Evaluation of a Nanocomposite Based on Reduced Graphene Oxide and Gold Nanoparticles as an Electrochemical Platform for Detection of Sulfamethazine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. Electroanalytical Procedures
2.3. Synthesis of GO, rGO and rGO-AuNPs Composite
2.4. Preparation of the Electrodes
2.5. Preparation of Swine Effluent Samples for SMZ Analysis
3. Results and Discussion
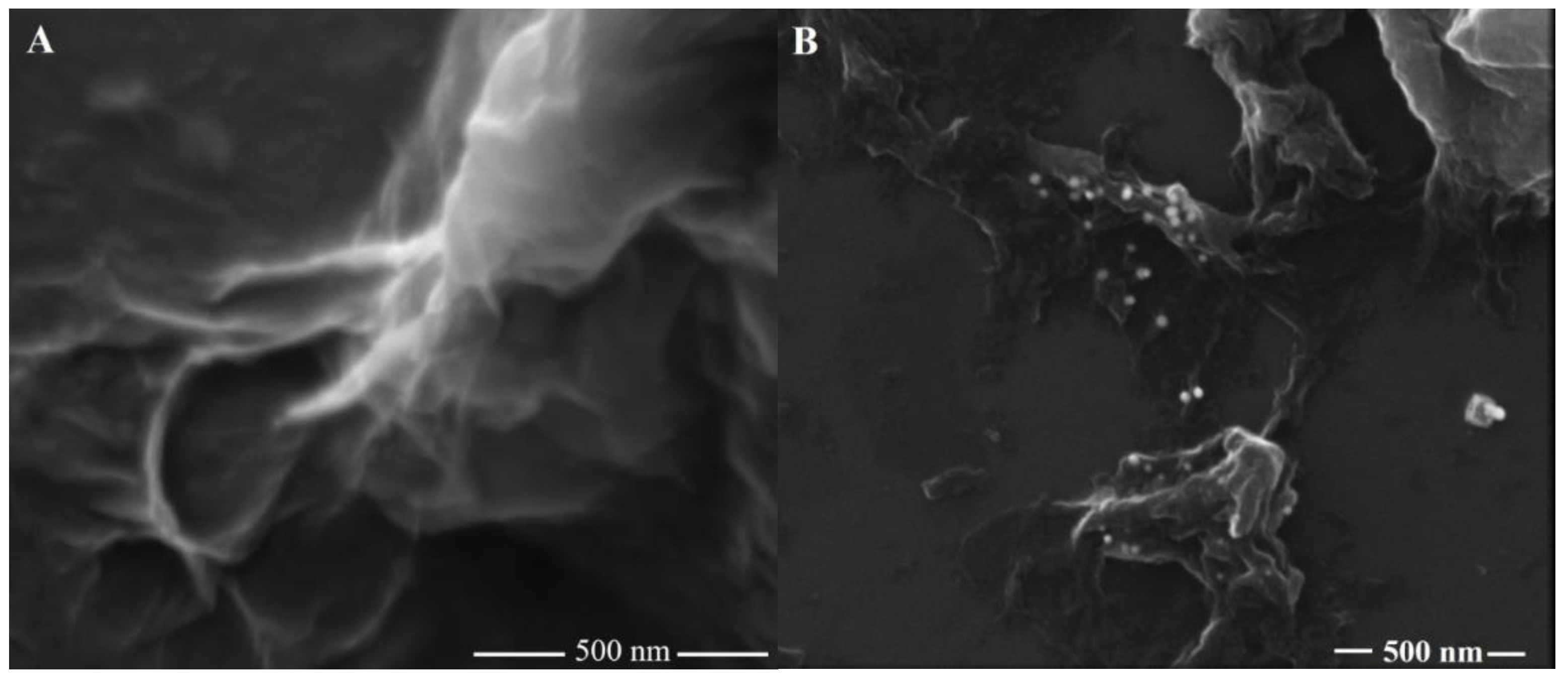
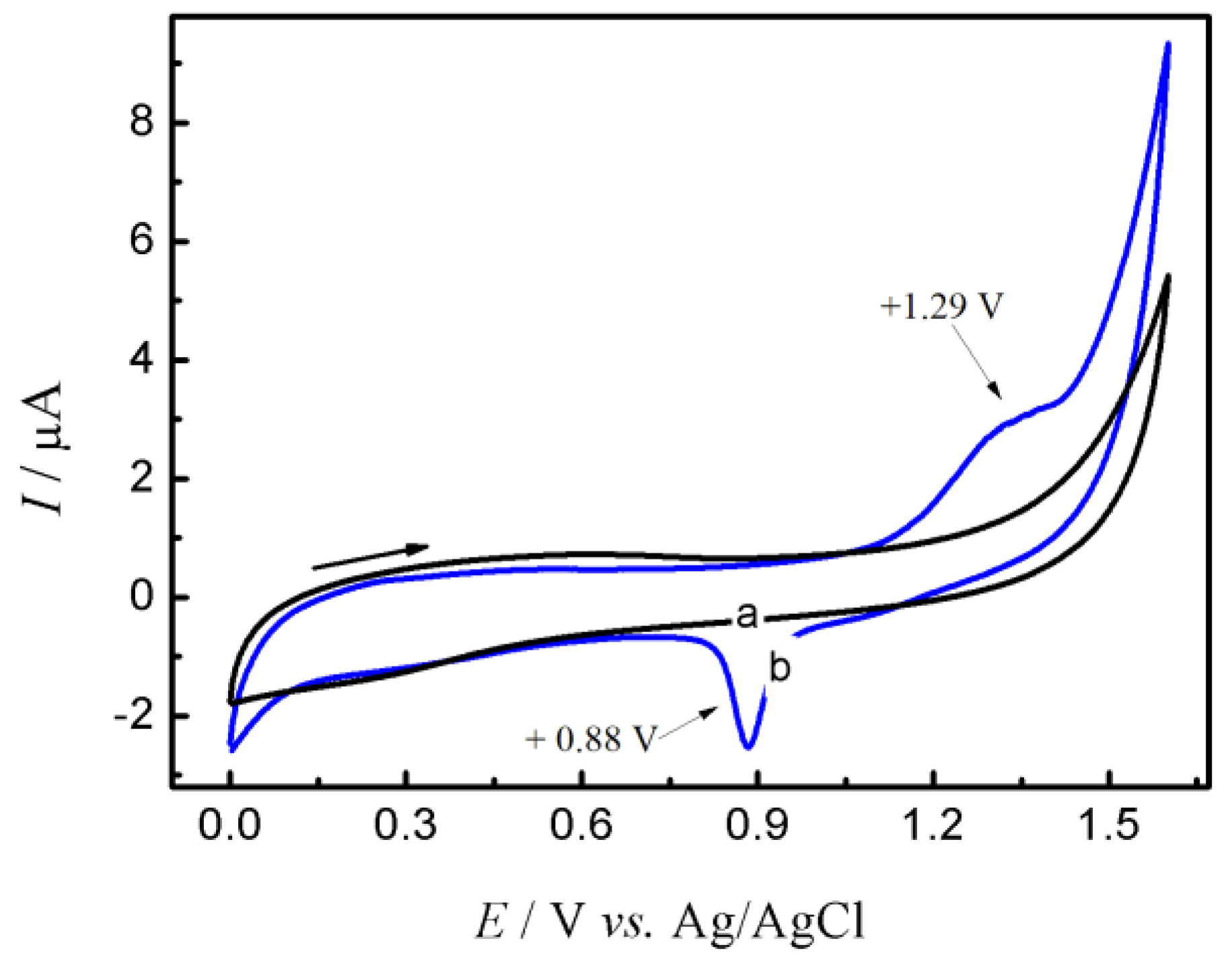
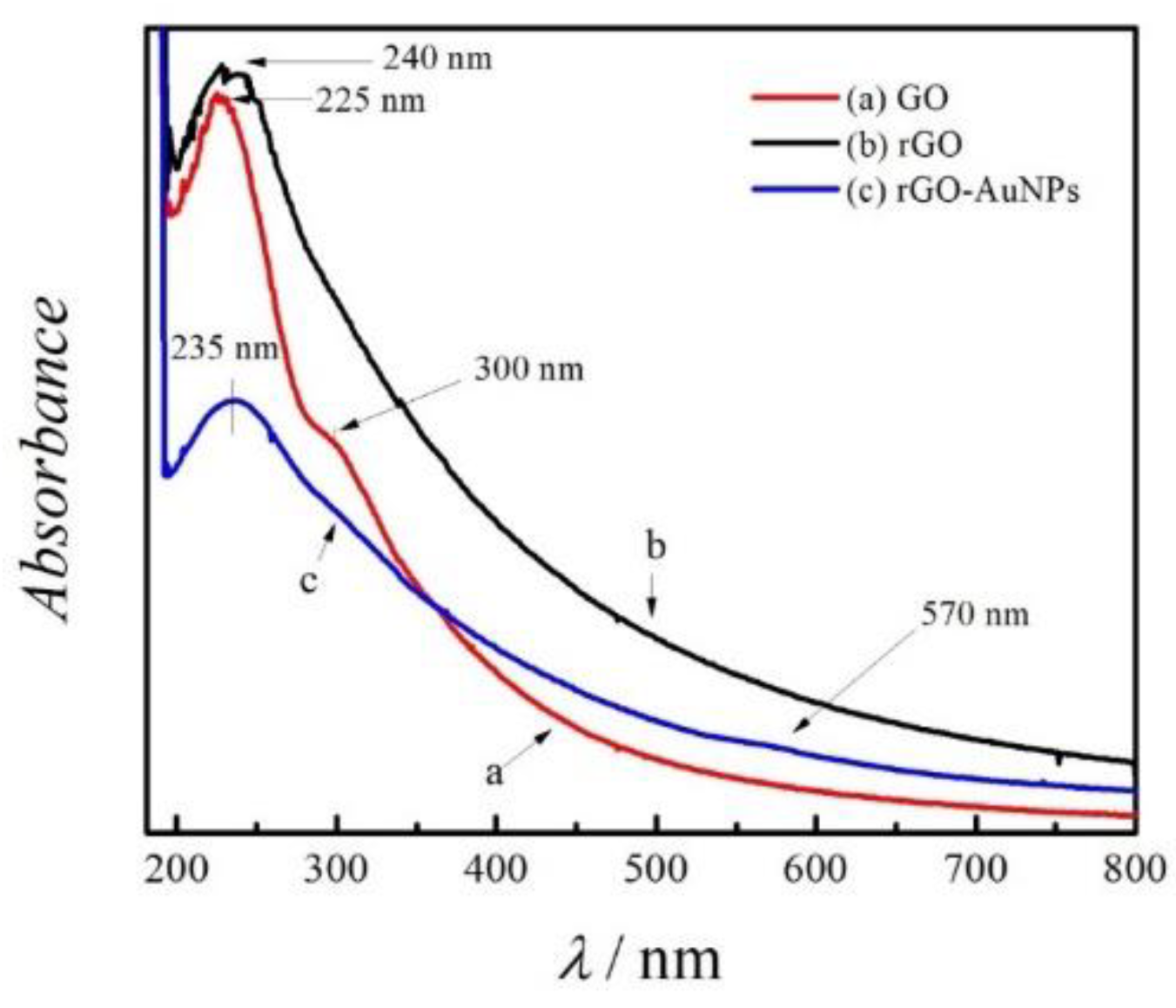
3.1. Characterization of the rGO-AuNPs Composite
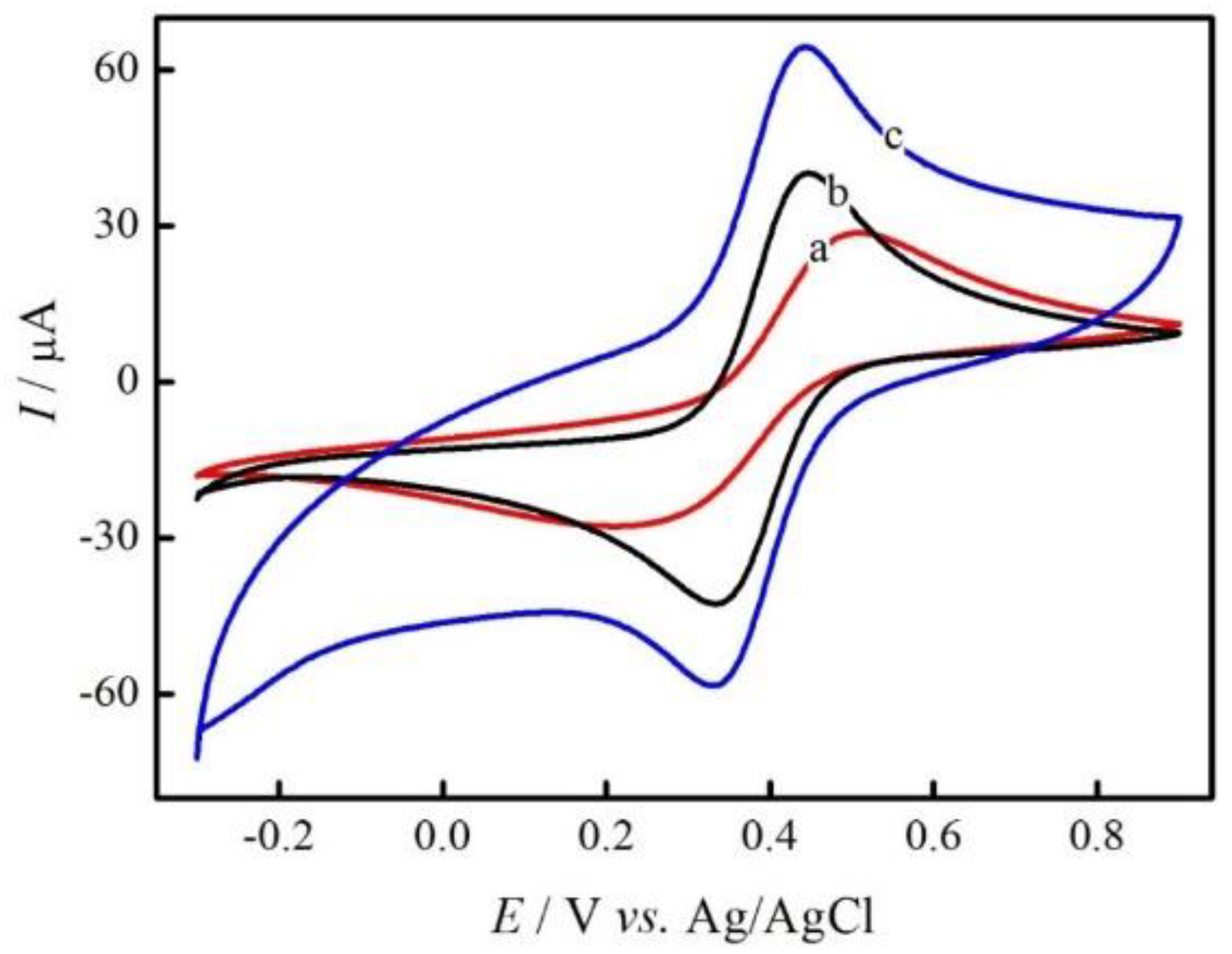
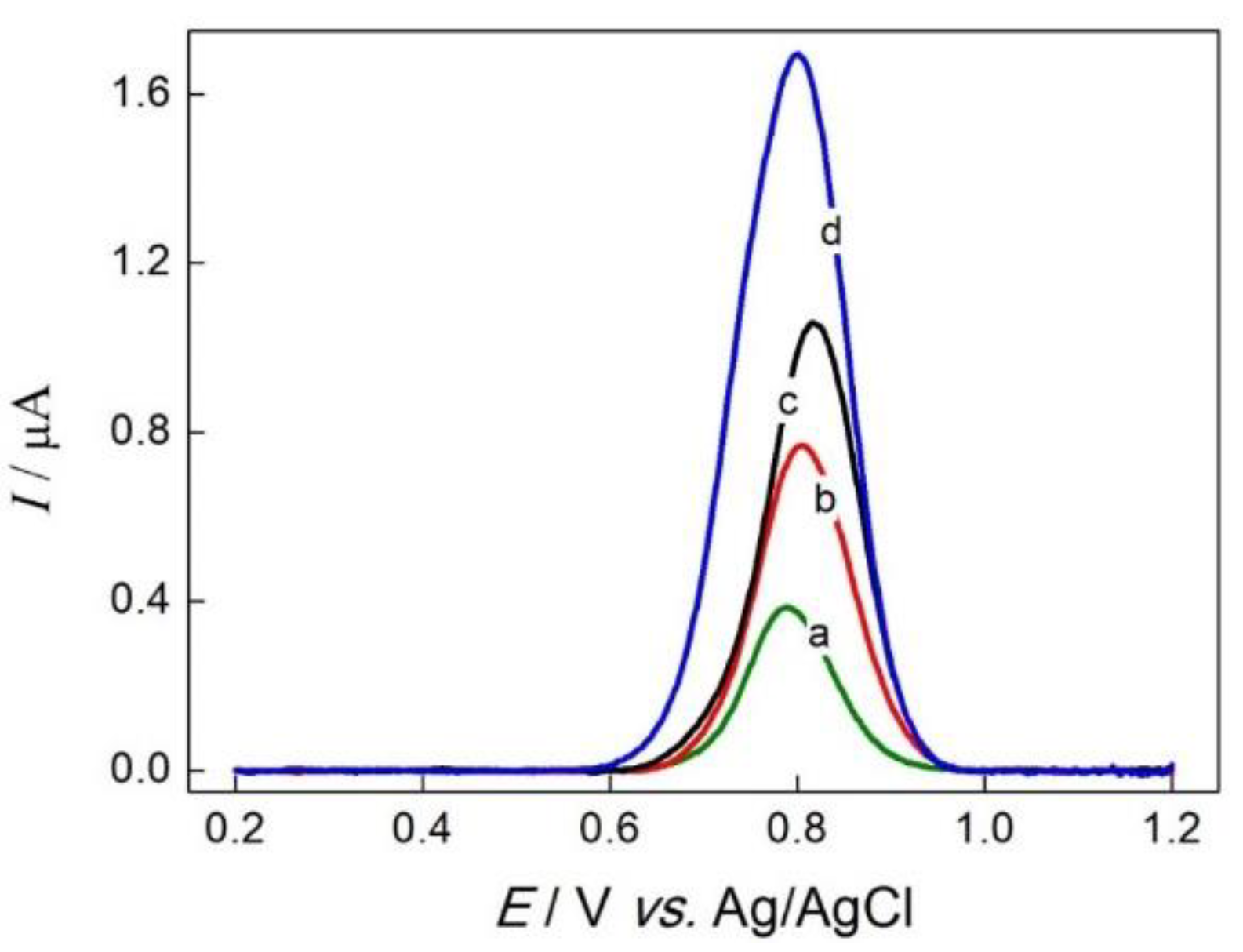
3.2. Electrochemical Behavior of GC/rGO-AuNPs Composite Electrode on the Sulfamethazine Oxidation Process
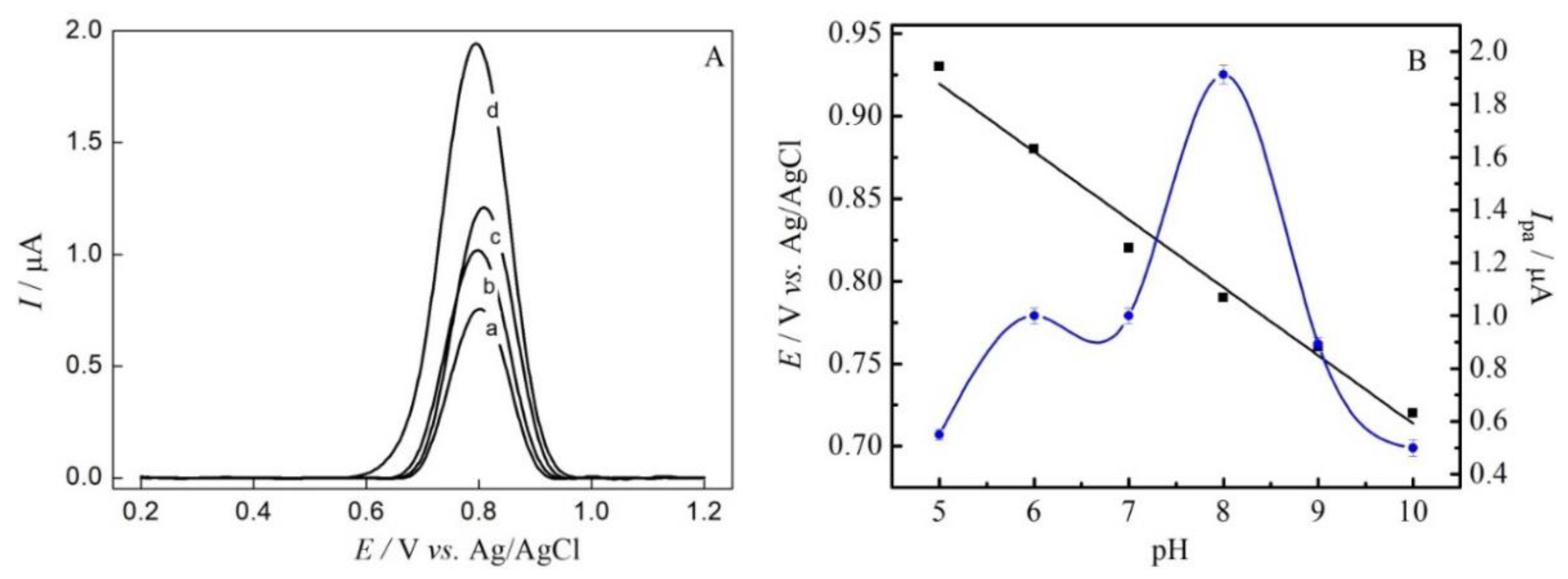
3.3. Effect of pH and Support Electrolyte on SMZ Oxidation
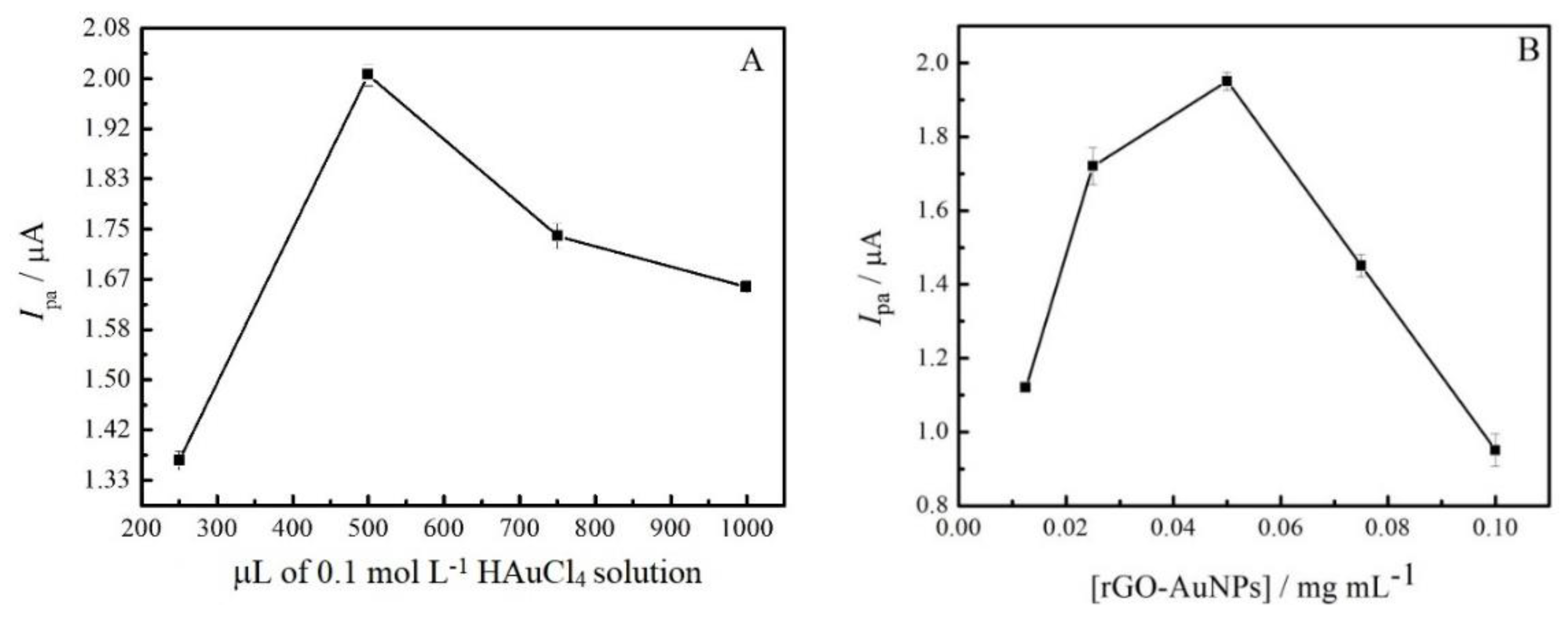
3.4. Optimization of GC/rGO-AuNPs Electrode Preparation
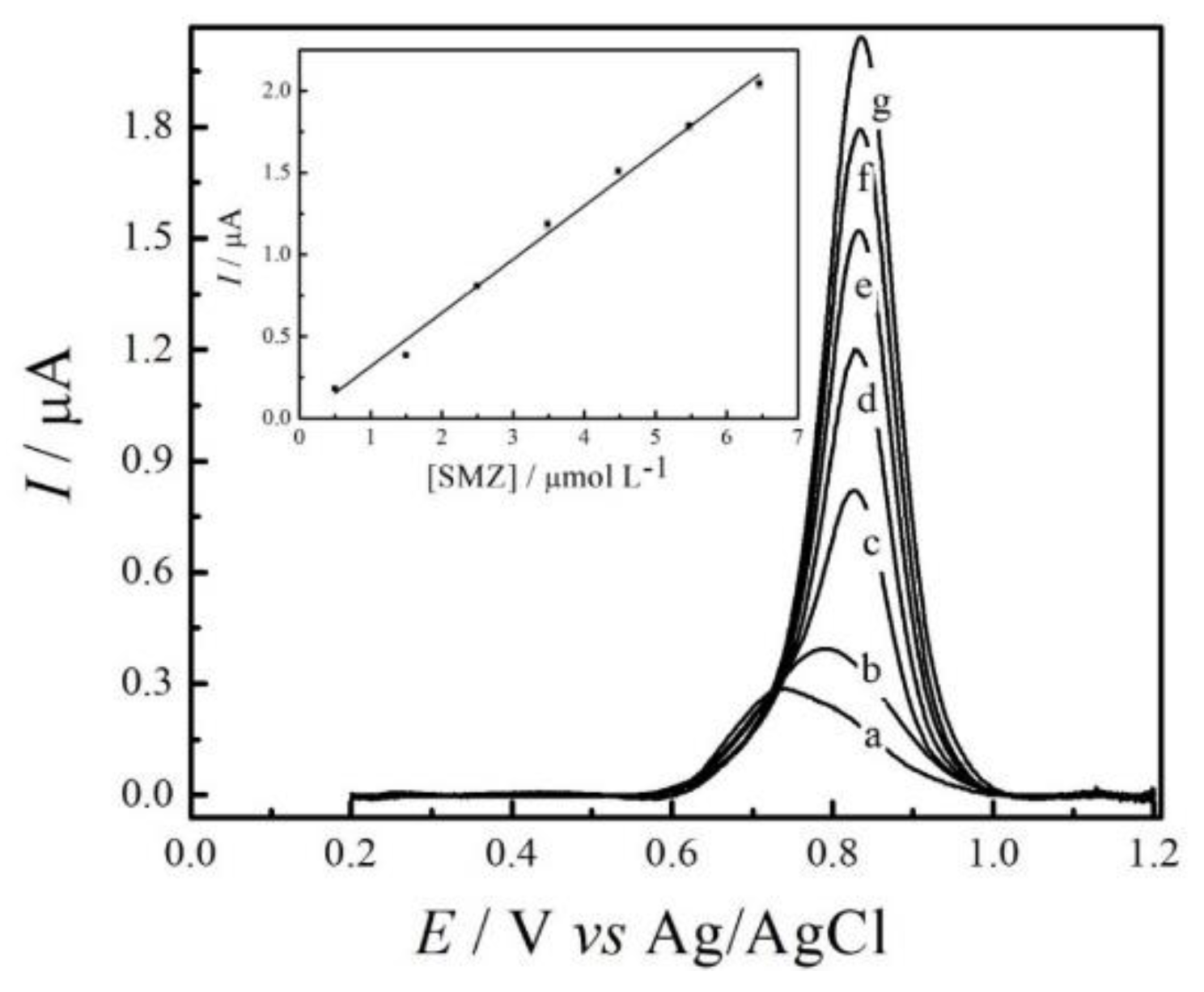
3.5. Calibration Curve
3.6. Influence of Interferences in the Determination of SMZ
3.7. Determination of SMZ in Synthetic Swine Effluent
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Zayas-Blanco, F.; García-Falcón, M.; Simal-Gándara, J. Determination of sulfamethazine in milk by solid phase extraction and liquid chromatographic separation with ultraviolet detection. Food Control 2004, 15, 375–378. [Google Scholar] [CrossRef]
- Dixon-Holland, D.E.; Katz, S.E. Competitive direct enzyme-linked immunosorbent screening assay for the detection of sulfamethazine contamination of animal feeds. J. Assoc. Off. Anal. Chem. 1991, 74, 784–789. [Google Scholar] [PubMed]
- Laurensen, J.J.; Nouws, J.F.M. Monitoring of chloramphenicol residues in muscle tissues by an immunoassay (La Carte® test). Vet. Q. 1990, 12, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Church, T.L.; Janzen, E.D.; Sisodia, C.S.; Radostits, O.M. Blood levels of sulfamethazine achieved in beef calves on medicated drinking water. Can. Vet. J. 1979, 20, 41–44. [Google Scholar] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. International Program on Chemical Safety. In Toxicological Evaluation of Certain food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 1993; ISBN 9241660325. [Google Scholar]
- Guzmán-Vázquez De Prada, A.; Martínez-Ruiz, P.; Reviejo, A.J.; Pingarrón, J.M. Solid-phase molecularly imprinted on-line preconcentration and voltammetric determination of sulfamethazine in milk. Anal. Chim. Acta 2005, 539, 125–132. [Google Scholar] [CrossRef]
- Cesarino, I.; Simões, R.P.; Lavarda, F.C.; Batagin-Neto, A. Electrochemical oxidation of sulfamethazine on a glassy carbon electrode modified with graphene and gold nanoparticles. Electrochim. Acta 2016, 192, 8–14. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, J.; Yang, Y.; Yin, J.; Zhang, J.; Shao, B. Transformation of sulfamethazine during the chlorination disinfection process: Transformation, kinetics, and toxicology assessment. J. Environ. Sci. 2018, 76, 48–56. [Google Scholar] [CrossRef]
- Washington, M.T.; Moorman, T.B.; Soupir, M.L.; Shelley, M.; Morrow, A.J. Monitoring tylosin and sulfamethazine in a tile-drained agricultural watershed using polar organic chemical integrative sampler (POCIS). Sci. Total Environ. 2018, 612, 358–367. [Google Scholar] [CrossRef]
- Peng, D.; Li, Z.; Wang, Y.; Liu, Z.; Sheng, F.; Yuan, Z. Enzyme-linked immunoassay based on imprinted microspheres for the detection of sulfamethazine residue. J. Chromatogr. A 2017, 1506, 9–17. [Google Scholar] [CrossRef]
- Su, Y.-L.; Cheng, S.-H. A novel electroanalytical assay for sulfamethazine determination in food samples based on conducting polymer nanocomposite-modified electrodes. Talanta 2018, 180, 81–89. [Google Scholar] [CrossRef]
- Rao, T.N.; Sarada, B.V.; Tryk, D.A.; Fujishima, A. Electroanalytical study of sulfa drugs at diamond electrodes and their determination by HPLC with amperometric detection. J. Electroanal. Chem. 2000, 491, 175–181. [Google Scholar] [CrossRef]
- He, B.; Chen, W.; Using, E.; Multiwalled, C.; Nanotubes, C.; He, B.; Chen, W.; Using, E.; Multiwalled, C.; Nanotubes, C. Voltammetric Determination of Sulfonamides with a Modified Glassy Carbon Electrode Using Carboxyl Multiwalled Carbon Nanotubes. J. Braz. Chem. Soc. 2016, 27, 2216–2225. [Google Scholar] [CrossRef]
- Fotouhi, L.; Zabeti, M. Electrochemical Oxidation of Sulfamethazine on Multi-Walled Nanotube Film Coated Glassy Carbon Electrode. Univ. Kashan 2014, 4, 161–166. [Google Scholar]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Huang, X.J. Voltammetric determination of mercury(II). TrAC Trends Anal. Chem. 2013, 51, 1–12. [Google Scholar] [CrossRef]
- Cesarino, I.; Hümmelgen, I.A. An additional tool towards overcoming absence of specificity of carbon nanostructure-based electrochemical sensors—Application to estriol and estradiol detection and distinction. J. Solid State Electrochem. 2015, 19, 3045–3050. [Google Scholar] [CrossRef]
- Cesarino, I.; Cincotto, F.H.; Machado, S.A.S. A synergistic combination of reduced graphene oxide and antimony nanoparticles for estriol hormone detection. Sens. Actuators B Chem. 2015, 210, 453–459. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, J.; Wu, H.; Liu, J.; Aksay, I.A.; Lin, Y. Graphene Based Electrochemical Sensors and Biosensors: A Review. Electroanalysis 2010, 22, 1027–1036. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold Nanoparticles in Chemical and Biological Sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef]
- Zayats, M.; Baron, R.; Popov, I.; Willner, I. Biocatalytic growth of Au nanoparticles: From mechanistic aspects to biosensors design. Nano Lett. 2005, 5, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, Y.; Jiang, Q.; Sun, Y.; Deng, L.; Liang, Z.; Du, Q.; Xing, J.; Zhao, Y.; Wang, P.C.; et al. Enhanced Gene Delivery and siRNA Silencing by Gold Nanoparticles Coated with Charge-Reversal Polyelectrolyte. ACS Nano 2010, 4, 5505–5511. [Google Scholar] [CrossRef] [PubMed]
- Rosi, N.L.; Giljohann, D.A.; Thaxton, C.S.; Lytton-Jean, A.K.R.; Han, M.S.; Mirkin, C.A. Oligonucleotide-Modified Gold Nanoparticles for Intracellular Gene Regulation. Science 2006, 312, 1027–1030. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.; Bhumkar, D.; Suresh, K.; Shinde, Y.; Gairola, S.; Jadhav, S.S. Gold nanoparticles as a potential carrier for transmucosal vaccine delivery. J. Biomed. Nanotechnol. 2011, 7, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, X.; Yu, X.; Zha, X.; Fu, Q.; Liu, B.; Wang, X.; Chen, Y.; Chen, Y.; Shan, Y.; et al. The effect of conjugation to gold nanoparticles on the ability of low molecular weight chitosan to transfer DNA vaccine. Biomaterials 2008, 29, 111–117. [Google Scholar] [CrossRef]
- Hall, W.P.; Ngatia, S.N.; Van Duyne, R.P. LSPR Biosensor Signal Enhancement Using Nanoparticle−Antibody Conjugates. J. Phys. Chem. C 2011, 115, 1410–1414. [Google Scholar] [CrossRef]
- Pissuwan, D.; Cortie, C.H.; Valenzuela, S.M.; Cortie, M.B. Functionalised gold nanoparticles for controlling pathogenic bacteria. Trends Biotechnol. 2010, 28, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Pivodová, V.; Franková, J.; Galandáková, A.; Ulrichová, J. In Vitro AuNPs’ Cytotoxicity and Their Effect on Wound Healing. Nanobiomedicine 2015, 2, 2–7. [Google Scholar] [CrossRef]
- Meir, R.; Shamalov, K.; Betzer, O.; Motiei, M.; Horovitz-Fried, M.; Yehuda, R.; Popovtzer, A.; Popovtzer, R.; Cohen, C.J. Nanomedicine for Cancer Immunotherapy: Tracking Cancer-Specific T-Cells in Vivo with Gold Nanoparticles and CT Imaging. ACS Nano 2015, 9, 6363–6372. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-Tethered Responsive Gold Nanoparticles Facilitate Intracellular Drug Delivery for Overcoming Multidrug Resistance in Cancer Cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef]
- Megiel, E. Surface modification using TEMPO and its derivatives. Adv. Colloid Interface Sci. 2017, 250, 158–184. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Souza, J.S.; Hirata, F.T.H.; Corio, P. Microwave-assisted synthesis of bismuth vanadate nanoflowers decorated with gold nanoparticles with enhanced photocatalytic activity. J. Nanopart. Res. 2019, 21, 35. [Google Scholar] [CrossRef]
- Schmidt, R.; Prado-Gonjal, J.; Morán, E. Microwave-Assisted Hydrothermal Synthesis of Nanoparticles. In CRC Concise Encyclopedia of Nanotechnology; Kharisov, B.I., Ortiz-Mendez, O.V.K.U., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2018; pp. 585–596. ISBN 9781466580343. [Google Scholar]
- Ali, G.A.M.; Megiel, E.; Romański, J.; Algarni, H.; Chong, K.F. A wide potential window symmetric supercapacitor by TEMPO functionalized MWCNTs. J. Mol. Liq. 2018, 271, 31–39. [Google Scholar] [CrossRef]
- He, D.; Shen, L.; Zhang, X.; Wang, Y.; Bao, N.; Kung, H.H. An efficient and eco-friendly solution-chemical route for preparation of ultrastable reduced graphene oxide suspensions. AIChE J. 2014, 60, 2757–2764. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.; Cesarino, I. Evaluation of a Nanocomposite Based on Reduced Graphene Oxide and Gold Nanoparticles as an Electrochemical Platform for Detection of Sulfamethazine. J. Compos. Sci. 2019, 3, 59. https://doi.org/10.3390/jcs3020059
Silva M, Cesarino I. Evaluation of a Nanocomposite Based on Reduced Graphene Oxide and Gold Nanoparticles as an Electrochemical Platform for Detection of Sulfamethazine. Journal of Composites Science. 2019; 3(2):59. https://doi.org/10.3390/jcs3020059
Chicago/Turabian StyleSilva, Martin, and Ivana Cesarino. 2019. "Evaluation of a Nanocomposite Based on Reduced Graphene Oxide and Gold Nanoparticles as an Electrochemical Platform for Detection of Sulfamethazine" Journal of Composites Science 3, no. 2: 59. https://doi.org/10.3390/jcs3020059
APA StyleSilva, M., & Cesarino, I. (2019). Evaluation of a Nanocomposite Based on Reduced Graphene Oxide and Gold Nanoparticles as an Electrochemical Platform for Detection of Sulfamethazine. Journal of Composites Science, 3(2), 59. https://doi.org/10.3390/jcs3020059





