Modification of Ground Tire Rubber—Promising Approach for Development of Green Composites
Abstract
:1. Introduction
2. Materials and Methods
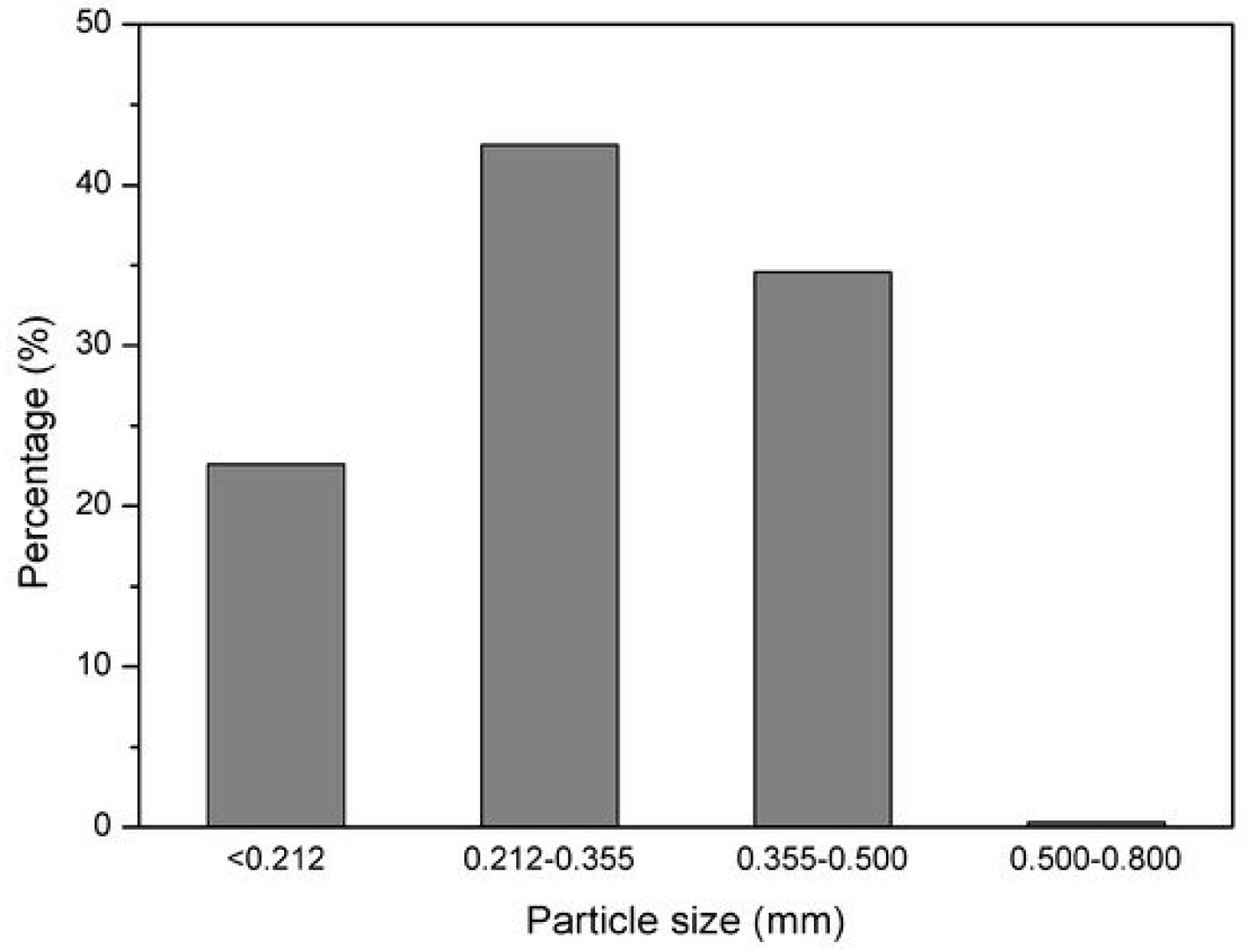
2.1. Materials
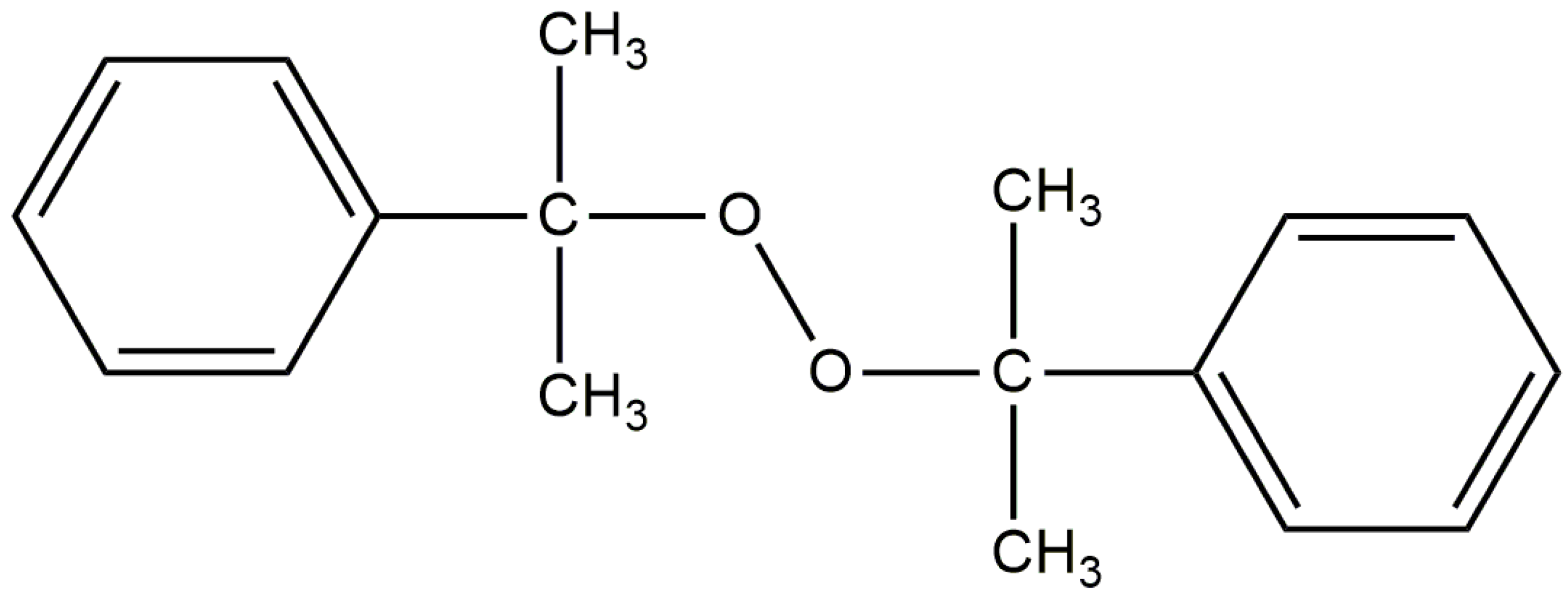
2.2. Sample Preparation
2.3. Measurements
3. Result and Discussion
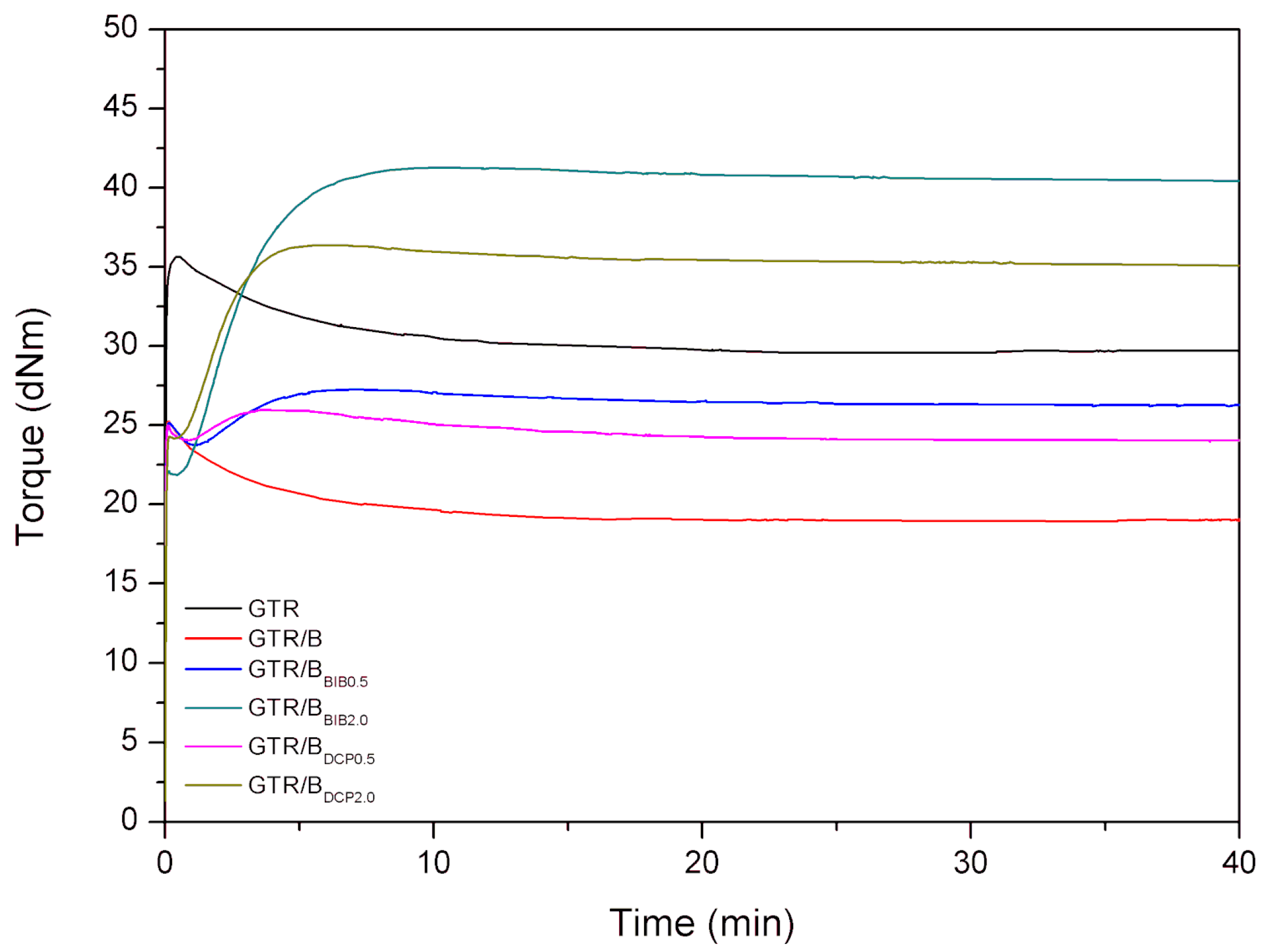
3.1. Curing Characteristics
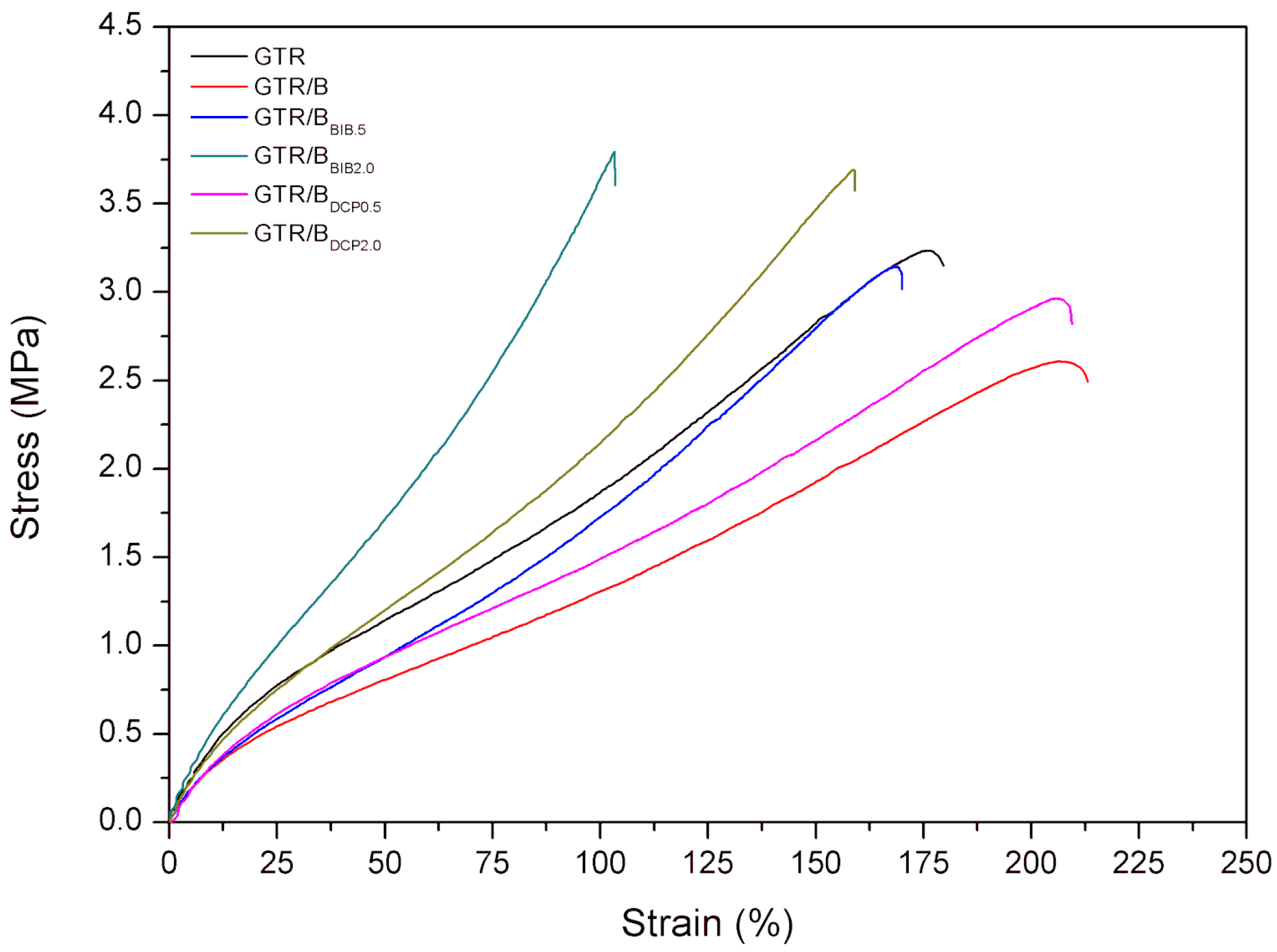
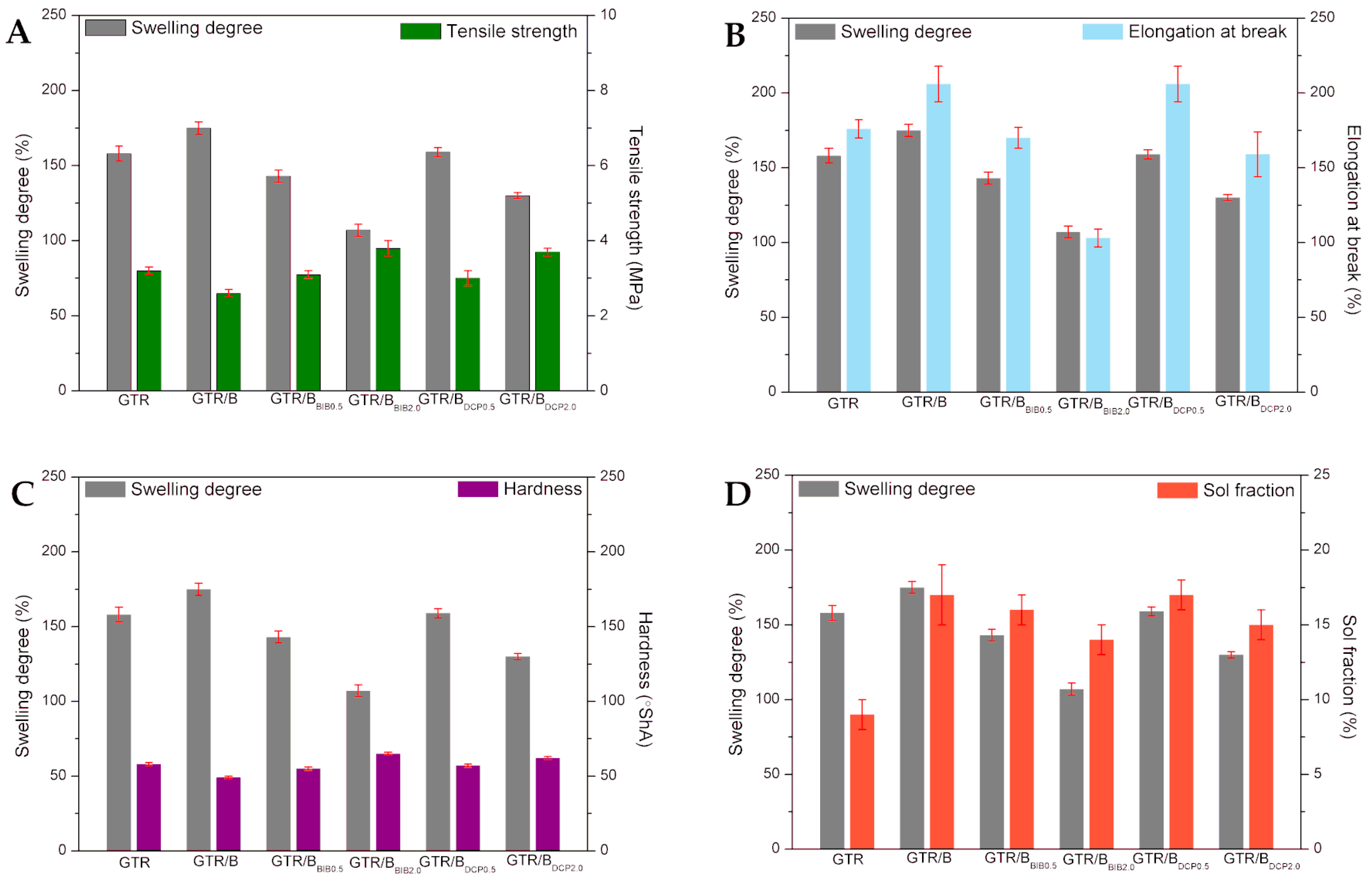
3.2. Physico-Mechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mitchell, N.C. Process of Reclaiming Rubber from Waste-Rubber Goods. U.S. Patent US419697, 21 January 1890. [Google Scholar]
- Garlick, F.W.; Bear, C.I.; Vail, W.A.; Wheeler, O.A. Art or Process of Reclaiming Scrap or Waste Vulcanized Rubber. U.S. Patent US866759, 24 September 1907. [Google Scholar]
- Lovette, N.G. Process for Reclaiming Rubber, Metal and Fabric from Whole Tires. U.S. Patent US4025990, 28 April 1976. [Google Scholar]
- Kim, J.K.; Lee, S.H. New technology of crumb rubber compounding for recycling of waste tires. J. Appl. Polym. Sci. 2000, 78, 1573–1577. [Google Scholar] [CrossRef]
- De, D.; Das, A.; De, D.; Dey, B.; Debnath, S.C.; Roy, B.C. Reclaiming of ground rubber tire (GRT) by a novel reclaiming agent. Eur. Polym. J. 2006, 42, 917–927. [Google Scholar] [CrossRef]
- Asaro, L.; Gratton, M.; Seghar, S.; Aït Hocine, N. Recycling of rubber wastes by devulcanization. Resour. Conserv. Recycl. 2018, 133, 250–262. [Google Scholar] [CrossRef]
- Formela, K.; Hejna, A.; Zedler, Ł.; Colom, X.; Cañavate, J. Microwave treatment in waste rubber recycling–Recent advances and limitations. Express Polym. Lett. 2019, 13, 565–588. [Google Scholar] [CrossRef]
- Adhikari, B.; De, D.; Maiti, S. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Nadal, M.; Rovira, J.; Díaz-Ferrero, J.; Schuhmacher, M.; Domingo, J.L. Human exposure to environmental pollutants after a tire landfill fire in Spain: Health risks. Environ. Int. 2016, 97, 37–44. [Google Scholar] [CrossRef]
- Jang, J.-W.; Yoo, T.-S.; Oh, J.-H.; Iwasaki, I. Discarded tire recycling practices in the United States, Japan and Korea. Resour. Conserv. Recycl. 1998, 22, 1–14. [Google Scholar] [CrossRef]
- Ogilvie, G.; Macdonald, K.; Karlik-Neale, M. Product Stewardship Case Study for End-of-Life Tyres; Raport of URS: Wellington, New Zealand, 2006. [Google Scholar]
- Torretta, V.; Rada, E.C.; Ragazzi, M.; Trulli, E.; Istrate, I.A.; Cioca, L.I. Treatment and disposal of tyres: Two EU approaches. A review. Waste Manag. 2015, 45, 152–160. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Mészáros, L.; Bárány, T. Ground tyre rubber (GTR) in thermoplastics, thermosets, and rubbers. J. Mater. Sci. 2013, 48, 1–38. [Google Scholar] [CrossRef]
- Ramarad, S.; Khalid, M.; Ratnam, C.T.; Chuah, A.L.; Rashmi, W. Waste tire rubber in polymer blends: A review on the evolution, properties and future. Prog. Mater. Sci. 2015, 72, 100–140. [Google Scholar] [CrossRef]
- Arastoopour, H.; Schocke, D.A.; Bernstein, B.; Bilgili, E. Process for Recycling of Rubber Materials. U.S. Patent US5904885, 18 May 1999. [Google Scholar]
- Saiwari, S.; Dierkes, W.K.; Noordermeer, J.W.M. Comparative investigation of the devulcanization parameters of tire rubbers. Rubber Chem. Technol. 2014, 87, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Sabzekar, M.; Chenar, M.P.; Mortazavi, S.M.; Kariminejad, M.; Asadi, S.; Zohuri, G. Influence of process variables on chemical devulcanization of sulfur-cured natural rubber. Polym. Degrad. Stab. 2015, 118, 88–95. [Google Scholar] [CrossRef]
- De, D.; De, D.; Singharoy, G.M. Reclaiming of ground rubber tire by a novel reclaiming agent. I. Virgin natural rubber/reclaimed GRT vulcanizates. Polym. Eng. Sci. 2007, 47, 1091–1100. [Google Scholar] [CrossRef]
- Thaicharoen, P.; Thamyongkit, P.; Poompradub, S. Thiosalicylic acid as a devulcanizing agent for mechano-chemical devulcanization. Korean J. Chem. Eng. 2010, 27, 1177–1183. [Google Scholar] [CrossRef]
- Ostad, M.S.; Ansarifar, A.; Karbalaee, S.; Athary Far, S. Devulcanization and recycling of waste automotive EPDM rubber powder by using shearing action and chemical additive. Prog. Rubber Plast. Recycl. Technol. 2015, 31, 87–116. [Google Scholar]
- Neto, J.R.A.; Visconte, L.L.Y.; Tavares, M.I.B.; Pacheco, E.B.A.V.; Furtado, C.R.G. Regeneration of vulcanized compounds based on butadiene-styrene copolymer. Int. J. Polym. Mater. Polym. Biomater. 2007, 56, 565–578. [Google Scholar] [CrossRef]
- Formela, K.; Cysewska, M.; Januszewicz, K. Effect of addition of reclaimed rubber on curing characteristics and mechanical properties of styrene-butadiene rubber. Przem. Chem. 2014, 93, 666–671. [Google Scholar]
- Sabzekar, M.; Zohuri, G.; Chenar, M.P.; Mortazavi, S.M.; Kariminejad, M.; Asadi, S. A new approach for reclaiming of waste automotive EPDM rubber using waste oil. Polym. Degrad. Stab. 2016, 129, 56–62. [Google Scholar] [CrossRef]
- Formela, K.; Klein, M.; Colom, X.; Saeb, M.R. Investigating the combined impact of plasticizer and shear force on the efficiency of low temperature reclaiming of ground tire rubber (GTR). Polym. Degrad. Stab. 2016, 125, 1–11. [Google Scholar] [CrossRef]
- Khang, T.H.; Ariff, Z.M. Vulcanization kinetics study of natural rubber compounds having different formulation variables. J. Therm. Anal. Calorim. 2012, 109, 1545–1553. [Google Scholar] [CrossRef]
- Yazdani, H.; Karrabi, M.; Ghasmi, I.; Azizi, H.; Bakhshandeh, G.R. Devulcanization of waste tires using a twin-screw extruder: The effects of processing conditions. J. Vinyl Addit. Technol. 2011, 17, 64–69. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, H.Y.; Lim, J.C.; Jeon, I.R.; Seo, K.H. Cure characteristics and physical properties of ground-rubber-filled natural rubber vulcanizates: Effects of the curing systems of the ground rubber and rubber matrix. J. Appl. Polym. Sci. 2007, 105, 2396–2406. [Google Scholar] [CrossRef]
- Ray, S.; Cooney, R.P. Chapter 7: Thermal degradation of polymer and polymer composites. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew Publishing (Elsevier): Norwich, NY, USA, 2013. [Google Scholar]
- Zedler, Ł.; Colom, X.; Saeb, M.R.; Formela, K. Preparation and characterization of natural rubber composites highly filled with brewers’ spent grain/ground tire rubber hybrid reinforcement. Compos. Part B Eng. 2018, 145, 182–188. [Google Scholar] [CrossRef]
- Morin, J.E.; Williams, D.E.; Farris, R.J. A novel method to recycle scrap tires: High-pressure high-temperature sintering. Rubber Chem. Technol. 2002, 75, 955–968. [Google Scholar] [CrossRef]
- Zhao, F.; Bi, W.; Zhao, S. Influence of crosslink density on mechanical properties of natural rubber vulcanizates. J. Macromol. Sci. Part B Phys. 2011, 50, 1460–1469. [Google Scholar] [CrossRef]
- Nandi, S.; Winter, H.H. Swelling behavior of partially cross-linked polymers: A ternary system. Macromolecules 2005, 38, 4447–4455. [Google Scholar] [CrossRef] [Green Version]

| Name | Abbreviation | Active Oxygen (%) * | The Half-Life Temperature (°C) * |
|---|---|---|---|
| Di-(2-tert-butyl-peroxyisopropyl)-benzene | BIB | 8.98 | 169 |
| Dicumyl peroxide | DCP | 5.80 | 162 |
| Components (phr) | Sample Code | |||||
|---|---|---|---|---|---|---|
| GTR | GTR/B a | GTR/BBIB0.5 b | GTR/BBIB2.0 b | GTR/BDCP0.5 c | GTR/BDCP2.0 c | |
| GTR | 100 | 100 | 100 | 100 | 100 | 100 |
| Bitumen 100/150 | - | 10 | 10 | 10 | 10 | 10 |
| BIB | - | - | 0.5 | 2.0 | - | - |
| DCP | - | - | - | - | 0.5 | 2.0 |
| Properties | Sample Code | |||||
|---|---|---|---|---|---|---|
| GTR | GTR/B | GTR/BBIB0.5 | GTR/BBIB2.0 | GTR/BDCP0.5 | GTR/BDCP2.0 | |
| Minimal torque (dNm) | - | - | 23.7 | 21.9 | - | 24.3 |
| Maximal torque (dNm) | - | - | 27.3 | 41.3 | - | 36.4 |
| ΔM (dNm) | - | - | 3.6 | 19.4 | - | 12.1 |
| Scorch time (t1, min) | - | - | 3.0 | 1.2 | - | 1.2 |
| Optimum cure time (t90, min) | 5 | 5 | 4.9 | 5.3 | 5 | 3.5 |
| Cure rate index (CRI, min−1) | - | - | 52.9 | 24.0 | - | 42.2 |
| Thermal aging resistance (R300, %) | - | - | 1.5 | 0.6 | - | 1.6 |
| Properties | Sample Code | |||||
|---|---|---|---|---|---|---|
| GTR | GTR/B | GTR/BBIB0.5 | GTR/BBIB2.0 | GTR/BDCP0.5 | GTR/BDCP2.0 | |
| Tensile strength (MPa) | 3.2 ± 0.1 | 2.6 ± 0.1 | 3.1 ± 0.1 | 3.8 ± 0.2 | 3.0 ± 0.2 | 3.7 ± 0.1 |
| Elongation at break (%) | 176 ± 6 | 206 ± 12 | 170 ± 7 | 103 ± 6 | 206 ± 12 | 159 ± 15 |
| M100 (MPa) | 1.8 | 1.3 | 1.5 | 3.6 | 1.4 | 2.4 |
| Hardness (Sh A) | 58 ± 1 | 49 ± 1 | 55 ± 1 | 65 ± 1 | 57 ± 1 | 62 ± 1 |
| Density at 25 °C (g/cm3) | 1.162 ± 0.010 | 1.142 ± 0.011 | 1.149 ± 0.012 | 1.18 ± 0.011 | 1.144 ± 0.010 | 1.149 ± 0.013 |
| Swelling degree (%) | 158 ± 5 | 175 ± 4 | 143 ± 4 | 107 ± 4 | 159 ± 3 | 130 ± 2 |
| Sol fraction (%) | 9 ± 1 | 17 ± 2 | 16 ± 1 | 14 ± 1 | 17 ± 1 | 15 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zedler, Ł.; Przybysz-Romatowska, M.; Haponiuk, J.; Wang, S.; Formela, K. Modification of Ground Tire Rubber—Promising Approach for Development of Green Composites. J. Compos. Sci. 2020, 4, 2. https://doi.org/10.3390/jcs4010002
Zedler Ł, Przybysz-Romatowska M, Haponiuk J, Wang S, Formela K. Modification of Ground Tire Rubber—Promising Approach for Development of Green Composites. Journal of Composites Science. 2020; 4(1):2. https://doi.org/10.3390/jcs4010002
Chicago/Turabian StyleZedler, Łukasz, Marta Przybysz-Romatowska, Józef Haponiuk, Shifeng Wang, and Krzysztof Formela. 2020. "Modification of Ground Tire Rubber—Promising Approach for Development of Green Composites" Journal of Composites Science 4, no. 1: 2. https://doi.org/10.3390/jcs4010002
APA StyleZedler, Ł., Przybysz-Romatowska, M., Haponiuk, J., Wang, S., & Formela, K. (2020). Modification of Ground Tire Rubber—Promising Approach for Development of Green Composites. Journal of Composites Science, 4(1), 2. https://doi.org/10.3390/jcs4010002







