Effect of MoSi2-Si3N4/SiC Multi-Layer Coating on the Oxidation Resistance of Carbon/Carbon Composites above 1770 K
Abstract
1. Introduction
2. Experimental Procedure
2.1. Coating Preparation
2.2. Oxidation Test
2.3. Characterization of the Coatings
3. Results and Discussion
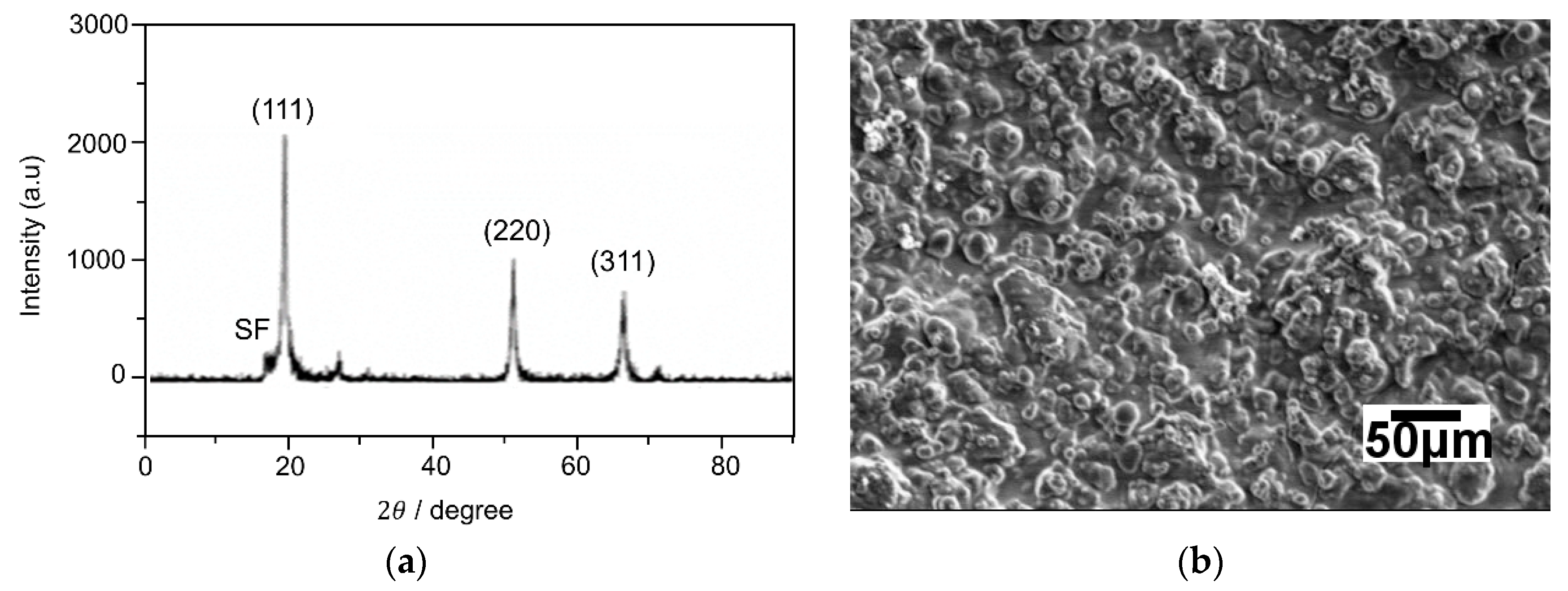
3.1. Microstructure of Coating before the Oxidation Test
- An increase in the interfacial bonding strength of the coating [25];
- A reduction in the thermal mismatch of the expansion between the coatings and the C/C substrate.
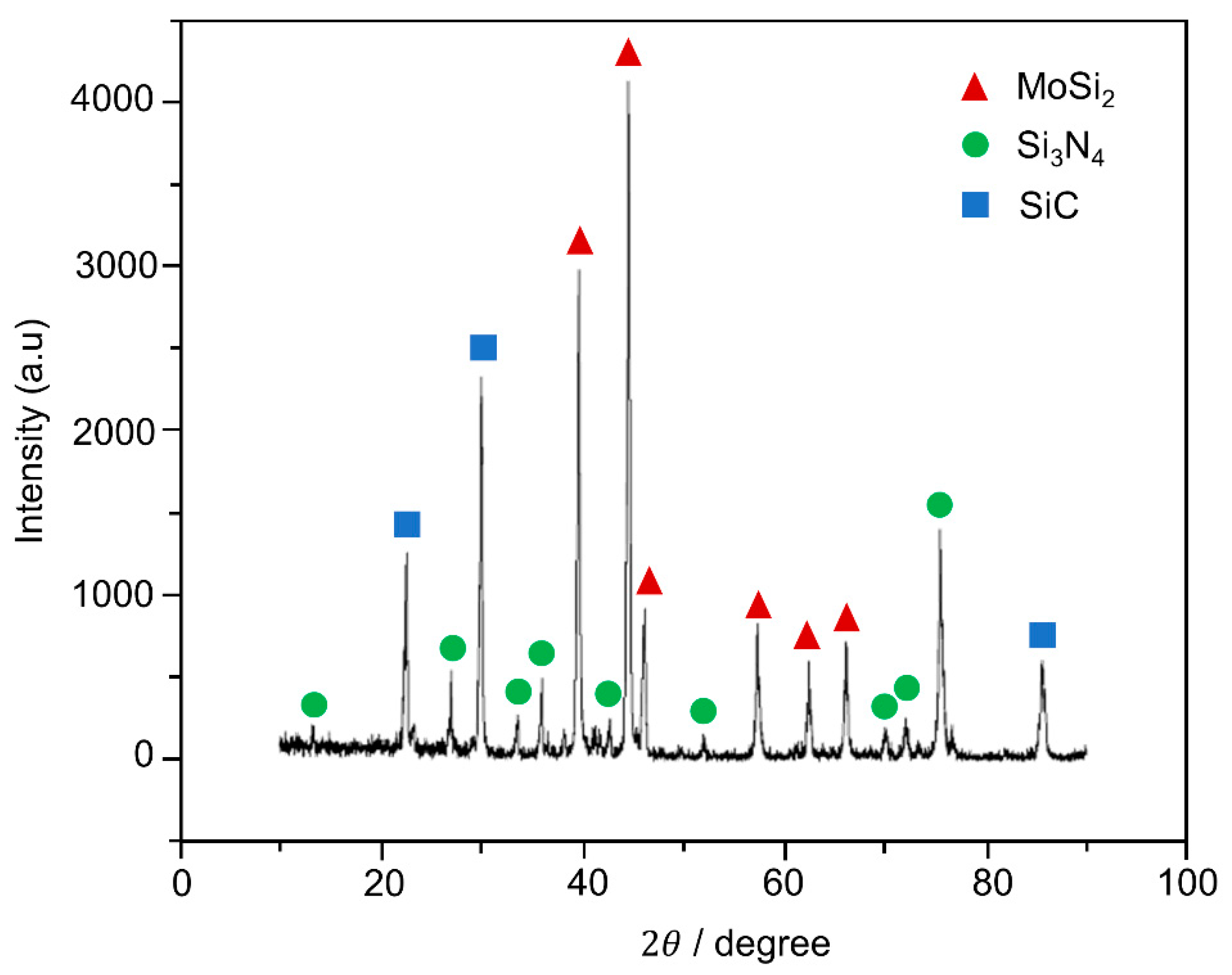
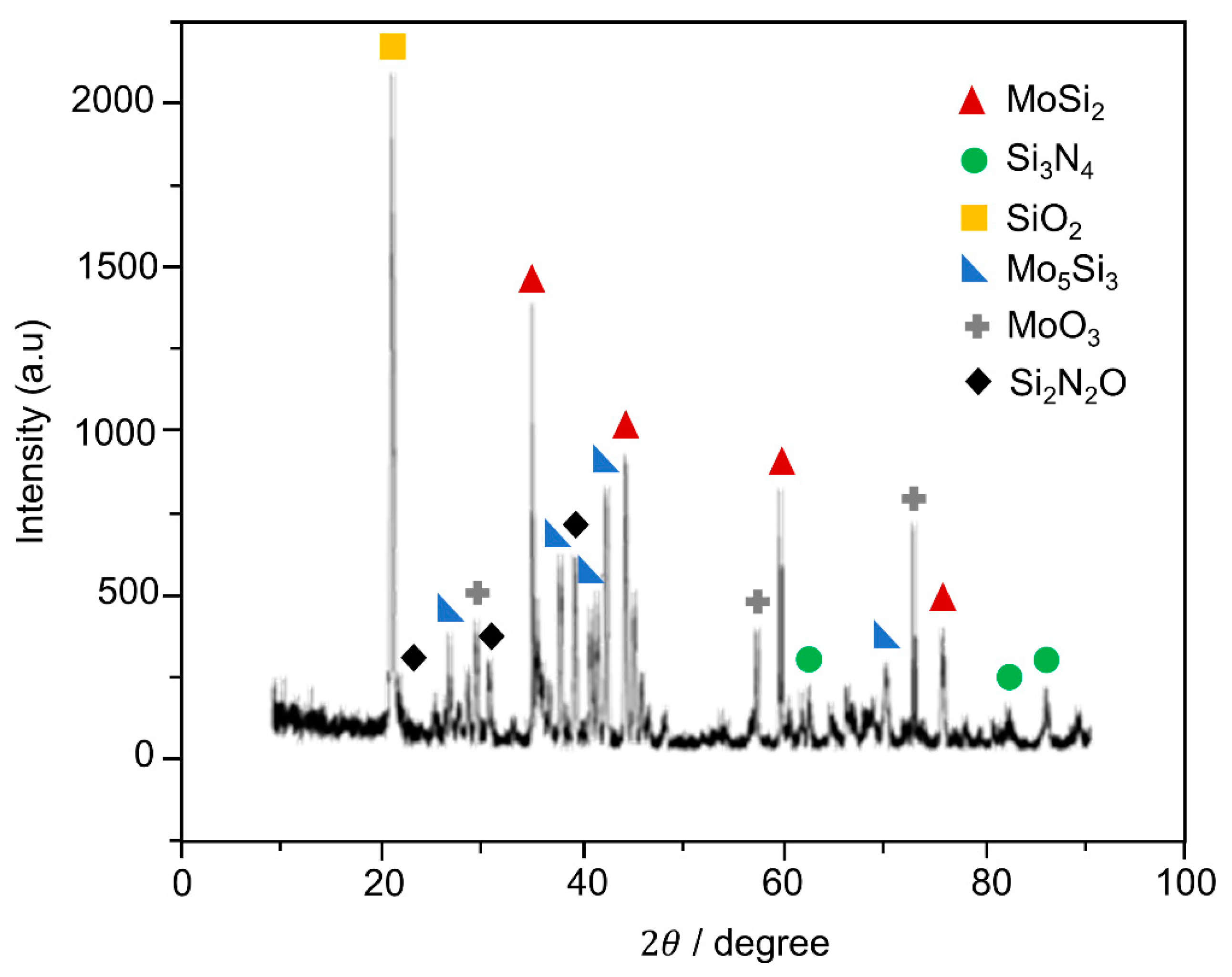
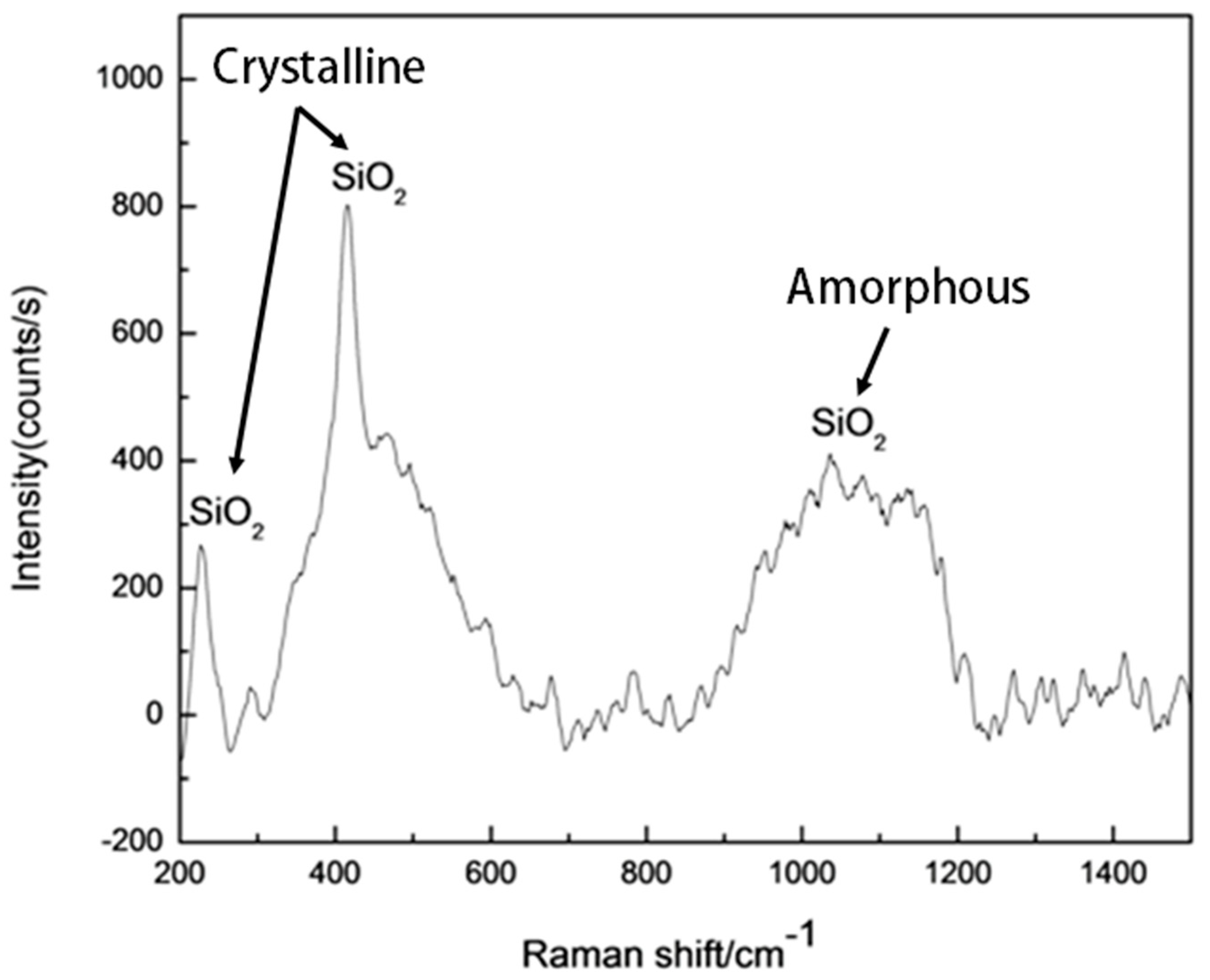
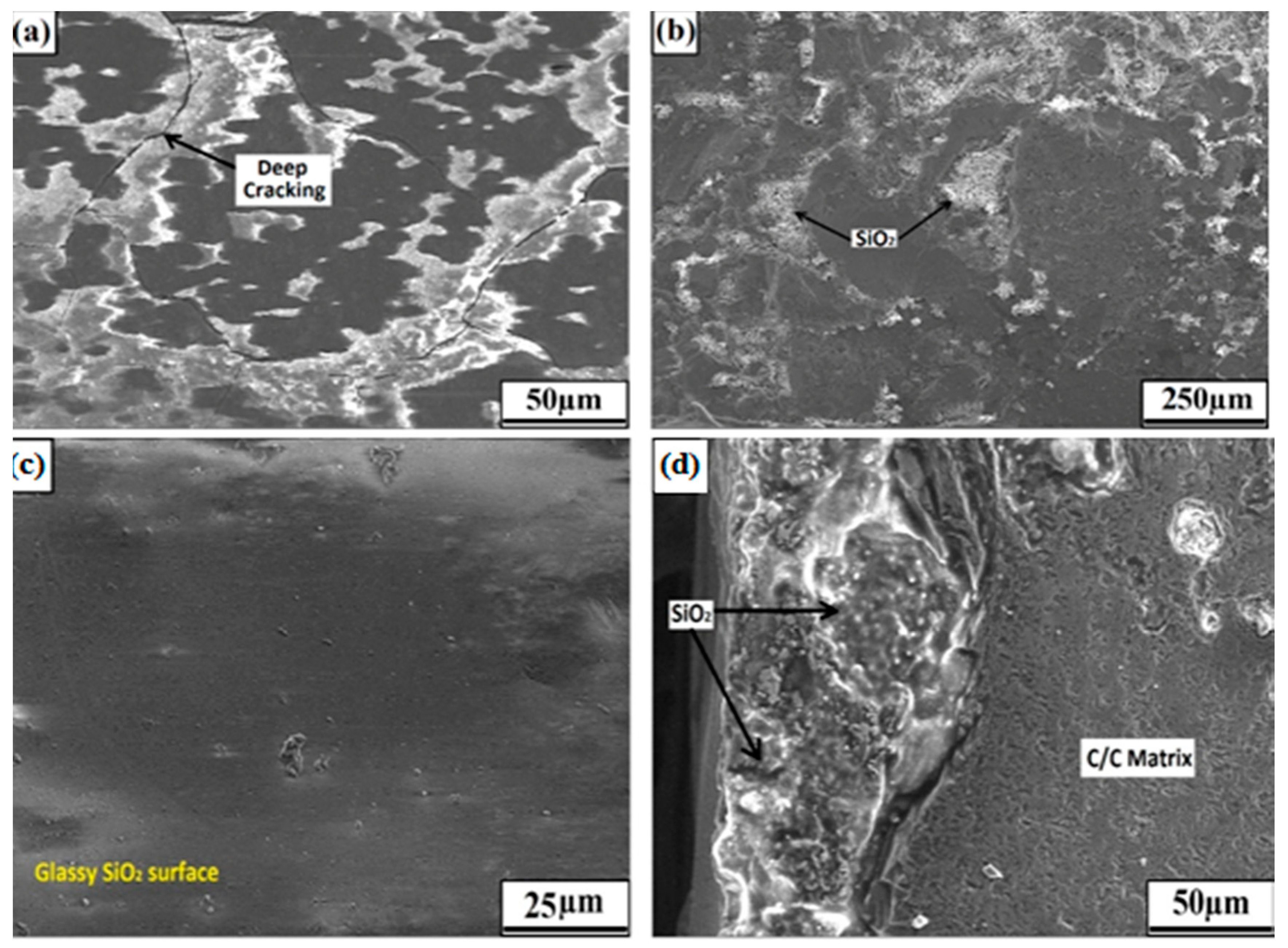
3.2. Microstructure of Coating Post-Oxidation Test: Oxidation Resistance Evaluation
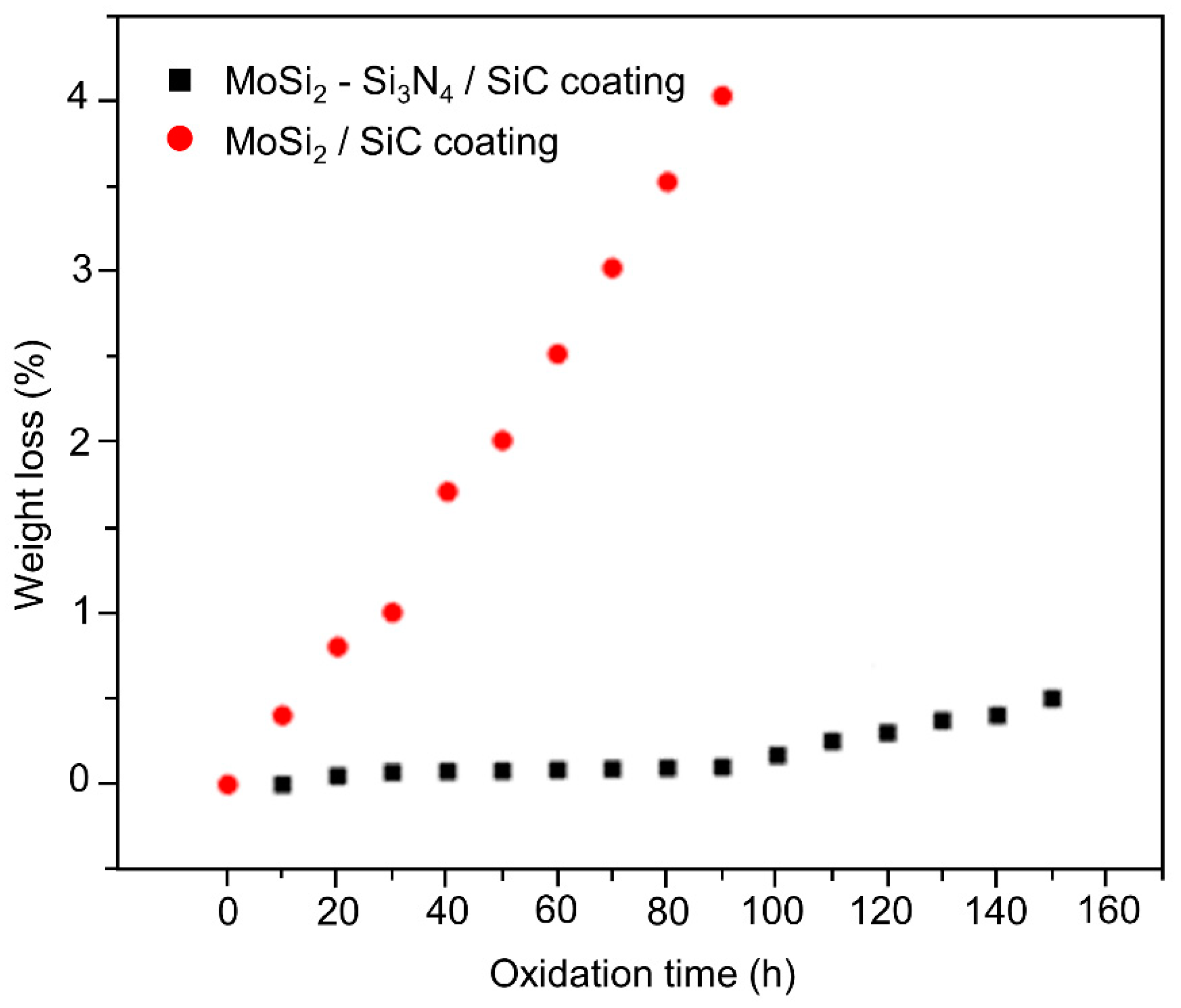
3.3. Thermal Cyclic Oxidation Resistance of the Coating at 1773 K
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krenkel, W.; Berndt, F. C/C–SiC composites for space applications and advanced friction systems. Mater. Sci. Eng. A 2005, 412, 177–181. [Google Scholar] [CrossRef]
- Katoh, Y.; Snead, L.L.; Henager, C.H., Jr.; Hasegawa, A.; Kohyama, A.; Riccardi, B.; Hegeman, H. Current status and critical issues for development of SiC composites for fusion applications. J. Nucl. Mater. 2007, 367, 659–671. [Google Scholar] [CrossRef]
- Kim, B.G.; Dong, S.; Park, S.D. Effects of thermal processing on thermal expansion coefficient of a 50 vol.% SiCp/Al composite. Mater. Chem. Phys. 2001, 72, 42–47. [Google Scholar] [CrossRef]
- Van der Berg, N.; Malherbe, J.B.; Botha, A.; Friedland, E. Thermal etching of SiC. Appl. Surf. Sci. 2012, 258, 5561–5566. [Google Scholar] [CrossRef]
- Gasch, M.; Ellerby, D.; Irby, E.; Beckman, S.; Gusman, M.; Johnson, S. Processing, properties and arc jet oxidation of hafnium diboride/silicon carbide ultra high temperature ceramics. J. Mater. Sci. 2004, 39, 5925–5937. [Google Scholar] [CrossRef]
- Kim, Y.-W.; Chun, Y.-S.; Nishimura, T.; Mitomo, M.; Lee, Y.-H. High-temperature strength of silicon carbide ceramics sintered with rare-earth oxide and aluminum nitride. Acta Mater. 2007, 55, 727–736. [Google Scholar] [CrossRef]
- Fu, Q.-G.; Li, H.-J.; Shi, X.-H.; Li, K.-Z.; Sun, G.-D. Silicon carbide coating to protect carbon/carbon composites against oxidation. Scr. Mater. 2005, 52, 923–927. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Qiang, X.; Li, K. Oxidation protective C/SiC/Si-SiC multilayer coating for carbon/carbon composites applying at 1873 K. J. Mater. Sci. Technol. 2010, 26, 1139–1142. [Google Scholar] [CrossRef]
- Chu, Y.; Li, H.; Fu, Q.; Wang, H.; Hou, X.; Zou, X.; Shang, G. Oxidation protection of C/C composites with a multilayer coating of SiC and Si+ SiC+ SiC nanowires. Carbon 2012, 50, 1280–1288. [Google Scholar] [CrossRef]
- Tian, X.; Guo, X.; Sun, Z.; Qu, J.; Wang, L. Oxidation resistance comparison of MoSi2 and B-modified MoSi2 coatings on pure Mo prepared through pack cementation. Mater. Corros. 2015, 66, 681–687. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, H.-J.; Qiang, X.-F.; Li, K.-Z.; Zhang, S.-Y. C/SiC/MoSi2–Si multilayer coatings for carbon/carbon composites for protection against oxidation. Corros. Sci. 2011, 53, 3840–3844. [Google Scholar] [CrossRef]
- Fu, Q.; Shan, Y.; Cao, C.; Li, H.; Li, K. Oxidation and erosion resistant property of SiC/Si–Mo–Cr/MoSi2 multi-layer coated C/C composites. Ceram. Int. 2015, 41, 4101–4107. [Google Scholar] [CrossRef]
- Feng, T.; Li, H.-J.; Fu, Q.-G.; Shen, X.-T.; Wu, H. Microstructure and oxidation of multi-layer MoSi2–CrSi2–Si coatings for SiC coated carbon/carbon composites. Corros. Sci. 2010, 52, 3011–3017. [Google Scholar] [CrossRef]
- Friedrich, C.; Gadow, R.; Speicher, M. Protective multilayer coatings for carbon–carbon composites. Surf. Coat. Technol. 2002, 151, 405–411. [Google Scholar] [CrossRef]
- Wu, H.; Li, H.-J.; Ma, C.; Fu, Q.-G.; Wang, Y.-J.; Wei, J.-f.; Tao, J. MoSi2-based oxidation protective coatings for SiC-coated carbon/carbon composites prepared by supersonic plasma spraying. J. Eur. Ceram. Soc. 2010, 30, 3267–3270. [Google Scholar] [CrossRef]
- Shi, X.; Wang, C.; Lin, H.; Huo, C.; Jin, X.; Shi, G.; Dong, K. Oxidation resistance of a La–Mo–Si–O–C coating prepared by supersonic atmosphere plasma spraying on the surface of SiC-coated C/C composites. Surf. Coat. Technol. 2016, 300, 10–18. [Google Scholar] [CrossRef]
- Li, H.-J.; Xue, H.; Wang, Y.-J.; Fu, Q.-G.; Yao, D.-J. A MoSi2–SiC–Si oxidation protective coating for carbon/carbon composites. Surf. Coat. Technol. 2007, 201, 9444–9447. [Google Scholar] [CrossRef]
- Fu, Q.; Zou, X.; Chu, Y.; Li, H.; Zou, J.; Gu, C. A multilayer MoSi2–SiC–B coating to protect SiC-coated carbon/carbon composites against oxidation. Vacuum 2012, 86, 1960–1963. [Google Scholar] [CrossRef]
- Feng, T.; Li, H.; Shi, X.; Yang, X.; Wang, S.; He, Z. Multi-layer CVD-SiC/MoSi2–CrSi2–Si/B-modified SiC oxidation protective coating for carbon/carbon composites. Vacuum 2013, 96, 52–58. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Zhuang, S.; Chen, Y.; Gu, S. Effect of WSi2 and Si3N4 contents on the thermal expansion behaviors of (Mo, W) Si2-Si3N4 composites. Ceram. Int. 2017, 43, 2847–2852. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Shen, Q.; Lin, H.; Feng, T.; Yao, X.; Fu, Q. Oxidation behavior and microstructure evolution of SiC-ZrB2-ZrC coating for C/C composites at 1673 K. Ceram. Int. 2016, 42, 13041–13046. [Google Scholar] [CrossRef]
- He, Z.; Li, H.; Shi, X.; Fu, Q.; Wu, H. Microstructure and oxidation resistance of SiC–MoSi2 multi-phase coating for SiC coated C/C composites. Prog. Nat. Sci. Mater. Int. 2014, 24, 247–252. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Zhang, H. Effect of Si3N4 content on microstructures and antioxidant properties of MoSi2/Si3N4 composite coatings on Mo substrate. Ceram. Int. 2015, 41, 13903–13907. [Google Scholar] [CrossRef]
- Hu, M.; Li, K.; Wang, J. Effect of Cr content on the microstructure and thermal properties of ZrSi2–CrSi2–SiC multiphase coating for the SiC coated C/C composites. Ceram. Int. 2016, 42, 19357–19364. [Google Scholar] [CrossRef]
- Srivastava, A.; Tripathi, P.; Nayak, M.; Lodha, G.; Nandedkar, R. Formation of Mo5Si3 phase in Mo/Si multilayers. J. Appl. Phys. 2002, 92, 5119–5126. [Google Scholar] [CrossRef]
- Fabrizi, A.; Cecchini, R.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Spigarelli, S.; Cabibbo, M. Comparative investigation of oxidation resistance and thermal stability of nano-structured Ti-B-N and Ti-Si-B-N coatings. J. Prot. Met. Phys. Chem. Surf. 2017, 53, 452–459. [Google Scholar] [CrossRef]
- Jianhui, Y.; Hongmei, X.U.; Houan, Z.; Siwen, T. MoSi2 oxidation resistance coatings for Mo5Si3/MoSi2 composites. Rare Met. 2009, 28, 418–422. [Google Scholar]
- Fu, Q.-G.; Jing, J.-Y.; Tan, B.-Y.; Yuan, R.-M.; Zhuang, L.; Li, L. Nanowire-toughened transition layer to improve the oxidation resistance of SiC–MoSi2–ZrB2 coating for C/C composites. Corros. Sci. 2016, 111, 259–266. [Google Scholar] [CrossRef]
- Huang, J.-F.; Wang, B.; Li, H.-J.; Liu, M.; Cao, L.-Y.; Yao, C.-Y. A MoSi2/SiC oxidation protective coating for carbon/carbon composites. Corros. Sci. 2011, 53, 834–839. [Google Scholar] [CrossRef]
- Iizuka, T.; Kita, H. Tribological behavior of Mo5Si3 particle reinforced Si3N4 matrix composites. Wear 2005, 258, 877–889. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Q.; Qu, J. Enhanced bonding strength and thermal cycling performance of MoSi2–CrSi2–SiC–Si coating for carbon/carbon composites by surface modification via blasting treatment. Ceram. Int. 2016, 42, 14021–14027. [Google Scholar] [CrossRef]
- Yoon, J.-K.; Lee, K.-H.; Kim, G.-H.; Han, J.-H.; Doh, J.-M.; Hong, K.-T. Low-temperature cyclic oxidation behavior of MoSi2/SiC nanocomposite coating formed on Mo substrate. Mater. Trans. 2004, 45, 2435–2442. [Google Scholar] [CrossRef]
- De la Pena, J.; Pech-Canul, M. Microstructure and kinetics of formation of Si2N2O and Si3N4 into Si porous preforms by chemical vapor infiltration (CVI). Ceram. Int. 2007, 33, 1349–1356. [Google Scholar] [CrossRef]
- ZhongLiu, W.; Peng, X.; Zhuan, L.; XiaoYu, Y.; Yang, L. Thermal cycling behavior and oxidation resistance of SiC whisker-toughened-mullite/SiC coated carbon/carbon composites in burner rig tests. Corros. Sci. 2016, 106, 179–187. [Google Scholar] [CrossRef]
- Lai, Z.; Meng, S.; Zhu, J.; Jeon, J. Microstructure of a Mo-Si-CN multi-layered anti-oxidation coating on carbon/carbon composites by fused slurry. Rare Met. 2009, 28, 460–464. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, I.; Wang, Y.; Elahi, H.; Siddiqui, M.A.; Ullah, M.; Qayyum, F. Effect of MoSi2-Si3N4/SiC Multi-Layer Coating on the Oxidation Resistance of Carbon/Carbon Composites above 1770 K. J. Compos. Sci. 2020, 4, 86. https://doi.org/10.3390/jcs4030086
Abbas I, Wang Y, Elahi H, Siddiqui MA, Ullah M, Qayyum F. Effect of MoSi2-Si3N4/SiC Multi-Layer Coating on the Oxidation Resistance of Carbon/Carbon Composites above 1770 K. Journal of Composites Science. 2020; 4(3):86. https://doi.org/10.3390/jcs4030086
Chicago/Turabian StyleAbbas, Imran, Yanxiang Wang, Hassan Elahi, Muhammad Ali Siddiqui, Mudaser Ullah, and Faisal Qayyum. 2020. "Effect of MoSi2-Si3N4/SiC Multi-Layer Coating on the Oxidation Resistance of Carbon/Carbon Composites above 1770 K" Journal of Composites Science 4, no. 3: 86. https://doi.org/10.3390/jcs4030086
APA StyleAbbas, I., Wang, Y., Elahi, H., Siddiqui, M. A., Ullah, M., & Qayyum, F. (2020). Effect of MoSi2-Si3N4/SiC Multi-Layer Coating on the Oxidation Resistance of Carbon/Carbon Composites above 1770 K. Journal of Composites Science, 4(3), 86. https://doi.org/10.3390/jcs4030086






