Photocatalytic Degradation of Organic Micropollutants in Water by Zr-MOF/GO Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Material Synthesis
2.3. Analytical Methods
2.4. Adsorption and Photodegradation Experiments
3. Results and Discussion
3.1. Material Characteristics
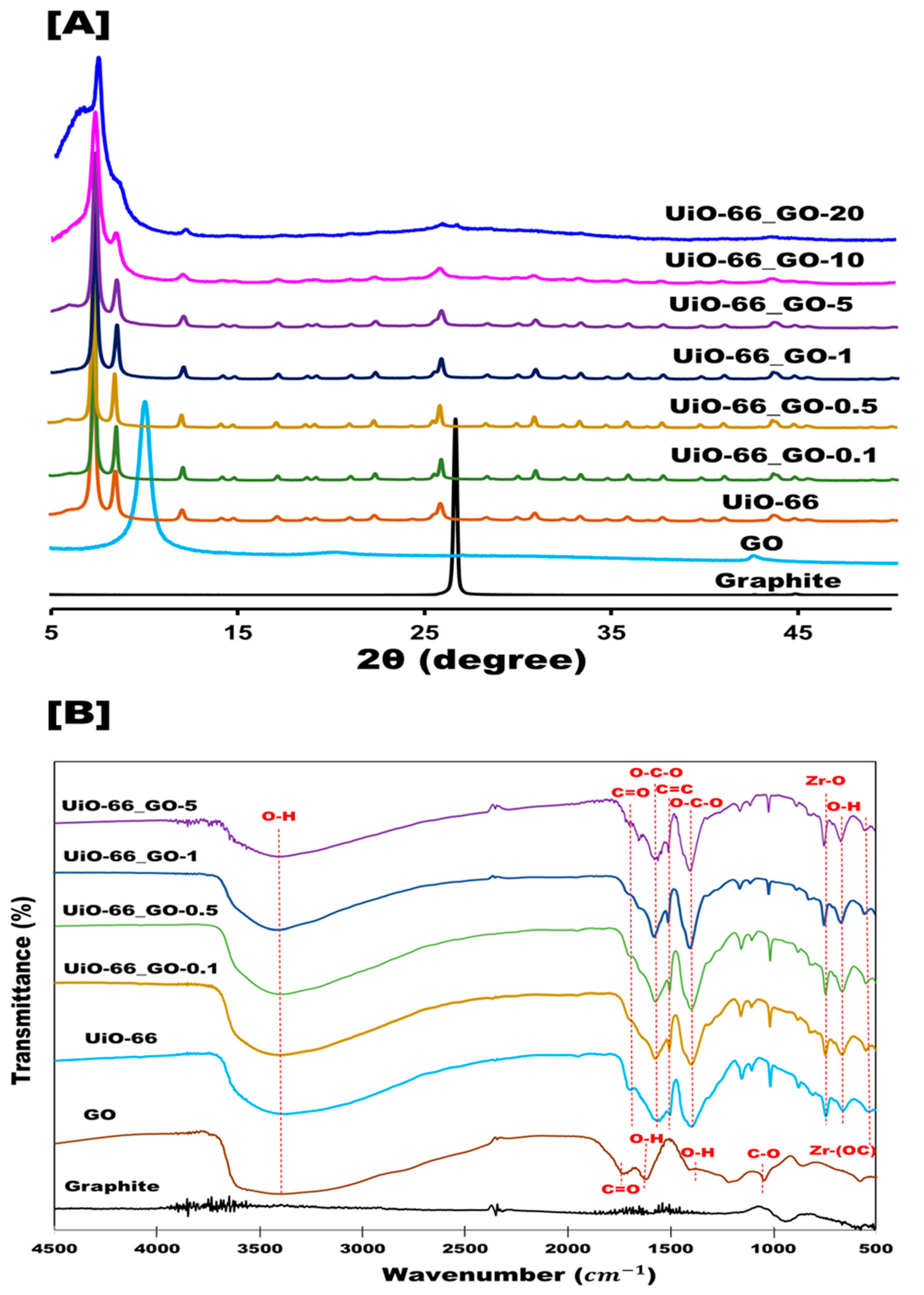
3.1.1. Crystalline Structure
3.1.2. Surface Functional Groups
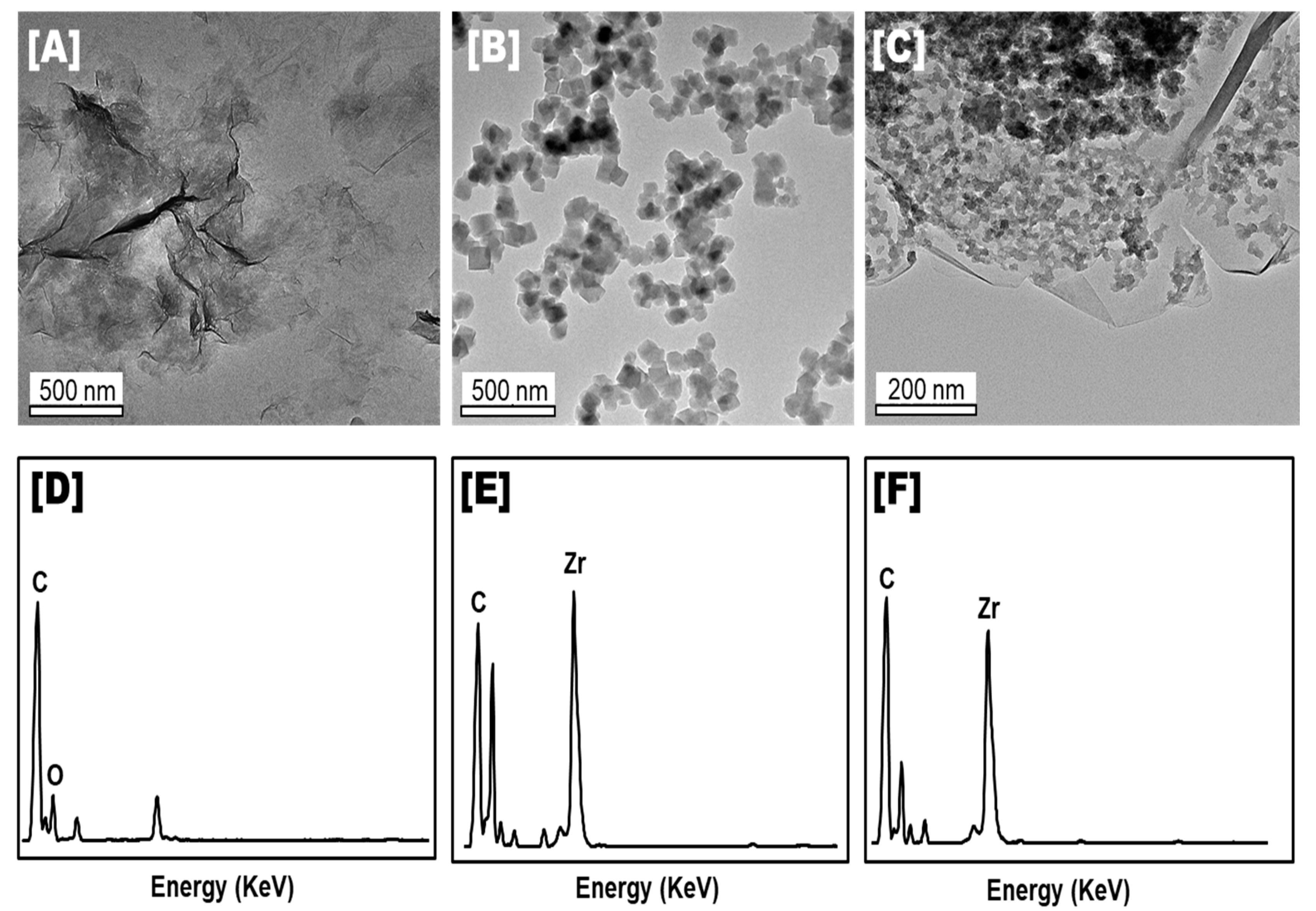
3.1.3. Morphology and Element Compositions
3.1.4. Specific Surface Area and Pore Volume
3.1.5. Light Absorption and Band Gap
3.2. Photocatalytic Performance
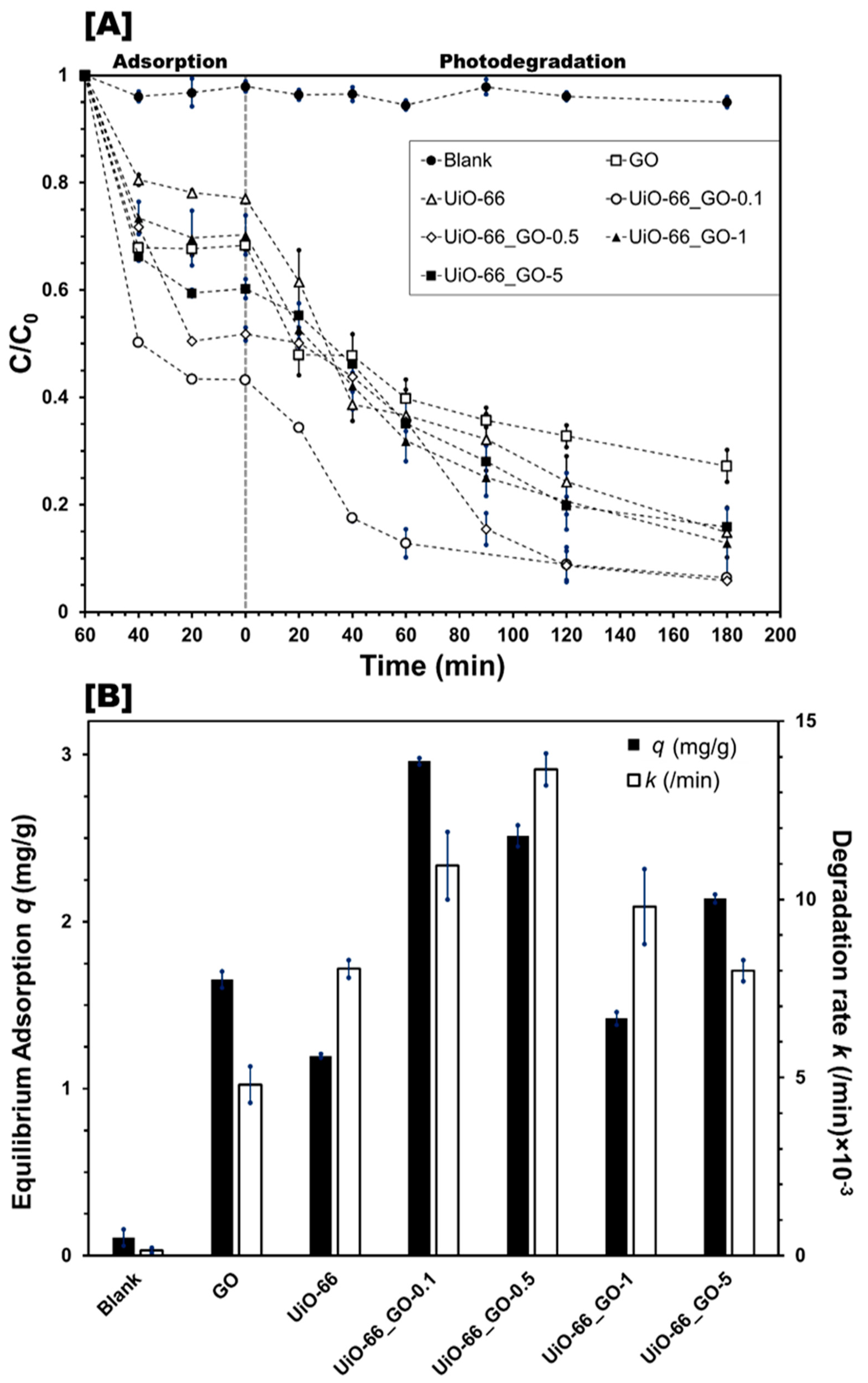
3.2.1. Effect of Photocatalyst Composite
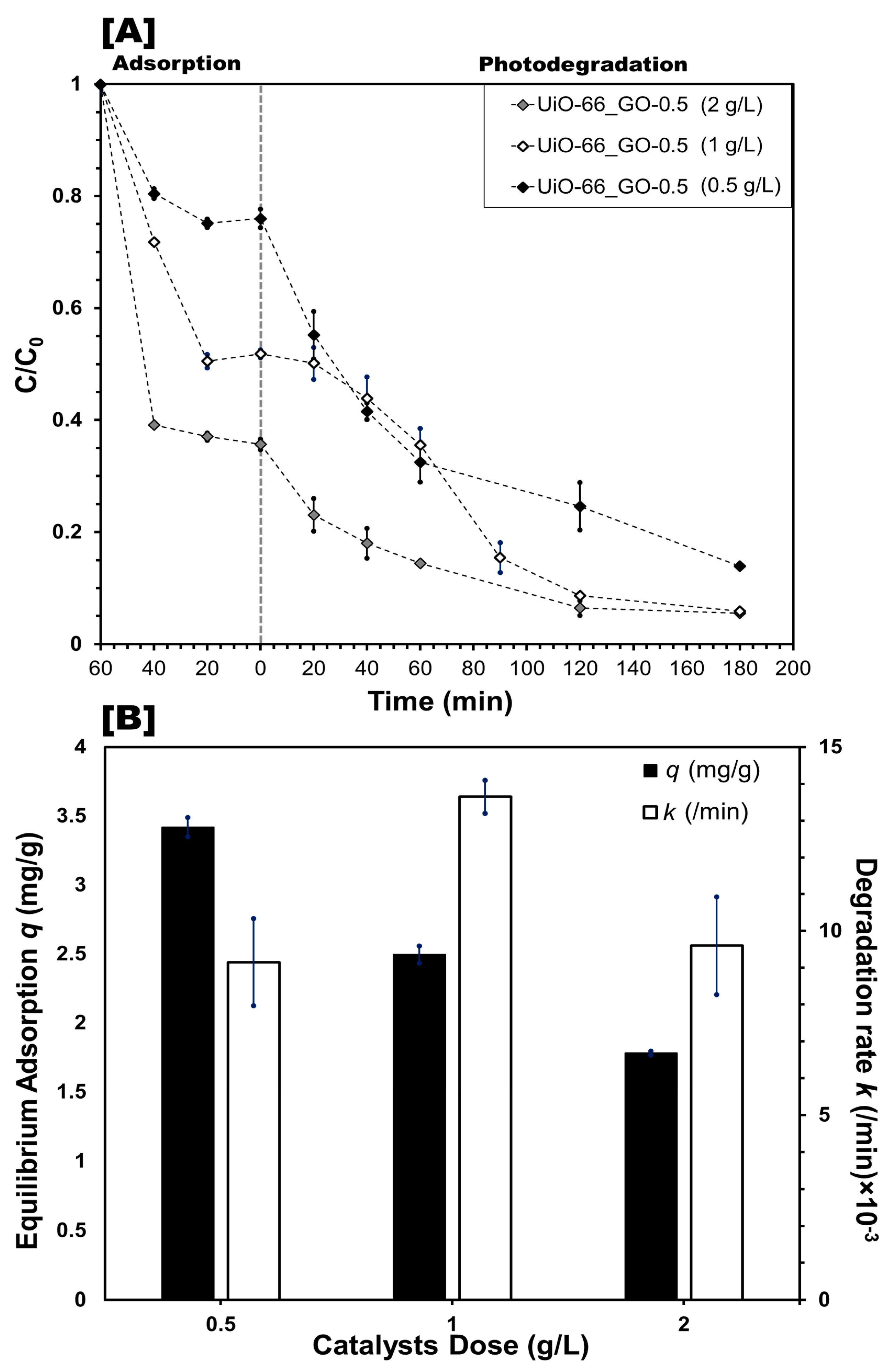
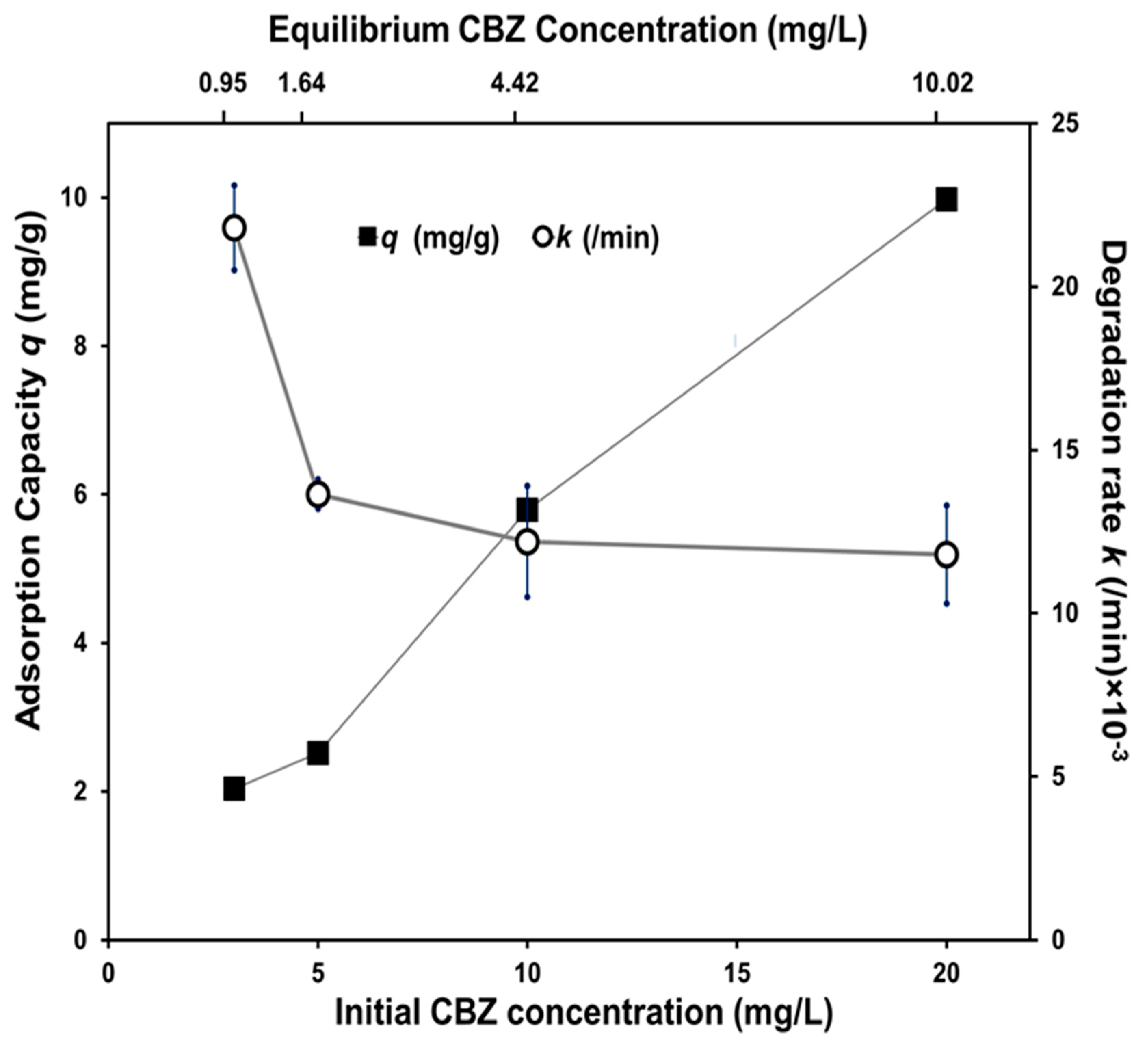
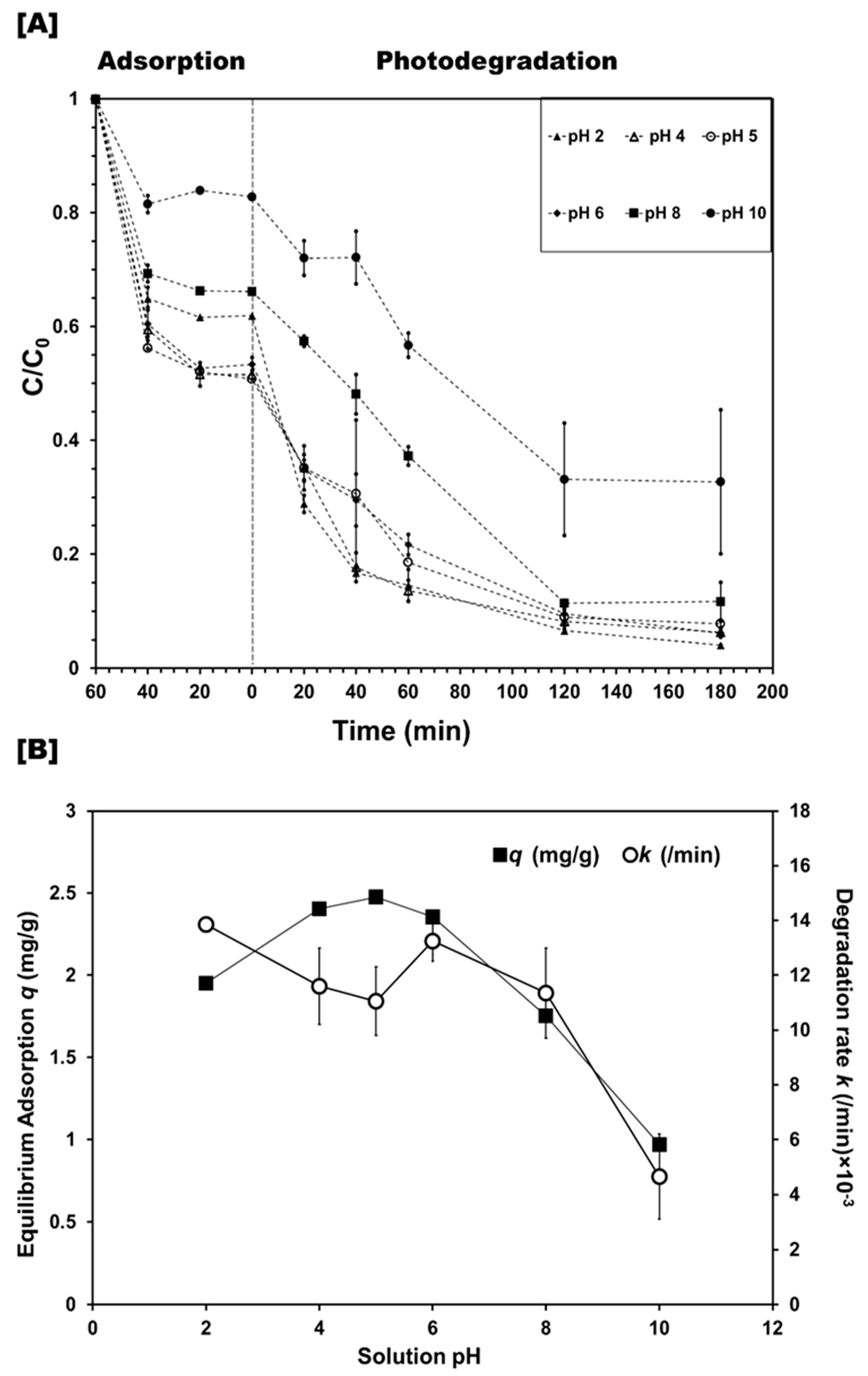
3.2.2. Effect of Photocatalyst Dose and Reaction Conditions
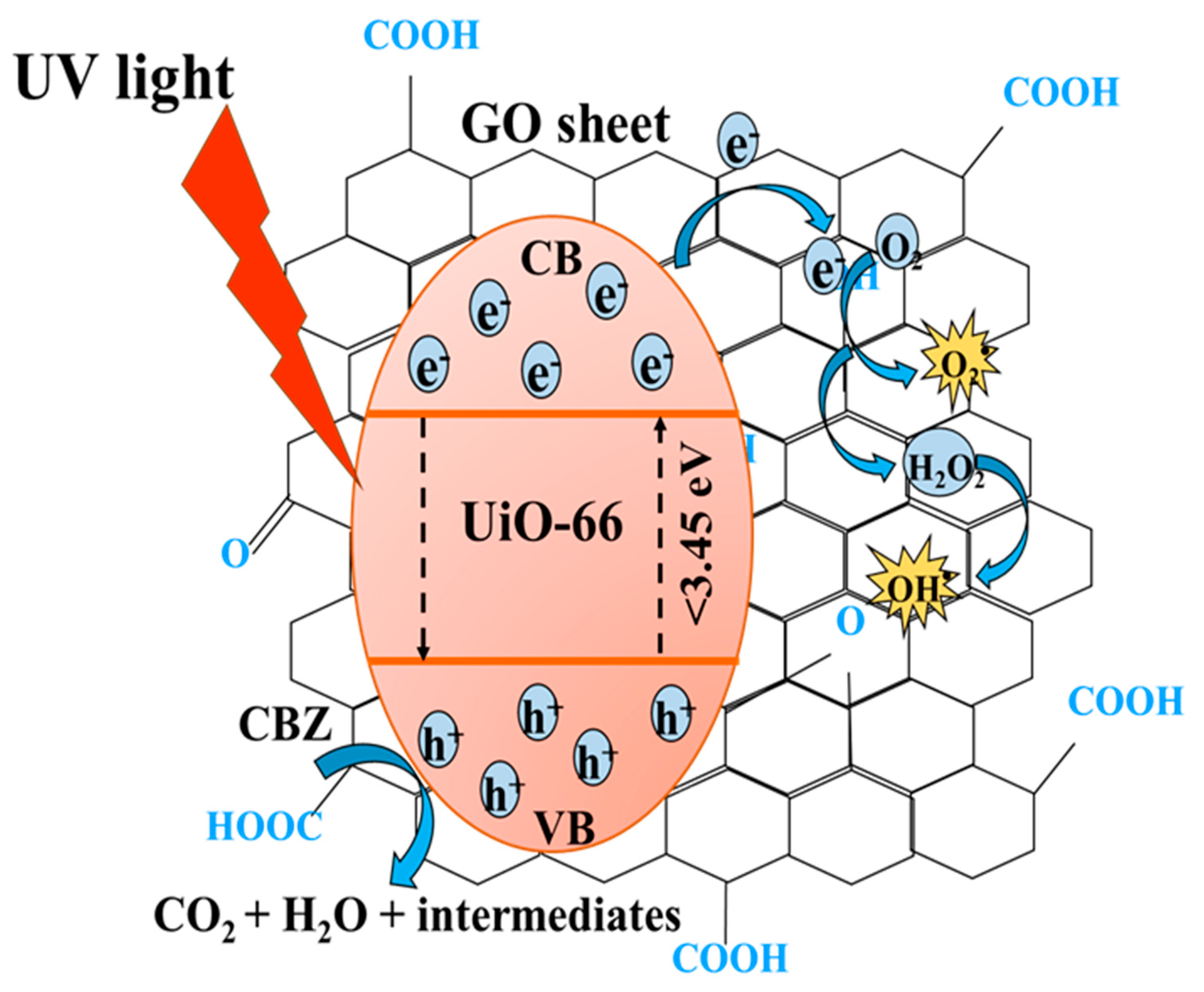
3.2.3. Identification of Reactive Species and Photodegradation Mechanism
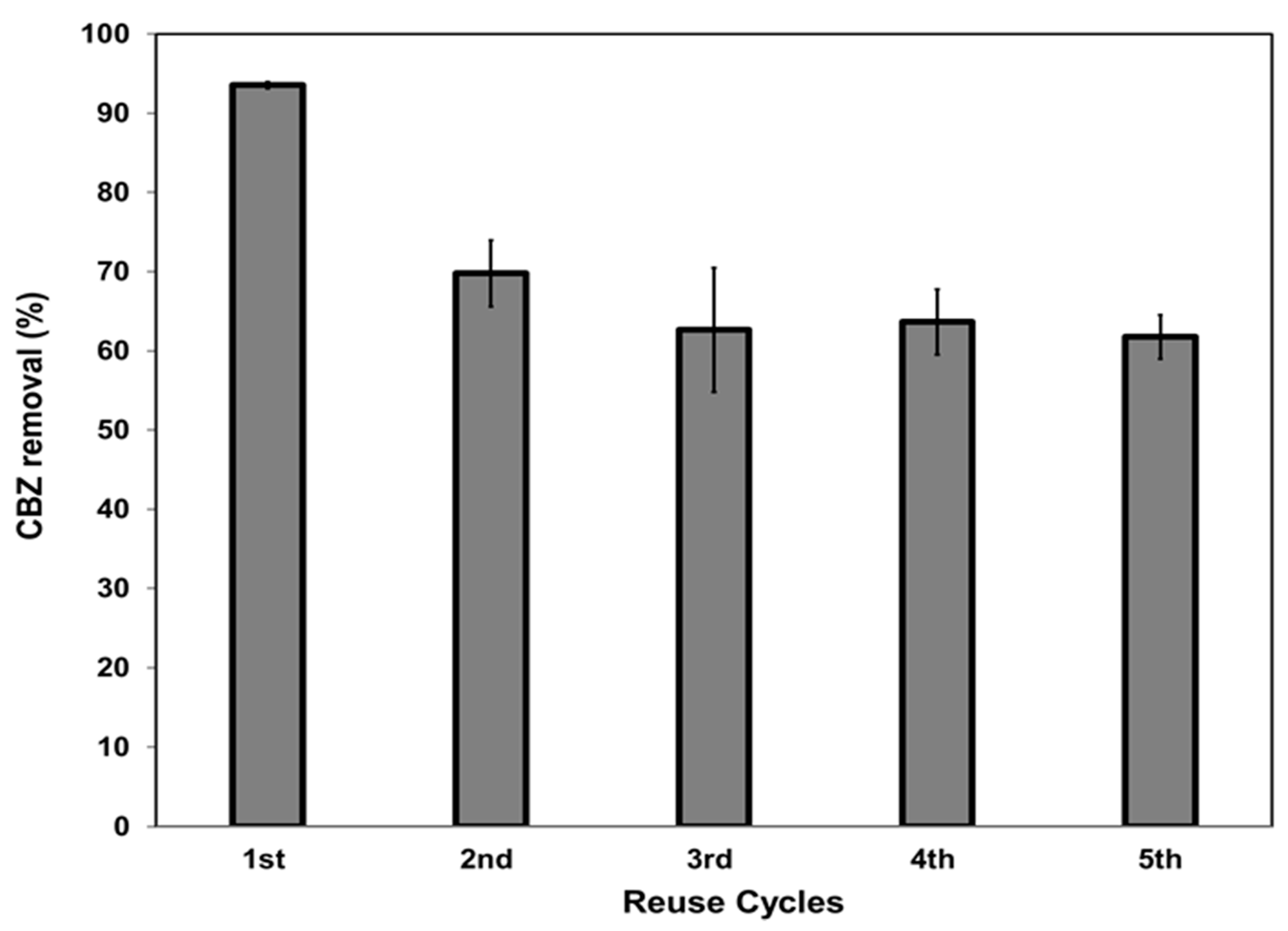
3.3. Recyclability of the Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uyguner-Demirel, C.S.; Birben, N.C.; Bekbolet, M. Elucidation of background organic matter matrix effect on photocatalytic treatment of contaminants using TiO2: A review. Catal. Today 2017, 284, 202–214. [Google Scholar] [CrossRef]
- Dong, S.; Feng, J.; Fan, M.; Pi, Y.; Hu, L.; Han, X.; Liu, M.; Sun, J.; Sun, J. Recent developments in heterogeneous photocatalytic water treatment using visible light-responsive photocatalysts: A review. RSC Adv. 2015, 5, 14610–14630. [Google Scholar] [CrossRef]
- Ateia, M.; Alalm, M.G.; Awfa, D.; Johnson, M.S.; Yoshimura, C. Modeling the degradation and disinfection of water pollutants by photocatalysts and composites: A critical review. Sci. Tot. Env. 2020, 698, 134197. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Ateia, M.; Wang, M.; Awfa, D.; Yoshimura, C. Disinfection mechanism of E. coli by CNT-TiO2 composites: Photocatalytic inactivation vs. physical separation. Chemosphere 2019, 235, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.P.; Qanbarzadeh, M.; Ateia, M.; Torkzadeh, H.; Maroli, A.S.; Cates, E.L. Rapid degradation and mineralization of perfluorooctanoic acid by a new petitjeanite Bi3O (OH)(PO4) 2 microparticle ultraviolet photocatalyst. Env. Sci. Tech. Lett. 2018, 5, 533–538. [Google Scholar] [CrossRef]
- Wen, M.; Mori, K.; Kuwahara, Y.; An, T.; Yamashita, H. Design and architecture of metal organic frameworks for visible light enhanced hydrogen production. Appl. Catal. B Environ. 2017, 218, 555–569. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Multifunctional metal–organic frameworks for photocatalysis. Small 2015, 3097–3112. [Google Scholar] [CrossRef]
- Sharma, V.K.; Feng, M. Water depollution using metal–organic frameworks-catalyzed advanced oxidation processes: A review. J. Hazard. Mater. 2019, 372, 3–16. [Google Scholar] [CrossRef]
- Wang, A.; Zhou, Y.; Wang, Z.; Chen, M.; Sun, L.; Liu, X. Titanium incorporated with UiO-66(Zr)-type Metal–Organic Framework (MOF) for photocatalytic application. RSC Adv. 2016, 6, 3671–3679. [Google Scholar] [CrossRef]
- Jin, Z.; Yang, H. Exploration of Zr–metal–organic framework as efficient photocatalyst for hydrogen production. Nanoscale Res. Lett. 2017, 12. [Google Scholar] [CrossRef]
- Denny, M.S.; Cohen, S.M. In situ modification of metal–organic frameworks in mixed-matrix membranes. Angew. Chem. Int. Ed. 2015, 54, 9029–9032. [Google Scholar] [CrossRef] [PubMed]
- Deria, P.; Mondloch, J.E.; Karagiaridi, O.; Bury, W.; Hupp, J.T.; Farha, O.K. Beyond post-synthesis modification: Evolution of metal–organic frameworks via building block replacement. Chem. Soc. Rev. 2014, 43, 5896–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Cahill, J.F.; Su, Y.; Prather, K.A.; Cohen, S.M. Postsynthetic ligand exchange as a route to functionalization of “inert” metal–organic frameworks. Chem. Sci. 2012, 3, 126–130. [Google Scholar] [CrossRef]
- Kalidindi, S.B.; Nayak, S.; Briggs, M.E.; Jansat, S.; Katsoulidis, A.P.; Miller, G.J.; Warren, J.E.; Antypov, D.; Corà, F.; Slater, B.; et al. Chemical and structural stability of zirconium-based metal–organic frameworks with large three-dimensional pores by linker engineering. Angew. Chem. Int. Ed. 2015, 54, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Cavka, J.H.; Olsbye, U.; Guillou, N.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability—Supporting Information. J. Am. Chem. Soc. 2008, 6, 1–19. [Google Scholar] [CrossRef]
- Musho, T.; Li, J.; Wu, N. Band gap modulation of functionalized metal–organic frameworks. Phys. Chem. Chem. Phys. 2014, 16, 23646–23653. [Google Scholar] [CrossRef]
- Akpinar, I.; Yazaydin, A.O. Adsorption of atrazine from water in metal–organic framework materials. J. Chem. Eng. Data 2018, 63, 2368–2375. [Google Scholar] [CrossRef]
- Lin, R.; Shen, L.; Ren, Z.; Wu, W.; Tan, Y.; Fu, H.; Zhang, J.; Wu, L. Enhanced photocatalytic hydrogen production activity via dual modification of MOF and reduced graphene oxide on CdS. Chem. Commun. 2014, 50, 8533–8535. [Google Scholar] [CrossRef]
- Xu, J.; He, S.; Zhang, H.; Huang, J.; Lin, H.; Wang, X.; Long, J. Layered metal–organic framework/graphene nanoarchitectures for organic photosynthesis under visible light. J. Mater. Chem. A 2015, 3, 24261–24271. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, X.; Liang, X.; Lei, C.; Gao, L.; Hao, R.; Lu, D.; Lei, Z. Fabrication of Ce doped UiO-66/graphene nanocomposites with enhanced visible light driven photoactivity for reduction of nitroaromatic compounds. Appl. Surf. Sci. 2017, 420, 276–285. [Google Scholar] [CrossRef]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic study of diclofenac and carbamazepine adsorption on functionalized silica-based porous materials. Chem. Eng. J. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Atkinson, D.E.; Brice-Bennett, S.; D’Souza, S.W. Antiepileptic medication during pregnancy: Does fetal genotype affect outcome? Pediatr. Res. 2007, 62, 120. [Google Scholar] [CrossRef] [PubMed]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Novel Magnetic Carbon Nanotube-TiO2 Composites for Solar Light Photocatalytic Degradation of Pharmaceuticals in the Presence of Natural Organic Matter. J. Wat. Proc. Eng. 2019, 31, 100836. [Google Scholar] [CrossRef]
- Painter, M.M.; Buerkley, M.A.; Julius, M.L.; Vajda, A.M.; Norris, D.O.; Barber, L.B.; Furlong, E.T.; Schultz, M.M.; Schoenfuss, H.L. Antidepressants at environmentally relevant concentrations affect predator avoidance behavior of larval fathead minnows (Pimephales promelas). Environ. Toxicol. Chem. 2009, 28, 2677–2684. [Google Scholar] [CrossRef] [Green Version]
- Ali, F.; Ali, J.; Shah, N.S.; Sayed, M.; Khan, H.M. Carbamazepine degradation by UV and UV-assisted AOPs: Kinetics, mechanism and toxicity investigations. Process Saf. Environ. Prot. 2018, 117, 307–314. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Photocatalytic degradation of organic micropollutants: Inhibition mechanisms by different fractions of natural organic matter. Wat. Res. 2020, 115643. [Google Scholar] [CrossRef]
- Hai, F.I.; Yang, S.; Id, M.B.A.; Id, V.S.; Shawkat, S.; Sanderson-smith, M.; Gorman, J.; Xu, Z.; Yamamoto, K. Carbamazepine as a possible anthropogenic marker in water: Occurrences, toxicological effects, treatment technologies. Water 2018, 10, 107. [Google Scholar] [CrossRef] [Green Version]
- Abdolhosseinzadeh, S.; Asgharzadeh, H.; Kim, H.S. Fast and fully-scalable synthesis of reduced graphene oxide. Nat. Publ. Gr. 2015, 5, 10160. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Guo, X.; Ying, Y.; Liu, D.; Zhong, C. Composite ultrafiltration membrane tailored by MOF@GO with highly improved water purification performance. Chem. Eng. J. 2017, 313, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Z.; Xiong, W.; Zhou, Y.; Peng, Y.; Li, X. One-step synthesis of Co-doped UiO-66 nanoparticle with enhanced removal efficiency of tetracycline: Simultaneous adsorption and photocatalysis. Chem. Eng. J. 2018, 353, 126–137. [Google Scholar] [CrossRef]
- Shen, L.; Liang, R.; Luo, M.; Jing, F.; Wu, L. Electronic effects of ligand substitution on metal–organic framework photocatalysts: The case study of UiO-66. Phys. Chem. Chem. Phys. 2015, 17, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-Q.; Zhang, Y.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Visible-light-driven oxidation of primary C–H bonds over CdS with dual co-catalysts graphene and TiO2. Sci. Rep. 2013, 3, 3314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, D.; Song, M. Preparation of fully exfoliated graphite oxide nanoplatelets in organic solvents. J. Mater. Chem. 2007, 17, 3678–3680. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, Y.; Lv, Z.; Song, F.; Zhong, Q. Preparation and enhanced CO2 adsorption capacity of UiO-66/graphene oxide composites. J. Ind. Eng. Chem. 2015, 27, 102–107. [Google Scholar] [CrossRef]
- Guo, H.; Wang, X.; Qian, Q.; Wang, F.; Xia, X. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Yang, C.; You, X.; Cheng, J.; Zheng, H.; Chen, Y. A novel visible-light-driven In-based MOF/graphene oxide composite photocatalyst with enhanced photocatalytic activity toward the degradation of amoxicillin. Appl. Catal. B Environ. 2017, 200, 673–680. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, X.; Li, Y. Controlled growth of dense and ordered metal–organic framework nanoparticles on graphene oxide. Chem. Commun. 2015, 51, 3874–3877. [Google Scholar] [CrossRef]
- Petit, C.; Bandosz, T.J. MOF-graphite oxide nanocomposites: Surface characterization and evaluation as adsorbents of ammonia. J. Mater. Chem. 2009, 19, 6521–6528. [Google Scholar] [CrossRef]
- Chen, C.; Chen, D.; Xie, S.; Quan, H.; Luo, X.; Guo, L. Adsorption behaviors of organic micropollutants on zirconium metal−organic framework UiO-66: Analysis of surface interactions. ACS Appl. Mater. Interfaces 2017, 9, 41043–41054. [Google Scholar] [CrossRef]
- Ding, J.; Yang, Z.; He, C.; Tong, X.; Li, Y.; Niu, X.; Zhang, H. UiO-66(Zr) coupled with Bi2MoO6 as photocatalyst for visible-light promoted dye degradation. J. Colloid Interface Sci. 2017, 497, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Shafiei-alavijeh, M.; Alivand, M.S.; Rashidi, A.; Samimi, A. Thermally stable UIO-66 nano-adsorbent with high gasoline vapor recovery. In Proceedings of the 7th International Congress on Nanoscience & Nanotechnology (ICNN2018), Tehran, Iran, 26–28 September 2018. [Google Scholar]
- Smith, S.J.D.; Ladewig, B.P.; Hill, A.J.; Lau, C.H.; Hill, M.R. Post-synthetic Ti exchanged UiO-66 metal−organic frameworks that deliver exceptional gas permeability in mixed matrix membranes. Sci. Rep. 2015, 5, 15–18. [Google Scholar] [CrossRef]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye adsorption on layered graphite oxide. J. Chem. Eng. Data 2011, 56, 138–141. [Google Scholar] [CrossRef]
- Liu, W.M.; Wang, X.Q.; Wang, C.M.; Chen, G.G.; Sun, W.L.; Zheng, C.T. Green and large-scale preparation of reduced graphene oxide for electroreduction of nitrobenzene to p-aminophenol. Micro Nano Lett. 2016, 11, 661–665. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Dubois, J.R.P. One-pot microwave-assisted synthesis of graphene/layered double hydroxide (LDH) nanohybrids. Nano-Micro Lett. 2015, 7, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pignatello, J.J. Characterization of aromatic compound sorptive interactions with black carbon (charcoal) assisted by graphite as a model. Environ. Sci. Technol. 2005, 39, 2033–2041. [Google Scholar] [CrossRef]
- Zhou, Y.; Apul, O.G.; Karanfil, T. Adsorption of halogenated aliphatic contaminants by graphene nanomaterials. Water Res. 2015, 79, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Peng, T.; Li, K.; Zeng, P.; Zhang, Q.; Zhang, X. Enhanced photocatalytic hydrogen production over graphene oxide−cadmium sulfide nanocomposite under visible light irradiation. J. Phys. Chem. C 2012, 116, 22720–22726. [Google Scholar] [CrossRef]
- Sun, D.; Liu, W.; Qiu, M.; Zhang, Y.; Li, Z. Introduction of a mediator for enhancing photocatalytic performance via post-synthetic metal exchange in metal−organic frameworks (MOFs). Chem. Commun. 2015, 66, 2056–2059. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Yu, J.; Jin, J.; Jaroniec, M. A noble metal-free reduced graphene oxide-cds nanorod composite for the enhanced visible-light photocatalytic reduction of CO2 to solar fuel. J. Mater. Chem. A 2014, 10, 3407–3416. [Google Scholar] [CrossRef]
- Cai, N.; Larese-casanova, P. Sorption of carbamazepine by commercial graphene oxides: A comparative study with granular activated carbon and multiwalled carbon nanotubes. J. Colloid Interface Sci. 2014, 426, 152–161. [Google Scholar] [CrossRef]
- Wang, C.; Li, H.; Liao, S.; Zheng, H.; Wang, Z.; Pan, B. Coadsorption, desorption hysteresis and sorption thermodynamics of sulfamethoxazole and carbamazepine on graphene oxide and graphite. Carbon N. Y. 2013, 65, 243–251. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO2 composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Giri, R.R.; Ozaki, H.; Ota, S.; Takanami, R.; Taniguchi, S. Degradation of common pharmaceuticals and personal care products in mixed solutions by advanced oxidation techniques. 2010, 7, 251–260. Int. J. Environ. Sci. Technol. 2010, 7, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Hu, A.; Zhang, X.; Oakes, K.D.; Peng, P.; Zhou, Y.N.; Servos, M.R. Hydrothermal growth of free standing TiO2 nanowire membranes for photocatalytic degradation of pharmaceuticals. J. Hazard. Mater. 2011, 189, 278–285. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Asghari, M.; Ramezanzadeh, B. Fabrication of an e ffi cient system for Zn ions removal from industrial wastewater based on graphene oxide nanosheets decorated with highly crystalline polyaniline nano fibers (GO-PANI): Experimental and ab initio quantum mechanics approaches. Chem. Eng. J. 2018, 337, 385–397. [Google Scholar] [CrossRef]
- Almasri, D.A.; Rhad, T.; Atieh, M.A.; Mckay, G.; Ahzi, S. High performance hydroxyiron modified montmorillonite nanoclay adsorbent for arsenite removal. Chem. Eng. J. 2018, 335, 1–12. [Google Scholar] [CrossRef]
- Abdi, J.; Vossoughi, M.; Mohammad, N. Synthesis of metal−organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal. Chem. Eng. J. 2017, 326, 1145–1158. [Google Scholar] [CrossRef]
- Alahiane, S.; Qourzal, S.; el Ouardi, M.; Abaamrane, A.; Assabbane, A. Factors influencing the photocatalytic degradation of reactive yellow 145 by TiO2-coated non-woven fibers. Am. J. Anal. Chem. 2014, 5, 445–454. [Google Scholar] [CrossRef] [Green Version]
- Mamun, K.; Asw, R.; Fahmida, K. Parameters affecting the photocatalytic degradation of dyes using TiO2: A review. Appl. Water Sci. 2017, 7, 1569–1578. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.A.; He, X.; Khan, H.M.; Shah, N.S.; Dionysiou, D.D. Oxidative degradation of atrazine in aqueous solution by UV/H2O2/Fe2+, UV/S2O82−/Fe2+ and UV/HSO5−/Fe2+ processes: A comparative study. Chem. Eng. J. 2013, 218, 376–383. [Google Scholar] [CrossRef]
- Moztahida, M.; Jang, J.; Nawaz, M.; Lim, S.; Sung, D. Effect of rGO loading on Fe3O4: A visible light assisted catalyst material for carbamazepine degradation. Sci. Total Environ. 2019, 667, 741–750. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Pan, B.; Xing, B. Adsorption and desorption of oxytetracycline and carbamazepine by multiwalled carbon nanotubes. Environ. Sci. Technol. 2009, 43, 9167–9173. [Google Scholar] [CrossRef]
- Castarlenas, S.; Téllez, C.; Coronas, J. Gas separation with mixed matrix membranes obtained from MOF UiO-66-graphite oxide hybrids. J. Memb. Sci. 2017, 526, 205–211. [Google Scholar] [CrossRef] [Green Version]

| Catalyst | SSABET a m2/g | Main Pore Size b nm | PVtotalc cm3/g | BJH Pore Volume Distribution d | Indirect Band Gap e eV | ||
|---|---|---|---|---|---|---|---|
| PVmicro(<2 nm) cm3/g, [%] | PVmeso(2–50 nm) cm3/g, [%] | PVmacro(> 50 nm) cm3/g, [%] | |||||
| GO | 56.4 | 10.94 | 0.588 | 0.027 [4.63] | 0.004 [0.68] | 0.557 [94.69] | N/A |
| UiO-66 | 419.9 | 3.94 | 0.448 | 0.177 [39.41] | 0.013 [2.99] | 0.258 [57.60] | 3.55 |
| UiO-66_GO-0.1 | 1004.6 | 3.95 | 0.879 | 0.577 [65.65] | 0.007 [0.83] | 0.295 [33.52] | 3.55 |
| UiO-66_GO-0.5 | 913.4 | 4.02 | 1.052 | 0.526 [50.04] | 0.004 [0.36] | 0.522 [49.6] | 3.45 |
| UiO-66_GO-1 | 778 | 3.92 | 0.811 | 0.385 [47.45] | 0.005 [0.56] | 0.522 [51.99] | 3.3 |
| UiO-66_GO-5 | 552.4 | 4.03 | 0.398 | 0.226 [56.79] | 0.005 [1.33] | 0.167 [41.87] | 3.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heu, R.; Ateia, M.; Awfa, D.; Punyapalakul, P.; Yoshimura, C. Photocatalytic Degradation of Organic Micropollutants in Water by Zr-MOF/GO Composites. J. Compos. Sci. 2020, 4, 54. https://doi.org/10.3390/jcs4020054
Heu R, Ateia M, Awfa D, Punyapalakul P, Yoshimura C. Photocatalytic Degradation of Organic Micropollutants in Water by Zr-MOF/GO Composites. Journal of Composites Science. 2020; 4(2):54. https://doi.org/10.3390/jcs4020054
Chicago/Turabian StyleHeu, Rina, Mohamed Ateia, Dion Awfa, Patiparn Punyapalakul, and Chihiro Yoshimura. 2020. "Photocatalytic Degradation of Organic Micropollutants in Water by Zr-MOF/GO Composites" Journal of Composites Science 4, no. 2: 54. https://doi.org/10.3390/jcs4020054
APA StyleHeu, R., Ateia, M., Awfa, D., Punyapalakul, P., & Yoshimura, C. (2020). Photocatalytic Degradation of Organic Micropollutants in Water by Zr-MOF/GO Composites. Journal of Composites Science, 4(2), 54. https://doi.org/10.3390/jcs4020054






