fill holes in ; erode ; get XOR as ; median filter (2 px kernel) on ; return ; |
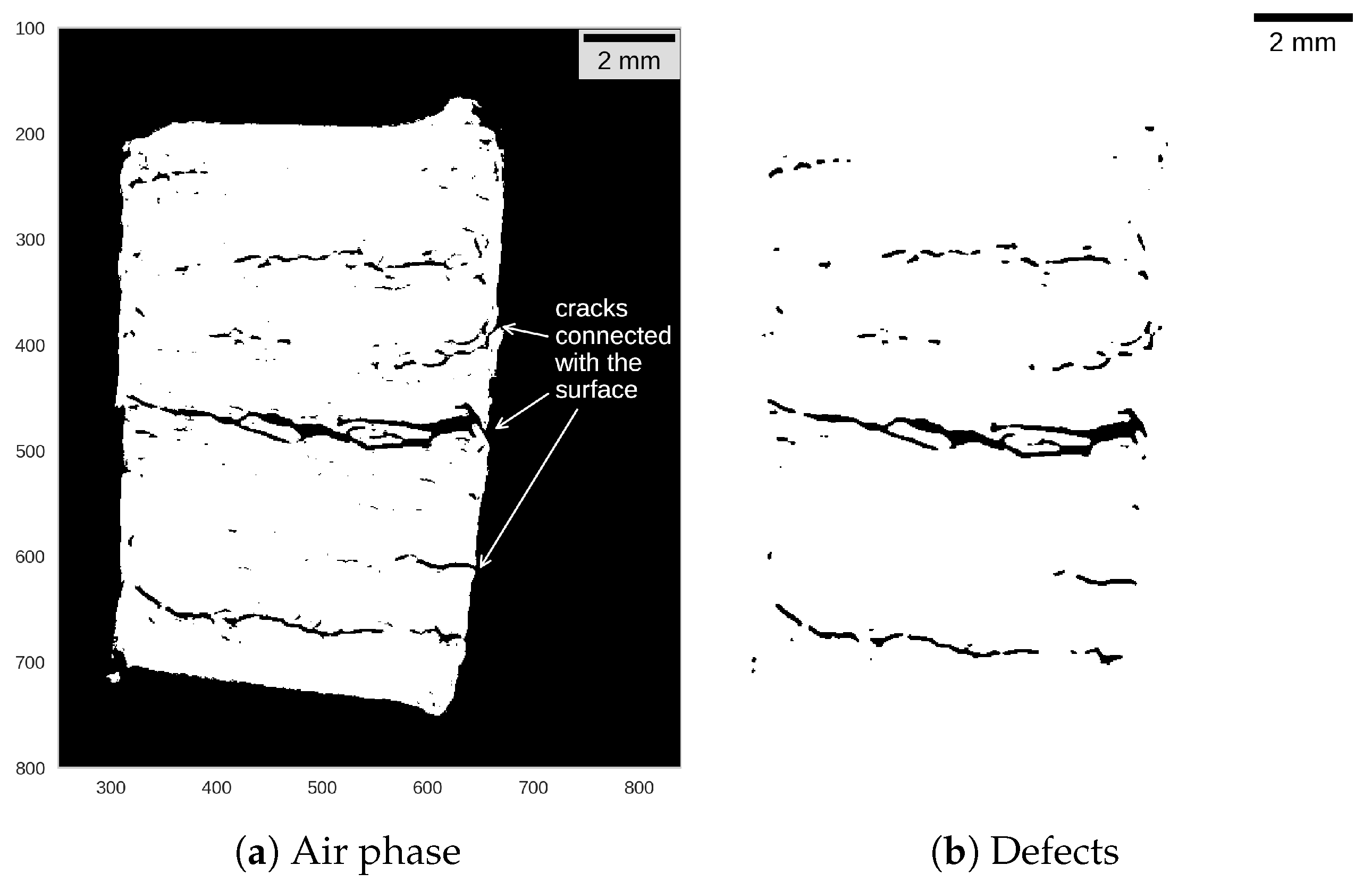
References
- Goodwin, A.A.; Howe, C.A.; Paton, R.J. The Role of Voids in Reducing the Interlaminar Shear Strength of RTM Laminates. In Proceedings of the Eleventh International Conference on Composite Materials, Gold Coast, QLD, Australia, 14–18 July 1997; pp. 11–19. [Google Scholar]
- Talreja, R. Manufacturing Defects in Composites and Their Effects on Performance; Elsevier: Amsterdam, The Netherlands, 2015; pp. 99–113. [Google Scholar] [CrossRef]
- Ricotta, M.; Quaresimin, M.; Talreja, R. Mode I Strain Energy Release Rate in composite laminates in the presence of voids. Compos. Sci. Technol. 2008, 68, 2616–2623. [Google Scholar] [CrossRef][Green Version]
- Suo, Y.; Wang, B.; Jia, P.; Gong, Y. The effect of fabrication defects on the mechanical behaviors of metal matrix composites. Mater. Today Commun. 2020, 25, 101663. [Google Scholar] [CrossRef]
- Drach, B.; Tsukrov, I.; Gross, T.; Dietrich, S.; Weidenmann, K.; Piat, R.; Böhlke, T. Numerical modeling of carbon/carbon composites with nanotextured matrix and 3D pores of irregular shapes. Int. J. Solids Struct. 2011, 48, 2447–2457. [Google Scholar] [CrossRef]
- Liotier, P.J.; Alain, V.; Christine, D. Characterization of 3D morphology and microcracks in composites reinforced by multi-axial multi-ply stitched preforms. Compos. Part Appl. Sci. Manuf. 2010, 41, 653–662. [Google Scholar] [CrossRef]
- Lambert, J.; Chambers, A.R.; Sinclair, I.; Spearing, S.M. 3D damage characterisation and the role of voids in the fatigue of wind turbine blade materials. Compos. Sci. Technol. 2012, 72, 337–343. [Google Scholar] [CrossRef]
- Mei, H.; Tan, Y.; Zhang, D.; Cheng, L. A novel delamination defects designed for understanding mechanical degradation in a laminated C/SiC composites. J. Alloys Compd. 2019, 770, 1138–1146. [Google Scholar] [CrossRef]
- ASTM D3171-2015 Standard Test Methods for Constituent Content of Composite Materials. Available online: https://www.astm.org/Standards/D3171.htm (accessed on 24 November 2020).
- Hamidi, Y.K.; Aktas, L.; Altan, M.C. Formation of microscopic voids in resin transfer molded composites. J. Eng. Mater. Technol. 2004, 126, 420–426. [Google Scholar] [CrossRef]
- McCombe, G.P.; Rouse, J.; Trask, R.S.; Withers, P.J.; Bond, I.P. X-ray damage characterisation in self-healing fibre reinforced polymers. Compos. Part Appl. Sci. Manuf. 2012, 43, 613–620. [Google Scholar] [CrossRef]
- Katunin, A.; Dańczak, M.; Kostka, P. Automated identification and classification of internal defects in composite structures using computed tomography and 3D wavelet analysis. Arch. Civ. Mech. Eng. 2015, 15, 436–448. [Google Scholar] [CrossRef]
- Tserpes, K.I.; Stamopoulos, A.G.; Pantelakis, S.G. A numerical methodology for simulating the mechanical behavior of CFRP laminates containing pores using X-ray computed tomography data. Compos. Part Eng. 2016, 102, 122–133. [Google Scholar] [CrossRef]
- Madra, A.; Hajj, N.; Benzeggagh, M. X-ray microtomography applications for quantitative and qualitative analysis of porosity in woven glass fiber reinforced thermoplastic. Compos. Part Appl. Sci. Manuf. 2014, 95. [Google Scholar] [CrossRef]
- Wang, P.; Lei, H.; Zhu, X.; Chen, H.; Wang, C.; Fang, D. Effect of manufacturing defect on mechanical performance of plain weave carbon/epoxy composite based on 3D geometrical reconstruction. Compos. Struct. 2018, 199, 38–52. [Google Scholar] [CrossRef]
- Friedman, H.P.; Rubin, J. On Some Invariant Criteria for Grouping Data. Source J. Am. Stat. Assoc. 1967, 62, 1159–1178. [Google Scholar] [CrossRef]
- Prodanov, D.; Feirabend, H.K. Automated characterization of nerve fibers labeled fluorescently: Determination of size, class and spatial distribution. Brain Res. 2008, 1233, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Elbischger, P.J.; Bischof, H.; Regitnig, P.; Holzapfel, G.A. Automatic analysis of collagen fiber orientation in the outermost layer of human arteries. Pattern Anal. Appl. 2004, 7, 269–284. [Google Scholar] [CrossRef]
- Godin, N.; Huguet, S.; Gaertner, R.; Salmon, L. Clustering of acoustic emission signals collected during tensile tests on unidirectional glass/polyester composite using supervised and unsupervised classifiers. NDT Int. 2004, 37, 253–264. [Google Scholar] [CrossRef]
- Godin, N.; Huguet, S.; Gaertner, R. Integration of the Kohonen’s self-organising map and k-means algorithm for the segmentation of the AE data collected during tensile tests on cross-ply composites. NDT Int. 2005, 38, 299–309. [Google Scholar] [CrossRef]
- Doan, D.D.; Ramasso, E.; Placet, V.; Zhang, S.; Boubakar, L.; Zerhouni, N. An unsupervised pattern recognition approach for AE data originating from fatigue tests on polymer-composite materials. Mech. Syst. Signal Process. 2015, 64–65, 465–478. [Google Scholar] [CrossRef]
- Madra, A.; Adrien, J.; Breitkopf, P.; Maire, E.; Trochu, F. A clustering method for analysis of morphology of short natural fibers in composites based on X-ray microtomography. Compos. Part Appl. Sci. Manuf. 2017, 102. [Google Scholar] [CrossRef]
- Van-Pham, D.T.; Nguyen, M.T.; Nguyen, C.N.; Le, T.T.D.; Pham, T.Y.N.; Nguyen, K.T.; Nishikawa, Y.; Tran-Cong-miyata, Q. Effects of processing parameters on mechanical properties and structure of banana fiber-reinforced composites. J. Renew. Mater. 2018, 6, 662–670. [Google Scholar] [CrossRef]
- Breitkopf, P.; Touzot, G.; Villon, P. Consistency Approach and Diffuse Derivation in Element Free Methods Based on Moving Least Squares Approximation. Comput. Assist. Mech. Eng. Sci. 1998, 5, 479–501. [Google Scholar]
- Lewiner, T.; Lopes, H.; Vieira, A.W.; Tavares, G. Efficient Implementation of Marching Cubes’ Cases with Topological Guarantees. J. Graph. Tools 2003, 8, 1–15. [Google Scholar] [CrossRef]
- Wildenschild, D.; Sheppard, A.P. X-ray imaging and analysis techniques for quantifying pore-scale structure and processes in subsurface porous medium systems. Adv. Water Resour. 2013, 51, 217–246. [Google Scholar] [CrossRef]
- Sibil, A.; Godin, N.; R’Mili, M.; Maillet, E.; Fantozzi, G. Optimization of Acoustic Emission Data Clustering by a Genetic Algorithm Method. J. Nondestruct. Eval. 2012, 31, 1573–4862. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
Sample Availability: The X-ray tomographic scans of the data are currently subject to a follow-up publication. Hence, they will be released for publication at a later date. |





| Specimen | Time [min] | Temperature [°C] | Pressure [kg/cm] |
|---|---|---|---|
| a1 | 10 | 150 | 100 |
| a2 | 10 | 160 | 100 |
| a3 | 15 | 170 | 100 |
| a4 | 15 | 170 | 100 |
| a5 | 15 | 170 | 100 |
| a6 | 20 | 170 | 75 |
| a7 | 20 | 170 | 125 |
| Name | a1 | a2 | a3 | a4 | a5 | a6 | a7 |
|---|---|---|---|---|---|---|---|
| resolution [m] | 21.76 | 22.43 | 23.49 | 23.07 | 22.88 | 23.04 | 23.36 |
| Specimen | Mesh Size [MB] | No. of Objects | No. of Nodes | No. of Triangles |
|---|---|---|---|---|
| a1 | 321 | 15,148 | ||
| a2 | 156 | 21,779 | ||
| a3 | 88 | 10,134 | ||
| a4 | 43 | 6506 | ||
| a5 | 30 | 2671 | ||
| a6 | 126 | 4527 | ||
| a7 | 163 | 13,873 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madra, A.; Van-Pham, D.-T.; Nguyen, M.-T.; Nguyen, C.-N.; Breitkopf, P.; Trochu, F. Automated Identification of Defect Morphology and Spatial Distribution in Woven Composites. J. Compos. Sci. 2020, 4, 178. https://doi.org/10.3390/jcs4040178
Madra A, Van-Pham D-T, Nguyen M-T, Nguyen C-N, Breitkopf P, Trochu F. Automated Identification of Defect Morphology and Spatial Distribution in Woven Composites. Journal of Composites Science. 2020; 4(4):178. https://doi.org/10.3390/jcs4040178
Chicago/Turabian StyleMadra, Anna, Dan-Thuy Van-Pham, Minh-Tri Nguyen, Chanh-Nghiem Nguyen, Piotr Breitkopf, and François Trochu. 2020. "Automated Identification of Defect Morphology and Spatial Distribution in Woven Composites" Journal of Composites Science 4, no. 4: 178. https://doi.org/10.3390/jcs4040178
APA StyleMadra, A., Van-Pham, D.-T., Nguyen, M.-T., Nguyen, C.-N., Breitkopf, P., & Trochu, F. (2020). Automated Identification of Defect Morphology and Spatial Distribution in Woven Composites. Journal of Composites Science, 4(4), 178. https://doi.org/10.3390/jcs4040178







