Utilization of Drinking Water Treatment Sludge as Cement Replacement to Mitigate Alkali–Silica Reaction in Cement Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Compositions and Mortar Preparation
2.3. Compressive Strength Test
2.4. Flexural Strength Test
2.5. Water Sorptivity Test
2.6. Accelerated ASR Test
3. Results
3.1. Compressive and Flexural Strength
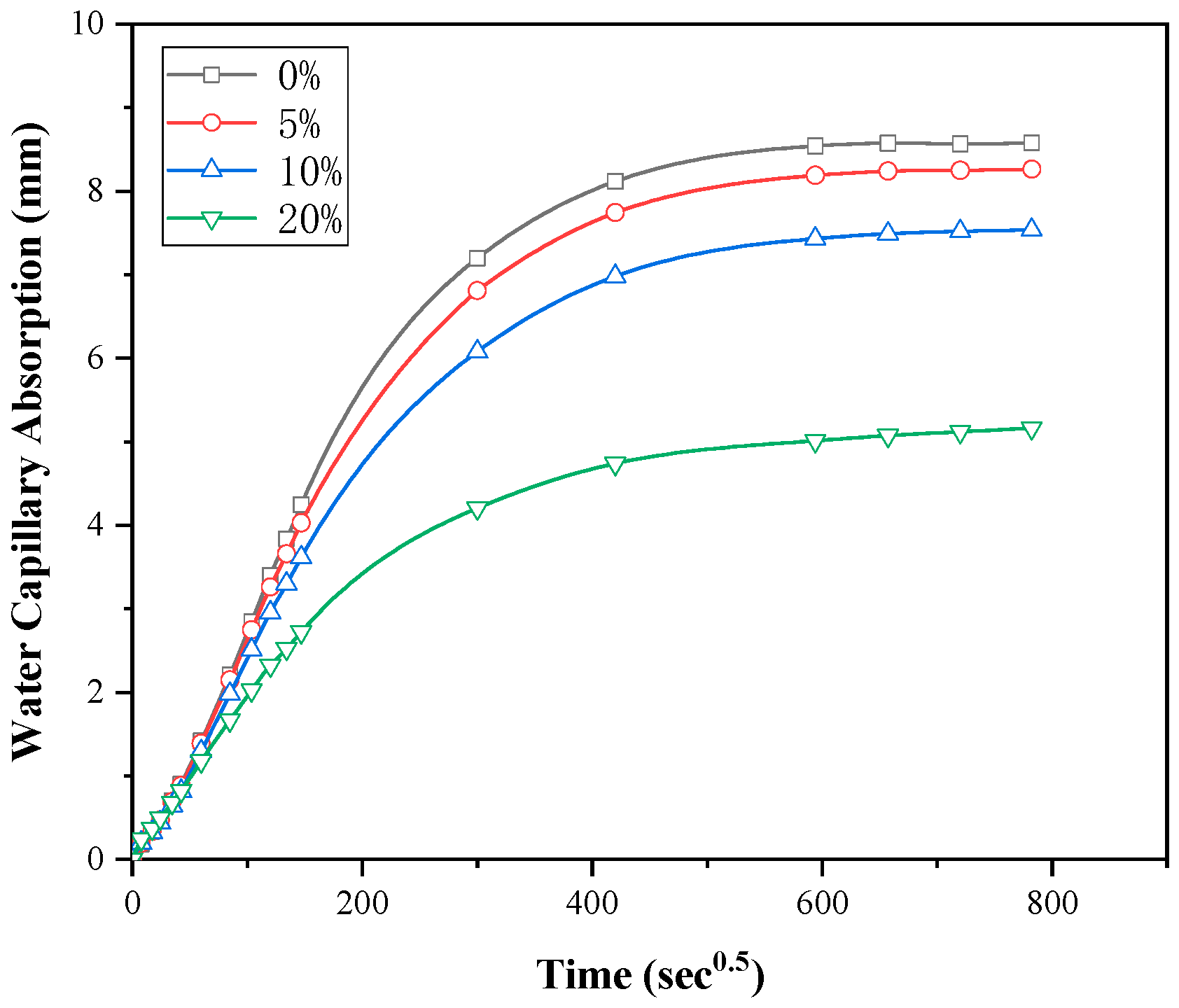
3.2. Water Sorptivity
3.3. ASR Assessment
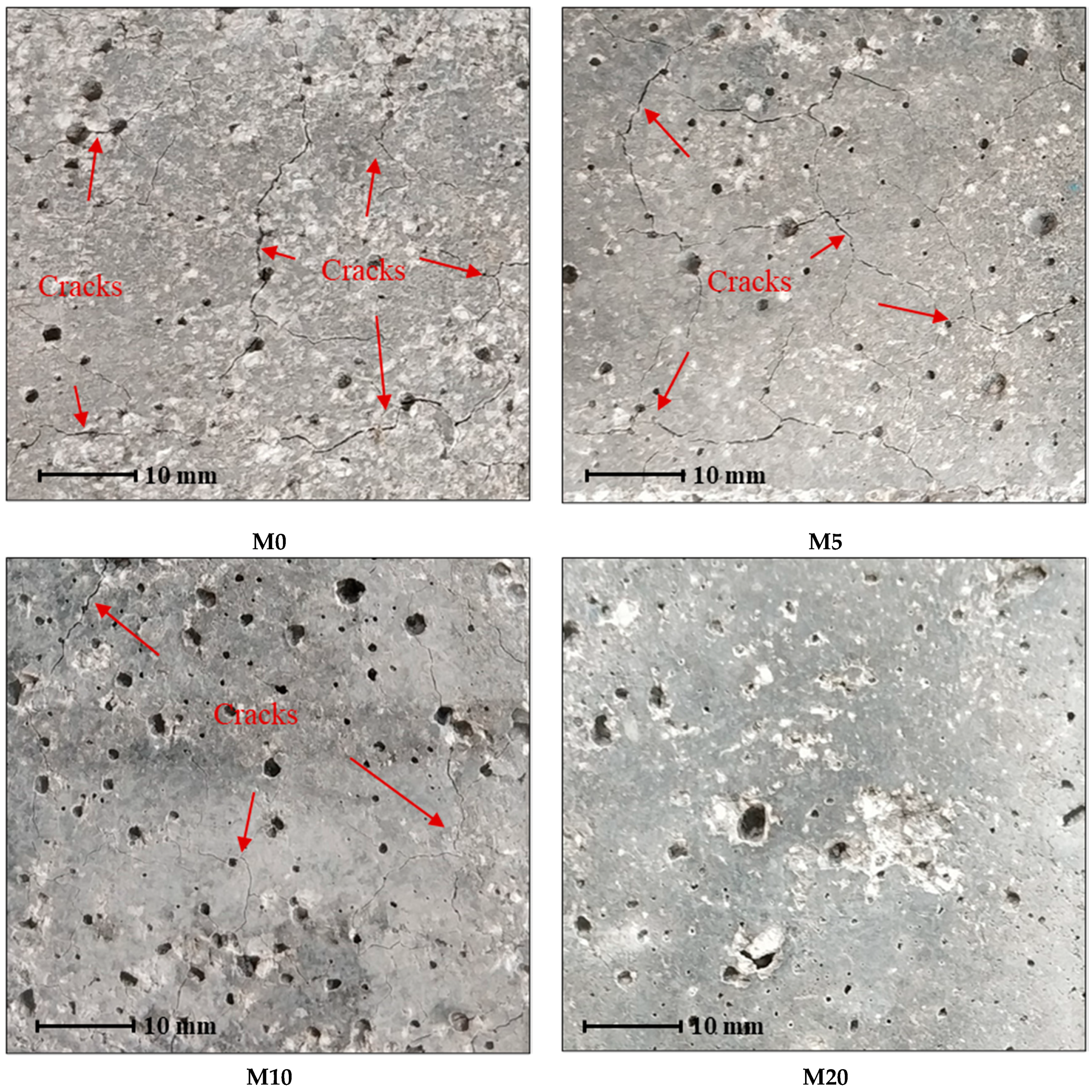
3.3.1. Visual Observation of Cracking under Accelerated ASR Test
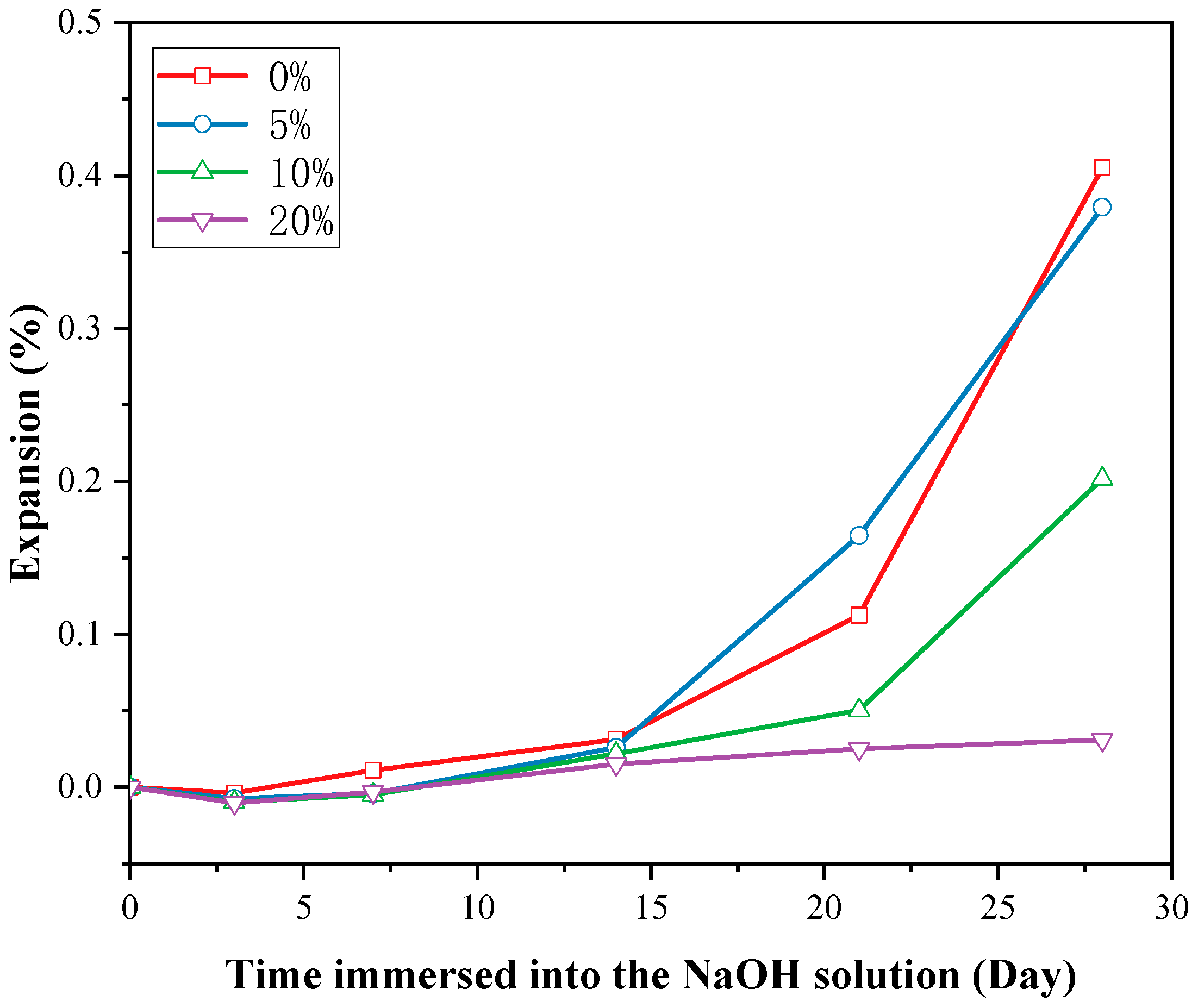
3.3.2. Expansion under Accelerated ASR Test
3.3.3. UPV changes under accelerated ASR test
4. Discussion
4.1. Effect of the Calcined DWTS on Mechanical Properties
4.2. Effect of the Calcined DWTS on Durability Performance
4.3. Effect of the Calcined DWTS on Mitigating ASR
5. Conclusions
- (1)
- The compressive strength and flexural strength of the mortars were improved when no more than 10% of the cement was replaced by the calcined DWTS in the composite. The optimal mix was 10% replacement, where the highest compressive strength and flexural strength of mortar were achieved.
- (2)
- The calcined DWTS was found to decrease the water capillary absorption of the mortars due to the large and open pores of the composites being confined by extra C-S-H formation from the pozzolanic reaction of the calcined DWTS. This confirms that inclusion of calcined DWTS could improve the durability of cementitious material.
- (3)
- Incorporating calcined DWTS into cementitious materials was able to mitigate the ASR effect. A 20% replacement of cement with the calcined DWTS appeared to successfully prevent ASR occurrence. The ASR mitigation mechanisms could be attributed to the formation of secondary C-S-H, the consumption of CH, the silica dissolution control by aluminium, and the reduction of permeability.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maier, P.L.; Durham, S.A. Beneficial use of recycled materials in concrete mixtures. Constr. Build. Mater. 2012, 29, 428–437. [Google Scholar] [CrossRef]
- Meesak, T.; Sujjavanich, S. Effectiveness of 3 different supplementary cementitious materials in mitigating alkali silica reaction. Mater. Today Proc. 2019, 17, 1652–1657. [Google Scholar] [CrossRef]
- Rajabipour, F.; Giannini, E.; Dunant, C.F.; Ideker, J.H.; Thomas, M.D. Alkali–silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cem. Concr. Res. 2015, 76, 130–146. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B. The combined effect of potassium, sodium and calcium on the formation of Alkali–silica reaction products. Cem. Concr. Res. 2020, 127, 105914. [Google Scholar] [CrossRef]
- Shi, Z.; Lothenbach, B. The role of calcium on the formation of Alkali–silica reaction products. Cem. Concr. Res. 2019, 126, 105898. [Google Scholar] [CrossRef]
- Geng, G.; Shi, Z.; Leemann, A.; Borca, C.; Huthwelker, T.; Glazyrin, K.; Pekov, I.V.; Churakov, S.; Lothenbach, B.; Dähn, R.; et al. Atomistic structure of Alkali–silica reaction products refined from X-ray diffraction and micro X-ray absorption data. Cem. Concr. Res. 2020, 129, 105958. [Google Scholar] [CrossRef]
- Leemann, A.; Shi, Z.; Wyrzykowski, M.; Winnefeld, F. Moisture stability of crystalline Alkali–silica reaction products formed in concrete exposed to natural environment. Mater. Des. 2020, 195, 109066. [Google Scholar] [CrossRef]
- Thomas, M. The effect of supplementary cementing materials on Alkali–silica reaction: A review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Oruji, S.; Brake, N.A.; Guduru, R.K.; Nalluri, L.; Günaydın-Şen, Ö.; Kharel, K.; Rabbanifar, S.; Hosseini, S.; Ingram, E. Mitigation of ASR expansion in concrete using ultra-fine coal bottom ash. Constr. Build. Mater. 2019, 202, 814–824. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Potential alkali silica reaction expansion mitigation of ferronickel slag aggregate by fly ash. Struct. Concr. 2018, 19, 1376–1386. [Google Scholar] [CrossRef] [Green Version]
- Nagrockienė, D.; Rutkauskas, A. The effect of fly ash additive on the resistance of concrete to alkali silica reaction. Constr. Build. Mater. 2019, 201, 599–609. [Google Scholar] [CrossRef]
- Duchesne, J.; Bérubé, M. The effectiveness of supplementary cementing materials in suppressing expansion due to asr. 2. Pore solution chemistry-reply. Cem. Concr. Res. 1994, 24, 221–230. [Google Scholar] [CrossRef]
- Canham, I.; Page, C.; Nixon, P. Aspects of the pore solution chemistry of blended cements related to the control of alkali silica reaction. Cem. Concr. Res. 1987, 17, 839–844. [Google Scholar] [CrossRef]
- Rayment, P. The effect of pulverised-fuel ash on the c/s molar ratio and alkali content of calcium silicate hydrates in cement. Cem. Concr. Res. 1982, 12, 133–140. [Google Scholar] [CrossRef]
- Shafaatian, S.M.; Akhavan, A.; Maraghechi, H.; Rajabipour, F. How does fly ash mitigate alkali–silica reaction (ASR) in accelerated mortar bar test (ASTM C1567)? Cem. Concr. Compos. 2013, 37, 143–153. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Monteiro, P.J.M. Structure and Intrinsic Mechanical Properties of Nanocrystalline Calcium Silicate Hydrate. ACS Sustain. Chem. Eng. 2020, 8, 12453–12461. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Xu, K.; Monteiro, P.J. Fibrillar calcium silicate hydrate seeds from hydrated tricalcium silicate lower cement demand. Cem. Concr. Res. 2020, 137, 106195. [Google Scholar] [CrossRef]
- Lagier, F.; Kurtis, K.E. Influence of Portland cement composition on early age reactions with metakaolin. Cem. Concr. Res. 2007, 37, 1411–1417. [Google Scholar] [CrossRef]
- Gruber, K.; Ramlochan, T.; Boddy, A.; Hooton, R.; Thomas, M. Increasing concrete durability with high-reactivity metakaolin. Cem. Concr. Compos. 2001, 23, 479–484. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Maniar, A.; Suganya, O. Strength development in concrete with wood ash blended cement and use of soft computing models to predict strength parameters. J. Adv. Res. 2015, 6, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Aquino, W.; Lange, D.A.; Olek, J. The influence of metakaolin and silica fume on the chemistry of alkali–silica reaction products. Cem. Concr. Compos. 2001, 23, 485–493. [Google Scholar] [CrossRef]
- Moser, R.D.; Jayapalan, A.R.; Garas, V.Y.; Kurtis, K.E. Assessment of binary and ternary blends of metakaolin and Class C fly ash for Alkali–silica reaction mitigation in concrete. Cem. Concr. Res. 2010, 40, 1664–1672. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Glasser, F.P. Alkali sorption by CSH and CASH gels: Part II. Role of alumina. Cement Concr. Res. 2002, 32, 1101–1111. [Google Scholar] [CrossRef]
- Bickmore, B.R.; Nagy, K.L.; Gray, A.K.; Brinkerhoff, A.R. The effect of Al (OH) 4− on the dissolution rate of quartz. Geochim. et Cosmochim. Acta 2006, 70, 290–305. [Google Scholar] [CrossRef]
- Chappex, T.; Scrivener, K.L. The influence of aluminium on the dissolution of amorphous silica and its relation to alkali silica reaction. Cem. Concr. Res. 2012, 42, 1645–1649. [Google Scholar] [CrossRef]
- Hay, R.; Ostertag, C.P. On utilization and mechanisms of waste aluminium in mitigating Alkali–silica reaction (ASR) in concrete. J. Clean. Prod. 2019, 212, 864–879. [Google Scholar] [CrossRef]
- Li, J.; Geng, G.; Myers, R.; Yu, Y.-S.; Shapiro, D.; Carraro, C.; Maboudian, R.; Monteiro, P.J. The chemistry and structure of calcium (alumino) silicate hydrate: A study by XANES, ptychographic imaging, and wide- and small-angle scattering. Cem. Concr. Res. 2019, 115, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Bureau of Meteorology, Australian Government. Water in Australia 2017–18; Bureau of Meteorology, Australian Government: Melbourne, Australia, 2018. [Google Scholar]
- Ahmad, T.; Ahmad, K.; Alam, M. Sustainable management of water treatment sludge through 3‘R’ concept. J. Clean. Prod. 2016, 124, 1–13. [Google Scholar] [CrossRef]
- Maiden, P.C.; Warnecke, M.; Boysen, R.; Hearn, M.; Chapman, M. Alum Sludge Reuse Investigation Working Technical Report. 2015. Available online: https://waterportal.com.au/swf/images/swf-files/10os-042-ghd-alum-recycling-and-reuse-project-final-report.pdf (accessed on 30 August 2020).
- Liu, Y.; Zhuge, Y.; Chow, C.W.; Keegan, A.; Li, D.; Pham, P.N.; Huang, J.; Siddique, R. Properties and microstructure of concrete blocks incorporating drinking water treatment sludge exposed to early-age carbonation curing. J. Clean. Prod. 2020, 261, 121257. [Google Scholar] [CrossRef]
- Rodríguez, N.H.; Ramírez, S.M.; Varela, M.B.; Guillem, M.; Puig, J.; Larrotcha, E.; Flores, J. Re-use of drinking water treatment plant (DWTP) sludge: Characterization and technological behaviour of cement mortars with atomized sludge additions. Cem. Concr. Res. 2010, 40, 778–786. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuge, Y.; Chow, C.W.; Keegan, A.; Li, D.; Pham, P.N.; Huang, J.; Siddique, R. Utilization of drinking water treatment sludge in concrete paving blocks: Microstructural analysis, durability and leaching properties. J. Environ. Manag. 2020, 262, 110352. [Google Scholar] [CrossRef] [PubMed]
- Benlalla, A.; Elmoussaouiti, M.; Dahhou, M.; Assafi, M. Utilization of water treatment plant sludge in structural ceramics bricks. Appl. Clay Sci. 2015, 118, 171–177. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuge, Y.; Chow, C.W.; Keegan, A.; Pham, P.N.; Li, D.; Qian, G.; Wang, L. Recycling drinking water treatment sludge into eco-concrete blocks with CO2 curing: Durability and leachability. Sci. Total. Environ. 2020, 746, 141182. [Google Scholar] [CrossRef] [PubMed]
- Gholampour, A.; Ho, V.D.; Ozbakkaloglu, T. Ambient-cured geopolymer mortars prepared with waste-based sands: Mechanical and durability-related properties and microstructure. Compos. Part B Eng. 2019, 160, 519–534. [Google Scholar] [CrossRef]
- Standards Australia. General Purpose and Blended Cements; Standards Australia: Sydney, Australia, 2010. [Google Scholar]
- Standards Australia. Methods for Sampling and Testing Aggregates; Standards Australia: Sydney, Australia, 2009. [Google Scholar]
- ASTM International. Standard Test Method for Flow of Hydraulic Cement Mortar. C1437; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Standards Australia. Masonry Units, Segmental Pavers and Flags—Methods of Test, Method 4: Determining Compressive Strength of Masonry Units; Standards Australia: Sydney, Australia, 2003. [Google Scholar]
- ASTM International. ASTM C 293: Standard Test Method for Flexural Strength of Concrete (Using Simple Beam With Center-Point Loading); ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM International. 1585-04. Standard Test Method for Measurement of Rate of Absorption of Water by Hydraulic-Cement Concretes; ASTM International: West Conshohocken, PA, USA, 2004. [Google Scholar]
- ASTM International. ASTM C 597: Standard Test Method for Pulse Velocity through Concrete; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Papadakis, V.G. Effect of fly ash on Portland cement systems: Part I. Low-calcium fly ash. Cem. Concr. Res. 1999, 29, 1727–1736. [Google Scholar] [CrossRef]



| Chemical Analysis (wt%) | GPC | Untreated DWTS | Calcined DWTS |
|---|---|---|---|
| Al2O3 | 3.57 | 28.27 | 47.68 |
| SiO2 | 20.78 | 26.43 | 31.11 |
| Fe2O3 | 3.99 | 7.66 | 4.94 |
| CaO | 61.8 | 5.36 | 4.32 |
| K2O | 0.82 | 1.23 | 0.97 |
| MgO | 2.65 | 1.11 | 0.96 |
| CuO | - | 0.71 | 0.29 |
| S/SO3 | 2.82 | 0.48 | 3.39 |
| Na2O | 0.06 | 0.06 | 0.19 |
| LOI | 3.26 | 29.5 | 6.15 |
| Specimen Notation | Cement (g) | Calcined DWTS (g) | Glass Sand (g) |
|---|---|---|---|
| M0 | 440 | 0 | 990 |
| M5 | 418 | 22 | 990 |
| M10 | 396 | 44 | 990 |
| M20 | 352 | 88 | 990 |
| Specimen Notation | Compressive Strength (MPa) | Flexural Strength (MPa) |
|---|---|---|
| M0 | 35 (±3.8) | 5.41 (±0.11) |
| M5 | 44 (±1.9) | 5.82 (±0.29) |
| M10 | 46 (±5.5) | 6.18 (±0.51) |
| M20 | 33 (±2.8) | 5.56 (±0.22) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, W.; Zhuge, Y.; Pham, P.N.; W. K. Chow, C.; Keegan, A.; Liu, Y. Utilization of Drinking Water Treatment Sludge as Cement Replacement to Mitigate Alkali–Silica Reaction in Cement Composites. J. Compos. Sci. 2020, 4, 171. https://doi.org/10.3390/jcs4040171
Duan W, Zhuge Y, Pham PN, W. K. Chow C, Keegan A, Liu Y. Utilization of Drinking Water Treatment Sludge as Cement Replacement to Mitigate Alkali–Silica Reaction in Cement Composites. Journal of Composites Science. 2020; 4(4):171. https://doi.org/10.3390/jcs4040171
Chicago/Turabian StyleDuan, Weiwei, Yan Zhuge, Phuong Ngoc Pham, Christopher W. K. Chow, Alexandra Keegan, and Yue Liu. 2020. "Utilization of Drinking Water Treatment Sludge as Cement Replacement to Mitigate Alkali–Silica Reaction in Cement Composites" Journal of Composites Science 4, no. 4: 171. https://doi.org/10.3390/jcs4040171
APA StyleDuan, W., Zhuge, Y., Pham, P. N., W. K. Chow, C., Keegan, A., & Liu, Y. (2020). Utilization of Drinking Water Treatment Sludge as Cement Replacement to Mitigate Alkali–Silica Reaction in Cement Composites. Journal of Composites Science, 4(4), 171. https://doi.org/10.3390/jcs4040171






