Polymeric Composites Based on Carboxymethyl Cellulose Cryogel and Conductive Polymers: Synthesis and Characterization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CMC Cryogel
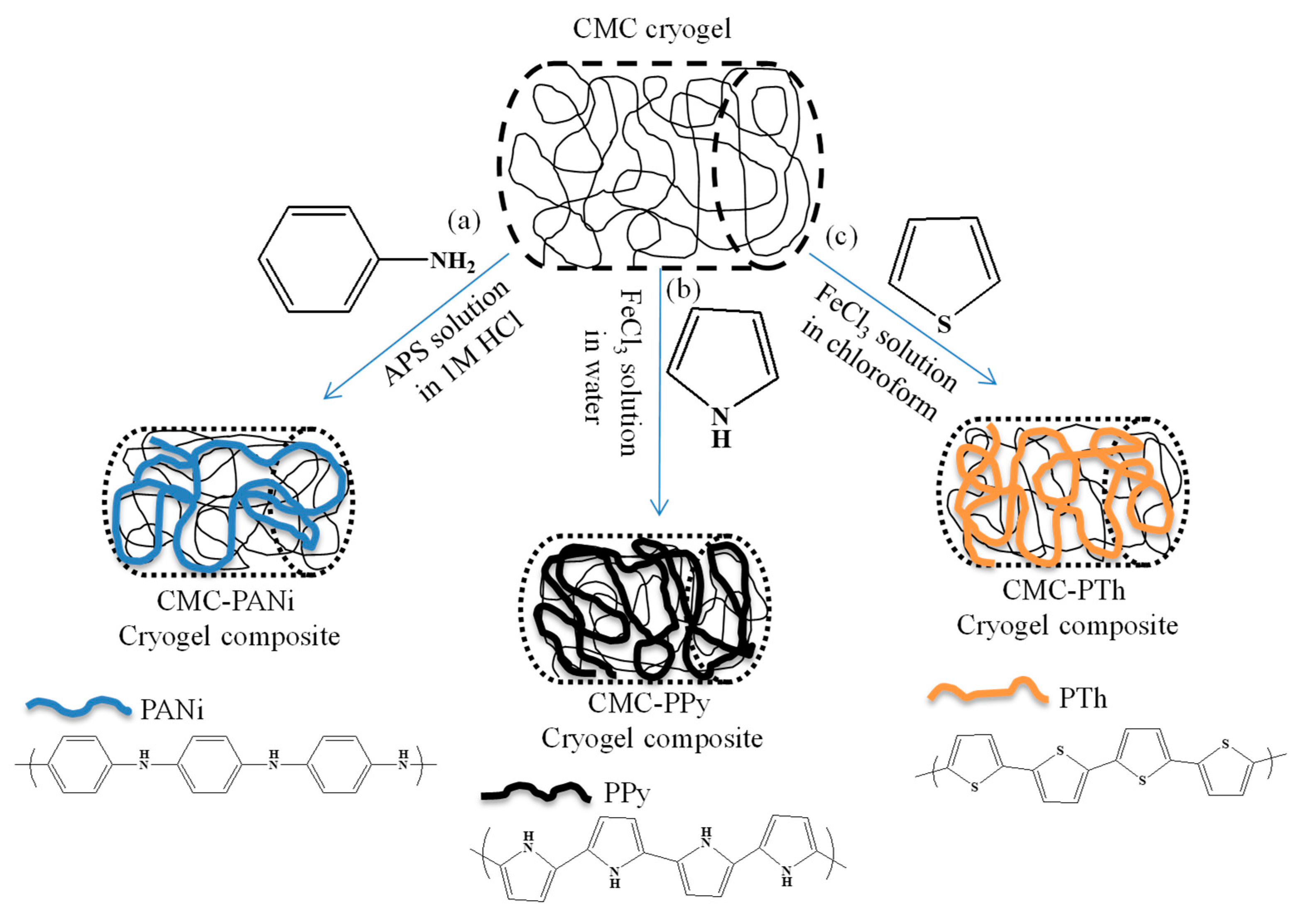
2.3. In Situ Synthesis of Conductive Polymers within CMC Cryogels
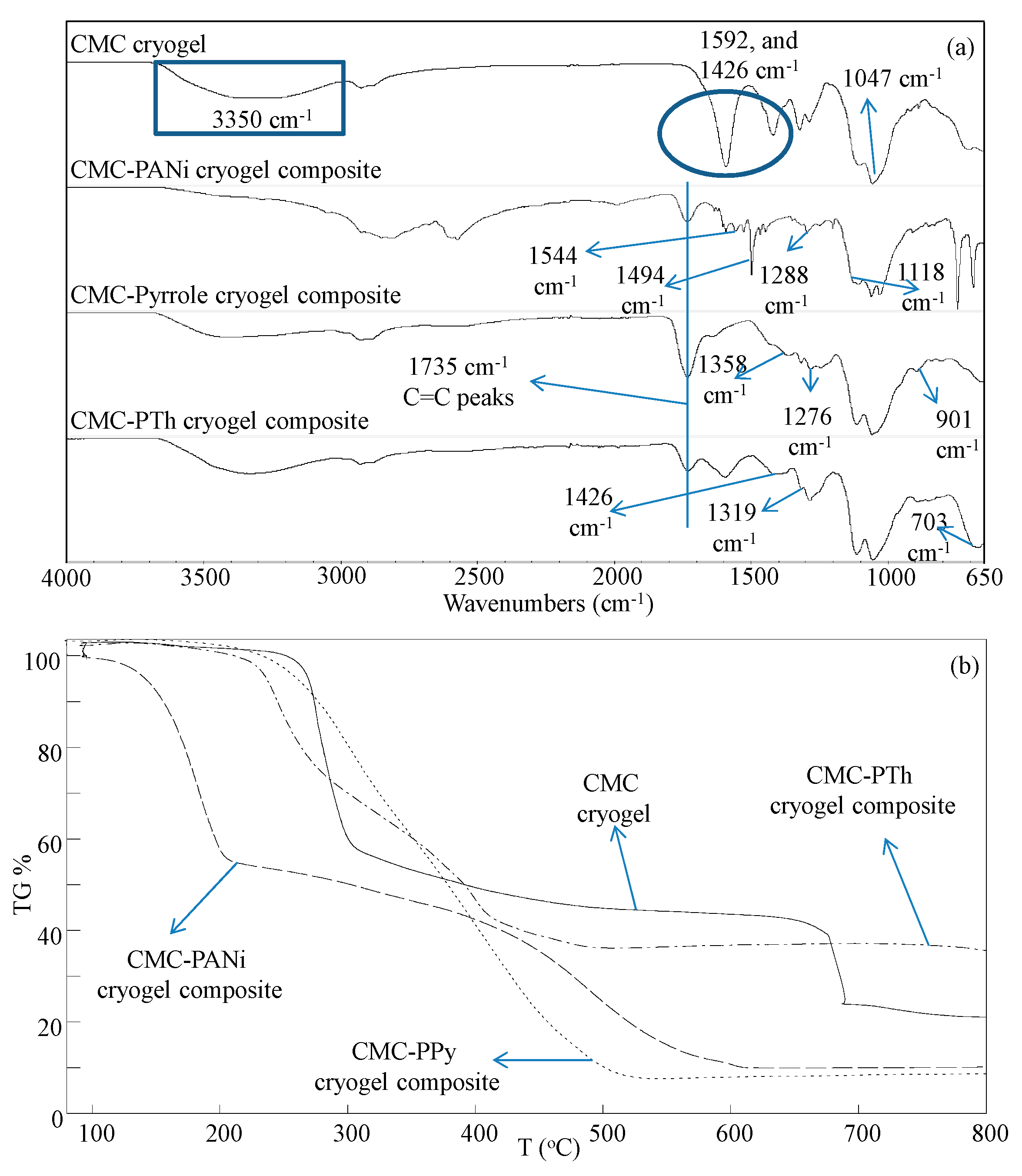
2.4. Characterization
2.5. Conductivity Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of In Situ Synthesized Conductive Polymer CMC Cryogel Composites
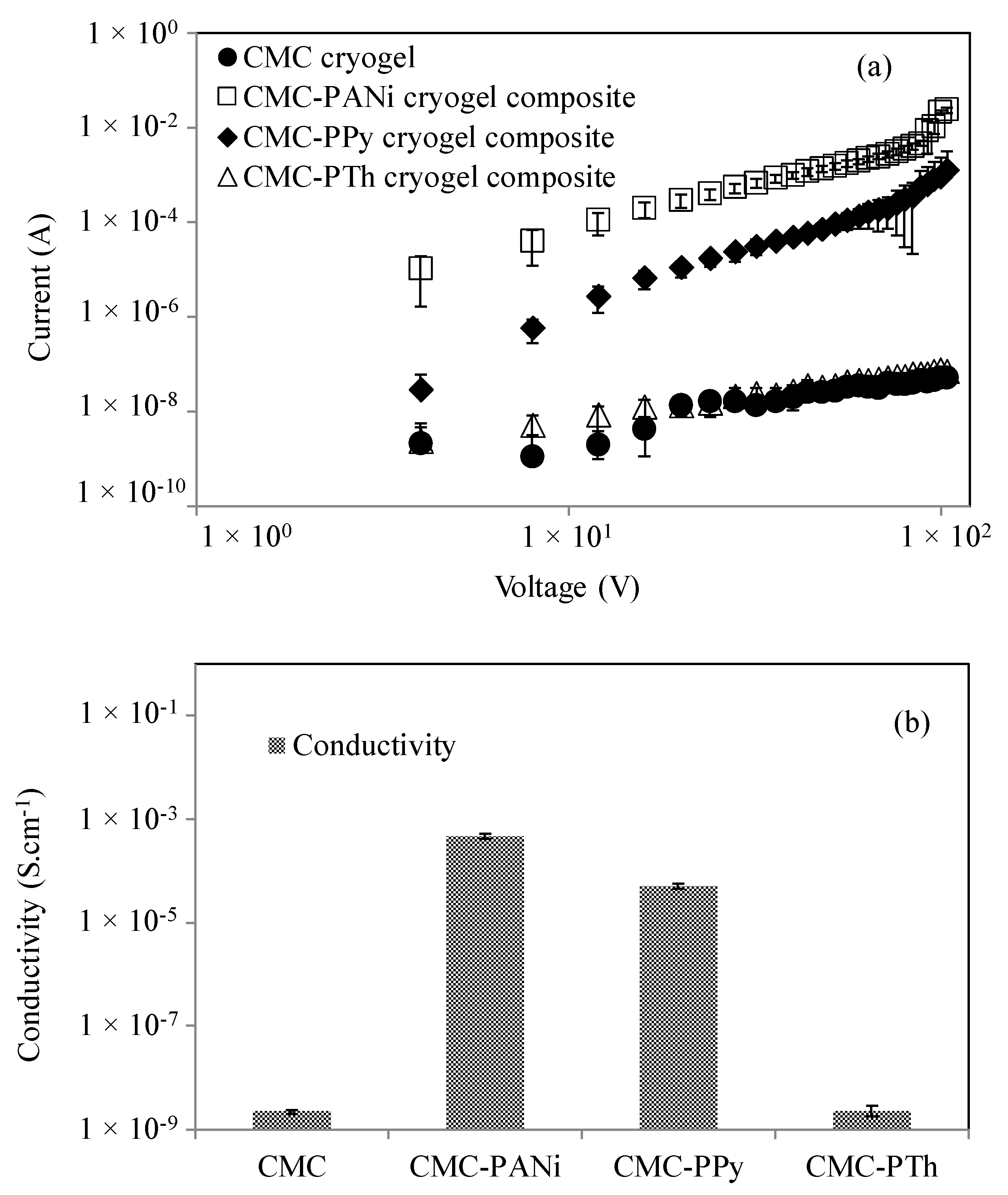
3.2. Conductivity Measurements
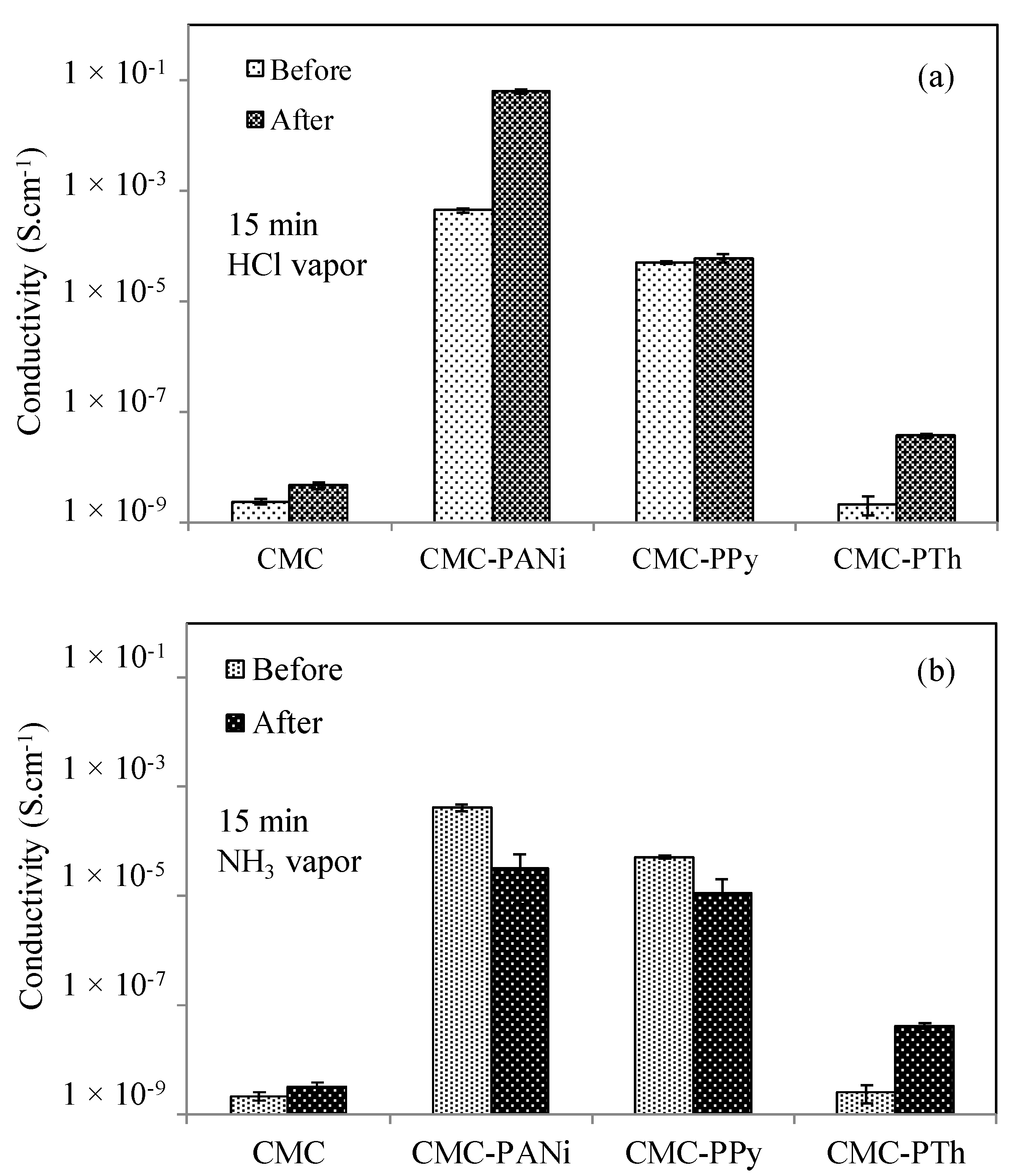
3.3. The Change in Conductivity Upon Exposure to HCl and NH3 Vapors
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shirakawa, H. The discovery of polyacetylene film: The dawning ofan era of conducting polymers (Nobel lecture). Angew. Chem. Int. Ed. 2001, 40, 2575–2580. [Google Scholar] [CrossRef]
- Heeger, A. Semiconducting and metallic polymers: The fourth gen-eration of polymeric materials. Synt. Metal. 2002, 125, 23–42. [Google Scholar] [CrossRef]
- Guo, B.; Glavas, L.; Albertsson, A.C. Biodegradable and electrically conducting polymers for biomedical application. Prog. Polym. Sci. 2013, 38, 1263–1286. [Google Scholar] [CrossRef]
- Cho, J.; Shin, K.H.; Jang, J. Micropatterning of conducting polymer tracks on plasma treated flexible substrate using vapor phase polymerization-mediated inkjet printing. Synth. Met. 2010, 160, 1119–1125. [Google Scholar] [CrossRef]
- Gonzalez-Macia, L.; Morrin, A.; Smyth, M.R.; Killard, A.J. Advanced printing and deposition methodologies for the fabrication of biosensors and biodevices. Analyst 2010, 135, 845–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ummartyotin, S.; Wu, C.; Sain, M.; Manuspiya, H. Deposition of PEDOT: PSS nanoparticles as a conductive microlayer anode in OLED devices by desktop inkjet printer. J. Nanomater. 2011, 2011, 606714. [Google Scholar] [CrossRef]
- Alshammary, B.; Walsh, F.C.; Herrasti, P.; Ponce de Leon, C. Electrodeposited conductive polymers for controlled drug release:polypyrrole. J. Solid State Electrochem. 2016, 20, 839–859. [Google Scholar] [CrossRef] [Green Version]
- Stenger-Smith, J.D. Intrinsically electrically conducting polymers, synthesis, characterization, and their applications. Prog. Polym. Sci. 1998, 23, 57–79. [Google Scholar] [CrossRef]
- Gurunathan, K.; Murugan, A.V.; Marimuthu, R.; Mulik, U.P.; Amalnerkar, D.P. Electrochemically synthesized conducting polymeric materials for applications towards technology in electronics, optoelectronics and energy storage devices. Mater. Chem. Phys. 1999, 61, 173–191. [Google Scholar] [CrossRef]
- Long, Y.Z.; Li, M.M.; Gu, C.Z.; Wan, M.X.; Duvail, J.L.; Liu, Z.W.; Fan, Z.Y. Recent advances in synthesis, physical properties and applications of con-ducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Wang, X.D.; Gu, X.S.; Yuan, C.W.; Chen, S.J.; Zhang, P.Y.; Zhang, T.Y.; Yao, J.; Chen, F.; Chen, G. Evaluation of biocompatibility of polypyrrole in vitro and in vivo. J. Biomed. Mater. Res. A 2004, 68, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Phar. Pharmacol. 2007, 59, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Banerjee, S.; Sailia, J.P.; Konwar, B.K. Swift heavy ion irradiation induced enhancement in the antioxidant activity and biocompatibility of polyaniline nanofiber. Nanaotechnology 2010, 21, 175102. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Dong, Y.Q.; Sengothi, K.; Tan, K.L.; Kang, E.T. In-vivo tissue response to polyaniline. Synt. Metal. 1999, 102, 1313–1314. [Google Scholar] [CrossRef]
- Zhao, H.C.; Zhu, B.; Sekine, J.; Luo, S.C.; Yu, H.H. Oligoethylene-glycol-functionalized polyoxythiophenes for cell engineering: Syntheses characterizations, and cell compatibilities. ACS Appl. Mater. Interfaces 2012, 4, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Garner, B.; Georgevich, A.; Hodgson, A.J.; Liu, L.; Wallace, G.G. Polypyrrole-heparin composites as stimulus-responsive substrates for endothelial cell growth. J. Biomed. Mater. Res. 1999, 44, 121–129. [Google Scholar] [CrossRef]
- Kim, D.H.; Wiler, J.A.; Anderson, D.J.; Kipke, D.R.; Martin, D.C. Conducting polymers on hydrogel-coated neural electrode provide sensitive neural recordings in auditory cortex. Acta Biomater. 2010, 6, 57–62. [Google Scholar] [CrossRef]
- Persson, K.M.; Karlsson, R.; Svennersten, K.; Loffler, S.; Jager, E.W.H.; Richter-Dahlfors, A.; Konradsson, P.; Berggren, M. Electronic control of cell detachment using a self-doped conducting polymer. Adv. Mater. 2011, 23, 4403–4408. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Serna, F.; Nickels, J.; Schmidt, C.E. Carboxylic acid-functionalized conductive polypyrrole as a bioactive platform for cell adhesion. Biomacromology 2006, 7, 1692–1965. [Google Scholar] [CrossRef] [Green Version]
- Nambiar, S.; Yeow, J.T.W. Conductive polymer-based sensors for biomedical applications. Biosens. Bioelectron. 2011, 26, 1825–1832. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Q.; Teng, W. Injectable, degradable, electroactive nanocomposite hydrogels containing conductive polymer nanoparticles for biomedical applications. Int. J. Nanomed. 2016, 11, 131–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, A.M.; KAdir, M.R.A.; Razak, S.I.A. Novel PLA-based conductive polymercomposites for biomedical applications. JOM 2017, 69, 2838–2843. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Akbarnejad, Z.; Eskandary, H.; Vergallo, C.; Nematollahi-Mahani, S.N.; Dini, L.; Darvishzadeh, F. Effects of extremely low-frequency pulsed electromagnetic fields (ELF-PEMFs) on glioblastoma cells (U87). Electreomag. Biol. Med. 2017, 36, 238–247. [Google Scholar] [CrossRef]
- Scanziani, M.; Häusser, M. Electrophysiology in the age of light. Nature 2009, 461, 930. [Google Scholar] [CrossRef]
- Clayton, R.H.; Bernus, O.; Cherry, E.M.; Dierckx, H.; Fenton, F.H.; Mirabella, L.; Panfilov, A.V.; Sachse, F.B.; Seemann, G.; Zhang, H. Models of cardiac tissue electrophysiology: Progress, challenges and open questions. Prog. Biophys. Mol. Biol. 2011, 104, 22–48. [Google Scholar] [CrossRef]
- Vasquez-Sancho, F.; Abdollahi, A.; Damjanovic, D.; Catalan, G. Flexoelectricity in Bones. Adv. Mater. 2018, 30, 1705316. [Google Scholar] [CrossRef]
- Cantu, T.; Walsh, K.; Pattani, V.P.; Moy, A.J.; Tunnel, J.W.; Irvin, J.A.; Betancourt, T. Conductive polymer-based nanoparticles for lase-mediated photothermal ablation of cancer: Synthesis, characterization, and in vitro evaluation. Int. J. Nanomed. 2017, 12, 615–632. [Google Scholar] [CrossRef] [Green Version]
- Hardy, J.G.; Lee, J.Y.; Schmidt, C.E. Biomimetic conducting polymer-based tissue scaffolds. Cur. Opin. Biotechnol. 2013, 24, 847–854. [Google Scholar] [CrossRef] [Green Version]
- Lassalle, N.; Vieil, E.; Correia, J.P.; Abrantes, L.M. Study of DNA hybridization on polypyrrole grafted with oligonucleotides by photocurrent spectroscopy. Biosens. Bioelectron. 2001, 16, 295–303. [Google Scholar] [CrossRef]
- Rozlosnik, N. New directions in medical biosensors employing poly(3,4-ethylenedioxy thiophene) derivative-based electrodes. Anal. Bioanal. Chem. 2009, 395, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Kleber, C.; Bruns, M.; Lienkamp, K.; Rühe, J.; Asplund, M. An interpenetrating, microstructurable and covalently attached conducting polymer hydrogel for neural interfaces. Acta Biomater. 2017, 58, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Shklovsky, J.; Reuveny, A.; Sverdlov, Y.; Krylov, S.; Shacham-Diamand, Y. Towards fully polymeric electroactive micro actuators with condutive polymer electrodes. Microelectron. Eng. 2018, 199, 58–62. [Google Scholar] [CrossRef]
- Zhou, J.; Mulle, M.; Zhang, Y.; Xu, X.; Li, E.Q.; Han, F.; Thoroddsen, S.T.; Lubineau, G. High-ampacity conductive polymer microfibers as fast response wearable heaters and electromechanical actuators. J. Mater. Chem. C. 2016, 4, 1238–1249. [Google Scholar] [CrossRef] [Green Version]
- Chapman, C.A.R.; Cuttaz, E.A.; Goding, J.A.; Green, R.A. Actively controlled local drug delivery using conductive polymer-based devices. Appl. Phys. Lett. 2020, 116, 010501. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Neoh, K.G.; Kang, E.T. Controlled release of heparin from polypyrrole-poly(vinyl Alcohol) assembly by electrical stimulation. J. Biomed. Mater. Res. Part A 2005, 73, 171–181. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Shastri, V.R.; Vacanti, J.P.; Langer, R. Stimulation of neurite outgrowth using an electrically conducting polymer. Proc. Natl. Acad. Sci. USA 1997, 94, 8948–8953. [Google Scholar] [CrossRef] [Green Version]
- Nezakati, T.; Tan, A.; Lim, J.; Cormia, R.D.; Teoh, S.H.; Seifalian, A.M. Ultra-low percolation threshold POSS-PCL/graphene electrically conductive polymer: Neural tissue engineering nanocomposites for neurosurgery. Mater. Sci. Eng. C 2019, 104, 109915. [Google Scholar] [CrossRef]
- Nezakati, T.; Seifalian, A.; Tan, A.; Seifalian, A.M. Conductive polymers: Opportunities and challenges in biomedical applications. Chem. Rev. 2018, 118, 6766–6843. [Google Scholar] [CrossRef]
- Sengel, S.B.; Sahiner, M.; Aktas, N.; Sahiner, N. Halloysite-carboxymethyl cellulose cryogel composite from natural sources. Appl. Cay. Sci. 2017, 140, 66–74. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S.; Aktas, N. Superporous cryogel/conductive composite systems for potential sensor applications. J. Polym. Res. 2017, 24, 126. [Google Scholar] [CrossRef]
- Sahiner, N.; Demirci, S. Conducting semi-interpenetrating polymeric composites via the preparation of poly(aniline), poly(thiophene), and poly(pyrrole) polymers within superporous poly(acrylic acid) cryogels. React. Funct. Polym. 2016, 105, 60–65. [Google Scholar] [CrossRef]
- Veara, R.A.; Romero, B.H.; Ahumada, E. Synthesis and characterization of polyaniline and poly-ortho-methoxyaniline behavior against carbon steel corrosion. J. Chill. Chem. Soc. 2003, 48, 1. [Google Scholar]
- Xu, C.; Sun, J.; Gao, L. Synthesis of novel hierarchical graphene/polypyrrole nano sheet composite and their superior electrochemical performance. J. Mater. Chem. 2011, 21, 11253–11258. [Google Scholar] [CrossRef]
- Olowu, R.A.; Arotiba, O.; Mailu, S.N.; Warto, T.T.; Baker, P.; Iwuoha, E. Electrochemical aptasensor for endocrine disrupting 17β-estradiol based on a poly(3,4-ethylenedioxylthiopene)-gold nanocomposite platform. Sensors 2010, 10, 9872–9890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, J.; Wang, L.; Bao, W. A process for desulfurization of coking benzene by a two-step method with reuse of sorbent/thiophene and its key procedures. Green Chem. 2015, 17, 3164–3175. [Google Scholar] [CrossRef]
- Park, H.S.; Ko, S.J.; Park, J.S.; Kim, J.Y.; Song, H.K. Redox-active charge carriers of conducting polymers as a tuner of conductivity and its potential window. Sci. Rep. 2013, 3, 2454. [Google Scholar] [CrossRef] [Green Version]

| Materials (Cryogel Composite) | Weigth of CMC Cryogels (g) | Loaded Amount of Monomer (g) | Amount of Composite Cryogel (g) | Yield of In Situ Polymerization (%) |
|---|---|---|---|---|
| CMC–PANi | 0.1 ± 0.01 | 1.98 ± 0.02 | 0.97 ± 0.11 | 46.2 ± 5.44 |
| CMC–PPy | 0.1 ± 0.01 | 1.94 ± 0.03 | 0.23 ± 0.01 | 10.9 ± 0.21 |
| CMC–PTh | 0.1 ± 0.01 | 1.92 ± 0.03 | 0.11 ± 0.01 | 5.18 ± 0.49 |
| Materials | Conductivity (S·cm-1) | |||||
|---|---|---|---|---|---|---|
| HCl Vapor | Change (fold) | NH3 Vapor | Change (fold) | |||
| Before | After | Before | After | |||
| CMC | 2.31 × 10−9 | 4.59 × 10−9 | 2 | 2.16 × 10−9 | 3.11 × 10−9 | 1.5 |
| CMC–PANi | 4.36 × 10−4 | 6.24 × 10−2 | 143 | 4.11 × 10−4 | 3.27 × 10−5 | 12 |
| CMC–PPy | 4.98 × 10−5 | 5.97 × 10−5 | 1.2 | 5.16 × 10−5 | 1.11 × 10−5 | 5 |
| CMC–PTh | 2.12 × 10−9 | 3.67 × 10−8 | 17 | 2.51 × 10−9 | 4.14 × 10−8 | 16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demirci, S.; Sutekin, S.D.; Sahiner, N. Polymeric Composites Based on Carboxymethyl Cellulose Cryogel and Conductive Polymers: Synthesis and Characterization. J. Compos. Sci. 2020, 4, 33. https://doi.org/10.3390/jcs4020033
Demirci S, Sutekin SD, Sahiner N. Polymeric Composites Based on Carboxymethyl Cellulose Cryogel and Conductive Polymers: Synthesis and Characterization. Journal of Composites Science. 2020; 4(2):33. https://doi.org/10.3390/jcs4020033
Chicago/Turabian StyleDemirci, Sahin, S. Duygu Sutekin, and Nurettin Sahiner. 2020. "Polymeric Composites Based on Carboxymethyl Cellulose Cryogel and Conductive Polymers: Synthesis and Characterization" Journal of Composites Science 4, no. 2: 33. https://doi.org/10.3390/jcs4020033
APA StyleDemirci, S., Sutekin, S. D., & Sahiner, N. (2020). Polymeric Composites Based on Carboxymethyl Cellulose Cryogel and Conductive Polymers: Synthesis and Characterization. Journal of Composites Science, 4(2), 33. https://doi.org/10.3390/jcs4020033






