Thermal Transformation of End-of-Life Latex to Valuable Materials
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Note
- Paraknowitsch, J.P.; Thomas, A. Doping carbons beyond nitrogen: An overview of advanced heteroatom doped carbons with boron, sulphur and phosphorus for energy applications. Energy Environ. Sci. 2013, 6, 2839–2855. [Google Scholar] [CrossRef]
- Peng, H.-J.; Zhang, Q. Designing host materials for sulfur cathodes: From physical confinement to surface chemistry. Angew. Chem. Int. Ed. 2015, 54, 11018–11020. [Google Scholar] [CrossRef] [PubMed]
- See, K.A.; Jun, Y.-S.; Gerbec, J.A.; Sprafke, J.K.; Wudl, F.; Stucky, G.D.; Seshadri, R. Sulfur-functionalized mesoporous carbons as sulfur hosts in Li-S batteries: Increasing the affinity of polysulfide intermediates to enhance performance. ACS Appl. Mater. Interfaces 2014, 6, 10908–10916. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dai, Y.; Wang, S.; Cheng, H.; Yu, J. In situ incorporation of a S, N doped carbon/sulfur composite for lithium sulfur batteries. Rsc Adv. 2015, 5, 78017–78025. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 2015, 6, 7760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Mahmood, N.; Yin, H.; Liu, F.; Hou, Y. Synthesis of phosphorus-doped graphene and its multifunctional applications for oxygen reduction reaction and lithium ion batteries. Adv. Mater. 2013, 25, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Y.; Zhu, D.J. Chemical doping of grapheme. Mater. Chem. 2011, 21, 3335–3345. [Google Scholar] [CrossRef]
- Shen, W.; Fan, W.J. Electrochemical performances of hydrothermal tannin-based carbons doped with nitrogen. Mater. Chem. A 2013, 1, 999–1013. [Google Scholar] [CrossRef]
- Jeon, J.-W.; Sharma, R.; Meduri, P.; Arey, B.W.; Schaef, H.T.; Lutkenhaus, J.L.; Lemmon, J.P.; Thallapally, P.K.; Nandasiri, M.I.; McGrail, B.P.; et al. In Situ One-Step Synthesis of Hierarchical Nitrogen-Doped Porous Carbon for High-Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 7214–7222. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hao, R.; Liao, H.; Hou, Y. Synthesis of amino-functionalized graphene as metal-free catalyst and exploration of the roles of various nitrogen states in oxygen reduction reaction. Nano Energy 2013, 2, 88–97. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped Graphene: Synthesis, characterization, and its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Kicinski, W.; Szala, M.; Bystrzejewski, M. Sulfur-doped porous carbons: Synthesis and applications. Carbon 2014, 68, 1–32. [Google Scholar] [CrossRef]
- Yang, Z.; Yao, Z.; Li, G.; Fang, G.; Nie, H.; Liu, Z.; Zhou, X.; Chen, X.; Huang, S. Sulfur-doped graphene as an efficient metal-free cathode catalyst for oxygen reduction. ACS Nano. 2012, 6, 205–211. [Google Scholar] [CrossRef]
- Zhang, L.; Niu, J.; Li, M.; Xia, Z.J. Catalytic Mechanisms of sulfur-doped graphene as efficient oxygen reduction reaction catalysts for fuel cells. Phys. Chem. C. 2014, 118, 3545–3553. [Google Scholar]
- Liang, J.; Jiao, Y.; Jaroniec, M.; Qiao, S.Z. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed. 2012, 51, 11496–11500. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Tang, Y.; Li, S.; Dong, H.; Li, K.; Han, M.; Lan, Y.-Q.; Bao, J.; Dai, Z.J. Metal–organic framework templated nitrogen and sulfur co-doped porous carbons as highly efficient metal-free electrocatalysts for oxygen reduction reactions. Mater. Chem. A 2014, 2, 6316–6319. [Google Scholar] [CrossRef]
- Jiang, X.; Li, C.; Chi, Y.; Yan, J. TG-FTIR study on urea-formaldehyde resin residue during pyrolysis and combustion. J. Hazard. Mater. 2010, 173, 205–210. [Google Scholar] [CrossRef]
- Giuntoli, J.; De Jong, W.; Arvelakis, S.; Spliethoff, H.; Verkooijen, A. Quantitative and kinetic TG-FTIR study of biomass residue pyrolysis: Dry distiller’s grains with solubles (DDGS) and chicken manure. J. Anal. Appl. Pyrolysis. 2009, 85, 301–312. [Google Scholar] [CrossRef]
- Wei, J.; Zhou, D.; Sun, Z.; Deng, Y.; Xia, Y.; Zhao, D. A controllable synthesis of rich nitrogen-doped ordered mesoporous carbon for co2 capture and supercapacitors. Adv. Funct. Mater. 2013, 23, 2322. [Google Scholar] [CrossRef]
- Li, L.; Liu, E.; Li, J.; Yang, Y.; Shen, H.; Huang, Z.; Xiang, X.; Li, W.J. A doped activated carbon prepared from polyaniline for high performance supercapacitors. Power Sources 2010, 195, 1516. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, R.; Candelaria, S.L.; Wang, J.; Qian, L.E.; Uchaker, P.; Li, Y.; Chen, G.C. Phosphorus/sulfur Co-doped porous carbon with enhanced specific capacitance for supercapacitor and improved catalytic activity for oxygen reduction reaction. J. Power Sources 2016, 314, 39–48. [Google Scholar] [CrossRef]
- Wu, M.; Dou, Z.J.C.; Cui, L. Nitrogen and sulfur co-doped graphene aerogels as an efficient metal-free catalyst for oxygen reduction reaction in an alkaline solution. Rsc Adv. 2016, 6, 22781. [Google Scholar] [CrossRef]
- Domínguez, C.; Pérez-Alonso, F.J.S.; Al-Thabaiti, A.; Basahel, S.N.; Obaid, A.Y.; Alyoubi, A.O.; Fuente, J.L.G.; Rojas, S. Effect of N and S co-doping of multiwalled carbon nanotubes for the oxygen reduction. Electrochim. Acta 2015, 157, 158–165. [Google Scholar] [CrossRef]
- Pan, F.; Duan, Y.; Zhang, X.; Zhang, J. A facile synthesis of nitrogen/sulfur co-doped graphene for the oxygen reduction reaction. J. ChemCatChem 2016, 8, 163–170. [Google Scholar] [CrossRef]
- Zhu, H.; Yan, J.; Jiang, X.; Lai, Y.; Cen, K. Study on pyrolysis of typical medical waste materials by using TG-FTIR analysis. J. Hazard. Mater. 2008, 153, 670–676. [Google Scholar] [CrossRef]
- Yang, S.; Zhi, L.; Tang, K.; Feng, X.; Maier, J.; Müllen, K. Efficient Synthesis of Heteroatom (N or S)-Doped Graphene Based on Ultrathin Graphene Oxide-Porous Silica Sheets for Oxygen Reduction Reactions. Adv. Funct. Mater. 2012, 22, 3634–3640. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Quan, X.; Chen, S.; Zhao, H.; Yu, H. Nitrogen and sulfur co-doped graphene/carbon nanotube as metal-free electrocatalyst for oxygen evolution reaction: The enhanced performance by sulfur doping. Electrochim. Acta 2016, 204, 169–175. [Google Scholar] [CrossRef]
- Niu, S.; Lv, W.; Zhou, G.; He, Y.; Li, B.; Yang, Q.-H.; Kang, F. N and S co-doped porous carbon spheres prepared using l-cysteine as a dual functional agent for high-performance lithium–sulfur batteries. Chem. Commun. 2015, 51, 17720–17723. [Google Scholar] [CrossRef]
- Gao, W.; Feng, X.; Zhang, T.; Huang, H.; Li, J.; Song, W. One-Step Pyrolytic Synthesis of Nitrogen and Sulfur Dual-Doped Porous Carbon with High Catalytic Activity and Good Accessibility to Small Biomolecules. Acs Appl. Mater. Interfaces 2014, 6, 19109–19117. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, X.; Huang, T.; Yu, A. Nitrogen-doped porous carbon nanofiber webs/sulfur composites as cathode materials for lithium-sulfur batteries. Electrochim. Acta 2014, 116, 210–216. [Google Scholar] [CrossRef]
- Shi, Q.; Peng, F.; Liao, S.; Wang, H.; Yu, H.; Liu, Z.; Zhang, B.; Su, D.J. Sulfur and nitrogen co-doped carbon nanotubes for enhancing electrochemical oxygen reduction activity in acidic and alkaline media. Mater. Chem. A 2013, 1, 14853–14857. [Google Scholar] [CrossRef]
- Blue Environment and Randell Environment Consulting. Waste Generation and Resource Recovery in Australia; Australian National Waste Reporting; Blue Environment and Randell Environment Consulting: Melbourne, Australia, 2013. [Google Scholar]
- (a) Waste Authority, Government of Western Australia, EMRC (2009). Available online: https://www.parliament.wa.gov.au/publications/tabledpapers.nsf/displaypaper/3811675a172c5a2337572bdac825767b000c60ba/$file/waste+authority+ar+2008-9.pdf. (b) Mattress Recycling Project Expansion: Final Report; Available online: https://www.environment.gov.au/system/files/resources/0a517ed7-74cb-418b-9319-7624491e4921/files/casestudy-softlanding.pdf (accessed on 4 November 2020).
- Georgouras, M. Soft Landing: Social Return on Investment Study; Centre for Social Impact: Sidney, Australia, 1992. [Google Scholar]
- Kemp, A.R. Composition and structure of heave latex. Ind. Eng. Chem. 1938, 30, 154–158. [Google Scholar] [CrossRef]
- Singh, S.; Wu, C.; Williams, J.P.T. Pyrolysis of waste materials using TGA-MS and TGA-FTIR as complementary characterisation techniques. Analyt. Appl. Pyrolys. 2012, 94, 99–107. [Google Scholar] [CrossRef]
- Tao, L.; Zhao, G.; Qian, J.; Qin, Y. TG-FTIR characterisation of pyrolysis of waste mixtures of paint and tar slag. J. Hazard. Mater. 2010, 175, 754–761. [Google Scholar] [CrossRef]
- Han, L.; Wang, Q.; Ma, Q.; Yu, C.; Luo, Z.; Cen, K. Influence of CaO additives on wheat-straw pyrolysis as determined by TG-FTIR analysis. J. Anal. Appl. Pyrolysis. 2010, 88, 199–206. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xiang, J.; Li, P.; Huang, D.; Jiang, L.; Zhang, A.; Zhang, J. FTIR study of pyrolysis products evolving from typical agricultural residues. J. Anal. Appl. Pyrolysis. 2010, 88, 117–123. [Google Scholar] [CrossRef]
- Hardy, A.; Van Werde, K.; Vanhoyland, G.; Van Bael, M.; Mullens, J.; Van Poucke, L.; Van Bael, M.K. Study of the decomposition of an aqueous metal–chelate gel precursor for (Bi,La)4Ti3O12 by means of TGA–FTIR, TGA–MS and HT-DRIFT. Acta 2003, 397, 143–153. [Google Scholar] [CrossRef]
- Ferrasse, J.-H.; Chávez, S.; Arlabosse, P.; Dupuy, N. Chemometrics as a tool for the analysis of evolved gas during the thermal treatment of sewage sludge using coupled TG–FTIR. Thermochim. Acta 2003, 404, 97–108. [Google Scholar] [CrossRef]
- Marcilla, A.; Menargues, A.G.S. TGA/FTIR study of the catalytic pyrolysis of ethylene–vinyl acetate co-polymers in the presence of MCM-41. Polym. Degrad. Stab. 2005, 89, 145–152. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Liang, T.D.; Chen, H.; Zheng, C. Pyrolysis of palm oil wastes for biofuel production. J. Energy Environ. 2006, 702, 315–323. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Jang, B.N.; Wilkie, C.A. A TGA/FTIR and mass spectral study on the thermal degradation of bisphenol A polycarbonate. Polym. Degrad. Stab. 2004, 86, 419–430. [Google Scholar] [CrossRef]
- Tudorachi, N.; Chiriac, A.P. TGA/FTIR/MS study on thermal decomposition of poly(succinimide) and sodium poly(aspartate). Polym. Test. 2011, 30, 397–407. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. Pyrolysis-thermogravimetric analysis of tyres and tyre components. Fuel 1995, 74, 1277–1283. [Google Scholar] [CrossRef]
- García, A.N.; Emarcilla, A.; Font, R. Thermogravimetric kinetic study of the pyrolysis of municipal solid waste. Acta 1995, 254, 277–304. [Google Scholar] [CrossRef]
- Buah, W.K.; Cunliffe, A.M.; Williams, P.T. Characterisation of products from the pyrolysis of municipal solid waste energy. Trans. IChemE Part B Process. Saf. Environ. Protec. 2007, 85, 450–457. [Google Scholar] [CrossRef]
- Sricharoenchaikul, V.; Atong, D. Thermal decomposition study on Jatropha curcas L. waste using TGA and fixed bed reactor. J. Anal. Appl. Pyrolysis 2009, 85, 155–162. [Google Scholar] [CrossRef]
- Duan, H.B.; Li, J.H. Thermal degradation behaviour of waste video cards using thermogravimetric analysis and pyrolysis gas chromatography/mass spectrometry techniques. J. Air Waste Manag. Assoc. 2010, 60, 540–547. [Google Scholar] [CrossRef]
- Na, D.; Yu-Feng, Z.; Yan, W. Thermogravimetric analysis and kinetic study of representative medical waste composition. Waste Manag. 2008, 28, 1572–1580. [Google Scholar]
- Yang, H.; Yan, R.; Chin, T.; Liang, D.T.; Chen, A.H.; Zheng, C. Thermogravimetric Analysis—Fourier Transform Infrared Analysis of Palm Oil Waste Pyrolysis. Energy Fuels 2004, 18, 1814–1821. [Google Scholar] [CrossRef]
- Giuntoli, J.; Arvelakis, S.; Spliethoff, H.; De Jong, W.; Verkooijen, A.H.M. Quantitative and Kinetic Thermogravimetric Fourier Transform Infrared (TG-FTIR) Study of Pyrolysis of Agricultural Residues: Influence of Different Pretreatments. Energy Fuels 2009, 23, 5695–5706. [Google Scholar] [CrossRef]
- Ischia, M.; Perazzolli, C.; Maschio, R.D.; Campostrini, R. Pyrolysis study of sewage sludge by TG-MS and TG-GC-MS coupled analyses. J. Anal. Calorim. 2006, 87, 567–574. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Z.; Zhang, Y.; Meng, B.; Zhou, A.; Qiu, J. Graphene Sheets from Graphitized Anthracite Coal: Preparation, Decoration, and Application. Energy Fuels 2012, 26, 5186–5192. [Google Scholar] [CrossRef]
- Orvis, A.L.; Allwein, S. Gas Sampling and Analysis by Fourier Transform Infrared Spectroscopy. In Proceedings of the Fire and Materials 2001 Conference, San Francisco, CA, USA, 22–24 January 2001; Copyright Interscience Communications Ltd.: London, UK, 2001; pp. 433–446. [Google Scholar]
- Filipczak, R.A.; Blake, D.; Speitel, L.; Lyon, R.E.; Williams, J.M.; Gill, W. Development and Testing of a Plastic Smoke Generation Source. In Proceedings of the Fire and Materials 2001 Conference, San Francisco, CA, USA., 22–24 January 2001; Copyright Interscience Communications Ltd.: London, UK, 2001; pp. 93–104. [Google Scholar]
- Sahajwalla, V.; Cayumil, R.; Khanna, R.; Ikram-Ul-Haq, M.; Rajarao, R.; Mukherjee, P.S.; Hill, A.J. Recycling polymer rich waste printed circuit boards at high temperatures: Recovery of value-added carbon resources. Sustain. Met. 2015, 1, 75–84. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.-I.; Li, C.-Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Raymundo-Pinero, E.; Cadek, M.; Beguin, F. A high-performance carbon for supercapacitors obtained by carbonization of a seaweed biopolymer. Adv. Funct. Mater. 2009, 19, 1032–1039. [Google Scholar] [CrossRef]
- Xu, B.; Yue, S.F.; Sui, Z.Y.; Zhang, X.T.; Hou, S.S.; Cao, G.P.; Yang, Y.S. What is the choice for supercapacitors: Graphene or graphene oxide? Energy Environ. Sci. 2011, 4, 2826–2830. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Leng, M.; Li, M.; Huang, X.L.; Xuen, J.M. Sulphur-functionalized graphene towards high performance supercapacitor. Nano Energy 2015, 12, 250–257. [Google Scholar] [CrossRef]
- Wen, Y.Y.; Wang, B.; Huang, C.C.; Wang, L.Z.; Hulicova-Jurcakova, D. Synthesis of phosphorus-doped graphene and its wide potential window in aqueous supercapacitors. Chem. Eur. J. 2015, 21, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.B.; Ren, J.; Zhang, Z.T.; Chen, X.L.; Guan, G.Z.; Qiu, L.B.; Zhang, Y.; Peng, H.S. Recent advancement of nanostructured carbon for energy applications. Chem. Rev. 2015, 115, 5159–5223. [Google Scholar] [CrossRef]
- Shen, W.Z.; Fan, W.B. Nitrogen-containing porous carbons: Synthesis and application. J. Mater. Chem. A. 2013, 1, 999–1013. [Google Scholar] [CrossRef]
- Marjanović, G.C.; Pašti, I.; Mentus, S. One-dimensional nitrogen-containing carbon nanostructures. Prog. Mater. Sci. 2015, 69, 61–182. [Google Scholar]
- Li, X.L.; Wang, H.L.; Robinson, J.T.; Sanchez, H.; Diankov, G.; Dai, H.J. Simultaneous Nitrogen Doping and Reduction of Graphene Oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944. [Google Scholar] [CrossRef]
- Pathak, H.; Tewari, A.N.; Sankhyan, S.; Dubey, D.S.; Mina, U.; Singh, V.K.; Jain, N.; Bhatia, A. Direct-seeded rice: Potential, performance and problems—A review. Curr. Adv. Agri. Sci. 2011, 3, 77–88. [Google Scholar]
- Sheng, Z.H.; Shao, L.; Chen, J.J.; Bao, W.J.; Wang, F.B.; Xia, X.H. Catalyst-Free Synthesis of Nitrogen-Doped Graphene via Thermal Annealing Graphite Oxide with Melamine and Its Excellent Electrocatalysis. Acs Nano. 2011, 5, 4350–4358. [Google Scholar] [CrossRef]
- Hou, S.C.; Cai, X.; Wu, H.W.; Yu, X.; Peng, M.; Yan, K.; Zou, D.C. Nitrogen-doped graphene for dye-sensitized solar cells and the role of nitrogen states in triiodide reduction. Energy Environ. Sci. 2013, 6, 3356–3362. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Park, S.H.; Woo, S.I. Enhanced electrochemical oxygen reduction reaction by restacking of N-doped single graphene layers. Rsc Adv. 2013, 3, 4246–4253. [Google Scholar] [CrossRef]
- Lai, L.F.; Potts, J.R.; Zhan, D.; Wang, L.; Poh, C.K.; Tang, C.H.; Gong, H.; Shen, Z.X.; Lin, J.Y.; Ruoff, R.S. Exploration of the active center structure of nitrogen-doped graphene-based catalysts for oxygen reduction reaction. Energy Environ. Sci. 2012, 5, 7936–7942. [Google Scholar] [CrossRef]
- Shen, Y.M.; Bai, J.F. A new kind CO2/CH4 separation material: Open ended nitrogen doped carbon nanotubes formed by direct pyrolysis of metal organic frameworks. Chem. Commun. 2010, 46, 1308–1310. [Google Scholar] [CrossRef]
- Shin, Y.; Fryxell, G.E.; Um, W.; Parker, K.; Mattigod, S.V.; Skaggs, R. Sulfur-Functionalized Mesoporous Carbon. Adv. Funct. Mater. 2007, 17, 2897–2901. [Google Scholar] [CrossRef]
- Böttger-Hiller, F.; Mehner, A.; Anders, S.; Kroll, L.; Cox, G.; Simond, F. Sulphur-doped porous carbon from a thiophene-based twin monomer. Chem. Commun. 2012, 48, 10568–10570. [Google Scholar] [CrossRef]
- Paraknowitsch, J.P.; Thomas, A.; Schmidt, J. Microporous sulfur-doped carbon from thienyl-based polymer network precursors. Chem. Commun. 2011, 47, 8283–8285. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bo, X.; Zhang, Y. Sulfur-doped ordered mesoporous carbon with high electrocatalytic activity for oxygen reduction. Electroch. Acta 2013, 108, 404–411. [Google Scholar] [CrossRef]
- Liu, X.; Antonietti, M. Moderating black powder chemistry for the synthesis of doped and highly porous graphene nanoplatelets and their use in electrocatalysis. Adv. Mater. 2013, 25, 6284–6290. [Google Scholar] [CrossRef]
- Jeon, I.-Y.; Zhang, S.; Zhang, L.; Choi, H.-J.; Seo, J.-M.; Xia, Z. Edge-selectively sulfurized graphene nanoplatelets as efficient metal-free electrocatalysts for oxygen reduction reaction: The electron spin effect. Adv. Mater. 2013, 25, 6138–6145. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Luo, B.; Li, X.; Jin, M.; Fang, Y.; Tang, Z.; Jia, Y.; Liang, M.; Thomas, A.; Yang, J.; et al. Graphenal Polymers for Energy Storage Studies. Energy Environ. Sci. 2012, 5, 9747–9751. [Google Scholar] [CrossRef]
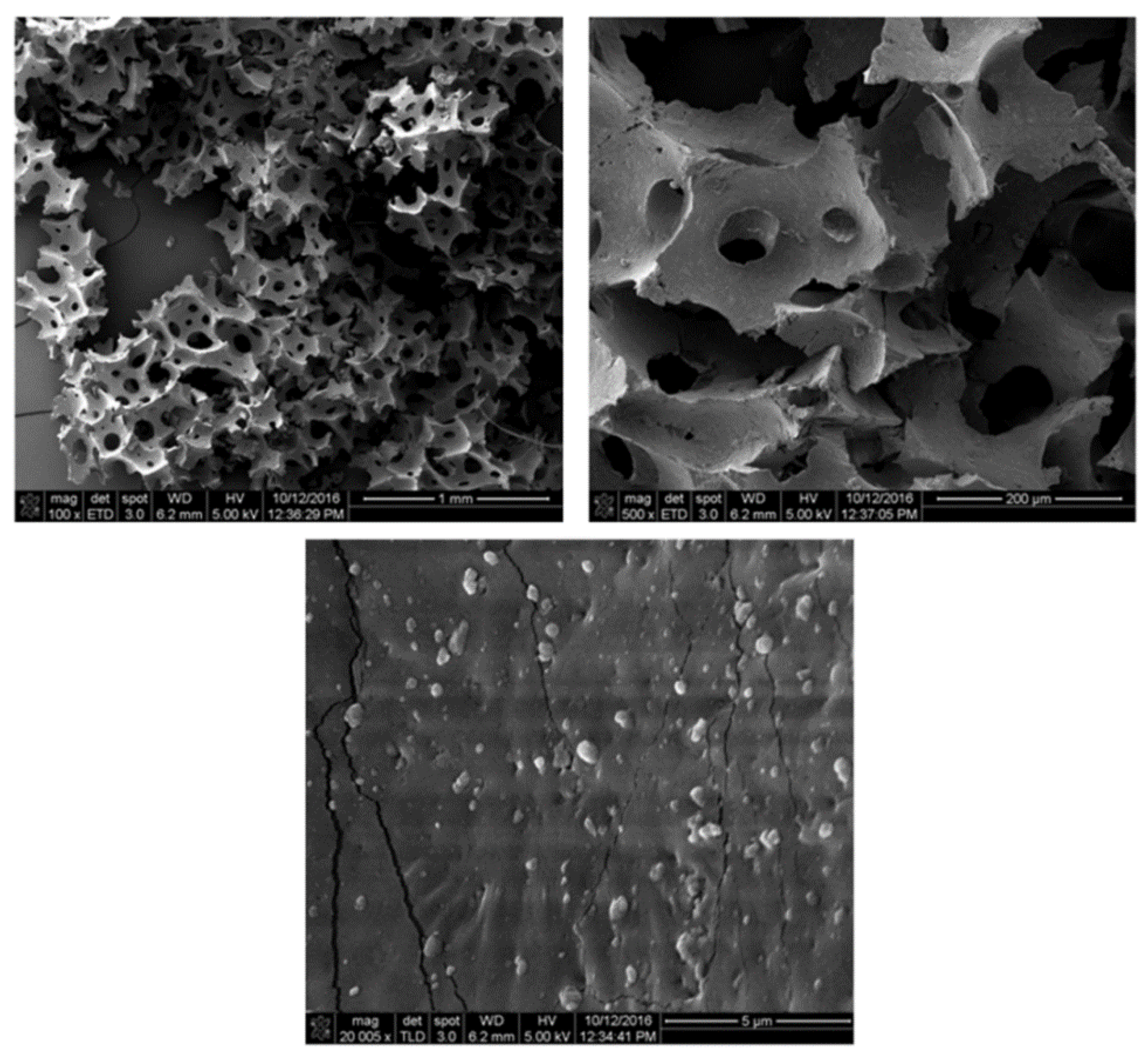
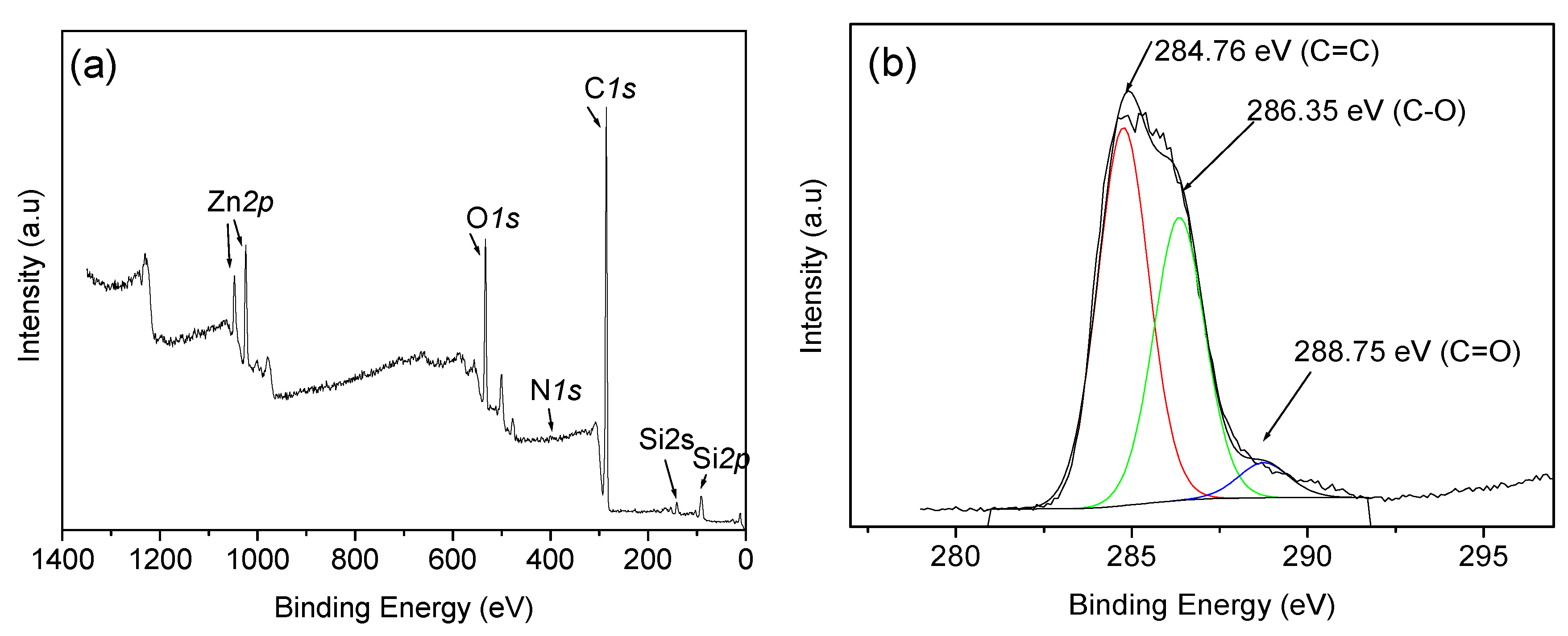

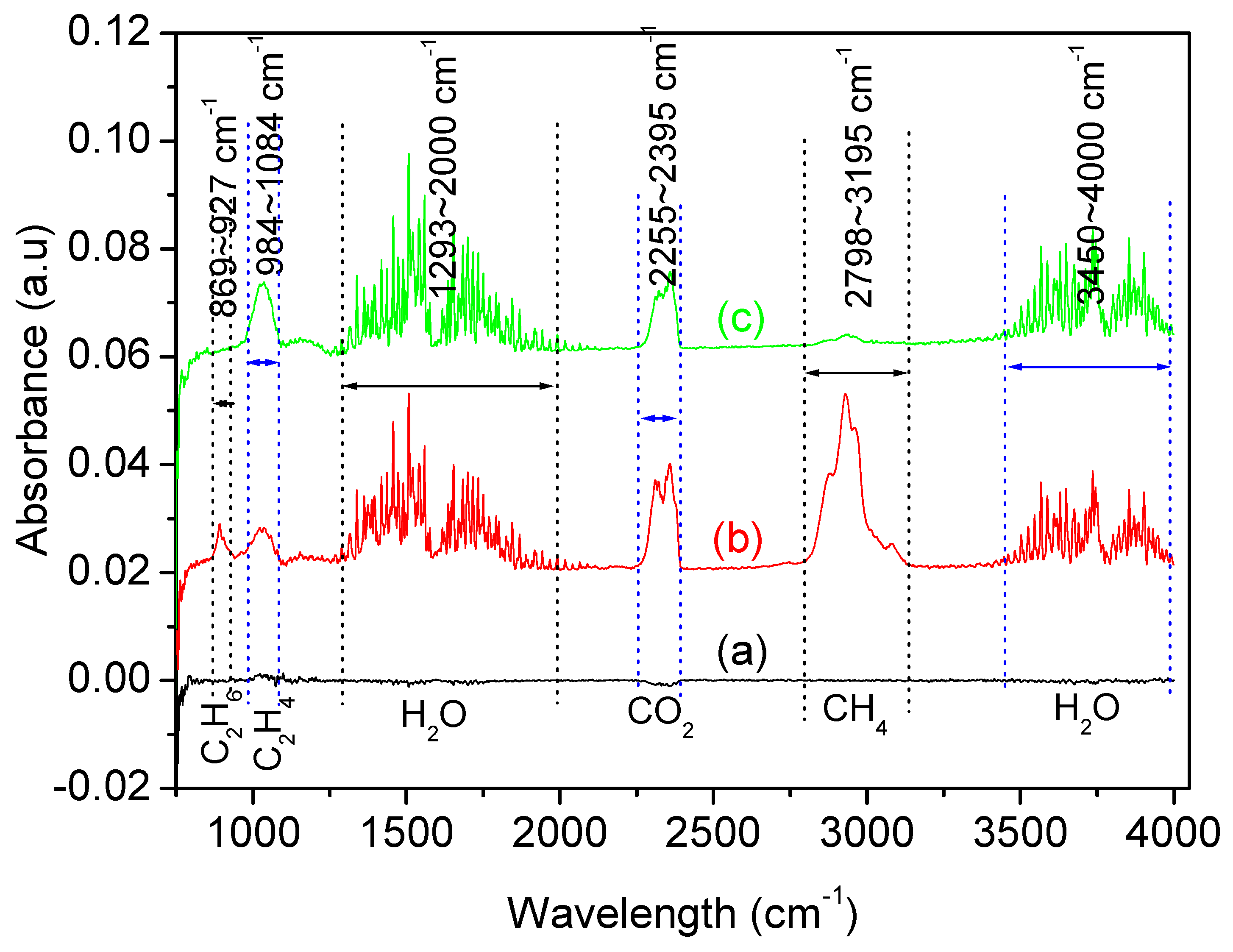

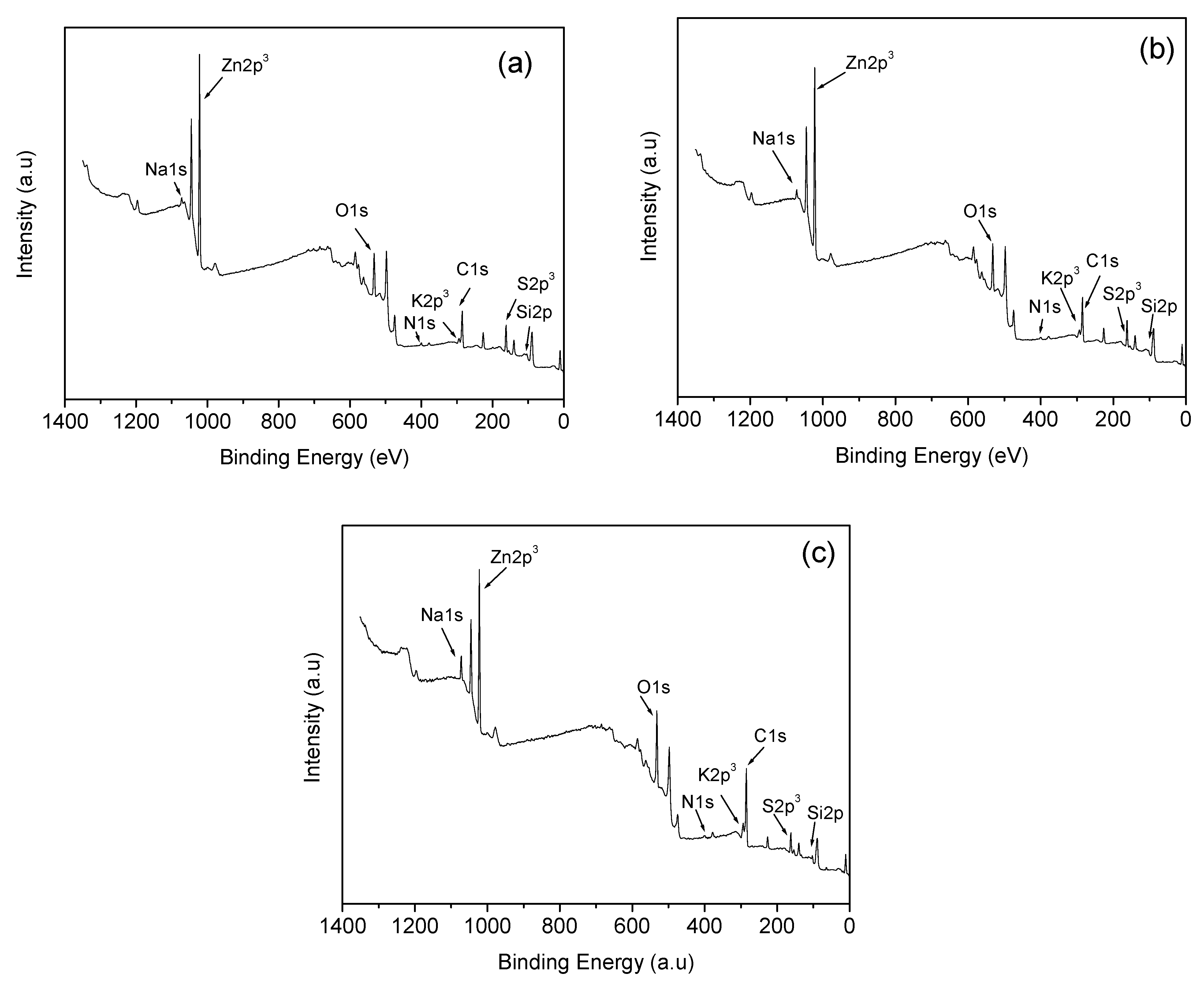
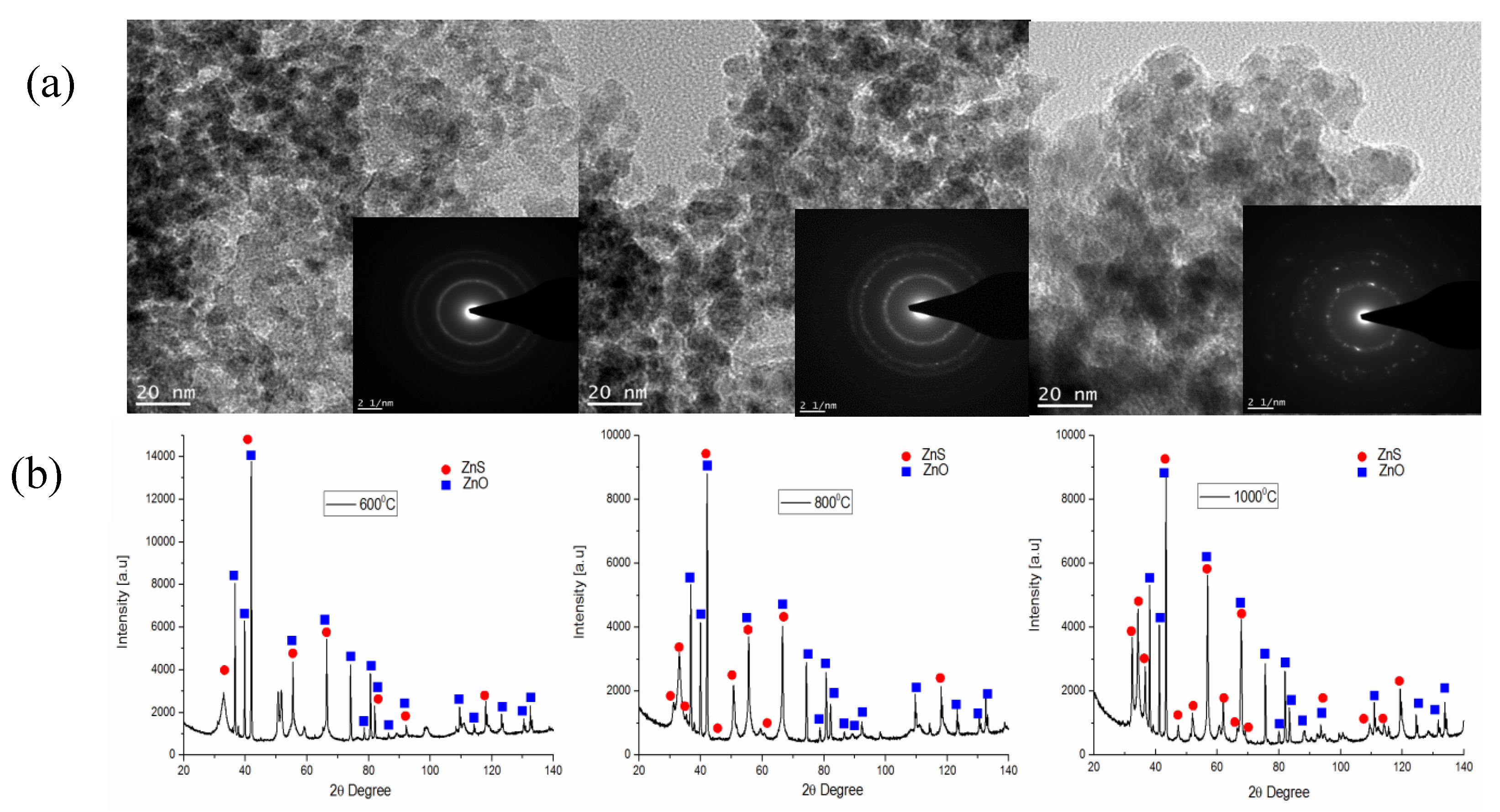

| Temperature (°C) | Type of Gas Produced (ppm/Kg) | Comparative Gas Ratio | |||||
|---|---|---|---|---|---|---|---|
| CO2 | CO | CH4 | CH4/CO | CH4/CO2 | CH41000/CH4800 | CH41000/CH4600 | |
| 600 | 687 | 503 | 3793 | 7.54 | 5.52 | ||
| 800 | 4606 | 1927 | 60380 | 31.34 | 13.10 | 3.07 | 48.97 |
| 1000 | 8780 | 3600 | 185760 | 51.6 | 21.16 | - | - |
| Materials | Chemical Composition by XPS Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Na | Zn | Si | K | S | N | |
| C-600 | 41.01 | 20.03 | 1.03 | 14.97 | 3.78 | 1.65 | 15.49 | 2.04 |
| C-800 | 47.88 | 17.95 | 1.4 | 12.84 | 2.66 | 2.24 | 13.55 | 1.48 |
| C−1000 | 56.4 | 19.33 | 2.48 | 7.36 | 3.62 | 2.86 | 6.65 | 1.29 |
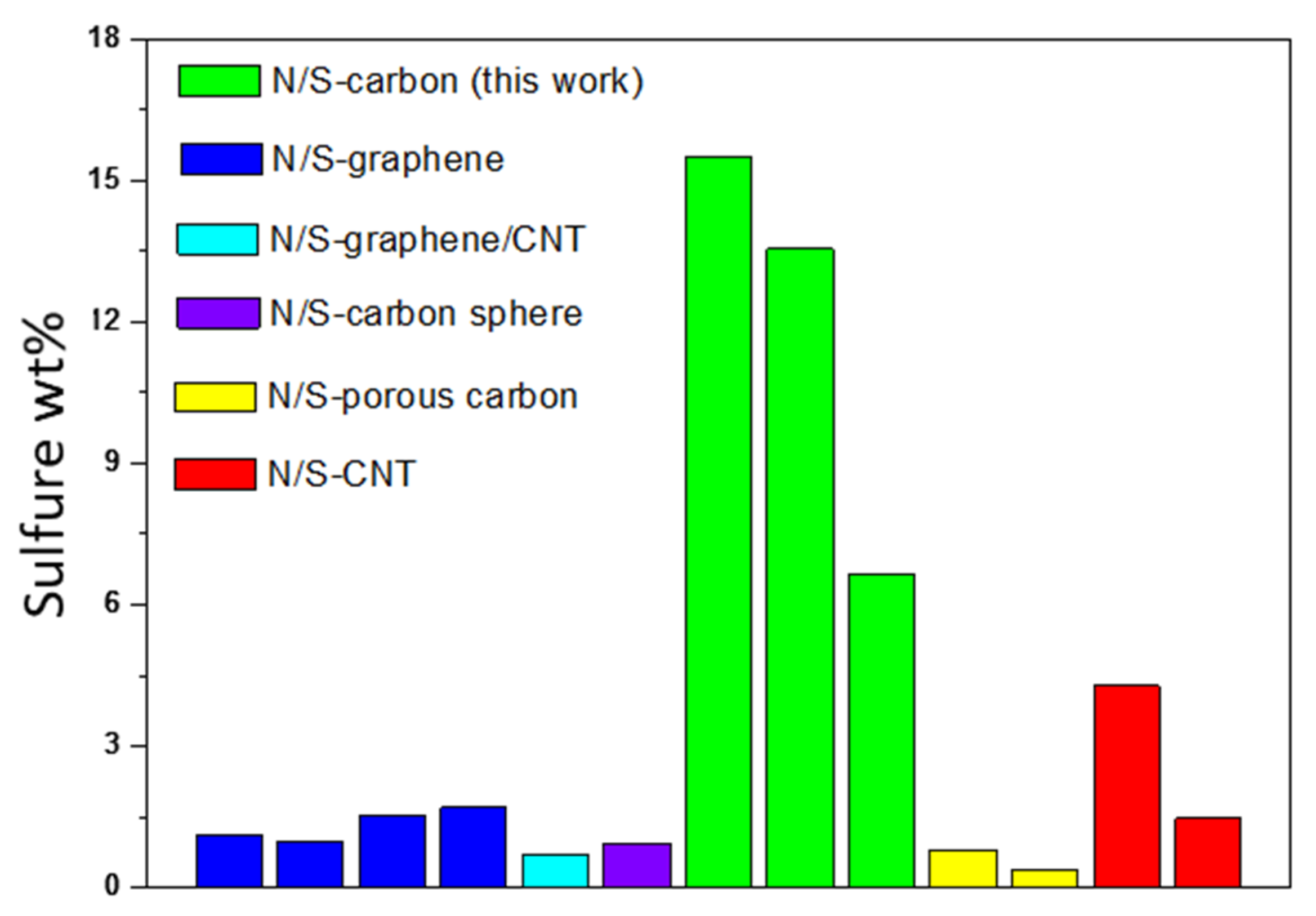
| Precursors | Process | Temperature | Time | Final Product | N % | S% | Ref. |
|---|---|---|---|---|---|---|---|
| Latex | Thermal treatment | 600, 800, 1000 °C | 15 min | S/N-doped carbon | 1.29, 1.48, 2.04 | 6.65, 13.55, 15.49 | This work |
| 5-Amino−1,3,4-thiadiazole-2-thiol (C2H3N3S2), and graphene oxide | 2 step process: Hydrothermal + Pyrolysis | 180 °C for hydrothermal + 600–900 °C for pyrolysis | 20 h + 2 h = 22 h | N/S-codoped graphene | 0.48–1.82 | 0.58–1.12 | 21 |
| NaCl, Cysteine [HO2CCH(NH2)CH2SH] | 3 step process: Freezing + pyrolysis + pyrolysis | −26 °C for freezing + 1st pyrolysis 750 °C + 2nd pyrolysis 1000 °C | 24 h + 2 h + 3 h = 29 h | N/S-codoped graphene | 1.85 | 0.99 | 23 |
| Benzyl disulphide, Graphene Oxide | Thermal anneal | 600 °C–1050 °C | 2 h | S-graphene | − | 1.3–1.53 | 13 |
| NH3 and H2S gas + Graphene-Silica | Thermal treatment | 500–1000 °C | 1 h | N/S-codoped graphene | 2.4–4.6 | 1.2–1.7 | 25 |
| Urea+ thiourea+ GO+ CNT | Hydrothermal | 180 °C | 12 h | N/S-codoped graphene/carbon nanotube | 2.4–4.6 | 0.67–0.71 | 26 |
| D-glucose, L-Cysteine, KOH, Sulfur powder | Hydrothermal + thermal activation + heat treatment | 180 °C for hydrothermal + 800 °C for activation + 155 °C for heat treatment | 24h for hydrothermal + 1 h for theral activation + 12 h for heat treatment = 37 h | N/S-codoped carbon sphere | 2.85 | 0.95 | 27 |
| Sodium Citrate + Cysteine | Pyrolysis | 800 °C | 2 h | N/S-codoped porous carbon | 2% | 0.8 | 28 |
| PPy precursors + KOH + S | Pyrolysis +KOH activation + heat treatment | 600 °C for Pyrolysis + 700 °C for activation + 155 °C for heat treatment | 1h for pyrolysis + 2h for KOH activation + 60h for heat treatment = 63 h | N/S-porous carbon fiber | 14.03 | − | 29 |
| Resorcinol and furaldehyde, phosphorus pentasulfide | Sol gel process + pyrolysis | 80 °C for slogel + 1000 °C for pyrolysis | 7 days for sol gel + 3 h for pyrolysis = 171 h | N/S-porous carbon | 1.3 N | 0.4 | 30 |
| Toluenethiol and sulphur, N-CNT | Hydrothermal + thermal | 180 °C for hydrothermal+ 900 °C for thermal | 5.5 h for hydrothermal + 4 h for thermal = 9.5 h | N/S-CNT | 0.18 | 3.02–4.29 | 20 |
| Urea, thiourea and CNT | Thermal | 800 °C | 1 h | N/S CNT | 1.15–3.43 | 0.62–1.47 | 22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haque, E.; Pahlevani, F.; Gorjizadeh, N.; Hossain, R.; Sahajwalla, V. Thermal Transformation of End-of-Life Latex to Valuable Materials. J. Compos. Sci. 2020, 4, 166. https://doi.org/10.3390/jcs4040166
Haque E, Pahlevani F, Gorjizadeh N, Hossain R, Sahajwalla V. Thermal Transformation of End-of-Life Latex to Valuable Materials. Journal of Composites Science. 2020; 4(4):166. https://doi.org/10.3390/jcs4040166
Chicago/Turabian StyleHaque, Enamul, Farshid Pahlevani, Narjes Gorjizadeh, Rumana Hossain, and Veena Sahajwalla. 2020. "Thermal Transformation of End-of-Life Latex to Valuable Materials" Journal of Composites Science 4, no. 4: 166. https://doi.org/10.3390/jcs4040166
APA StyleHaque, E., Pahlevani, F., Gorjizadeh, N., Hossain, R., & Sahajwalla, V. (2020). Thermal Transformation of End-of-Life Latex to Valuable Materials. Journal of Composites Science, 4(4), 166. https://doi.org/10.3390/jcs4040166







