Synthesis and Characterization of Graphite Composite Foams for Oil Spill Recovery Application
Abstract
1. Introduction
2. Experimental Part
2.1. Synthesis of Composite Silicone Foams
2.2. Morphological Analysis
2.3. Sorption Capacity Tests
3. Sorption Kinetics Models
- pseudo-first order (PFO) model
- pseudo-second order (PSO) model
- Elovich model
3.1. Pseudo-First Order Kinetic Model
3.2. Pseudo-Second Order Kinetic Model
3.3. Elovich Model
4. Results and Discussion
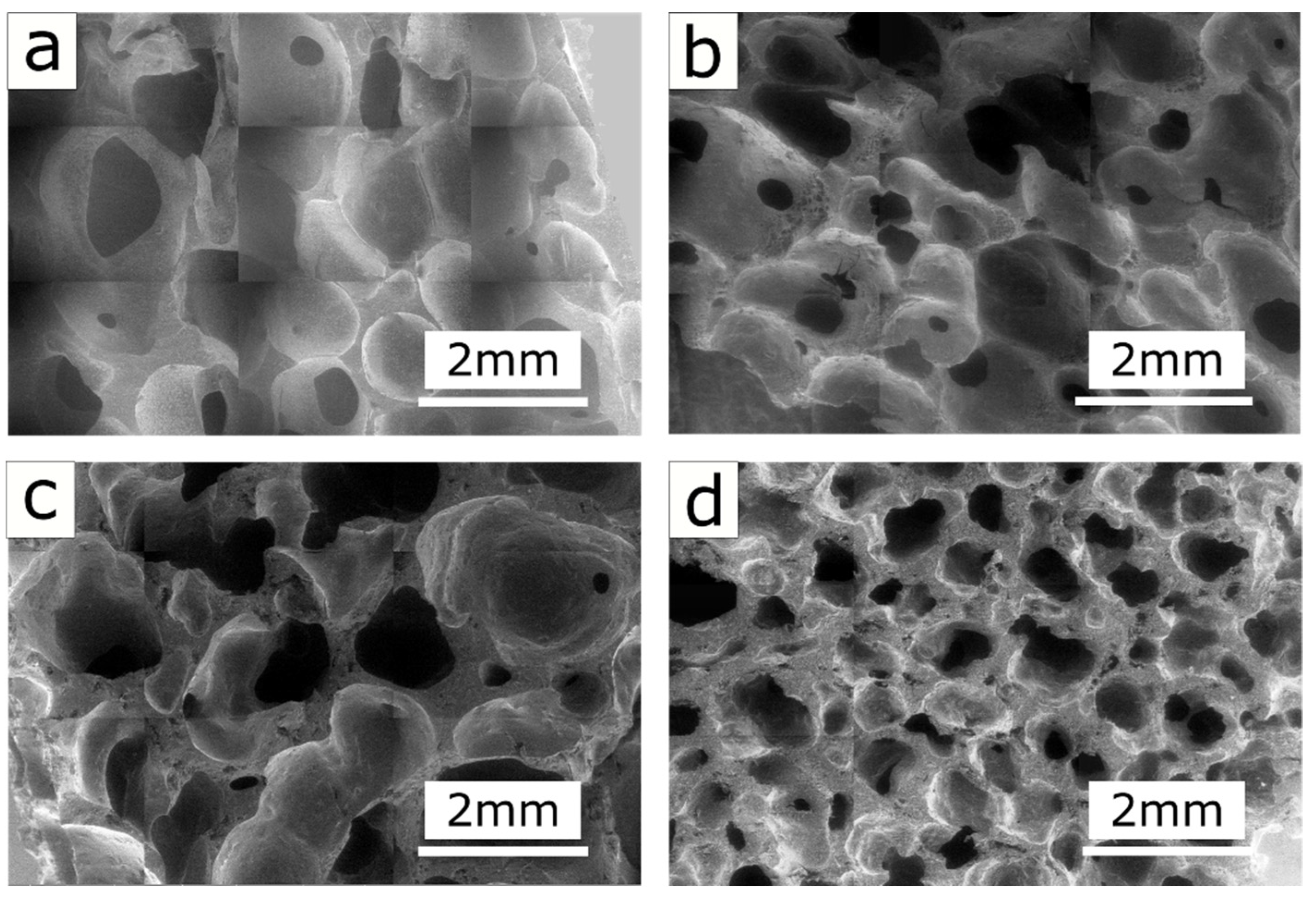
4.1. Morphological Analysis
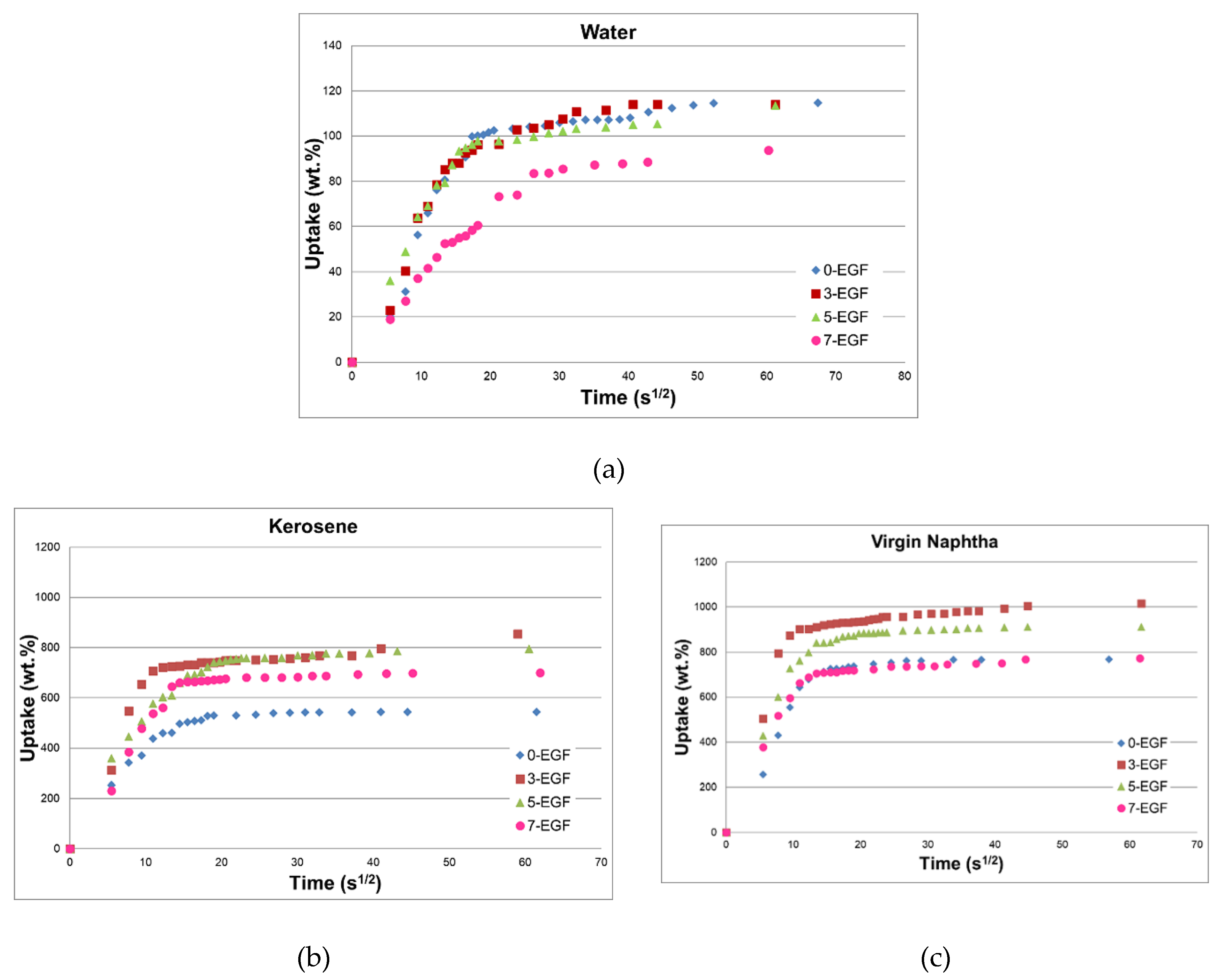
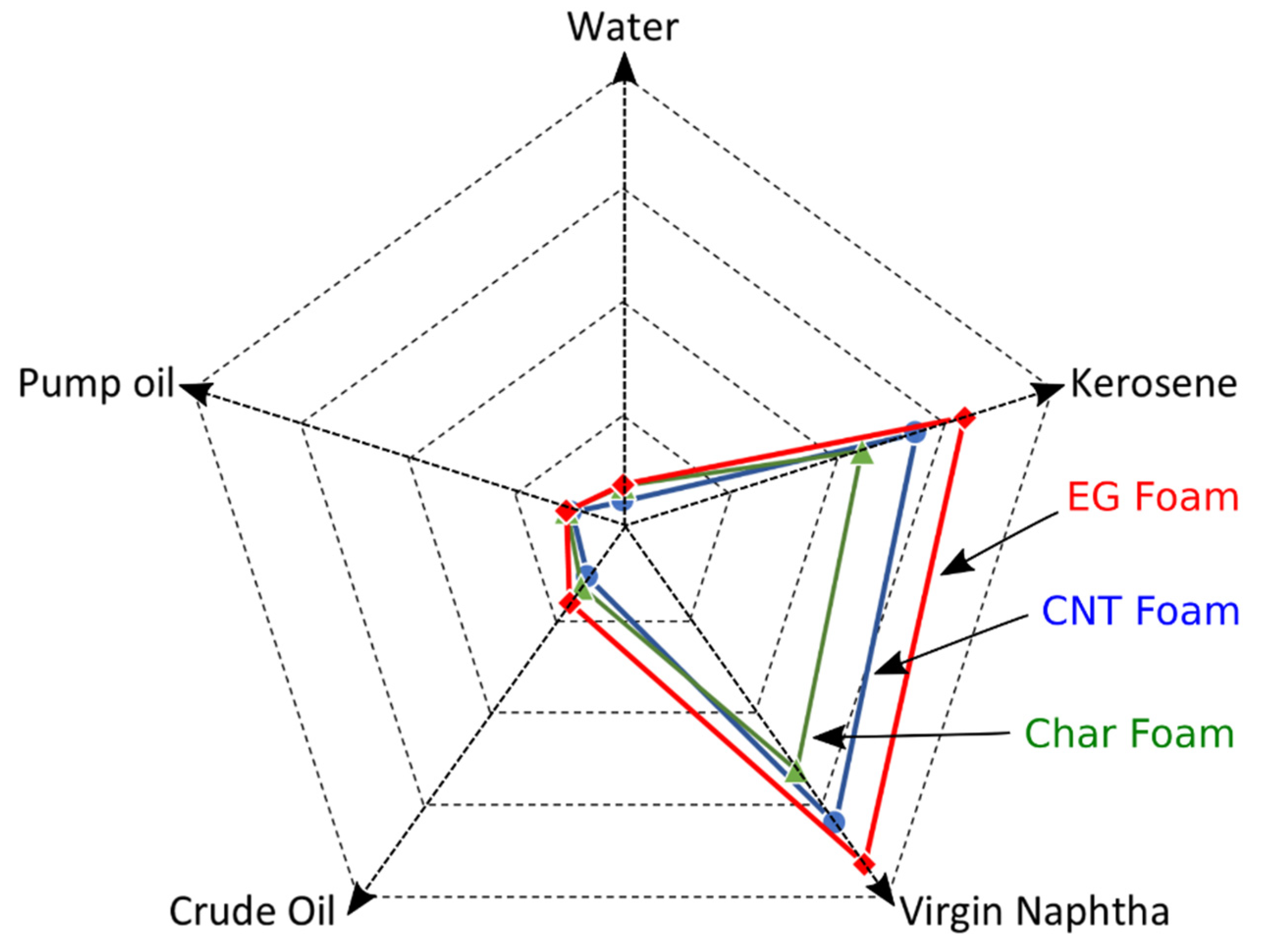
4.2. Sorption Performances
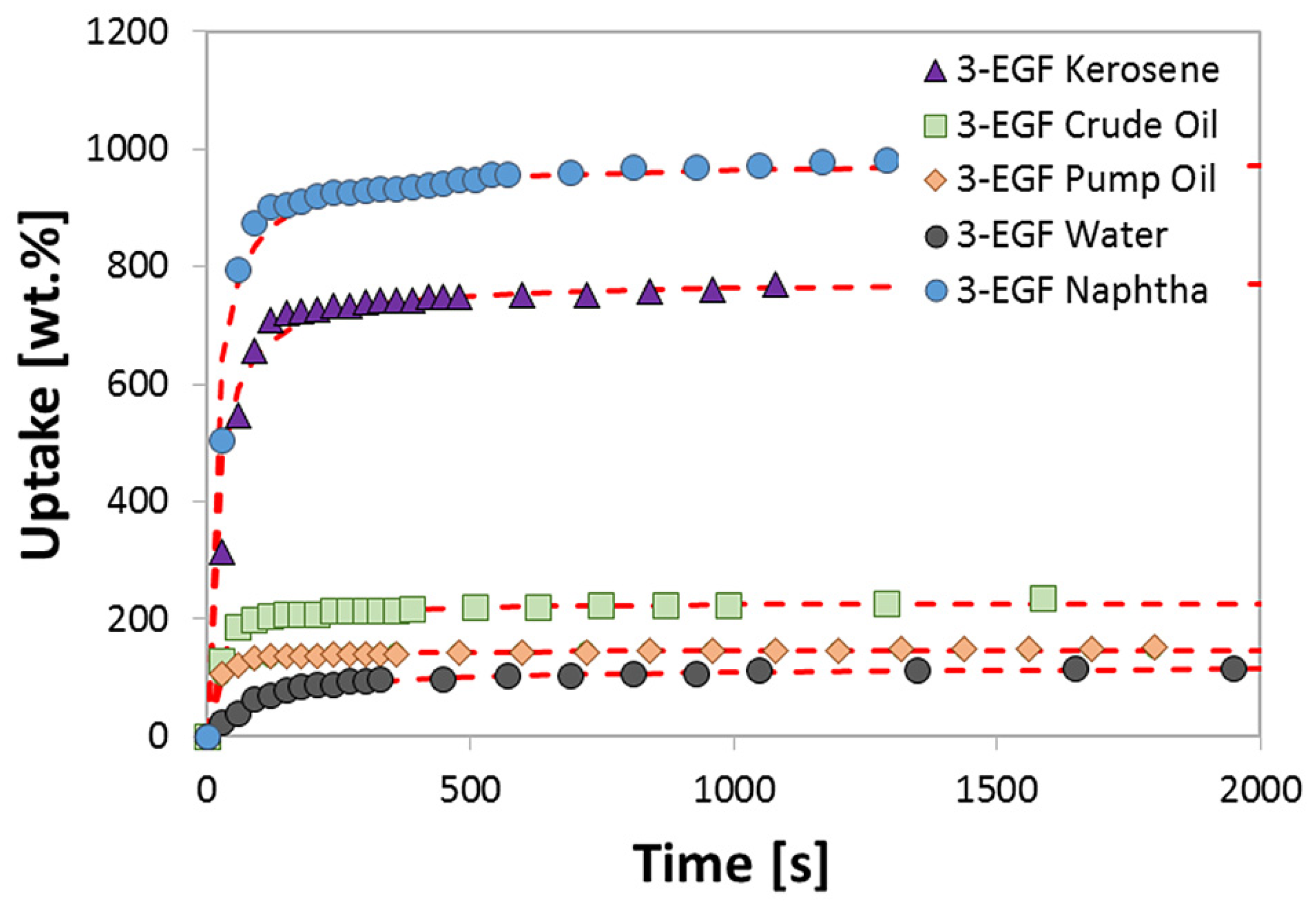
4.3. Kinetic Modeling
5. Conclusions
- By increasing the EG amount, the foams’ absorption capacity was reduced.
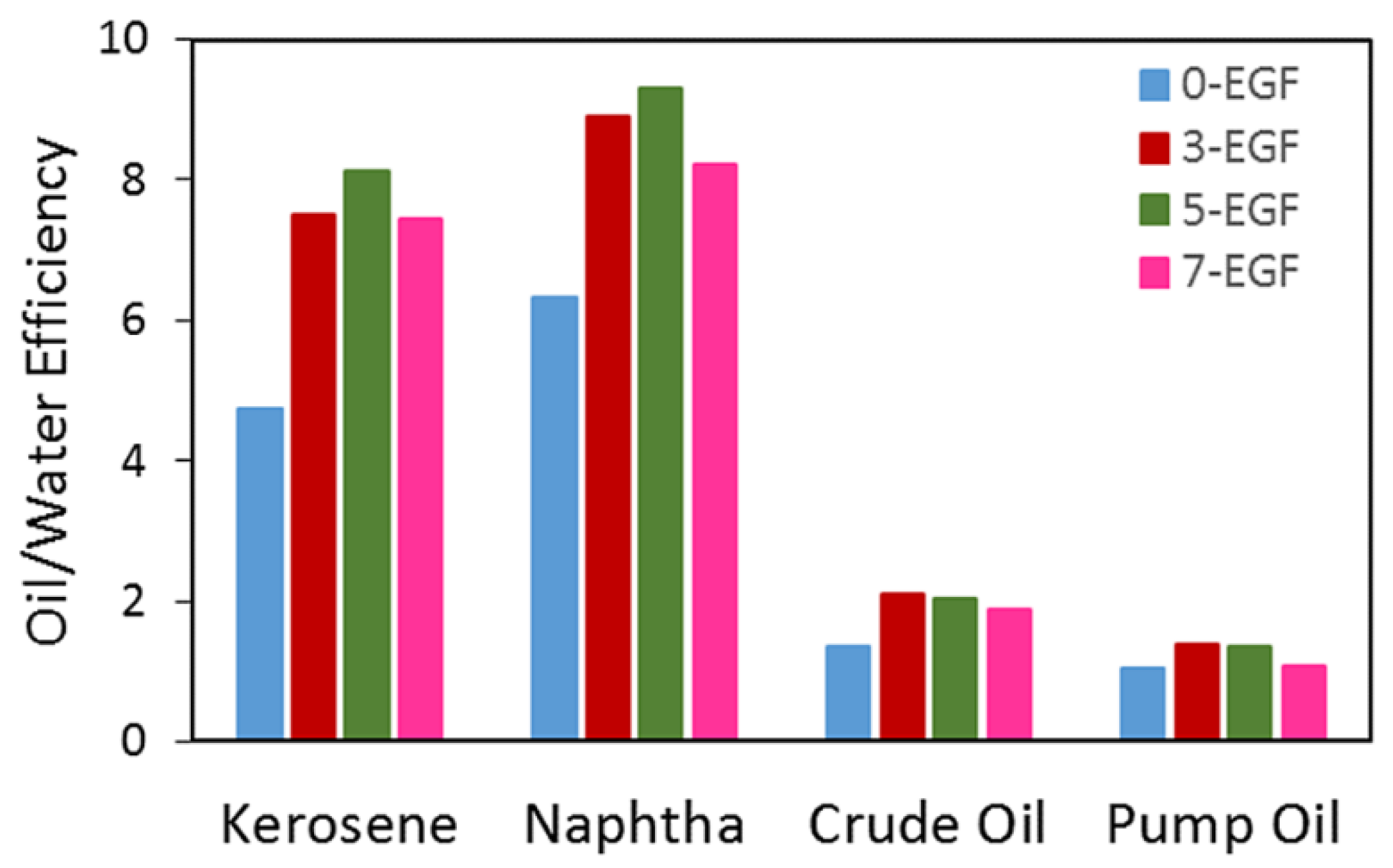
- The 3-EGF foam exhibited the highest sorption uptake, in particular in light oils (854 and 1016 wt.% in kerosene and virgin naphtha, respectively). This result is attributed to its larger pore size (1.25 mm vs. 0.59 mm for 3-EGF and 7-EGF, respectively), and consequently its lower density.
- The small pore diameter of 7-EGF did not allow the polluting oil to fill the entire available foam volume.
- In heavy oils the recovery performance was maintained below 250 wt.% (240 and 157 wt.% in crude oil and pump oil, respectively). In fact, the high viscosity of the heavy oils hindered the oil entry into the pores.
- Moreover, the silicone composite hydrophobicity (114 wt.%) allowed for sufficient oil selectivity and oil/water efficiency reaching values above seven for kerosene and virgin naphtha.
Author Contributions
Funding
Conflicts of Interest
References
- Eurostat. Energy Production and Imports. 2020. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php/Energy_production_and_imports (accessed on 17 October 2020).
- Italian Government. Quadro Strategico Nazionale Sezione C: Fornitura Di Gas Naturale Per Il Trasporto E Per Altri Usi. 2020. Available online: file:///C:/Users/MDPI/AppData/Local/Temp/ALLEGATO%20III%20-%20SEZ%20C1%20-1.pdf (accessed on 17 October 2020).
- Nel Mar Mediterraneo record di inquinamento da idrocarburi. Available online: https://www.adnkronos.com/sostenibilita/risorse/2014/07/23/nel-mar-mediterraneo-record-inquinamento-idrocarburi_0J3mSPyBIgkZ7MEqjRg5oJ.html?refresh_ce (accessed on 17 October 2020).
- European Commission. I Porti Marittimi Europei Nel 2030: Le Sfide Che Ci Attendono Importanza Dei Porti. 2013. Available online: https://ec.europa.eu/commission/presscorner/detail/it/MEMO_13_448 (accessed on 17 October 2020).
- Kabiri, S.; Tran, D.N.H.; Altalhi, T.; Losic, D. Outstanding adsorption performance of graphene-carbon nanotube aerogels for continuous oil removal. Carbon 2014, 80, 523–533. [Google Scholar] [CrossRef]
- Parmar, K.R.; Dora, D.T.K.; Pant, K.K.; Roy, S. An ultra-light flexible aerogel-based on methane derived CNTs as a reinforcing agent in silica-CMC matrix for efficient oil adsorption. J. Hazard. Mater. 2019, 375, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Shang, Y.Y.; Sun, P.Z.; Li, Z.; Li, X.M.; Wei, J.Q.; Wang, K.L.; Wu, D.H.; Cao, A.Y.; Zhu, H.W. Oil spill cleanup from sea water by carbon nanotube sponges. Front. Mater. Sci. 2013, 7, 170–176. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Milone, C.; Proverbio, E. Carbon-based sponges for oil spill recovery. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 155–175. [Google Scholar]
- Adebajo, M.O.; Frost, R.L.; Kloprogge, J.T.; Carmody, O.; Kokot, S. Porous materials for oil spill cleanup: A review of synthesis and absorbing properties. J. Porous Mater. 2003, 10, 159–170. [Google Scholar] [CrossRef]
- Pinto, J.; Athanassiou, A.; Fragouli, D. Effect of the porous structure of polymer foams on the remediation of oil spills. J. Phys. D Appl. Phys. 2016, 49, 145601–145608. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Tai, N.-H.; Lee, S.-B.; Kuo, W.-S. Superhydrophobic and superoleophilic properties of graphene-based sponges fabricated using a facile dip coating method. Energy Environ. Sci. 2012, 5, 7908. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, J.; Wu, T.; Wang, X.; Huang, G.; Liu, Y.; Qiu, H.; Li, Y.; Wang, W.; Gao, J. Cost-effective reduced graphene oxide-coated polyurethane sponge as a highly efficient and reusable oil-absorbent. ACS Appl. Mater. Interfaces 2013, 5, 10018–10026. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Li, H.; Wang, K.; Wei, J.; Jia, Y.; Li, Z.; Fan, L.; Cao, A.; Zhu, H.; Wu, D. Recyclable carbon nanotube sponges for oil absorption. Acta Mater. 2011, 59, 4798–4804. [Google Scholar] [CrossRef]
- Gui, X.; Zeng, Z.; Lin, Z.; Gan, Q.; Xiang, R.; Zhu, Y.; Cao, A.; Tang, Z. Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation. ACS Appl. Mater. Interfaces 2013, 5, 5845–5850. [Google Scholar] [CrossRef] [PubMed]
- Camilli, L.; Pisani, C.; Gautron, E.; Scarselli, M.; Castrucci, P.; D’Orazio, F.; Passacantando, M.; Moscone, D.; De Crescenzi, M. A three-dimensional carbon nanotube network for water treatment. Nanotechnology 2014, 25. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Huang, J.Q.; Zhang, Q.; Luo, W.L.; Wei, F. Improvement of oil adsorption performance by a sponge-like natural vermiculite-carbon nanotube hybrid. Appl. Clay Sci. 2011, 53, 1–7. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Proverbio, E.; Milone, C. Synthesis of reusable silicone foam containing carbon nanotubes for oil spill remediation. J. Appl. Polym. Sci. 2018, 135, 46067. [Google Scholar] [CrossRef]
- Ghasemi, O.; Mehrdadi, N.; Baghdadi, M.; Aminzadeh, B. An investigation on absorption properties of exfoliated graphite for oil spill from Caspian Sea water. Eurasian Chem. Commun. 2019, 1, 318–333. [Google Scholar] [CrossRef]
- Vasquez, L.; Campagnolo, L.; Athanassiou, A.; Fragouli, D. Expanded graphite-polyurethane foams for water–oil filtration. ACS Appl. Mater. Interfaces 2019, 11, 30207–30217. [Google Scholar] [CrossRef]
- Bentini, R.; Pola, A.; Rizzi, L.G.; Athanassiou, A.; Fragouli, D. A highly porous solvent free PVDF/expanded graphite foam for oil/water separation. Chem. Eng. J. 2019, 372, 1174–1182. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Freni, A.; Proverbio, E. Synthesis of SAPO-34 zeolite filled macrocellular foams for adsorption heat pump applications: A preliminary study. Appl. Therm. Eng. 2017. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Elovich, S.Y.; Larionov, O.G. Theory of adsorption from nonelectrolyte solutions on solid adsorbents - 2. Experimental verification of the equation for the adsorption isotherm from solutions. Bull. Acad. Sci. USSR Div. Chem. Sci. 1962, 11, 198–203. [Google Scholar] [CrossRef]
- Yaneva, Z.; Koumanova, B.; Georgieva, N. Study of the mechanism of nitrophenols sorption on expanded perlite ‒ equilibrium and kinetics modelling. Maced. J. Chem. Chem. Eng. 2012, 31, 101–114. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Abdul Rahim, S.H.; Proverbio, E.; Milone, C. Assessment of sorption kinetics of carbon nanotube-based composite foams for oil recovery application. J. Appl. Polym. Sci. 2019, 136. [Google Scholar] [CrossRef]
- Verdejo, R.; Barroso-Bujans, F.; Rodriguez-Perez, M.A.; Antonio de Saja, J.; Arroyo, M.; Lopez-Manchado, M.A. Carbon nanotubes provide self-extinguishing grade to silicone-based foams. J. Mater. Chem. 2008, 18, 3933–3939. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Freni, A.; Proverbio, E. Morphological and functional aspects of zeolite filled siloxane composite foams. J. Appl. Polym. Sci. 2018, 135, 45683. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Assessment of the hydration/dehydration behaviour of MgSO4∙7H2O filled cellular foams for sorption storage applications through morphological and thermo-gravimetric analyses. Sustain. Mater. Technol. 2018, 17. [Google Scholar] [CrossRef]
- Gulbin, Y. On estimation and hypothesis testing of the grain size distribution by the Saltykov method. Image Anal. Stereol. 2008, 27, 163–174. [Google Scholar] [CrossRef]
- Underwood, E.E. Stereology, or the quantitative evaluation of microstructures. J. Microsc. 1969, 89, 161–180. [Google Scholar] [CrossRef]
- Duong, H.T.T.; Burford, R.P. Effect of foam density, oil viscosity, and temperature on oil sorption behavior of polyurethane. J. Appl. Polym. Sci. 2006, 99, 360–367. [Google Scholar] [CrossRef]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon nanotube sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef]
- Yongmei, Z. Bio-Inspired Wettability Surfaces: Developments in Micro- and Nanostructures; Pan Stanford Publishing: Singapore, 2015; ISBN 9789814463607. [Google Scholar]
- Zhao, X.; Li, L.; Li, B.; Zhang, J.; Wang, A. Durable superhydrophobic/superoleophilic PDMS sponges and their applications in selective oil absorption and in plugging oil leakages. J. Mater. Chem. A 2014, 2, 18281–18287. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Khaskhoussi, A.; Proverbio, E.; Milone, C. Thermo-physical characterization of carbon nanotube composite foam for oil recovery applications. Nanomaterials 2020, 10, 86. [Google Scholar] [CrossRef]
- Sidik, S.M.; Jalil, A.A.; Triwahyono, S.; Adam, S.H.; Satar, M.A.H.; Hameed, B.H. Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal. Chem. Eng. J. 2012, 203, 9–18. [Google Scholar] [CrossRef]
- Sokker, H.H.; El-Sawy, N.M.; Hassan, M.A.; El-Anadouli, B.E. Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization. J. Hazard. Mater. 2011, 190, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Younis, S.A.; Moustafa, Y.M. Synthesis of urea-modified MnFe2O4 for aromatic micro-pollutants adsorption from wastewater: Mechanism and modeling. Clean Technol. Environ. Policy 2017, 19, 527–540. [Google Scholar] [CrossRef]
- Cherukupally, P.; Hinestroza, J.H.; Farnood, R.; Bilton, A.M.; Park, C.B. Adsorption mechanisms of emulsified crude oil droplets onto hydrophilic open-cell polymer foams. In Proceedings of the 32nd International Conference of the Polymer Processing Society, Lyon, France, 25–29 July 2016; Volume 1914, p. 170003. [Google Scholar] [CrossRef]
- Cheu, S.C.; Kong, H.; Song, S.T.; Johari, K.; Saman, N.; Che Yunus, M.A.; Mat, H. Separation of dissolved oil from aqueous solution by sorption onto acetylated lignocellulosic biomass—equilibrium, kinetics and mechanism studies. J. Environ. Chem. Eng. 2016, 4, 864–881. [Google Scholar] [CrossRef]
- Piperopoulos, E.; Calabrese, L.; Mastronardo, E.; Proverbio, E.; Milone, C. Sustainable reuse of char waste for oil spill recovery foams. Water Air Soil Pollut. 2020, 231, 293. [Google Scholar] [CrossRef]

| Code | Siloxane | Solvent | Catalyst | Filler | ||
|---|---|---|---|---|---|---|
| PDMS (wt.%) | PMHS (wt.%) | Ethanol (wt.%) | Water (wt.%) | Sn(II)-EH (wt.%) | EG (wt.%) | |
| 0-EGF | 47.6 | 23.8 | 4.8 | 11.9 | 11.9 | 0 |
| 3-EGF | 45.0 | 22.5 | 4.5 | 11.2 | 11.2 | 3.5 |
| 5-EGF | 45.0 | 22.5 | 4.5 | 11.2 | 11.2 | 5.5 |
| 7-EGF | 45.0 | 22.5 | 4.5 | 11.2 | 11.2 | 7.0 |
| - | Pump Oil | Crude Oil | Kerosene | Virgin Naphtha | Water |
|---|---|---|---|---|---|
| ρ(kg/m3) | 858 | 890 | 780 | 630 | 1000 |
| µ(Pa · s) | 0.1231 | 0.2710 | 0.0019 | 0.0012 | 0.0010 |
| - | Sorption Rate | Sorption Capacity | Constants |
|---|---|---|---|
| Pseudo-first Order | K1 (s−1) | ||
| Pseudo-second Order | K2 (g·mg−1·s−1) | ||
| Elovich | α (mg·g−1·s−1) β (mg·g−1) |
| Sample | PS (mm) | ρa (kg/m3) |
|---|---|---|
| 0-EGF | 1.75 | 280.3 |
| 3-EGF | 1.25 | 309.4 |
| 5-EGF | 1.20 | 339.6 |
| 7-EGF | 0.59 | 531.4 |
| Adsorption Liquid | 0-EGF (wt.%) | 3-EGF (wt.%) | 5-EGF (wt.%) | 7-EGF (wt.%) |
|---|---|---|---|---|
| Water | 115 ± 3 | 114 ± 2 | 98 ± 3.0 | 94 ± 5.0 |
| Kerosene | 544 ± 4 | 854 ± 4 | 796 ± 5 | 700 ± 12.5 |
| Virgin Naphtha | 726 ± 4 | 1016 ± 5 | 911 ± 4 | 772 ± 13.2 |
| Crude Oil | 155 ± 3 | 240 ± 2 | 200 ± 2 | 177 ± 9.0 |
| Pump Oil | 119 ± 3 | 157 ± 3 | 131 ± 2 | 101 ± 4.3 |
| Adsorption Liquid | Pseudo-First Order | Pseudo-Second Order | Elovich | |||
|---|---|---|---|---|---|---|
| Water | qe | 1073.99 | qe | 1160.66 | Omega | 0.00278 |
| K1 | 0.0060 | K2 | 9.974 × 10−6 | alpha | 18.538 | |
| h0,1 | 6.465 | h0,2 | 13.437 | Re | 0.336 | |
| R2 | 0.939 | R2 | 0.998 | R2 | 0.969 | |
| Kerosene | qe | 7804.433 | qe | 5428.224 | Omega | 0.00085 |
| K1 | 0.0189 | K2 | 8.149 × 10−6 | alpha | 354.882 | |
| h0,1 | 147.390 | h0,2 | 240.123 | Re | 0.217 | |
| R2 | 0.705 | R2 | 0.999 | R2 | 0.9511 | |
| Virgin Naphtha | qe | 6983.01 | qe | 7327.496 | Omega | 0.000488 |
| K1 | 0.01470 | K2 | 2.242 × 10−5 | alpha | 308.691 | |
| h0,1 | 102.649 | h0,2 | 1204.247 | Re | 0.267 | |
| R2 | 0.824 | R2 | 0.998 | R2 | 0.9294 | |
| Crude oil | qe | 4058.10872 | qe | 1606.2186 | Omega | 0.002630 |
| K1 | 0.0340 | K2 | 0.00033 | alpha | 197.043 | |
| h0,1 | 138.343 | h0,2 | 849.520 | Re | 0.206 | |
| R2 | 0.728 | R2 | 0.999 | R2 | 0.759 | |
| Pump Oil | qe | 773.096 | qe | 1199.678 | Omega | 0.00590 |
| K1 | 0.0125 | K2 | 5.071 × 10−5 | alpha | 613.967 | |
| h0,1 | 9.654 | h0,2 | 72.985 | Re | 0.143 | |
| R2 | 0.745 | R2 | 0.999 | R2 | 0.769 | |
| Adsorption Liquid | Pseudo-First Order | Pseudo-Second Order | Elovich | |||
|---|---|---|---|---|---|---|
| Water | qe | 84.56 | qe | 120.25 | Omega | 0.0343 |
| K1 | 0.0040 | K2 | 8.735 × 10−5 | alpha | 2.4657 | |
| h0,1 | 0.3395 | h0,2 | 1.2633 | Re | 0.3026 | |
| R2 | 0.821 | R2 | 0.999 | R2 | 0.934 | |
| Kerosene | qe | 580.04 | qe | 748.31 | Omega | 0.0058 |
| K1 | 0.0090 | K2 | 0.00028 | alpha | 63.479 | |
| h0,1 | 5.2477 | h0,2 | 157.282 | Re | 0.201 | |
| R2 | 0.886 | R2 | 0.999 | R2 | 0.820 | |
| Virgin Naphtha | qe | 276.66 | qe | 1013.41 | Omega | 0.0086 |
| K1 | 0.0060 | K2 | 2.841 × 10−5 | alpha | 1707.177 | |
| h0,1 | 1.6771 | h0,2 | 29.181 | Re | 0.1187 | |
| R2 | 0.359 | R2 | 0.999 | R2 | 0.4683 | |
| Crude oil | qe | 145.99 | qe | 238.87 | Omega | 0.0173 |
| K1 | 0.0116 | K2 | 0.000106 | alpha | 19.413 | |
| h0,1 | 1.700 | h0,2 | 6.0310 | Re | 0.2693 | |
| R2 | 0.764 | R2 | 0.998 | R2 | 0.7896 | |
| Pump Oil | qe | 42.05 | qe | 154.35 | Omega | 0.2174 |
| K1 | 0.0044 | K2 | 0.000157 | alpha | 3.10 × 1011 | |
| h0,1 | 0.1855 | h0,2 | 3.7482 | Re | 0.0320 | |
| R2 | 0.361 | R2 | 0.999 | R2 | 0.229 | |
| Adsorption Liquid | Pseudo-First Order | Pseudo-Second Order | Elovich | |||
|---|---|---|---|---|---|---|
| Water | qe | 98.857 | qe | 102.24 | Omega | 0.0384 |
| K1 | 0.007 | K2 | 0.00053 | alpha | 3.3306 | |
| h0,1 | 0.653 | h0,2 | 5.523 | Re | 0.2605 | |
| R2 | 0.954 | R2 | 0.996 | R2 | 0.968 | |
| Kerosene | qe | 562.537 | qe | 772.5942 | Omega | 0.0063 |
| K1 | 0.006 | K2 | 6.543 × 10−5 | alpha | 46.461 | |
| h0,1 | 3.633 | h0,2 | 39.0581 | Re | 0.210 | |
| R2 | 0.960 | R2 | 0.999 | R2 | 0.990 | |
| Virgin Naphtha | qe | 540.575 | qe | 897.0989 | Omega | 0.0058 |
| K1 | 0.0093 | K2 | 0.000135 | alpha | 98.1594 | |
| h0,1 | 5.0479 | h0,2 | 108.7349 | Re | 0.1951 | |
| R2 | 0.874 | R2 | 0.999 | R2 | 0.916 | |
| Crude oil | qe | 129.403 | qe | 200.420 | Omega | 0.0306 |
| K1 | 0.0048 | K2 | 6.410 × 10−5 | alpha | 17.525 | |
| h0,1 | 0.627 | h0,2 | 2.575 | Re | 0.183 | |
| R2 | 0.782 | R2 | 0.997 | R2 | 0.917 | |
| Pump Oil | qe | 47.843 | qe | 126.1734 | Omega | 0.0941 |
| K1 | 0.008 | K2 | 0.0015 | alpha | 4863.059 | |
| h0,1 | 0.3832 | h0,2 | 24.5413 | Re | 0.0834 | |
| R2 | 0.818 | R2 | 0.999 | R2 | 0.949 | |
| Adsorption Liquid | Pseudo-First Order | Pseudo-Second Order | Elovich | |||
|---|---|---|---|---|---|---|
| Water | qe | 81.926 | qe | 96.9305 | Omega | 0.0533 |
| K1 | 0.0035 | K2 | 6.504 × 10−5 | alpha | 1.567 | |
| h0,1 | 0.2845 | h0,2 | 0.6111 | Re | 0.2554 | |
| R2 | 0.899 | R2 | 0.995 | R2 | 0.934 | |
| Kerosene | qe | 657.46 | qe | 701.9896 | Omega | 0.0053 |
| K1 | 0.0120 | K2 | 5.584 × 10−5 | alpha | 25.206 | |
| h0,1 | 7.9172 | h0,2 | 27.5199 | Re | 0.2720 | |
| R2 | 0.957 | R2 | 0.999 | R2 | 0.9638 | |
| Virgin Naphtha | qe | 258.6308 | qe | 749.1983 | Omega | 0.017805 |
| K1 | 0.0071 | K2 | 0.00012 | alpha | 81789.43 | |
| h0,1 | 1.8506 | h0,2 | 71.8545 | Re | 0.0761 | |
| R2 | 0.6692 | R2 | 0.999 | R2 | 0.7495 | |
| Crude oil | qe | 75.0781 | qe | 173.5510 | Omega | 0.0508 |
| K1 | 0.0083 | K2 | 0.000525 | alpha | 353.114 | |
| h0,1 | 0.6212 | h0,2 | 15.81438 | Re | 0.1158 | |
| R2 | 0.732 | R2 | 0.999 | R2 | 0.7697 | |
| Pump Oil | qe | 32.6895 | qe | 99.2060 | Omega | 0.174785 |
| K1 | 0.0066 | K2 | 0.00065 | alpha | 258926.2 | |
| h0,1 | 0.2148 | h0,2 | 6.4201 | Re | 0.0601 | |
| R2 | 0.528 | R2 | 0.999 | R2 | 0.6215 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brancato, V.; Piperopoulos, E.; Mastronardo, E.; Calabrese, L.; Milone, C.; Proverbio, E. Synthesis and Characterization of Graphite Composite Foams for Oil Spill Recovery Application. J. Compos. Sci. 2020, 4, 154. https://doi.org/10.3390/jcs4040154
Brancato V, Piperopoulos E, Mastronardo E, Calabrese L, Milone C, Proverbio E. Synthesis and Characterization of Graphite Composite Foams for Oil Spill Recovery Application. Journal of Composites Science. 2020; 4(4):154. https://doi.org/10.3390/jcs4040154
Chicago/Turabian StyleBrancato, Vincenza, Elpida Piperopoulos, Emanuela Mastronardo, Luigi Calabrese, Candida Milone, and Edoardo Proverbio. 2020. "Synthesis and Characterization of Graphite Composite Foams for Oil Spill Recovery Application" Journal of Composites Science 4, no. 4: 154. https://doi.org/10.3390/jcs4040154
APA StyleBrancato, V., Piperopoulos, E., Mastronardo, E., Calabrese, L., Milone, C., & Proverbio, E. (2020). Synthesis and Characterization of Graphite Composite Foams for Oil Spill Recovery Application. Journal of Composites Science, 4(4), 154. https://doi.org/10.3390/jcs4040154










