pH-Sensitive Hydrogel from Polyethylene Oxide and Acrylic acid by Gamma Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PEO/AAc (PA) Hydrogels
2.3. FTIR-ATR Spectral Analysis
2.4. Scanning Electron Microscopy (SEM)
2.5. Measurement of Gel Fraction of Prepared Hydrogel
2.6. Measurement of Swelling Ratio in Distilled Water
2.7. Preparation of Buffer Solutions of Different pH
2.8. Measurement of Mechanical Properties (Tensile Strength) of Prepared Hydrogels
3. Results and Discussion
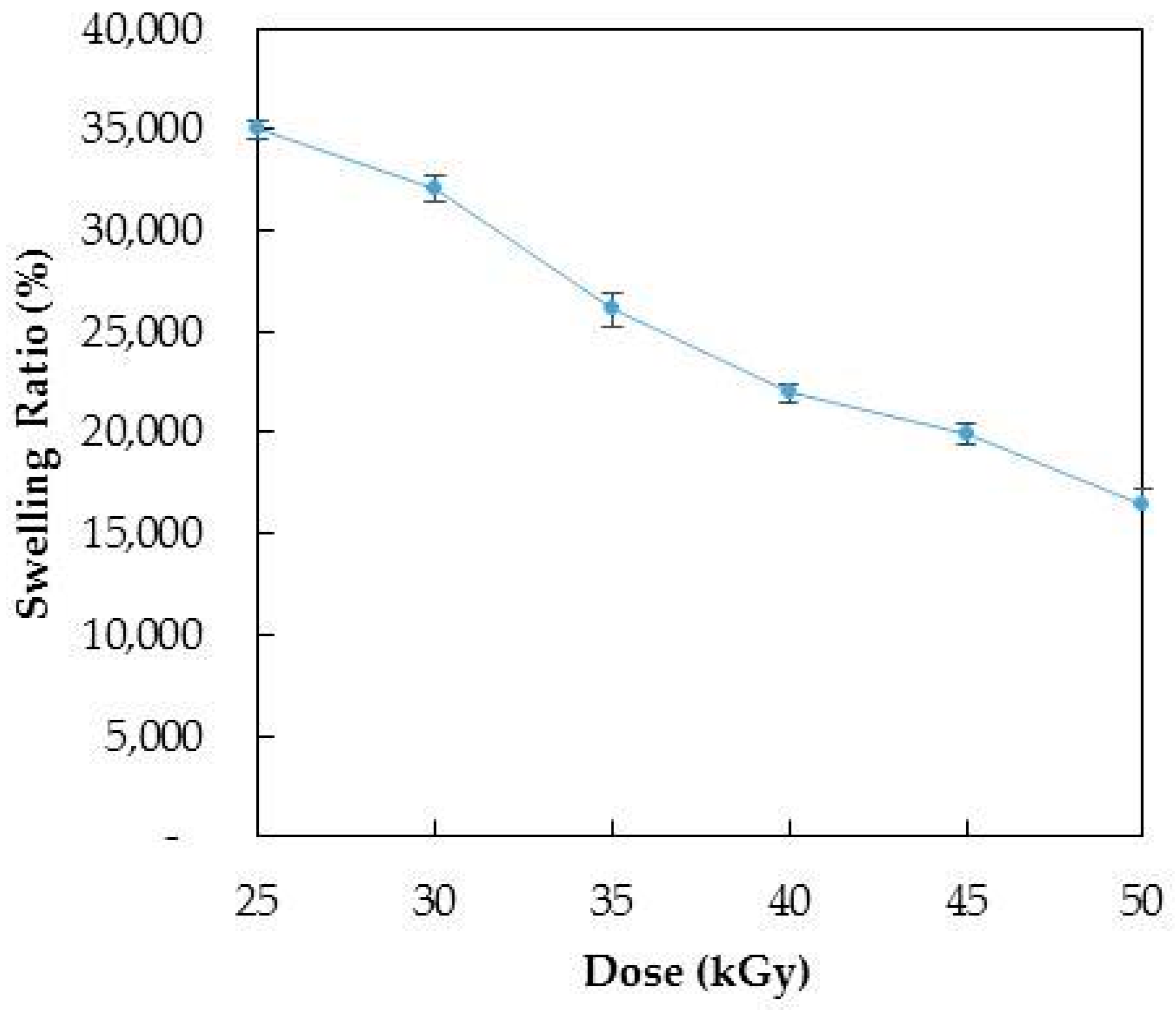
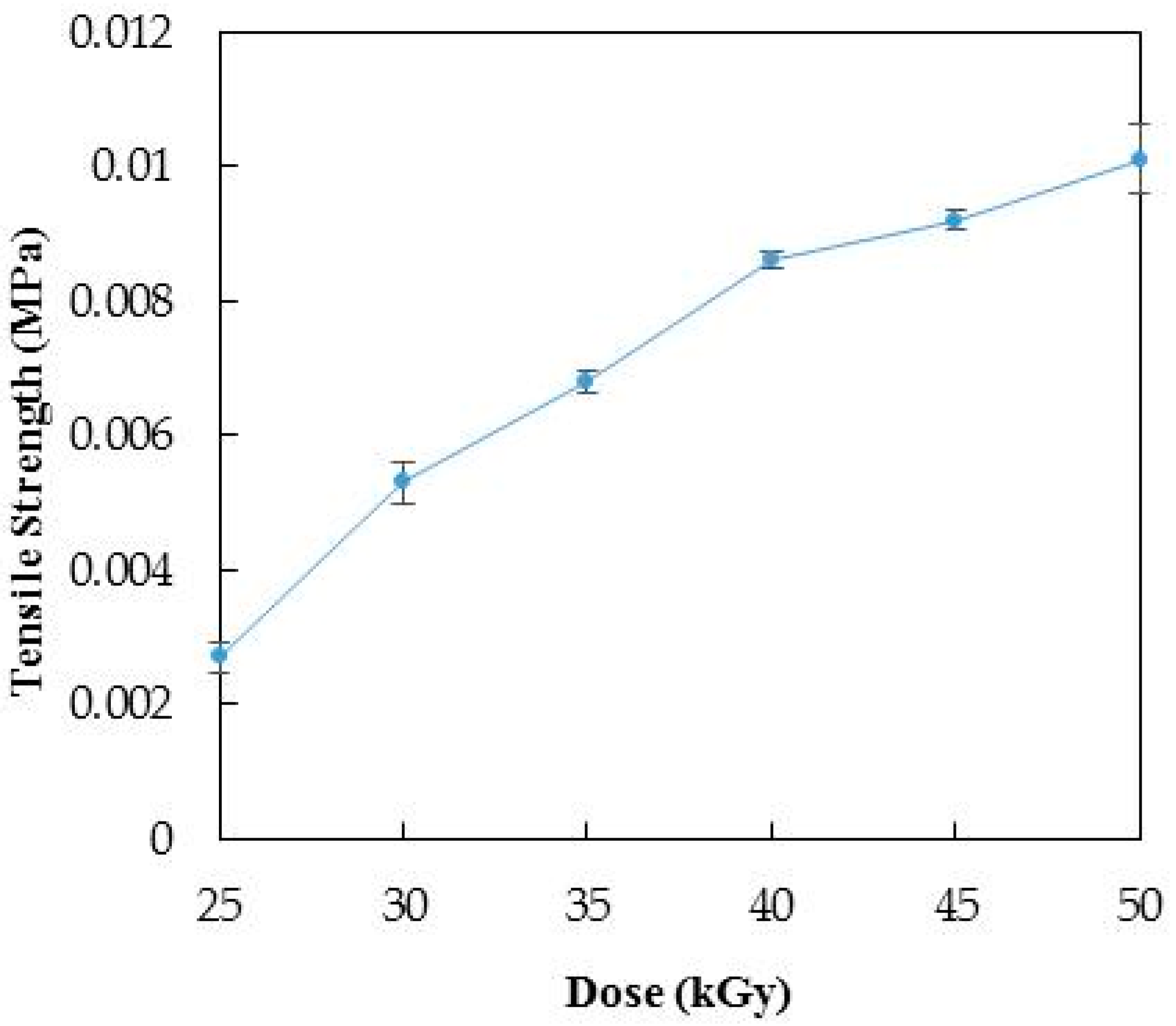
3.1. Optimization of Radiation Dose
3.2. PEO/AAc(PA) Hydrogels Synthesis at Optimized Dose
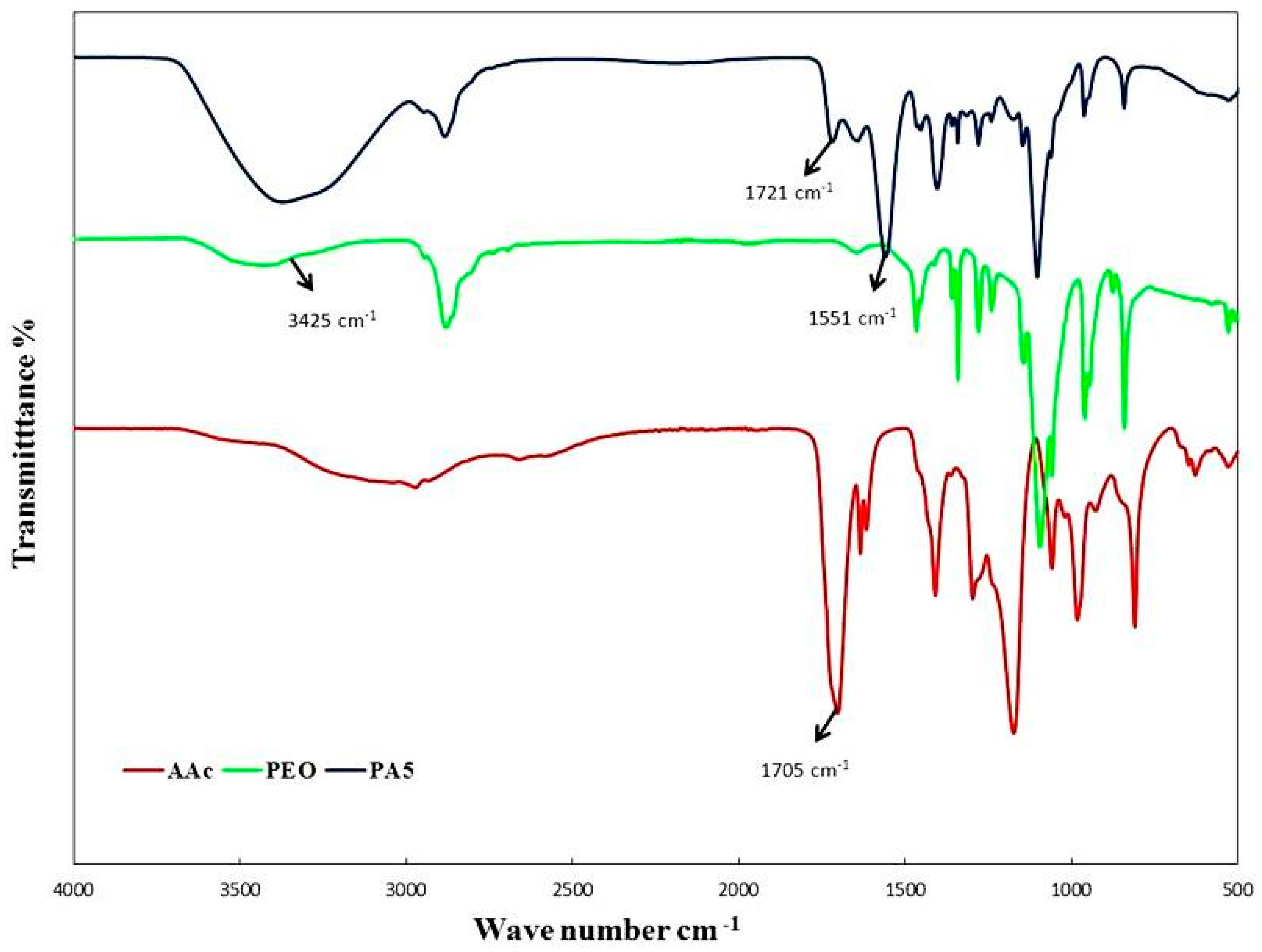
3.3. FT-IR Analysis of Hydrogels
3.4. Morphological Analysis by Scanning Electron Microscopy
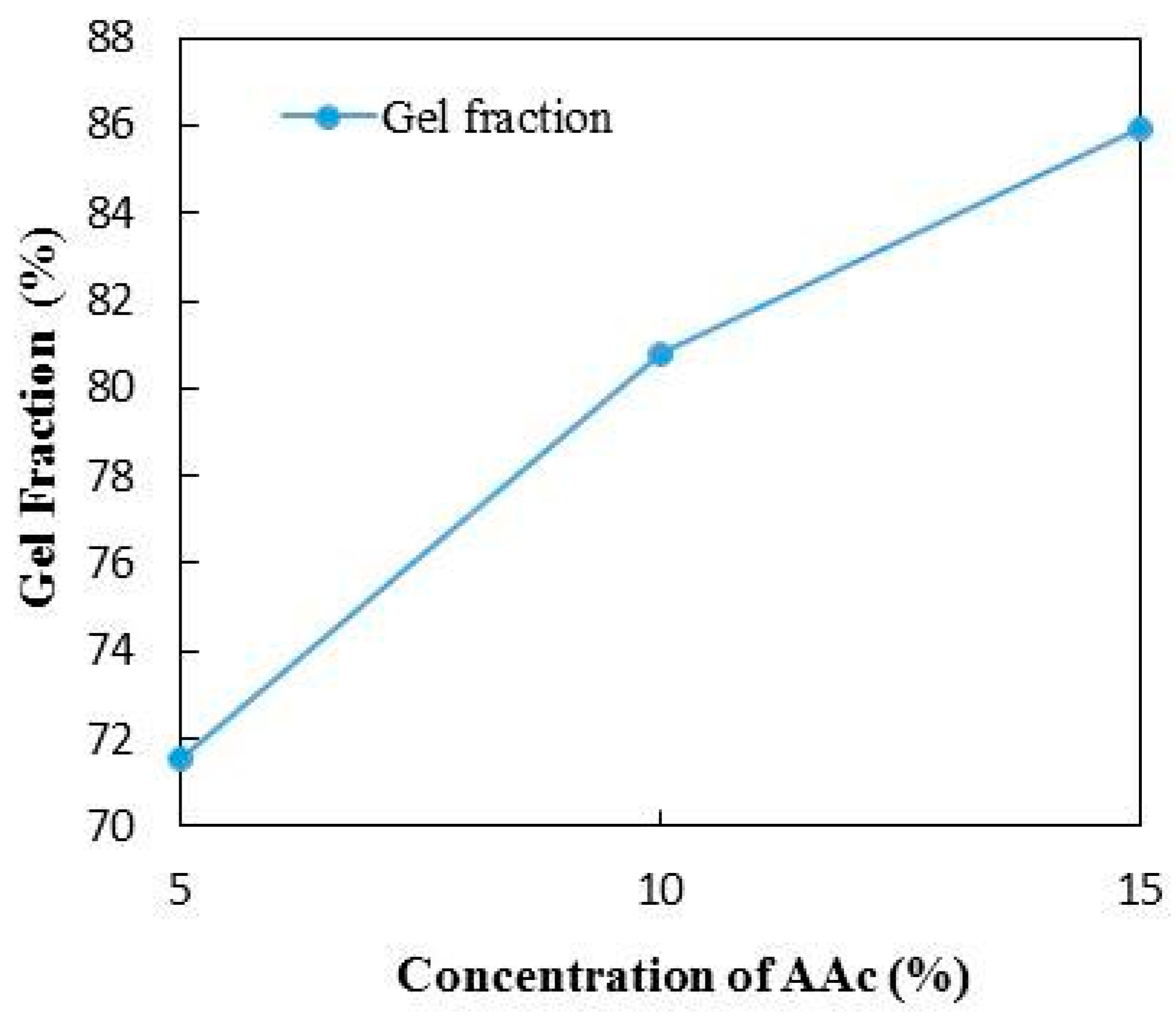
3.5. Gel Fraction of PA Hydrogel
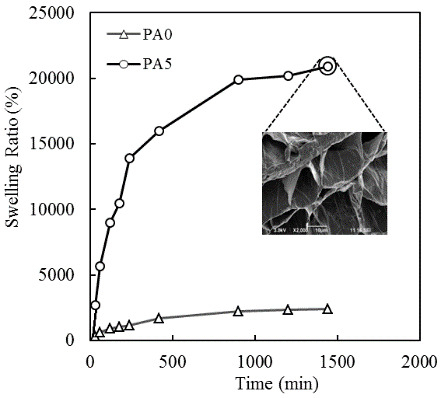
3.6. Swelling Ratio of PA Hydrogel in Distill Water
3.7. Effect of pH on Swelling Ratio of PA Hydrogels
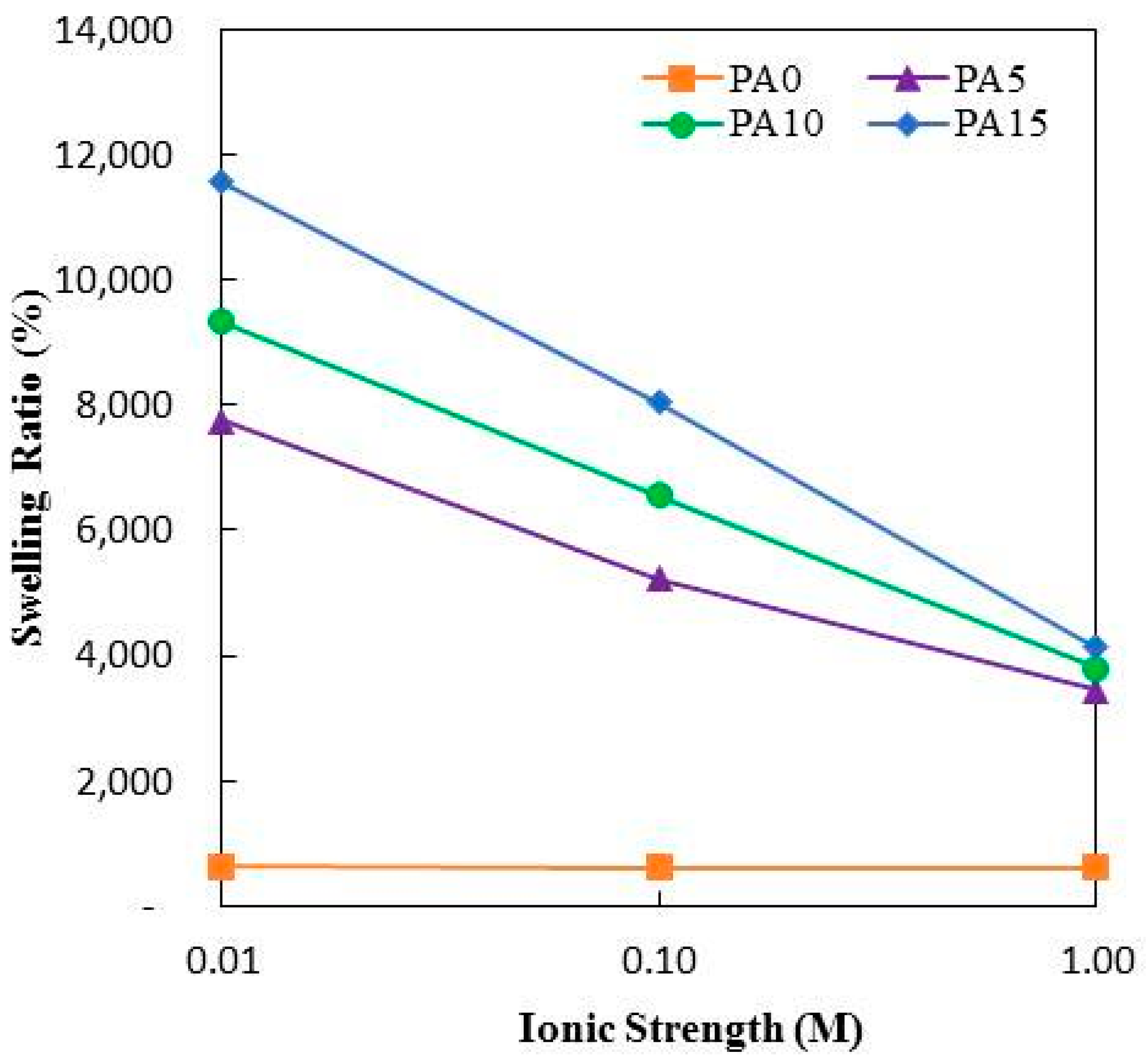
3.8. Effect of Ionic Strength on Swelling Ratio of PA Hydrogels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mahdavinia, G.R.; Pourjavadi, A.; Zohuriaan-Mehr, M. A convenient one-step preparation of chitosan-poly(sodium acrylate-co-acrylamide) hydrogel hybrids with super-swelling properties. J. Appl. Polym. Sci. 2006, 99, 1615–1619. [Google Scholar] [CrossRef]
- Garcia, D.M.; Escobar, J.L.; Noa, Y.; Bada, N.; Hernaez, E.; Katime, I. Timolol maleate release from pH-sensible poly (2- hydroxyethyl methacrylate-co-methacrylic acid) hydrogels. Eur. Polym. J. 2004, 40, 1683–1690. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Pereyra, J.; Martinez, M.; Barbero, C.; Bruno, M.; Acevedo, D. Hydrogel-Graphene Oxide Nanocomposites as Electrochemical Platform to Simultaneously Determine Dopamine in Presence of Ascorbic Acid Using an Unmodified Glassy Carbon Electrode. J. Compos. Sci. 2019, 3, 1. [Google Scholar] [CrossRef]
- Takafuji, M.; Alam, M.A.; Goto, H.; Ihara, H. Microspherical hydrogel particles based on silica nanoparticle webbed polymer networks. J. Colloid Interface Sci. 2015, 455, 32–38. [Google Scholar] [CrossRef]
- Sadeghi, M.; Hosseinaden, H. Synthesis and superswelling behavior of carboxymethylcellulose-poly (sodium acrylate-co-acrylamide) hydrogel. J. Appl. Polym. Sci. 2008, 108, 1142–1151. [Google Scholar] [CrossRef]
- Alam, M.A.; Takafuji, M.; Ihara, H. Silica nanoparticle-crosslinked thermosensitive hybrid hydrogels as potential drug-release carriers. Polym. J. 2014, 46, 293–300. [Google Scholar] [CrossRef]
- Jovasevic, J.; Dimitrisevic, S.; Filipovic, J.; Tomic, S.; Micic, M. Swelling, Mechanical and Antimicrobial Studies of Ag/P(HEMA/IA)/PVP Semi-IPN Hydrogel Hybrids. Acta Phys. Pol. A 2011, 120, 279–283. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ohashi, H.; Maekawa, Y.; Katakai, R.; Yoshida, M. Thermo- and pH-sensitive gel membranes based on poly-(acryloyl-L-proline methyl ester)- graft-poly(acrylic acid) for selective permeation of metal ions. Radiat. Phys. 2005, 72, 595–600. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef]
- Khare, A.R.; Peppas, N.A. Swelling/deswelling of anionic copolymer gels. Biomaterials 1995, 16, 559–567. [Google Scholar] [CrossRef]
- Rizwan, M.; Yahya, R.; Aziz Hassan, A.; Yar, M.; Azzahari, A.D.; Vidhya Selvanathan, V.; Faridah Sonsudin, F.; Abouloula, C.N. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef]
- Donini, C.; Robinson, D.N.; Colombo, P.; Giordano, F.; Peppas, N.A. Preparation of poly (methacrylic acid-g poly(ethylene glycol)) nanospheres from methacrylic monomers for pharmaceutical applications. Int. J. Pharm. 2002, 245, 83–91. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Murthy, P.S.K.; Raju, K.M. Synthesis, characterization and effect of reaction parameters on swelling properties of acrylamide–sodium methacrylate superabsorbent copolymers. React. Funct. Polym. 2005, 63, 11–26. [Google Scholar] [CrossRef]
- Thakur, A.; Wanchoo, R.K.; Singh, P. Structural parameters and swelling behavior of ph sensitive poly (acrylamide-coacrylicacid) hydrogels. Chem. Biochem. Eng. Q. 2011, 25, 181–194. [Google Scholar]
- Gundogan, N.; Okay, O.; Oppermann, W. Swelling, Elasticity and Spatial Inhomogeneity of Poly(N,N-dimethylacrylamide) Hydrogels Formed at Various Polymer Concentrations. Macromol. Chem. Phys. 2004, 205, 814–823. [Google Scholar] [CrossRef]
- Jipa, I.M.; Stroescu, M.; Stoica-Guzun, A.; Dobre, T.; Jinga, S.; Zaharescu, T. Effect of gamma irradiation on biopolymer composite films of poly(vinyl alcohol) and bacterial cellulose. Nucl. Instrum. Methods Phys. Res. Sect. B 2012, 278, 82–87. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Micic, M. Smart poly (oligo(propylene glycol) methacrylate) hydrogel prepared by gamma radiation. Nucl. Instrum. Methods. Phys. Res. Sect. B 2015, 342, 206–214. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulanski, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Clough, R.L. High-energy radiation and polymers: A review of commercial processes and emerging applications. Nucl. Instrum. Methods Phys. Res. Sect. B 2001, 185, 8–33. [Google Scholar] [CrossRef]
- Rahman, M.A.; Khan, M.A.; Tareq, S.M. Preparation and characterization of polyethylene oxide (PEO)/gelatin blend for biomedical application: Effect of gamma radiation. J. Appl. Polym. Sci. 2010, 117, 2075–2082. [Google Scholar] [CrossRef]
- Afroz, S.; Afrose, F.; Alam, A.; Khan, R.A.; Alam, M.A. Synthesis and characterization of polyethylene oxide (PEO)—N,N-dimethylacrylamide (DMA) hydrogel by gamma radiation. Adv. Compos. Hybrid. Mater. 2019, 2, 133–141. [Google Scholar] [CrossRef]
- Ray, D.; Sahoo, P.K.; Mohanta, G.P. Designing of superporous crosslinked hydrogels containing acrylic- based polymer network. Asian J. Pharma. 2008, 123, 123–127. [Google Scholar] [CrossRef]
- Chauhan, S. Acrylic acid and methacrylic acid based hydrogels-A review. Pelagia Res. Libr. Der Chem. Sin. 2015, 6, 61–72. [Google Scholar]
- Khoylou, F.; Naimian, F. Radiation synthesis of superabsorbent polyethylene oxide/tragacanth hydrogel. Radiat. Phys. Chem. 2009, 78, 195–198. [Google Scholar] [CrossRef]
- Kowalski, G.; Kijowska, K.; Witczak, M.; Kuterasiński, Ł.; Łukasiewicz, M. Synthesis and Effect of Structure on Swelling Properties of Hydrogels Based on High Methylated Pectin and Acrylic Polymers. Polymers 2019, 11, 114. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Nanohydrogel Formation within the Halloysite Lumen for Triggered and Sustained Release. ACS Appl. Mater. Interfaces 2018, 10, 8265–8273. [Google Scholar] [CrossRef] [PubMed]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Fakhrullin, R.; Lazzara, G. Core/Shell Gel Beads with Embedded Halloysite Nanotubes for Controlled Drug Release. Coatings 2019, 9, 70. [Google Scholar] [CrossRef]
- Nho, Y.C.; Lim, Y.M.; Lee, Y.M. Preparation, properties and biological application of pH-sensitive poly(ethylene oxide) (PEO) hydrogels grafted with acrylic acid(AAc) using gamma-ray irradiation. Radiat. Phys. Chem 2004, 71, 239–242. [Google Scholar] [CrossRef]
- Hassan, M.R.; Chowdhury, A.B.M.A.; Islam, M.T. γ-Irradiated Polyvinyl Alcohol (PVA) and Acrylic Acid Blend Hydrogels: Swelling and Absorption properties. Chem. Technol. Ind. J. 2016, 11, 107–117. [Google Scholar]
- Dafader, N.C.; Akhter, T.; Haque, M.E.; Swapna, S.P.; Islam, S.; Huq, D. Effect of acrylic acid on the properties of polyvinylpyrrolidone hydrogel prepared by the application of gamma radiation. Afr. J. Biotechnol. 2012, 11, 13049–13057. [Google Scholar] [CrossRef]
- Lim, Y.M.; Lee, Y.M. Preparation and Characterization of pH-Sensitive Poly (ethylene oxide) Grafted Methacrylic Acid and Acrylic Acid Hydrogels by γ-ray Irradiation. Macromol. Res. 2005, 13, 327–333. [Google Scholar] [CrossRef]
- Bhuiyan, M.A.Q.; Rahman, M.S.; Rahaman, M.S.; Shajahan, M.; Dafader, N.C. Improvement of swelling behaviour of poly (vinyl pyrrolidone) and acrylic acid blend hydrogel prepared by the application of gamma radiation. Org. Chem. Curr. Res. 2015, 4, 100–138. [Google Scholar]
- Ranjha, N.M.; Qureshi, U.F. Preparation and characterization of crosslinked acrylic acid/hydroxypropyl methyl cellulose hydrogels for drug delivery. Int. J. Pharm. Pharm. Sci. 2014, 6, 400–410. [Google Scholar]
- Ranjha, N.M.; Doelker, E. pH sensitive non crosslinked Poly (alcohol-co-acrylic acid) hydrogels for site specific drug deliver. Saudi Pharm. J. 1999, 7, 137–143. [Google Scholar]
- Yang, S.; Zhang, Y.; Wang, L. Composite thin film by hydrogen bonding assembly of polymer brush and poly(vinylpyrrolidone). Langmuir 2006, 22, 338–343. [Google Scholar] [CrossRef]
- Wang, L.; Dong, W.; Xu, Y. Synthesis and characterization of hydroxypropyl methylcellulose and ethyl acrylate graft copolymers. Carbohydr. Polym. 2007, 68, 626–636. [Google Scholar] [CrossRef]
- Nita, L.E.; Chiriac, A.P.; Nistor, M.T.; Neamtu, I. Hydrogel based on poly(n, n-dimethylacrylamide-co-3, 9-divinyl-2,4,8,10-tetraoxaspiro (5.5) undecane with dual sensitive behavior:synthesis and Characterisation. Rev. Roum. Chim. 2013, 58, 137–143. [Google Scholar]
- Tomar, R.S.; Gupta, I.; Singhal, R.; Nagpal, A.K. Synthesis of poly(acrylamide-co-acrylic acid)-based super-absorbent hydrogels by gamma radiation: Study of swelling behaviour and network parameters, Designed Monomers and Polymers. Des. Monomers Polym. 2007, 10, 49–66. [Google Scholar] [CrossRef]
- Ahmed, Z.; Stepto, R.F.T. Effect of monomer functionality and concentration on gelation in non-linear random polymerization. Polym. J. 1982, 14, 767–772. [Google Scholar] [CrossRef]
- Ranjha, N.M.; Mudassir, J.; Sheikh, Z.Z. Synthesis and Characterization of pH-Sensitive Pectin/Acrylic Acid Hydrogels for Verapamil Release Study. Iranian Polym. J. 2011, 20, 147–159. [Google Scholar]
- Wu, F.; Zhang, Y.; Liu, L.; Yao, J. Synthesis and characterization of a novel cellulose-g-poly(acrylic acid-co-acrylamide) superabsorbent composite based on flax yarn waste. Carbohydr. Polym. 2012, 87, 2519–2525. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Salimi, H.; Kurdtabar, M. Hydrolyzed collagen-based hydrogel with salt and pH-responsiveness properties. J. Appl. Polym. Sci. 2007, 106, 2371–2379. [Google Scholar] [CrossRef]
- Drozdov, A.D.; de Claville Christiansen, J. Modeling the effects of pH and ionic strength on swelling of polyelectrolyte gels. J. Chem. Phys. 2015, 142, 114904–114921. [Google Scholar] [CrossRef] [PubMed]




| Sample | PEO (%) | AAc (%) | Dose (kGy) |
|---|---|---|---|
| PA0 | 5 | 0 | 30 |
| PA5 | 5 | 5 | 30 |
| PA10 | 5 | 10 | 30 |
| PA15 | 5 | 15 | 30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monir, T.S.B.; Afroz, S.; Khan, R.A.; Miah, M.Y.; Takafuji, M.; Alam, M.A. pH-Sensitive Hydrogel from Polyethylene Oxide and Acrylic acid by Gamma Radiation. J. Compos. Sci. 2019, 3, 58. https://doi.org/10.3390/jcs3020058
Monir TSB, Afroz S, Khan RA, Miah MY, Takafuji M, Alam MA. pH-Sensitive Hydrogel from Polyethylene Oxide and Acrylic acid by Gamma Radiation. Journal of Composites Science. 2019; 3(2):58. https://doi.org/10.3390/jcs3020058
Chicago/Turabian StyleMonir, Tania Sabnam Binta, Sadia Afroz, Ruhul A. Khan, Muhammed Yusuf Miah, Makoto Takafuji, and Md. Ashraful Alam. 2019. "pH-Sensitive Hydrogel from Polyethylene Oxide and Acrylic acid by Gamma Radiation" Journal of Composites Science 3, no. 2: 58. https://doi.org/10.3390/jcs3020058
APA StyleMonir, T. S. B., Afroz, S., Khan, R. A., Miah, M. Y., Takafuji, M., & Alam, M. A. (2019). pH-Sensitive Hydrogel from Polyethylene Oxide and Acrylic acid by Gamma Radiation. Journal of Composites Science, 3(2), 58. https://doi.org/10.3390/jcs3020058